Significance
The regular distribution of mesenchymal cells, the formation of epithelial monolayers, or their collapse into spheroidal tumors illustrates the broad range of possible organizations of cells in tissues. Unveiling a physical picture of their emergence and dynamics is of critical importance to understand tissue morphogenesis or cancer progression. Although the role of cell–substrate and cell–cell adhesion in the organization of cell colonies has been widely studied, the impact of the cell-type–specific contact inhibition of locomotion (CIL) remains unclear. Here, we include this interaction in simulations of active particles and find a number of structures and collective dynamics that recapitulate existing tissue phenotypes. We give analytical predictions for the epithelial–mesenchymal transition and the formation of 3D aggregates as a function of cell–cell adhesion and CIL strengths. Thus, our findings shed light on the physical mechanisms underlying multicellular organization.
Keywords: self-propelled particles, cell–cell adhesion, contact inhibition of locomotion, cell monolayers, collective motion
Abstract
Cells in tissues can organize into a broad spectrum of structures according to their function. Drastic changes of organization, such as epithelial–mesenchymal transitions or the formation of spheroidal aggregates, are often associated either to tissue morphogenesis or to cancer progression. Here, we study the organization of cell colonies by means of simulations of self-propelled particles with generic cell-like interactions. The interplay between cell softness, cell–cell adhesion, and contact inhibition of locomotion (CIL) yields structures and collective dynamics observed in several existing tissue phenotypes. These include regular distributions of cells, dynamic cell clusters, gel-like networks, collectively migrating monolayers, and 3D aggregates. We give analytical predictions for transitions between noncohesive, cohesive, and 3D cell arrangements. We explicitly show how CIL yields an effective repulsion that promotes cell dispersal, thereby hindering the formation of cohesive tissues. Yet, in continuous monolayers, CIL leads to collective cell motion, ensures tensile intercellular stresses, and opposes cell extrusion. Thus, our work highlights the prominent role of CIL in determining the emergent structures and dynamics of cell colonies.
Cell colonies exhibit a broad range of phenotypes. In terms of structure, collections of cells can arrange into distributions of single cells, assemble into continuous monolayers or multilayered tissues, or even form 3D agglomerates. In terms of dynamics, cell motility may simply be absent or produce random, directed, or collective migration of cells. Transitions between these states of tissue organization are characteristic of morphogenetic events and are also central to tumor formation and dispersal (1–4). Therefore, a physical understanding of the collective behavior of cell colonies will shed light on the regulation of many multicellular processes involved in development and disease.
However, a complete physical picture of multicellular organization is not yet available, partly due to the challenge of modeling the complex interactions between cells. Here, we address this problem by means of large-scale simulations of self-propelled particles (SPP) endowed with interactions capturing generic cellular behaviors. Models of SPP with aligning interactions have been used to investigate collective cell motions in tissue monolayers (5–18). We extend this approach to unveil how the different structures and collective dynamics of cell colonies emerge from cell–cell interactions.
In addition to an excluded-volume repulsion, cells generally feature a short-range attraction as a consequence of their active cortical contractility transmitted through cell–cell junctions. With no additional interactions, this attraction would typically lead to cohesive tissues. However, not all cell types form cohesive tissues. Whereas epithelial cells tend to form continuous monolayers, mesenchymal cells separate after division despite the presence of cell–cell junctions. This observation calls for an extra effective repulsion to drive the separation, which may ultimately have a deep impact on the overall organization of the colony.
Such a repulsive interaction mediated by adhesion is indeed present in many cell types upon cell–cell contact and is known as contact inhibition of locomotion (CIL) after Abercrombie and Heaysman (19). Upon a cell–cell collision, the cell front adheres to the colliding cell, which hinders further cell protrusions. Subsequently, repolarization of the cell’s cytoskeleton creates a new front away from the adhesion zone, and the two cells thus separate (20, 21). This interaction has been shown to be crucial in determining the collective behavior of cell groups in several contexts (22). For example, CIL guides the directional migration of neural crest cells (23) and also ensures the correct dispersion of Cajal–Retzius cells in the cerebral cortex (24) or of hemocytes in the embryo (25).
