Significance
The ability to convert cells into desired cell types enables tissue engineering, disease modeling, and regenerative medicine; however, methods to generate desired cell types remain difficult, uncertain, and laborious. We developed a strategy to screen gene regulatory elements on a genome scale to discover paths that trigger cell fate changes. The proteins used in this study cooperatively bind DNA and activate genes in a synergistic manner. Subsequent identification of transcriptional networks does not depend on prior knowledge of specific regulators important in the biological system being tested. This powerful forward genetic approach enables direct cell state conversions as well as other challenging manipulations of cell fate.
Keywords: artificial transcription factor, genome-scale library, cell fate, reprogramming, gene regulatory networks
Abstract
Artificial transcription factors (ATFs) are precision-tailored molecules designed to bind DNA and regulate transcription in a preprogrammed manner. Libraries of ATFs enable the high-throughput screening of gene networks that trigger cell fate decisions or phenotypic changes. We developed a genome-scale library of ATFs that display an engineered interaction domain (ID) to enable cooperative assembly and synergistic gene expression at targeted sites. We used this ATF library to screen for key regulators of the pluripotency network and discovered three combinations of ATFs capable of inducing pluripotency without exogenous expression of Oct4 (POU domain, class 5, TF 1). Cognate site identification, global transcriptional profiling, and identification of ATF binding sites reveal that the ATFs do not directly target Oct4; instead, they target distinct nodes that converge to stimulate the endogenous pluripotency network. This forward genetic approach enables cell type conversions without a priori knowledge of potential key regulators and reveals unanticipated gene network dynamics that drive cell fate choices.
Expression of certain transcription factors (TFs) can profoundly alter gene regulatory dynamics of a cell to the extent that the cell may transition to a completely different state. For example, the TFs Oct4 (POU domain, class 5, TF 1), Sox2 [SRY (sex-determining region Y)-box 2], Klf4 (Kruppel-like factor 4), and c-Myc (myelocytomatosis oncogene), have been widely used to reprogram somatic cells to an induced pluripotent stem (iPS) cell state (1–3). Similarly, other TF combinations can reprogram somatic cells to adopt specific cell states, such as myocytes, cardiomyocytes, neurons, and hepatocytes (4–7). However, state-of-the-art methods to find regulators of cell fate conversions rely on trial and error and empirical exploration of a small subset of combinations of different transcriptional regulators (8). Such efforts are highly constrained by the number of combinations that can be tested and are labor intensive and cost prohibitive. Conventional approaches often rely on the assumption that the factors that maintain a particular cell state are the same factors that reprogram gene networks to drive cell fate conversion, an assumption that may not necessarily be correct, especially when the intended conversion does not occur naturally during development. Moreover, TFs function in a specific cellular milieu and trigger appropriate gene expression in response to specific cues that might not occur in the cellular systems where they are being tested. The epigenetic landscape and heterochromatic regions of the cell may also present barriers to accessibility to key regulatory regions (9). To overcome such barriers to cell fate conversions, we developed a library of artificial transcription factors (ATFs) that stimulate transcriptional circuits independently of the original cell state.
ATFs are DNA-binding molecules designed to control gene expression in a predetermined manner (10). Rather than taking the conventional approach of testing candidate factors curated from studying embryonic development or differential expression analysis, unbiased screening of a genome-scale ATF library can be a highly effective and orthogonal approach to sample thousands of sites in parallel and activate cell fate-defining transcriptional networks. Use of a library also yields ATFs that can access genomic loci without having to first identify accessible regions upstream of desired target genes. Because ATFs do not rely on endogenously expressed cofactors and are not restrained by feedback circuits that limit the function of ectopically expressed natural factors, they can serve as powerful agents to perturb the homeostatic state of any cell type. The target genes of specific ATFs that evoke changes in cell states can enable the unbiased identification of gene regulatory networks that govern cell fate conversion.
TFs are modular by nature, and each domain can be tailored to create ATFs that target and regulate genes and networks in a preprogrammed manner (11–14). The DNA-binding domain (DBD) confers sequence specificity in targeting genomic loci. The effector domain provides the ATF with function, be it transcriptional activation, repression, or modification of chromatin. Importantly, an interaction domain (ID) can be incorporated in the design such that the ATF can interact with other factors in the cell (10). Principles of cooperative assembly and synergistic activation were integrated in the design of our genome-scale ATF library (15, 16).
We used the following three criteria to choose among an array of DBD scaffolds: (i) ability to target multiple nodes of a gene network, (ii) regulatory potency of resulting ATFs, and (iii) efficiency of delivery into cells. Applying these criteria to the repurposed nuclease-inactivated CRISPR/Cas9 system, TAL-effectors, programmable small-molecule polyamides, and zinc fingers, we concluded that the zinc finger scaffold, engineered to enable cooperative and combinatorial assembly on DNA, would be the most effective DBD scaffold for the creation of an ATF library designed to trigger cell fate conversions (see Materials and Methods for details on choice of DBD).
To demonstrate unbiased ability to change cell identity, we used our ATF library to screen for factors that induced pluripotency in mouse embryonic fibroblasts (MEFs) without exogenous delivery of Oct4. We created a library of 2.62 × 106 ATFs that encompass five times the number of factors as the entire sequence space of all 10-bp binding sites on double-stranded DNA (524,809 unique sequences). RNA-sequencing (RNA-seq) data, epigenetic landscapes, and comprehensive ATF binding profiles by cognate site identification (CSI) sequencing (CSI-seq) and ChIP-sequencing (ChIP-seq) were analyzed to determine the key nodes through which ATFs activate the pluripotency network. Surprisingly, bioinformatic analysis reveals that the ATFs take fibroblasts through a different path to pluripotency than exogenously expressed Oct4. We demonstrate that this forward genetic approach enables the pursuit of elusive cell fate conversions in an unbiased manner.
Results
ATF Architecture.
To determine the best architecture for a zinc finger ATF library, we tested the impact of each modular domain on the level of induction. The zinc finger backbone is derived from human EGR1/ZIF268 (early growth response 1), a well-studied scaffold for zinc finger ATFs (17–19). We fused VP64, a tetrameric repeat of the 11-aa activation region of VP16, a potent transactivation domain from the herpes simplex virus to the C terminus to the zinc fingers (Fig. 1A) (20). Although a variety of zinc finger-based libraries have been described before (21–27), a distinguishing and important feature of our ATF design is inclusion of a 15-aa peptide that serves as an ID, allowing dimerization of the ATF with another ATF through the hydrophobic surface of the first zinc finger of EGR1 (28). The inclusion of the ID in our ATF design adds a layer of control to the ATF library by allowing the ATFs to harness cooperative binding and synergistic activation (16).
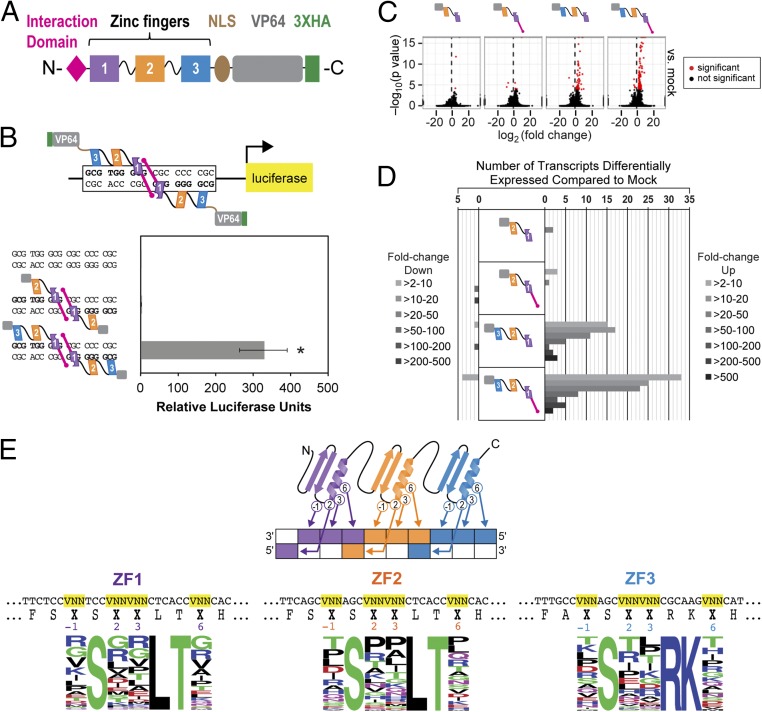
Fig. 1.