Here, we model cellular interactions by means of an attraction due to intercellular adhesion and a soft repulsion associated to the reduction of cell–substrate adhesion area. In addition, CIL is modeled as an interaction orienting cell motility away from cell–cell contacts. We analytically show how CIL acts as an effective repulsive force that hinders the formation of cohesive cell monolayers or 3D tissues at increasing cell–cell adhesion. We then explicitly predict the transitions between noncohesive, cohesive, and overlapped organizations of the colonies as a function of cell–cell adhesion and CIL strength. In simulations, we identify states with different structures and emergent dynamics, including ordered or dynamic arrangements of clusters, gel-like networks, active gas and polar liquid states, and 3D aggregates. The results may be interpreted in biological terms by associating each state to common phenotypes, namely grid-like distributions of mesenchymal cells, collectively migrating epithelial monolayers, and cellular spheroids. Both the soft character of the potential and the CIL interactions are key in producing structures and collective behaviors observed in cell colonies. In particular, the former enables the formation of 3D tissues via cell extrusion. In turn, CIL gives rise to self-organized collective motion in continuous cell monolayers. In line with ref. 17, we find that this effective repulsion induces tensile stresses in cell monolayers.
Model
We model a 2D colony of cells as a suspension of overdamped self-propelled disks. Neglecting translational noise, the equation of motion of cell with position and polarity reads
| [1] |
for contacting nearest-neighbor cells , with and . Here, is the magnitude of the cell self-propulsion force, and and are cell–substrate and cell–cell friction constants, respectively.
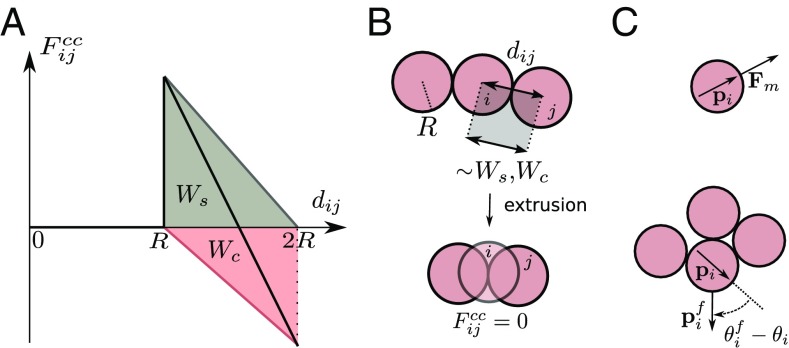
The central force includes a soft repulsion associated to the reduction of the cell–substrate adhesion area when two cells are closer than their spread size , and an attractive force that accounts for active contractility transmitted through cell–cell adhesions. is assumed to increase linearly with decreasing intercellular distance up to . Hence, , with the cell–substrate adhesion energy (gray in Fig. 1A). No further reduction of the cell–substrate contact area is allowed for . As a result, cells can approach at smaller distances under compression. In this regime cells do not exert any force on the substrate and are considered to be extruded from the monolayer (Fig. 1 A and B). Cell extrusions may lead to 3D tissues, whose structure and dynamics are not described by our 2D model. is assumed to increase linearly with distance up to . Hence, , with the cell–cell adhesion energy (red in Fig. 1A). Accordingly, the total interaction force (black in Fig. 1A) reads
| [2] |
Fig. 1.
A model of self-propelled particles with cell-like interactions. (A) Central cell–cell force (black), including a soft repulsion due to reduction of cell–substrate adhesion area (gray) and attraction due to active contractility through cell–cell adhesions (red). (B) Cell extrusion for intercellular distances , resulting in vanishing cell–cell forces in the plane. (C) Cellular self-propulsion force in the direction of the cell polarity . CIL rotates the polarity toward the direction pointing away from cell–cell contacts.
In turn, CIL tends to orient the cell polarity in the direction pointing away from the weighted average position of the contacting cells (Fig. 1C and SI Appendix). Similarly to ref. 18, we model this interaction via a harmonic potential for the polarization angle that, in addition to rotational noise, yields
| [3] |
Here, is the cellular repolarization rate upon cell–cell contact, whereas is a typified Gaussian white noise, and is the rotational diffusion coefficient of cell motion.