ATF designed with three zinc fingers, activation domain, and interaction domain (ID) to maximize transcriptional effect. (A) Architecture of the ATF. From N to C terminus, the ATF consists of an ID, three zinc fingers, a nuclear localization signal (NLS) from EGR1, and a VP64 activation domain. (B) The three-zinc finger ATF induces expression 329-fold over the mock control, whereas the two-zinc finger ATF induces expression twofold in a luciferase assay performed in HEK293 cells. The DBD comes from either the first two or all three zinc fingers of EGR1. Each ATF is also comprised of an ID, NLS, and VP64 (n = 4, P < 0.01 by one-way ANOVA with post hoc Tukey test). (C) RNA-seq results in HEK293 cells show that the three-zinc finger ATF with an ID up-regulates expression of the greatest number of genes compared with the mock-transfected control. All ATFs in this assay have an NLS and VP64 (n = 1; P < 0.0005). (D) Among the up-regulated genes, 18 genes are expressed 50-fold or more by the three-zinc finger ATF with an ID (n = 1; P < 0.0005). (E) Design of the ATF library. The residues that confer specificity (−1, 2, 3, and 6 positions) were randomized to amino acids represented by VNN codons, where V is A, C, or G. A library with a complexity of 2.6 × 106 ATFs was created. Sequencing of 100 ATFs from the library shows representation of all 16 amino acids. X in the amino acid sequence represents any residue.
Because we wanted to create an ATF library of high complexity while minimizing nonessential modules, it was necessary to determine the minimum number of zinc fingers required to have a transcriptional effect. Toward this end, we compared a two-zinc finger ATF with a three-zinc finger ATF in a luciferase assay with a palindromic cognate site for EGR1 upstream of the luciferase reporter. The two-zinc finger ATF could only activate the luciferase reporter 2-fold over background, whereas the three-zinc finger ATF was capable of activating 329-fold over background (Fig. 1B). Incorporation of the ID further increased activation of the three-zinc finger ATF almost by an order of magnitude (SI Appendix, Fig. S3A).
To determine how the ATFs impact genome-wide transcription, we performed RNA-seq in human cells expressing one of the four ATFs with different architectures. The ATFs either had the first two or all three zinc fingers of EGR1 as the DBD, with and without the ID. Compared with the mock-transfected control, the two-zinc finger ATFs had little impact on altering the transcriptional profile (Fig. 1 C and D, and SI Appendix, Fig. S3 B and C). On the other hand, the three-zinc finger ATF with the ID altered the expression of 104 transcripts (100 up-regulated, 4 down-regulated) (Fig. 1D and SI Appendix, Fig. S3C). Most of the genes were up-regulated compared with the mock-transfected control, and the repressed genes could be attributed to indirect effects of the ATFs. As the three-zinc finger ATF with the ID was capable of binding as a monomer or dimer, most of the subset of genes up-regulated by the three-zinc finger ATF without the ID could also be activated by the three-zinc finger ATF with the ID, and the ID increased the level of induction for a subset of genes (SI Appendix, Fig. S3B).
Genome-Scale ATF Library Design.
Taking all these results into consideration, a scaffold that includes from N to C terminus: an ID, three zinc fingers derived from EGR1, NLS from EGR1, VP64 activation domain, and a 3× hemagglutinin (HA) tag was used to construct the ATF scaffold. The library of different DNA sequence-targeting ATFs was created by incorporating VNN codons, where V is A, C, or G, at the DNA recognition residues (−1, 2, 3, and 6). Use of VNN codons prevents incorporation of premature stop codons within the ORF and permits the incorporation of 16 different amino acids (Fig. 1E). The library was cloned into a second-generation lentiviral system to ensure efficient delivery to mammalian cells. The ATF is driven by the constitutively active EF-1α promoter, which resists silencing in mammalian cells compared with other constitutive promoters (Fig. 2A) (29). The sequence space for all 10-bp sequences on double-stranded DNA is 524,809 different sequence permutations. We created an ATF library with a complexity of 2.62 × 106, five times the targeted sequence space. Sanger sequencing of 100 clones confirmed success of our design with incorporation of all 16 aa at each recognition residue, suggesting diverse representation in the library (Fig. 1E and SI Appendix, Table S7).
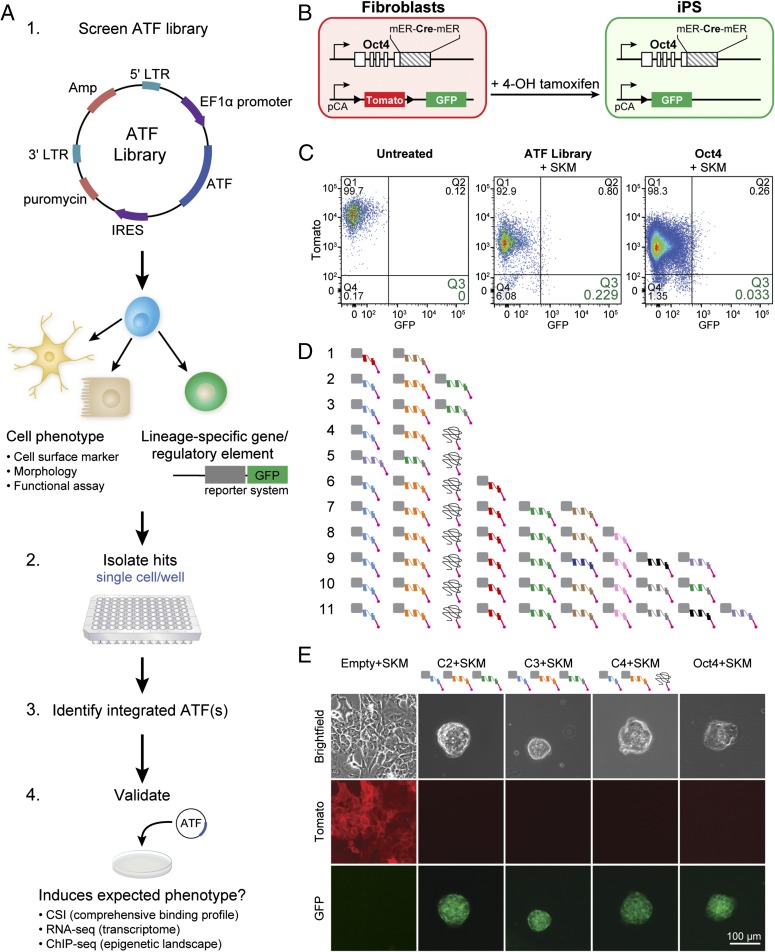
Fig. 2.
ATF library activates pluripotency network. (A) Genetic screen with an ATF library. (1) The ATF library was cloned into a second-generation lentiviral vector. The screen is performed in cells with a robust change in phenotype or a lineage-specific reporter. (2) Positive outcomes are isolated as single cells, such that combinations of ATFs, if any, can be captured (3). Integrated ATFs are identified from single cells (4). Identified ATFs are retested for validation. Once validated, downstream experiments can be performed to identify ATF target genes. (B) Testing the ATF library in mouse embryonic fibroblasts (MEFs) isolated from a transgenic mouse line that allows lineage tracing of endogenous Oct4 transcription. Upon induction of endogenous Oct4, tamoxifen-inducible Cre recombinase (mER-Cre-mER) is coexpressed. The recombinase removes Tomato from the ROSA locus, and transmembrane-bound GFP is expressed. Consequently, Tomato+GFP– MEFs become Tomato–GFP+, and GFP expression is maintained in all their cell progeny. (C) Flow cytometry results at day 15 after introduction of TFs. Tomato–GFP+ MEFs transduced with the ATF library+SKM were isolated as single cells for further analysis. MEFs treated with Oct4+SKM (positive control) and untreated MEFs (negative control) were used for comparison. Double-positive (Tomato+GFP+; Q2) cells were also collected. Percentages are displayed under the quadrant number. (D) ATFs were identified from 11 single cells by two-step nested PCR of genomic DNA. Unique ATFs are depicted with a different color. One ATF had a frameshift mutation shortly after the ID, coding for a protein that does not have a zinc finger structure. A few ATFs, notably the light blue and orange ATFs, are expressed in most of the cells analyzed. Three ATFs are made up of two fingers (light blue, red, and pink). All cells except number 4 were collected as Tomato–GFP+ cells. Cell 4 was Tomato+GFP+ at day 15. (E) Three combinations of ATFs (C2, C3, and C4) successfully induced pluripotency with SKM. Micrographs of MEFs transduced with an Empty+SKM are Tomato+GFP–. iPS cells generated with ATFs+SKM are similar to those generated with Oct4+SKM and are Tomato–GFP+. Two ATFs in each combination are the same (light blue and orange). (Scale bar, 100 μm.)
ATF Library Activates the Pluripotency Network.
We asked whether ATFs in the library could replace the key regulator of pluripotency, Oct4, in the mixture of TFs that triggers the pluripotency network, Oct4, Sox2, Klf4, and c-Myc (Oct4+SKM). To test a library, capable of sampling thousands of sites in the genome, it was necessary to have a robust readout of positive phenotypes (Fig. 2A). Toward this end, we used MEFs isolated from a transgenic mouse line that allows lineage tracing of endogenous Oct4 transcription (Fig. 2B) (30). In these cells, tamoxifen-inducible Cre recombinase (mER-Cre-mER) is expressed when the endogenous pluripotency associated gene, Oct4, is transcribed. In the presence of 4-hydroxytamoxifen, the recombinase removes Tomato from the ROSA locus, and transmembrane-bound GFP is expressed. Consequently, Tomato+GFP– MEFs become Tomato–GFP+ cells after endogenous Oct4 is activated, and GFP expression is maintained in all their cell progeny (Fig. 2B).
The ATF library was transduced in MEFs (multiplicity of infection of 3) with Sox2, Klf4, and c-Myc (SKM). As a positive control, we delivered Oct4+SKM to MEFs (SI Appendix, Fig. S4A). To account for reprogramming events induced by SKM, alone, we delivered lentivirus lacking an ORF in place of the ATF (Empty+SKM). The lentivirus with the “empty” ORF accounted for any false-positive events that could arise from lentiviral delivery or integration of a strong constitutive promoter at relevant genomic sites. We also included a control with the ATF library alone, as well as untreated MEFs. Although the widely used pluripotency-inducing combination of Oct4+SKM resulted in Tomato–GFP+ expression in 0.033% cells, the ATF library+SKM induced reporter activation in 0.229% of the cells (Fig. 2C). No Tomato–GFP+ cells were observed for untreated MEFs and cells treated with ATF library alone or Empty+SKM. Although the percentage of cells with Oct4 activated in ATF-treated cells was higher than in Oct4+SKM cells, this higher percentage of Tomato–GFP+ cells can be attributed to more cell death in the ATF-treated cells. Tomato–GFP+ cells bearing members of the ATF library+SKM were isolated as single cells for further analysis. Of the ATF library+SKM cells, a small fraction (0.8%) of cells were Tomato+GFP+. Because the half-life of Tomato fluorescent protein is ∼24 h, there is a period after Oct4 is activated when the cells are double positive. We sorted these cells separately to determine whether the ATFs expressed in the double-positive cells were different from those expressed in the Tomato–GFP+ cells.
Single-Cell Retrieval of Active ATF Combinations.
Because different combinations of ATFs can potentially act in concert to activate the pluripotency network, we identified the ATFs from individual single cells to capture ATF combinations that activate endogenous Oct4 transcription and induce GFP expression. Preliminary evaluation of iPS colonies derived from the screen with mixed combinations of ATFs showed high levels of expression for endogenous pluripotency genes (SI Appendix, Fig. S4C). Eleven different cells that were GFP+ were isolated and subjected to two-step nested PCR of genomic DNA. Sequencing of the PCR products revealed 11 unique combinations of ATFs (Fig. 2D). The range of ATFs varied between 2 and 10 ATFs within a single cell. ZFATF1 (light blue) and ZFATF2 (orange) appeared in most of the combinations; however, an additional ATF was necessary for reproducible conversion to a bona fide iPS cell state (SI Appendix, Table S8). One ATF from cells 4–11 had a frameshift mutation near the N terminus, resulting in a 163-aa protein product that does not code for a zinc finger protein (ZFATF5). Only the ID as well as the first 19 aa of the first zinc finger remained intact (SI Appendix, Fig. S4B).
All ATF combinations identified in the screen for endogenous Oct4 expression were revalidated to determine whether they were true positives. Among the 11 ATF combinations, C2, C3, and C4 reproducibly generate colonies of iPS cells when expressed with Sox2, Klf4, and c-Myc (Fig. 2E and SI Appendix, Fig. S4E and Table S8). Interestingly, C4 was identified from the Tomato+GFP+ cells, in which Oct4 was activated before the Tomato signal dissipated. During the validation step, MEFs expressing C4+SKM became iPS cells ∼28 d later than the iPS cells generated by the other ATF combinations or Oct4+SKM. The doubling times for the iPS cells generated with C2+SKM or C3+SKM were comparable to that of iPS cells generated with Oct4+SKM; however, the doubling time for iPS cells expressing C4+SKM was slightly longer (SI Appendix, Fig. S4D). ATF-induced iPS cells demonstrated capacity for self-renewal and have been cultured beyond 65 passages.
Cells Generated with Different ATF Combinations Are Pluripotent.
ATF-induced Oct4+ cells were further characterized for markers of pluripotency. Immunofluorescence was performed to confirm expression of pluripotency markers, OCT4, SOX2, and NANOG (Fig. 3A and SI Appendix, Fig. S5A). The capacity for ATF-derived iPS cells to differentiate into all three germ layers was assessed by formation of teratomas (Fig. 3B) and embryoid bodies (EBs) (SI Appendix, Fig. S5 B–E). Immunocytochemistry of myosin light polypeptide 2 (mesoderm), forkhead box A2 (endoderm), and βIII tubulin (ectoderm) confirmed expression of germ layer markers at the protein level, and RT-qPCR of T, Nkx2.5, and Kdr (mesoderm); Ttr, Afp, and FoxA2 (endoderm); Nes, Nefl, and Sox17 (ectoderm) confirmed differentiation into all three germ layers at the transcriptional level. Beating cardiomyocytes were also observed from embryoid outgrowths from EBs derived from ATF-induced iPS cells (Movie S1).
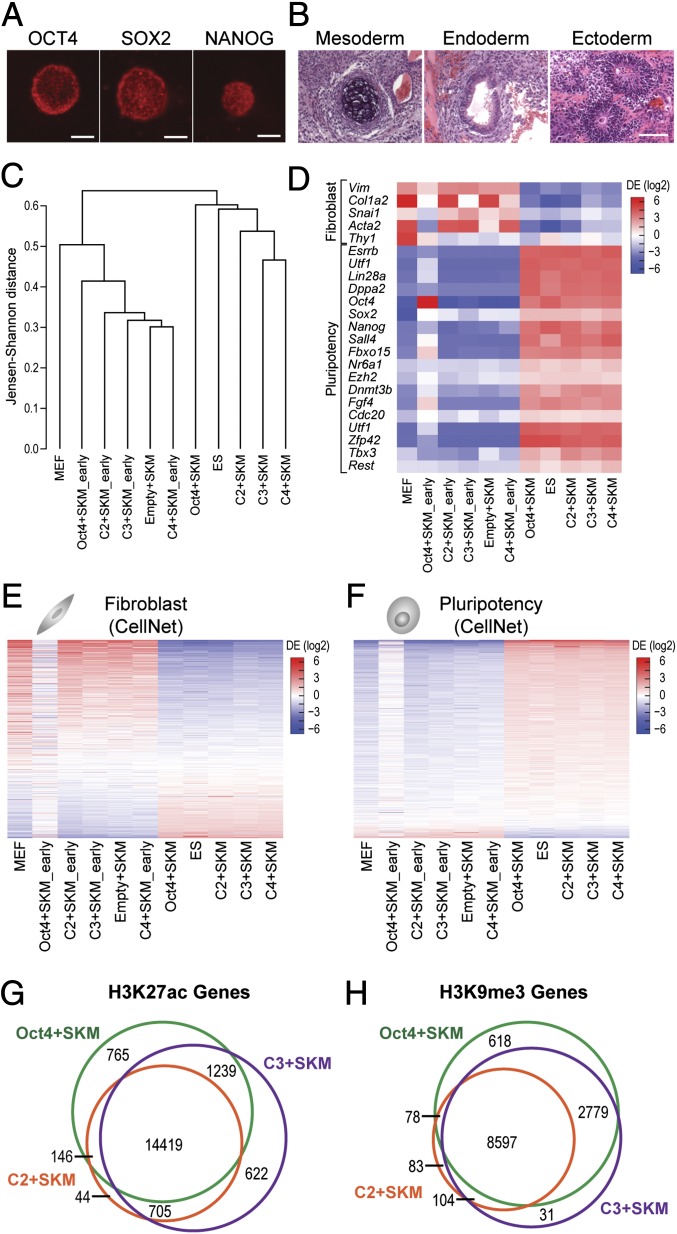
Fig. 3.