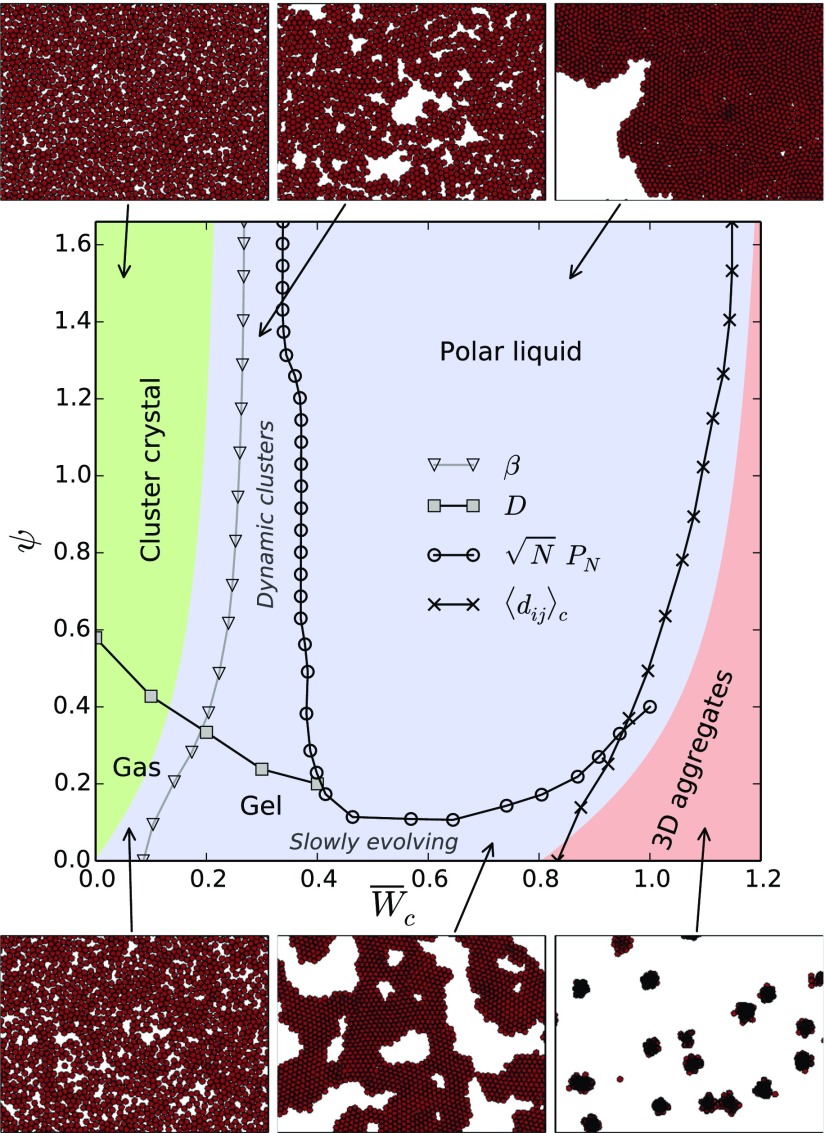
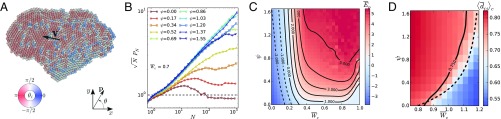
The parameters of the model may be reduced to five dimensionless quantities: the packing fraction of cells , cell–cell and cell–substrate adhesion energies and , cell–cell friction , and a parameter that compares the timescale of cytoskeletal repolarization associated to CIL to the rotational diffusion. Hereafter, we set , , and and focus on the effects of intercellular adhesion and CIL on the organization of cell colonies. The results are summarized in the phase diagram in Fig. 2. Including cell–cell friction leads to jammed configurations of cohesive tissues (SI Appendix), in line with ref. 16. In turn, cell density does not affect the phase transitions but modifies the dynamical behavior of the cell colony (SI Appendix). Thus, cell proliferation may drive the colony through different dynamical states (SI Appendix).