iPS cells generated with ATFs are pluripotent. (A) Immunofluorescence staining of C2+SKM iPS colonies with OCT4, SOX2, and NANOG. (Scale bars, 100 μm.) (B) Teratoma assay results show differentiation into mesoderm, endoderm, and ectoderm. (Scale bar, 100 μm.) (C) iPS cells generated with ATFs cluster with mouse ES cells and iPS cells generated with Oct4+SKM. Samples marked early are MEFs transduced with the indicated factors before conversion into iPS cells between days 18 and 27 (n = 3 or 4). (D) A heat map of fibroblast and pluripotency markers of iPS cells generated with ATFs shows down-regulation of fibroblast genes and up-regulation of pluripotency genes. Scale displays differential expression log2(ratio relative to mean). (E) A heat map of 853 genes from the CellNet fibroblast gene regulatory network (GRN) show iPS cells generated with ATFs obtain transcriptional profiles similar to that of other pluripotent cells (Oct4+SKM iPS cells and ES cells). Scale displays differential expression log2(ratio relative to mean). (F) A heat map of 705 genes from the CellNet pluripotency GRN show iPS cells generated with ATFs have expression profiles similar to that of other pluripotent cells. Scale displays differential expression log2(ratio relative to mean). (G) H3K27ac marks, specifying active regions of chromatin, appear in a common set of genes for Oct4+SKM iPS cells, C2+SKM iPS cells, and C3+SKM iPS cells. H3K27ac peaks were annotated to genes with Homer to create Venn diagrams for genes. ChIP enrichment among treatments was determined to be significant at an FDR < 0.1 by DiffBind (n = 2). (H) H3K9me3 marks, specifying repressed regions of chromatin, appear in a common set of genes for Oct4+SKM iPS cells, C2+SKM iPS cells, and C3+SKM iPS cells. H3K9me3 peaks were annotated to genes with Homer to create Venn diagrams for genes. ChIP enrichment among treatments was determined to be significant at an FDR < 0.1 by DiffBind (n = 2).
From morphological and select gene marker analysis, we expanded our validation to global transcriptome analysis of the ATF-treated iPS cells. Comparison of genome-wide transcriptional profiles showed that the transcriptomes of ATF-induced iPS cells cluster tightly with mouse ES cells as well as iPS cells generated with Oct4+SKM (Fig. 3C and SI Appendix, Fig. S6A). RNA profiles of cells at early stages of reprogramming clustered with MEFs and the Empty+SKM control.
ATF-induced iPS cells show an up-regulation of pluripotency markers and a down-regulation of fibroblast markers (Fig. 3D). Using the 853 genes that make up the fibroblast gene regulatory network (GRN) and the 705 genes that make up the pluripotency GRN from CellNet (31), we compared the expression profiles of ATF-induced iPS cells to those of other pluripotent cells and MEFs (Fig. 3 E and F). Our genome-wide analysis indicates that the profiles of our ATF-induced iPS cells highly correlated with profiles of pluripotent cells generated using exogenous Oct4. It is important to note that, at early stages of reprogramming, ATF-treated cells have a remarkably different profile compared with Oct4+SKM-treated cells (Fig. 3F). These differences suggest other underlying regulators beyond what is characterized in the GRNs of CellNet guide cells to pluripotency. Once fully reprogrammed to the pluripotent state, global transcriptome profiles shows ATF-induced iPS cells share more similarity among themselves than with Oct4+SKM or ES cells (Fig. 3C and SI Appendix, Fig. S6 B and C). ATF expression at early stages of reprogramming was readily detectable by RNA-seq; however, once converted to iPS cells, the lentiviral elements controlling the expression of ATFs are silenced, a further confirmation that the cells were fully reprogrammed. Once reprogrammed, the converted cells maintain the iPS cell state even in the absence of regulators that triggered the initial regulatory nodes that led to cell fate conversion.
Signature Epigenetic Landscapes at ATF-Activated Pluripotency Genes.
The genome-wide chromatin modification landscapes in ATF-induced iPS cells were compared with iPS cells generated with Oct4+SKM. Specifically, ChIP-seq was performed on histone 3 lysine 27 acetylation (H3K27ac), the marker delineating active promoters and superenhancers that define cell identity (32, 33), and histone 3 lysine 9 trimethylation (H3K9me3), the marker that is strongly correlated to repressed regions of the genome that are bound by heterochromatin protein 1 (34). For the analysis of the epigenetic changes induced by the ATFs, ChIP-seq peaks were identified using MACS2 after alignment to the mouse genome with Bowtie2 (35, 36). Analysis of aggregate patterns of the active and repressive histone marks by deepTools package shows strong H3K27ac peaks upstream of the gene and in the gene body for expressed genes, whereas broad H3K9me3 peaks marked genes that were not expressed (SI Appendix, Fig. S7 C and D) (37).
The differentially marked histone modifications were determined by using DiffBind (38). Peaks with a false-discovery rate (FDR) < 0.1 were categorized into unique and overlapping sets for iPS cells generated with ATF combinations+SKM or Oct4+SKM. Remarkably, despite differences in transcriptome profiles during early stages (Fig. 3F), pluripotent cells shared similar sets of peaks for H3K27ac regardless of whether they were generated with ATFs or with natural factors (Fig. 3G). Likewise, repressive H3K9me3 peaks were similar for ATF-induced iPS cells and Oct4-induced iPS cells, although there was greater overlap for Oct4+SKM and C3+SKM (Fig. 3H). Taken together, the active H3K27ac marks and the repressive H3K9me3 marks confirm that the ATFs+SKM induced significant remodeling of the chromatin structure in MEFs to a state that distinguishes them as pluripotent cells.
DNA Sequence Specificity Landscapes of Pluripotency-Inducing ATFs.
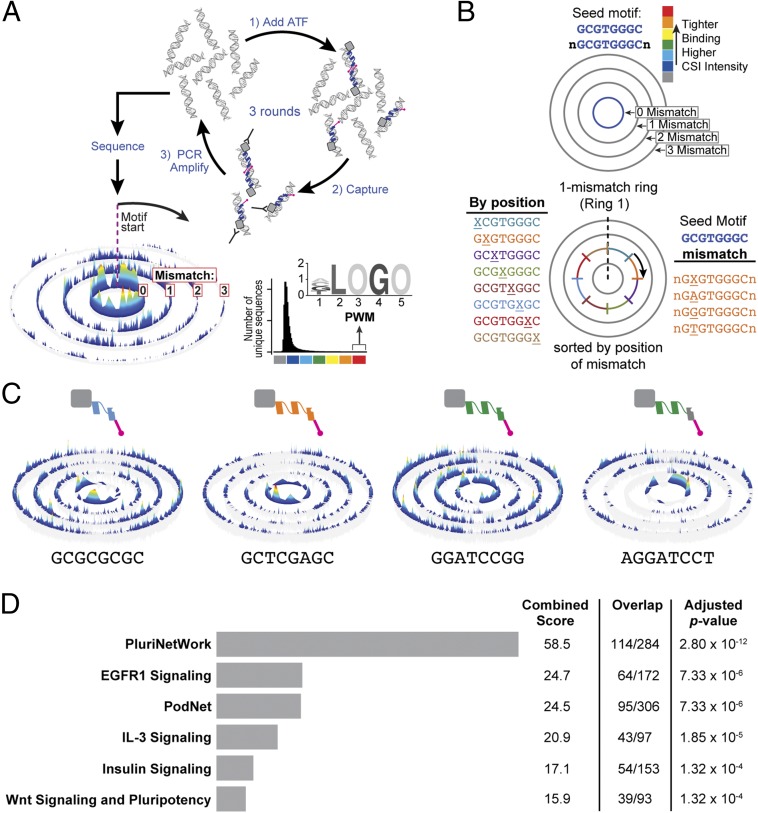
DNA targets of ATFs were examined by CSI, a method that captures the comprehensive binding profile of a DNA-binding factor (39–41). CSI enables the discovery of sequence specificity across all possible randomized 25-bp sequence permutations (Fig. 4A) (41). In brief, a DNA-binding protein is incubated with DNA sequences, protein–DNA complexes are isolated, and the bound DNA sequences are PCR amplified for the next round of selection. Two to three rounds of enrichment efficiently captures the broad spectrum of DNA sequence preferences of a given protein or ATF. The enriched cognate sites are identified via massively parallel high-throughput sequencing. The comprehensive binding site specificity of the factor is displayed as specificity and energy landscape (SEL) (Fig. 4 A and B) (40, 41).