Fig. 2.
Phase behavior of cell colonies as a function of cell–cell adhesion and cell repolarization rate associated to CIL. Colors indicate the predicted regions for noncohesive (green), cohesive (blue), and overlapped (red) organizations. In addition to capturing these structural transitions, simulations allow us to identify dynamically distinct states such as an active gas, a cluster crystal, a gel-like percolated network, dynamic clusters, and an active polar liquid, as illustrated in snapshots.
Results
Noncohesive Phase.
We first study the transition between a cohesive phase in which cells remain in contact, , and a noncohesive phase in which they lose contact. Loss of cell contact is possible only if the maximal attractive force at , , is overcome by the component of the cells’ self-propulsion force along the interparticle axis. This component depends on the relative alignment of self-propulsion forces, and hence on CIL. When averaged over orientations, self-propulsion forces yield an effective central repulsion between cells that depends on their repolarization rate (SI Appendix). In the relevant limit (Discussion), it reads
| [4] |
Then, within this mean-field approximation, the condition gives a prediction for the transition between the noncohesive (green in Fig. 2) and cohesive (blue in Fig. 2) phases. This sets a critical adhesion energy
| [5] |
above which cells are expected to be in contact, or, alternatively, a critical CIL rate above which cohesiveness is lost. Therefore, at low cell–cell adhesion, CIL promotes cell dispersal, thereby hindering the formation of cohesive tissues.
In simulations, we quantify this transition in terms of particle number fluctuations. Phase-separated self-propelled disks feature giant number fluctuations (9, 26, 27). There, the standard deviation of the number of particles in a given region scales as for large , with , whereas a system at equilibrium would feature . Similarly, we compute the exponent (Fig. 3A) and identify the regions with as phase separated and thus cohesive. Consequently, we identify the transition to the cohesive phase from the onset of giant number fluctuations (triangles in Fig. 2), which qualitatively agrees with the mean-field analytical prediction.
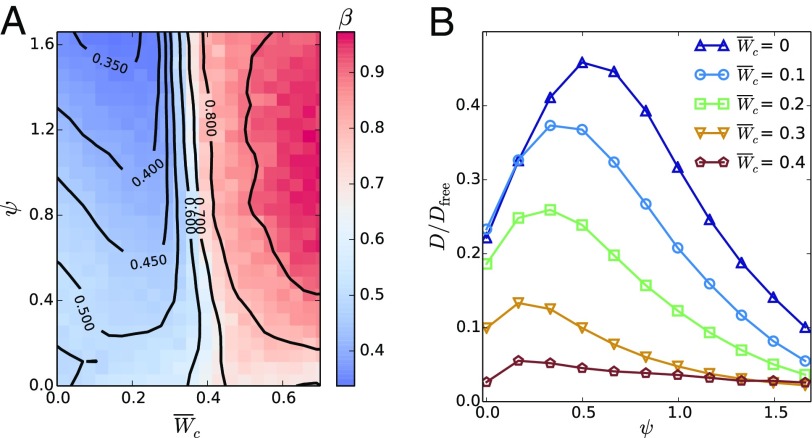
Fig. 3.
Number fluctuations and diffusion in cell colonies. (A) Exponent of number fluctuations as a function of cell–cell adhesion and CIL repolarization rate . Phase-separated states feature giant number fluctuations () whose onset identifies the transition to the cohesive phase (triangles in Fig. 2). In the noncohesive phase, colonies of slowly repolarizing cells (low ) feature equilibrium-like fluctuations (), whereas faster repolarizations (higher ) induce a hyperuniform distribution of cells (). (B) Cell diffusion coefficient as a function of for some values of . For increasing repolarization rate , initially increases but then decreases as clusters form. The maximum of identifies the onset of clustering (squares in Fig. 2). is the translational diffusion coefficient of a persistent random walker with rotational diffusion (28).