Fig. 4.
ATFs target pluripotency genes. (A) Cognate site identification (CSI). This method of determining the sequence specificity of DNA-binding factors involves incubating the ATF with randomized permutations of 25-bp sequences. The ATF–DNA complexes are captured with an HA antibody, and bound DNA is PCR amplified for the next round of selection. Three rounds of selection are performed, and all three rounds are multiplexed and sequenced to obtain specificity and energy landscapes (SELs) and position weight matrices (PWMs). (B) SELs display the comprehensive binding preferences based on a chosen seed motif. The height of the peak is associated with affinity. Sequences that are 1–2 bp longer than the seed are arranged in concentric rings. Each ring outward from the 0-mismatch ring displays sequences to the corresponding number of mismatches. Within the mismatch rings, sequences are arranged by position of the mismatch, and then alphabetically. (C) SELs of four ATFs validated in this study. The first two ATFs (light blue and orange) appear in all three combinations. Each SEL displayed shows data after three rounds of enrichment. (D) Gene set enrichment analysis of genes with a CSI score of >20 within ±1 kb of the TSS shows overrepresentation of genes from PluriNetWork for Mus musculus. The top-100–scoring 10-bp motifs for each ATF from C2 was used for this analysis. Binding sites were annotated to genes with Homer. Genes with a sum CSI score of >20 (equivalent to six or more ATF binding sites) were analyzed with Enrichr (WikiPathways). The combined score from Enrichr is calculated by multiplying the log of the P value by the z score (deviation from the expected rank). The adjusted P value corresponds to the Benjamini–Hochberg corrected P value.
The top-five 10-bp binding sites for each ATF of C2 were used to identify target genes. Binding sites within ±1 kb of the transcriptional start site (TSS) were considered in the analysis. We chose this stringent window based on ATF design principles (42), the tendency of sequence-specific TFs to exhibit a peak −300 bp relative to the TSS (43), and evidence that the predictive power of TF binding on gene regulation drops significantly when the binding sites examined are beyond 2 kb from the TSS (44, 45). Gene set enrichment analysis of all target genes with Enrichr (46) showed an overrepresentation of genes found in PluriNetWork for Mus musculus (SI Appendix, Fig. S7E) (47). In addition to high-affinity sites, a wide range of binding sites with moderate to lower affinities are used to regulate the expression of different genes (48, 49). Therefore, rather than relying solely on single consensus motifs or position weighted matrices, the top-100–scoring 10-bp binding sites for each ATF were used to identify target genes (Fig. 4C and Dataset S1). Analysis with Enrichr for 2,897 genes with a sum CSI score of 20 or greater, in other words, six or more medium- to high-affinity ATF binding sites within ±1 kb of the TSS showed an overrepresentation of PluriNetWork genes (Fig. 4D) (47). This analysis was also performed for C3 and C4, and similar results were obtained, suggesting that the different ATF combinations activate similar nodes of the pluripotency network. To our surprise, Oct4 was not the primary target as it ranks 4,788 for the sum CSI score for C2, 3,929 for C3, and 5,225 for C4. Other regulators in the pluripotency circuitry with significantly higher sum CSI scores would serve as primary targets of the ATFs, and these genes, subsequently, trigger the activation of endogenous Oct4.
ATF-Triggered Networks.
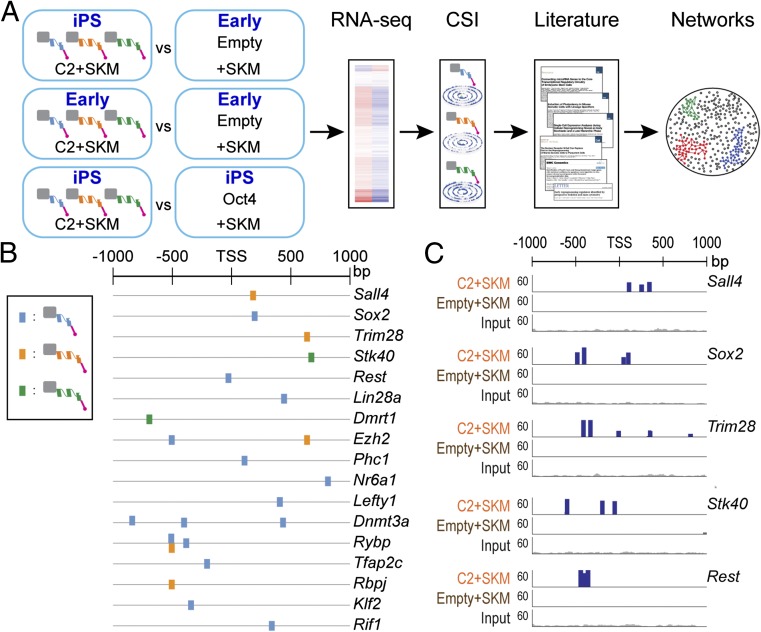
To map the ATF target genes in the pluripotency network, CSI data were integrated with RNA-seq data. Genes, expressed greater than twofold more in ATF-induced iPS cells compared with Empty+SKM cells, were filtered with those having ATF binding sites within ±1 kb of the TSS using the top-five–scoring motifs from CSI (Fig. 5A). Two other pairwise comparisons (C2/C3/C4+SKM at the early stage versus iPS stage and C2/C3/C4+SKM iPS cells versus Oct4+SKM iPS cells) were included for differential expression analysis. Of the genes up-regulated greater than twofold that also bear ATF binding sites, 17 were implicated in inducing pluripotency from previous studies (Fig. 5B and Dataset S2) (47, 50–57). To determine the direct ATF targets, we performed ChIP-seq on C2+SKM early cells before complete conversion to iPS cells and consequent silencing of the exogenously delivered ATFs. ChIP-seq of ATFs tagged with HA suggests binding of the ATFs to these targets (Fig. 5C and SI Appendix, Fig. S7A). Presence of H3K27ac peaks at the ATF target genes also indicates that they are actively expressed (SI Appendix, Fig. S7B).
Fig. 5.
ATFs activate key regulators of the pluripotency network. (A) Workflow for determining ATF target genes for C2+SKM. Three pairwise comparisons were made: (1) C2+SKM iPS cells vs. Empty+SKM cells, (2) C2+SKM early cells vs. Empty+SKM cells, (3) C2+SKM iPS cells vs. Oct4+SKM iPS cells. Genes up-regulated greater than twofold (P < 0.05) in the cells transduced with ATFs with ATF binding sites within a ±1-kb window of the TSS were determined to be potential targets. Binding sites were identified by using the top-five–scoring 10-bp motifs from CSI. These target genes were used to build the network in Fig. 6A with information from the literature and the STRING database. (B) Differentially expressed pluripotency genes with ATF binding sites within ±1 kb of the transcriptional start site (TSS). ATF binding sites were derived from the top-five–scoring 10-bp motifs from CSI for C2+SKM. (C) ChIP-seq signal for HA tag on ATFs for five predicted targets in Fig. 5B. Additional ChIP-seq traces are in SI Appendix, Fig. S5A. Traces display total reads for C2+SKM and Empty+SKM cells at an intermediate stage before reprogramming to a pluripotent state.
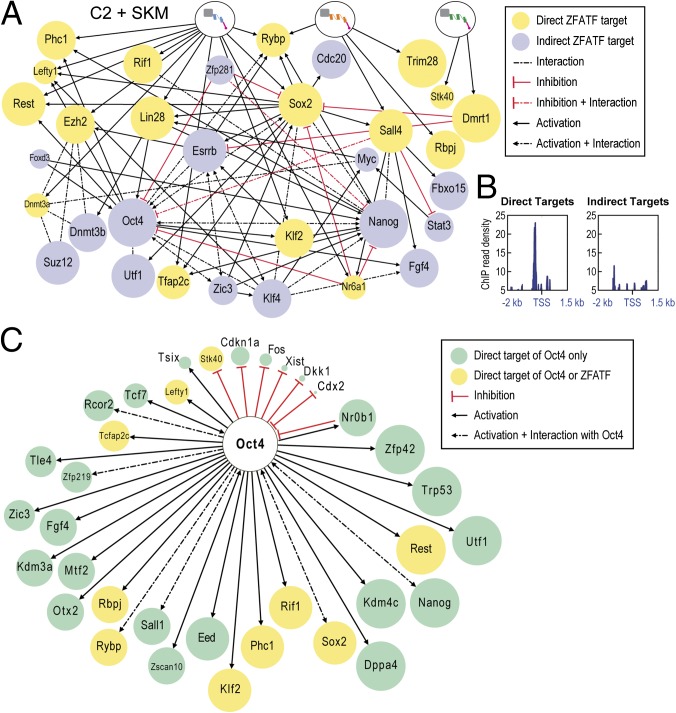
A gene regulatory network based on the CSI results and differential expression data were built using information from the literature and the STRING database (Fig. 6A) (58). Greater ATF occupancy near the TSS for the 17 predicted targets in the gene regulatory network suggests they are direct targets (Fig. 6B). A comparison of direct OCT4 targets with the ATF target genes reveals striking differences (Fig. 6C) (47). These differences suggest that ATFs activate the pluripotency network through different nodes than the exogenously expressed Oct4. Although the primary targets may differ at the outset, the eventual iPS cells show remarkable convergence in the transcriptome profiles and epigenetic landscapes.
Fig. 6.
Transcriptional networks activated by ATFs and Oct4. (A) Nodes of the pluripotency network activated by the ATFs of C2. The size of the node reflects level of expression in C2+SKM iPS cells. (B) ChIP read density in C2+SKM early cells for HA-tagged ATFs for 17 direct target genes (Left) and 15 indirect target genes (Right) for nodes in Fig. 6A. Traces represent coverage across a window of −2 to +1.5 kb relative to the TSS. (C) Direct targets of Oct4 (47). The size of the node reflects level of expression in Oct4+SKM iPS cells.
Discussion
Zinc finger, TAL effector, and CRISPR/Cas9 libraries have been tested for loss-of-function phenotypes, acquisition of resistance to a drug, or up-regulation of specific genes (21, 22, 27, 59–63); however, this study reports a gain-of-function screen with a genome-scale ATF library to reprogram fibroblasts to iPS cells, a feat that requires drastic transcriptional and epigenetic changes. Rather than engineering ATFs to target unique sites in the genome, we chose the zinc finger DBD primarily because of its ability to target a range of different DNA sites as well as the ability to interact with methylated and heterochromatic DNA. Furthermore, we deliberately engineered in a synthetic protein–protein interaction module that endows the ATFs with a unique ability to sample binding sites as cooperative dimers and thereby stimulate target gene expression to greater extent due to transcriptional synergy (15). Based on design principles, we generated and screened a zinc finger ATF library of high complexity, not previously tested in mammalian cells. Furthermore, the capacity for the ATFs to cooperatively bind target genes provides the library with a unique feature to sample a larger set of binding sites cooperatively and activate to greater extent due to synergy. Conventional zinc finger libraries consisted of ATFs created by shuffling a limited number of zinc finger units, previously characterized to bind specific triplets of nucleotides (64). The library used in this study uses a much larger repertoire of residues, incorporating 16 of the 20 possible amino acids in the recognition residues, greatly expanding the target space of the ATFs. Our design, which is consistent with a survey of natural zinc fingers found in eukaryotes, finds that nature uses all amino acids in the recognition residues (65).
Application of the ATF library in this gain-of-function screen demonstrates that zinc finger ATFs can perturb the transcriptional profile of a cell to levels that are sufficiently robust to induce a dramatic phenotypic change. Like natural TFs, the ATFs in this study target 9- to 10-bp sites and can bind cooperatively as homodimers and heterodimers to target a diverse range of regulatory elements simultaneously. As each ATF in the library will have unique sequence preferences and varying degrees of affinity for DNA, a wide range of outcomes can be elicited upon introduction of these ATFs. More importantly, unlike natural TFs, ATFs do not necessarily rely on endogenous partner proteins, and thus, transcriptional networks can be stimulated from any homeostatic state. Moreover, rather than first identifying regulatory regions that might permit ATF binding, the use of a library permits the identification of molecules that can bind at functionally relevant loci to regulate the expression of critical genes that drive desired phenotypes or cell states.
Because we are using a large library of ATFs, capable of sampling thousands of genes in parallel, there is a potential for our ATFs to activate endogenous Oct4 directly; however, in our analysis, we find that the ATFs seem to activate Oct4 indirectly. It is interesting to note that two ATFs that recur in different combinations (ZFATF1 and ZFATF2), target sites that are prevalent in CpG islands. These ATFs could potentially amplify the output of transcriptional networks that are stimulated by combinations of pluripotency factors working alongside the exogenously expressed ATFs and SKM. Intriguingly, during the early stages of conversion, cells expressing ATFs+SKM exhibited a different transcriptome profile from those expressing Oct4+SKM; however, in the final iPS cell state, the expression profiles of all of the pluripotent cell types were similar at the molecular level. Although the functional pluripotency of the ATF-derived iPS cells are described, direct comparison with teratomas or EBs from OSKM-derived iPS cells remains to be studied. The differences in molecular signatures at the early stage suggest that the MEFs take different dedifferentiation routes to the same pluripotent state. Thus, this unbiased forward genetic approach can reveal unanticipated network linkages and identify ways to interface with a cell fate-defining gene regulatory network. More remarkably, our results show that continued expression of ATFs is not required to maintain a stable cell state once homeostatic systems are set.
In addition to providing a means to perform a forward genetic screen, we demonstrate that our ATF library is a powerful resource for everyone who is interested in inducing cell fate conversion in the absence of a priori knowledge of natural TFs that might govern the desired cell type or phenotype. Furthermore, we describe a strategy toward identifying cell fate-defining transcriptional networks. By integrating expression data with in vitro binding site data, we were able to identify the nodes of the transcriptional network implicated in the induction of pluripotency. This technology enables the pursuit of elusive cell phenotypes or direct conversions, considered challenging to achieve by conventional methods. In summary, this study provides compelling support of our design principles and demonstrates that our ATF library can be used in a gain-of-function screen for complex cell fate conversions.
Materials and Methods
Design: Choice of DBD Scaffold.
In designing an ATF library for cell fate conversions, we first focused on choosing a DNA-binding scaffold that would be most conducive for performing a forward genetic screen. As noted above, the following criteria were considered.
i) Ability to target multiple nodes.
Although nuclease-inactivated CRISPR/Cas9 (66) and TAL-effectors (67) require at least than 10–20 bp to bind DNA, zinc fingers can be designed to target a wider range of sequences. The breadth of binding sites of zinc fingers is an important advantage. A zinc finger ATF can have thousands of binding sites in the genome, much like natural TFs, and perturb the transcriptome on a genomic scale. By contrast, highly specific DBDs such as TAL-effectors or RNA-guided CRISPR/Cas systems that target single genomic loci are less likely to perturb multiple nodes in a network and alter the homeostatic state to induce a change in cell fate.
ii) Regulatory potency.
Among the DBDs, zinc fingers have the unique ability to target both active and silenced regions of the genome, a feature important for cell fate conversions. Certain naturally occurring families of C2H2 zinc fingers bind methylated DNA and heterochromatin (65, 68), making zinc fingers well suited for activating epigenetically silenced regions unlike TAL-effectors, which are sensitive to DNA methylation (61, 69). Although CRISPR/Cas9 systems can activate silenced genes, they show limited ability to increase the expression of genes that are already expressed at moderate or high levels (63, 70). Additionally, compared with zinc fingers and TAL-effectors that can up-regulate genes to biologically relevant levels (42, 71, 72), the magnitude of transcriptional change induced by nuclease-inactivated CRISPR/Cas9 systems with a single guide is not as robust (73). Recent modifications to the CRISPR/Cas9 system have improved their impact on the level of expression of target genes; however, these modifications come at the expense of increasing their size (70) or introducing additional effector molecules that can be recruited by Cas9 (74, 75) or the guide RNA (63, 76).
iii) Efficient delivery.
Because cell fate conversions occur at low frequencies, efficient delivery of the ATFs to cells is critical for genome-scale gain-of-function screens. Each zinc finger unit is ∼30 aa, and three zinc fingers (90 aa) can be linked in tandem to target a 9- to 10-bp site (77). Compared with Cas9 (∼1,380 aa) or TAL-effectors (∼520 aa), designed to target a binding site of the same size, zinc fingers are much smaller and can be efficiently delivered to mammalian cells. Programmable molecules such as polyamides may provide a smaller ATF; however, the rules governing polyamide cell permeability are still not well understood, making delivery problematic (78). In brief, the zinc finger DBD emerges as an optimal scaffold for design of complex ATF libraries that can trigger cell fate-defining gene networks.
Zinc Finger ATF Library.