Within the noncohesive phase (green in Fig. 2), the colony forms an active gas state with equilibrium-like statistics () at low CIL repolarization rates (Movie S1). At larger , cells get hyperuniformly distributed, with (Fig. 3A), forming a crystal of small cell clusters (Movie S2). This state is reminiscent of the equilibrium cluster crystals formed by purely repulsive soft spheres (29). In our case, an effective repulsion arises from antialigned propulsion forces via CIL (Eq. 4). Similarly to ref. 30, we set a dynamical criterion for the clustering transition based on the cell diffusion coefficient obtained from the long-time mean-squared displacement (MSD), . Increasing the repolarization rate initially enhances diffusion by promoting cluster evaporation. However, the stronger effective repulsion at larger progressively prevents cells from escaping the clusters, hence reducing diffusion until it is eventually solely due to intercluster hopping events (31) (see SI Appendix for a discussion on the dependence of D on ). Consequently, we locate the clustering transition (squares in Fig. 2) from the maximum of at each (Fig. 3B). Increasing cell–cell adhesion favors clustering, thereby enabling the short-range CIL-associated repulsion responsible for the crystalline order.
Cohesive Phase.
Increasing cell–cell adhesion beyond the transition to the cohesive phase (blue in Fig. 2), the colony initially forms a percolating structure of clusters. At low CIL repolarization rate , cells arrange in a network with very slow, subdiffusive dynamics, as shown by the MSD with (Fig. 4A). Thus, due to cell–cell adhesion, the colony forms a near-equilibrium attractive gel (32) with few cell rearrangements (Movie S3). At larger repolarization rates (above squares in Fig. 2) (Fig. 3B), the effective CIL-associated repulsion yields smaller, dynamic, and locally crystalline clusters (Movie S4). They arise from a kinetic balance between the CIL-enhanced evaporation and the adhesion-induced condensation of clusters that prevents the completion of phase separation into a continuous dense phase.
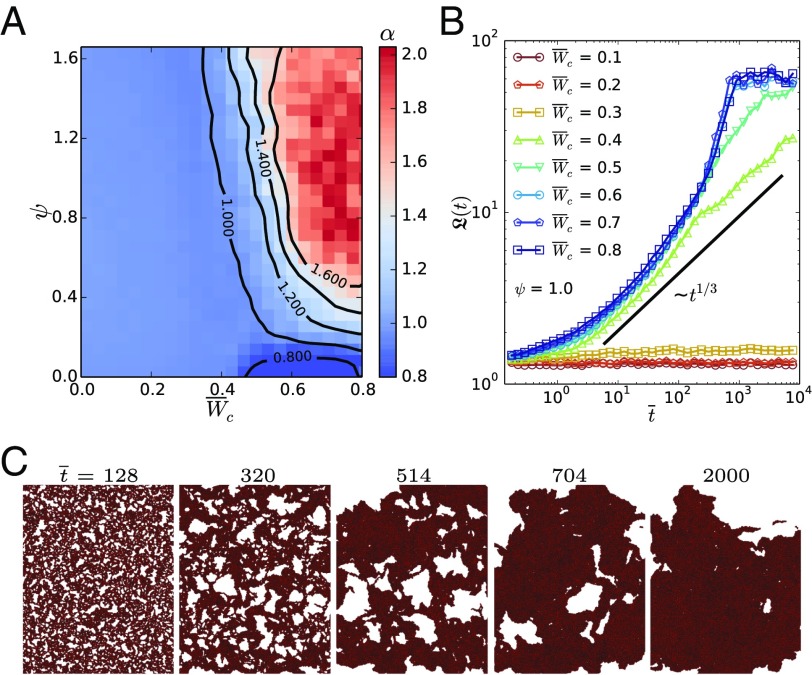
Fig. 4.
Dynamics and phase-separation kinetics in cell colonies. (A) MSD exponent as a function of cell–cell adhesion and CIL repolarization rate . The colony forms a gel-like network with subdiffusive dynamics () at low . Faster CIL gives rise to collective cell motion, indicated by almost ballistic dynamics (). (B) Evolution of the average domain size , computed from the structure factor (SI Appendix), for different at . Dimensionless time reads . The colony phase separates for . CIL yields faster phase-separation kinetics than the diffusive coarsening dynamics of passive systems, for which (33). (C) Illustration of the phase separation from an initial random configuration toward the active polar liquid at and .