The scaffold of the ATF is comprised from N to C terminus: a 15-aa ID, the DNA binding domain of human EGR1, NLS from EGR1, VP64, and 3× HA tag. The ATF library was created by amplifying oligos with VNN codons at the −1, 2, 3, and 6 positions relative to the recognition helix of each zinc finger. The ATF library was cloned into the second-generation pSIN vector by ligation-independent cloning as described in SI Appendix, SI Materials and Methods.
Cell Culture.
Oct4: CremER-Cre-mER; mTmG MEFs were grown in DMEM supplemented with 10% (vol/vol) FBS on plates coated with 0.1% gelatin. Mouse E14T ES cells and iPS cells were grown in knockout DMEM supplemented with 15% (vol/vol) FBS, 1% nonessential amino acids, 2 mM l-glutamine, 1 × 103 units/mL leukemia inhibitory factor, 1 mM sodium pyruvate, and 100 µM β-mercaptoethanol. During reprogramming of Oct4:CremER-Cre-mER; mTmG MEFs, 4-hydroxytamoxifen was added at 100 nM concentration. Cells were maintained in a humidified 37 °C incubator with 5% CO2. Additional details are in SI Appendix, SI Materials and Methods.
Luciferase Assay.
The palindromic EGR1 binding site 5′-GCG-TGG-GCG-CGC-CCC-CGC-3′ was cloned upstream of the luciferase gene in the pGL3 basic vector (Promega). Luciferase assay (Promega; E4030) was performed according to the manufacturer’s guidelines. Additional details are in SI Appendix, SI Materials and Methods.
Retrovirus production.
Oct4, Sox2, Klf4, and c-Myc were packaged into retrovirus with Plat-E cells as described in ref. 79.
Lentivirus production.
ATFs and the empty control were packaged into lentivirus with HEK293FT cells using a second-generation lentiviral system. Details are described in SI Appendix, SI Materials and Methods.
Identification of ATFs from Single Cells.
Cells with a positive phenotype for Oct4 lineage tracing activation were isolated as single cells into a 96-well plate. Nested two-step PCR is described in SI Appendix, SI Materials and Methods.
EB Formation.
For EB formation, pluripotent cells were seeded into ultralow-adhesion dishes at a concentration of 1 × 105 cells/mL in knockout DMEM supplemented with 15% (vol/vol) FBS, 1% nonessential amino acids, 2 mM l-glutamine, 1 mM sodium pyruvate, and 100 µM β-mercaptoethanol. Media was changed the day after seeding and every 2 d thereafter. EBs were collected on days 7, 11, and 14 for quantitative RT-PCR (RT-qPCR) and immunofluorescence. Cells were maintained in a humidified 37 °C incubator with 5% CO2.
Immunofluorescence.
EBs were plated on poly-l-lysine on day 14 to culture EB outgrowths. iPS cells were plated on glass slides coated with 0.1% gelatin for immunofluorescence. Antibody sources and dilutions are described in SI Appendix, SI Materials and Methods.
RT-qPCR.
RNA was extracted from cells with RNeasy Mini Kit (Qiagen; 74104). RNA was converted into cDNA with SuperScript III First-Strand Synthesis System (Thermo Fisher; 18080051). qPCR was performed with Bullseye EvaGreen qPCR Mix with low ROX (Midwest Scientific; BEQPCR-LR). Primer sets are listed in SI Appendix, SI Materials and Methods.
ChIP.
For ChIP, 5 × 106 cells were fixed in 1.5% (vol/vol) formaldehyde for 15 min. Harvested cells were flash frozen, and then sonicated and lysed. Lysates were precleared and immunoprecipitated overnight with H3K27ac antibody (Abcam; ab4729), H3K9me3 antibody (Abcam; ab8898), or HA antibody (Abcam; ab9110) at 4 °C. Immunoprecipitated histone marks were purified with protein G magnetic beads (Life Technologies; 10004D) and a series of five washes. Cross-links of protein–DNA complexes were reversed by incubating at 65 °C for 6 h. Eluted DNA was treated with RNase A and Proteinase K. Additional details are in SI Appendix, SI Materials and Methods.
RNA-Seq Analysis.
Reads were aligned with Bowtie2, version 2.2.5, to either the human genome hg19 (HEK293) or mouse genome mm10 (MEFs or cells derived from MEFs). Counts were quantified with Cufflinks, and differential expression was determined by Cuffdiff (80).
ChIP-Seq Analysis.
Reads were annotated to the mouse genome mm10 with Bowtie2, version 2.2.5. Output sam files were converted to bam files, sorted, and indexed with Samtools 1.3. H3K27ac, H3K9me3, and HA peaks were called with MACS2 2.2.1. Differential peak signals were determined by DiffBind 1.16.2. ChIP peaks were visualized with Integrative Genomics Viewer (IGV). Additional details are in SI Appendix, SI Materials and Methods.
CSI.
CSI was performed by incubating cell lysates containing zinc finger ATFs with randomized permutations of 25-bp sequences. The ATF–DNA complexes were immunoprecipitated with HA magnetic beads. Three rounds of enrichment were performed, and all three rounds of enrichment were sequenced. Experimental details and bioinformatic analysis are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sandra Tseng and Graham Erwin for help with ChIP-seq analysis. We thank José Rodríguez-Martínez for CSI analysis and Christina Shafer with RNA-seq analysis. We also acknowledge Mitchell Probasco with help with flow cytometry, Jennifer Bolin for help with Illumina sequencing, and Bret Duffin for the teratoma assay. We thank Laura Vanderploeg for help with figure graphics. We are also grateful to Judith Kimble, Sushmita Roy, Garrett Lee, and Fang Wan for helpful discussions. This work was supported by the NIH Grant HL099773, W. M. Keck Medical Research Award, and Progenitor Cell Biology Consortium Jump-Start Award 5U01HL099997-05 (Subaward 101330A). A.E. was supported by the Morgridge Biotechnology Wisconsin Distinguished Fellowship Award and the Stem Cell and Regenerative Medicine Training Award. A.S.K. was supported by Genomic Sciences Training Program Grant 5T32HG002760. D.B. was supported by a National Science Foundation–Nanoscale Science and Engineering Center grant.
Footnotes
Conflict of interest statement: A.Z.A. is the sole member of VistaMotif, LLC, and founder of the nonprofit WINStep Forward.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq and ChIP-seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE89221).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611142114/-/DCSupplemental.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 5.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475(7356):390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 8.Cohen DE, Melton D. Turning straw into gold: Directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12(4):243–252. doi: 10.1038/nrg2938. [DOI] [PubMed] [Google Scholar]
- 9.Becker JS, Nicetto D, Zaret KS. H3K9me3-dependent heterochromatin: Barrier to cell fate changes. Trends Genet. 2016;32(1):29–41. doi: 10.1016/j.tig.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eguchi A, Lee GO, Wan F, Erwin GS, Ansari AZ. Controlling gene networks and cell fate with precision-targeted DNA-binding proteins and small-molecule-based genome readers. Biochem J. 2014;462(3):397–413. doi: 10.1042/BJ20140400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansari AZ, Mapp AK. Modular design of artificial transcription factors. Curr Opin Chem Biol. 2002;6(6):765–772. doi: 10.1016/s1367-5931(02)00377-0. [DOI] [PubMed] [Google Scholar]
- 12.Mapp AK, Ansari AZ, Ptashne M, Dervan PB. Activation of gene expression by small molecule transcription factors. Proc Natl Acad Sci USA. 2000;97(8):3930–3935. doi: 10.1073/pnas.97.8.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mapp AK, Ansari AZ. A TAD further: Exogenous control of gene activation. ACS Chem Biol. 2007;2(1):62–75. doi: 10.1021/cb600463w. [DOI] [PubMed] [Google Scholar]
- 14.Ansari AZ. Chemical crosshairs on the central dogma. Nat Chem Biol. 2007;3(1):2–7. doi: 10.1038/nchembio0107-2. [DOI] [PubMed] [Google Scholar]
- 15.Ptashne M. A Genetic Switch. 3rd Ed Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2004. [Google Scholar]
- 16.Moretti R, Ansari AZ. Expanding the specificity of DNA targeting by harnessing cooperative assembly. Biochimie. 2008;90(7):1015–1025. doi: 10.1016/j.biochi.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- 18.Segal DJ, Barbas CF. Custom DNA-binding proteins come of age: Polydactyl zinc-finger proteins. Curr Opin Biotechnol. 2001;12(6):632–637. doi: 10.1016/s0958-1669(01)00272-5. [DOI] [PubMed] [Google Scholar]
- 19.Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem. 2010;79:213–231. doi: 10.1146/annurev-biochem-010909-095056. [DOI] [PubMed] [Google Scholar]
- 20.Triezenberg SJ, Kingsbury RC, McKnight SL. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- 21.Bae K-H, et al. Human zinc fingers as building blocks in the construction of artificial transcription factors. Nat Biotechnol. 2003;21(3):275–280. doi: 10.1038/nbt796. [DOI] [PubMed] [Google Scholar]
- 22.Blancafort P, Magnenat L, Barbas CF. Scanning the human genome with combinatorial transcription factor libraries. Nat Biotechnol. 2003;21(3):269–274. doi: 10.1038/nbt794. [DOI] [PubMed] [Google Scholar]
- 23.Carroll D, Morton JJ, Beumer KJ, Segal DJ. Design, construction and in vitro testing of zinc finger nucleases. Nat Protoc. 2006;1(3):1329–1341. doi: 10.1038/nprot.2006.231. [DOI] [PubMed] [Google Scholar]
- 24.Greisman HA, Pabo CO. A general strategy for selecting high-affinity zinc finger proteins for diverse DNA target sites. Science. 1997;275(5300):657–661. doi: 10.1126/science.275.5300.657. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, et al. Induction of stable drug resistance in human breast cancer cells using a combinatorial zinc finger transcription factor library. PLoS One. 2011;6(7):e21112. doi: 10.1371/journal.pone.0021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park K-S, et al. Phenotypic alteration of eukaryotic cells using randomized libraries of artificial transcription factors. Nat Biotechnol. 2003;21(10):1208–1214. doi: 10.1038/nbt868. [DOI] [PubMed] [Google Scholar]
- 27.Tschulena U, Peterson KR, Gonzalez B, Fedosyuk H, Barbas CF. Positive selection of DNA-protein interactions in mammalian cells through phenotypic coupling with retrovirus production. Nat Struct Mol Biol. 2009;16(11):1195–1199. doi: 10.1038/nsmb.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang BS, Grant RA, Pabo CO. Selected peptide extension contacts hydrophobic patch on neighboring zinc finger and mediates dimerization on DNA. Nat Struct Biol. 2001;8(7):589–593. doi: 10.1038/89617. [DOI] [PubMed] [Google Scholar]
- 29.Teschendorf C, Warrington KH, Siemann DW, Muzyczka N. Comparison of the EF-1 alpha and the CMV promoter for engineering stable tumor cell lines using recombinant adeno-associated virus. Anticancer Res. 2002;22(6A):3325–3330. [PubMed] [Google Scholar]
- 30.Greder LV, et al. Analysis of endogenous Oct4 activation during induced pluripotent stem cell reprogramming using an inducible Oct4 lineage label. Stem Cells. 2012;30(11):2596–2601. doi: 10.1002/stem.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahan P, et al. CellNet: Network biology applied to stem cell engineering. Cell. 2014;158(4):903–915. doi: 10.1016/j.cell.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whyte WA, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153(2):307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hnisz D, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155(4):934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz DC, Ayyanathan K, Negorev D, Maul GG, Rauscher FJ. SETDB1: A novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002;16(8):919–932. doi: 10.1101/gad.973302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramírez F, Dündar F, Diehl S, Grüning BA, Manke T. deepTools: A flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014;42(Web server issue):W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross-Innes CS, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481(7381):389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren CL, et al. Defining the sequence-recognition profile of DNA-binding molecules. Proc Natl Acad Sci USA. 2006;103(4):867–872. doi: 10.1073/pnas.0509843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson CD, et al. Specificity landscapes of DNA binding molecules elucidate biological function. Proc Natl Acad Sci USA. 2010;107(10):4544–4549. doi: 10.1073/pnas.0914023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tietjen JR, Donato LJ, Bhimisaria D, Ansari AZ. Sequence-specificity and energy landscapes of DNA-binding molecules. Methods Enzymol. 2011;497:3–30. doi: 10.1016/B978-0-12-385075-1.00001-9. [DOI] [PubMed] [Google Scholar]
- 42.Rebar EJ, et al. Induction of angiogenesis in a mouse model using engineered transcription factors. Nat Med. 2002;8(12):1427–1432. doi: 10.1038/nm1202-795. [DOI] [PubMed] [Google Scholar]
- 43.Koudritsky M, Domany E. Positional distribution of human transcription factor binding sites. Nucleic Acids Res. 2008;36(21):6795–6805. doi: 10.1093/nar/gkn752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng C, Gerstein M. Modeling the relative relationship of transcription factor binding and histone modifications to gene expression levels in mouse embryonic stem cells. Nucleic Acids Res. 2012;40(2):553–568. doi: 10.1093/nar/gkr752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitfield TW, et al. Functional analysis of transcription factor binding sites in human promoters. Genome Biol. 2012;13(9):R50. doi: 10.1186/gb-2012-13-9-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen EY, et al. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14(1):128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Som A, et al. The PluriNetWork: An electronic representation of the network underlying pluripotency in mouse, and its applications. PLoS One. 2010;5(12):e15165. doi: 10.1371/journal.pone.0015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farley EK, et al. Suboptimization of developmental enhancers. Science. 2015;350(6258):325–328. doi: 10.1126/science.aac6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crocker J, et al. Low affinity binding site clusters confer hox specificity and regulatory robustness. Cell. 2015;160(1-2):191–203. doi: 10.1016/j.cell.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharov AA, et al. Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics. 2008;9(1):269. doi: 10.1186/1471-2164-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marson A, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134(3):521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heng J-CD, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Buganim Y, et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150(6):1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shu J, et al. Induction of pluripotency in mouse somatic cells with lineage specifiers. Cell. 2013;153(5):963–975. doi: 10.1016/j.cell.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lujan E, et al. Early reprogramming regulators identified by prospective isolation and mass cytometry. Nature. 2015;521(7552):352–356. doi: 10.1038/nature14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krentz AD, et al. Interaction between DMRT1 function and genetic background modulates signaling and pluripotency to control tumor susceptibility in the fetal germ line. Dev Biol. 2013;377(1):67–78. doi: 10.1016/j.ydbio.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132(6):1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szklarczyk D, et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y, et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature. 2014;509(7501):487–491. doi: 10.1038/nature13166. [DOI] [PubMed] [Google Scholar]
- 60.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343(6166):80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y, et al. A library of TAL effector nucleases spanning the human genome. Nat Biotechnol. 2013;31(3):251–258. doi: 10.1038/nbt.2517. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Ehrhardt K, Zhang MQ, Bleris L. Assembly and validation of versatile transcription activator-like effector libraries. Sci Rep. 2014;4:4857. doi: 10.1038/srep04857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konermann S, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517(7536):583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez B, et al. Modular system for the construction of zinc-finger libraries and proteins. Nat Protoc. 2010;5(4):791–810. doi: 10.1038/nprot.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Najafabadi HS, et al. C2H2 zinc finger proteins greatly expand the human regulatory lexicon. Nat Biotechnol. 2015;33(5):555–562. doi: 10.1038/nbt.3128. [DOI] [PubMed] [Google Scholar]
- 66.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boch J, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326(5959):1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 68.Filion GJP, et al. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol Cell Biol. 2006;26(1):169–181. doi: 10.1128/MCB.26.1.169-181.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valton J, et al. Overcoming transcription activator-like effector (TALE) DNA binding domain sensitivity to cytosine methylation. J Biol Chem. 2012;287(46):38427–38432. doi: 10.1074/jbc.C112.408864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chavez A, et al. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 2015;12(4):326–328. doi: 10.1038/nmeth.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao X, et al. Reprogramming to pluripotency using designer TALE transcription factors targeting enhancers. Stem Cell Rep. 2013;1(2):183–197. doi: 10.1016/j.stemcr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bailus BJ, et al. Protein delivery of an artificial transcription factor restores widespread Ube3a expression in an Angelman syndrome mouse brain. Mol Ther. 2016;24(3):548–555. doi: 10.1038/mt.2015.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esvelt KM, et al. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods. 2013;10(11):1116–1121. doi: 10.1038/nmeth.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilbert LA, et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159(3):647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, Vale RD. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159(3):635–646. doi: 10.1016/j.cell.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zalatan JG, et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160(1-2):339–350. doi: 10.1016/j.cell.2014.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pabo CO, Sauer RT. Transcription factors: Structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- 78.Edelson BS, et al. Influence of structural variation on nuclear localization of DNA-binding polyamide-fluorophore conjugates. Nucleic Acids Res. 2004;32(9):2802–2818. doi: 10.1093/nar/gkh609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 80.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.