Complete phase separation occurs at larger cell–cell adhesion, . The coarsening dynamics (Fig. 4 B and C) are much faster than in a passive system, for which particle domains grow by diffusion as (33). By orienting cell motility toward free space, CIL induces an advective coarsening of the cell domains that enables a fast phase separation of cell colonies.
Upon phase separation, the colony forms a continuous cell monolayer that exhibits self-organized collective motion (Movie S5). This is reflected in the MSD exponent that evolves from diffusive () toward almost ballistic () above (Fig. 4A). CIL induces a coupling between cell polarity and density fluctuations in the fluid phase that gives rise to a macroscopic polarization via a spontaneous symmetry breaking. The outward motion of cells at the boundary of the monolayer creates free space behind them, which polarizes neighboring cells before the leading cell can reorient back. Through this mechanism, self-organized collective cell motion emerges from CIL, leading to an active polar liquid state.
The polar order is stable if the confinement imposed by neighbors restores the position and orientation of a cell before its polarity turns toward a new free direction. The repolarization occurs within a timescale , and the characteristic time of position relaxation in a dense environment is , with the stiffness of a two-neighbor confinement. Thus, an approximate stability criterion reads , which is satisfied for the whole parameter range in Fig. 2 (SI Appendix).
As illustrated in Fig. 5A, isolated fluid monolayers may acquire a global polarity and consequently perform persistent random walks with a persistence much larger than that of single cells (Movie S6). For randomly oriented cells, the average polarity of cells scales as . If cell polarities align, the average polarity of a small region of cells decreases slower with its size, so that . The larger the repolarization rate is, the faster the increase of polarity with (SI Appendix). At sufficiently large sizes, multiple misaligned polarity domains appear that restore the random scaling (Fig. 5B). Hence, we define the onset of macroscopic polarization (circles in Fig. 2) by the condition that has a maximum at , namely that connected clusters consisting of up to 75 cells may form a single polarity domain. The appropriate choice of depends on system size. However, for the sizes explored, the transition line (circles in Fig. 2) is hardly sensitive to values around (SI Appendix). In conclusion, by ensuring a complete phase separation while still allowing for cell rearrangements, sufficiently strong cell–cell adhesion and CIL are required to form a polar, collectively moving cell monolayer.
Fig. 5.
Collective motion, mechanics, and dewetting of cell monolayers. (A) Snapshot of a globally polarized, collectively migrating cell monolayer. (B) Rescaled average polarity of a monolayer of cells for different CIL repolarization rates at a cell–cell adhesion . corresponds to randomly oriented cells. CIL induces a global polarity () that gives rise to collective motion. The appearance of several polarity domains reduces the average polarity of large cell groups. The transition to the active polar liquid state (circles in Fig. 2) is defined by the condition that the maximum of is at . (C) Average cell–cell potential energy as a function of cell–cell adhesion and CIL repolarization rate . CIL-associated repulsion induces tensile stresses () in cell monolayers. (D) Average distance between contacting cells as a function of and . The transition between cell monolayers and 3D aggregates is predicted to occur at a vanishing average cell–cell force (dashed line) and is identified by the condition (solid line, crosses in Fig. 2).
Finally, the effective potential energy of cell–cell interactions gives information on the mechanics of the colony. Positive (negative) potential energies correspond to tensile (compressive) intercellular stresses. Noncohesive colonies at low cell–cell adhesion feature average attractive interactions leading to the formation of clusters. In turn, by polarizing border cells outward, CIL induces tensile stresses in cell monolayers (Fig. 5C), in agreement with ref. 17.
Overlapped Phase.
We finally focus on the transition to 3D tissues. When the average total cell–cell force is attractive, cells eventually overcome the energy barrier associated to the soft repulsive potential (Fig. 1A), which corresponds to cell extrusion events. Extruded cells are confined at distances smaller than , where they exert neither cell–cell nor traction forces. Thus, our model can predict the onset of the transition to 3D cell arrangements. Assuming a homogeneous distribution of cells, and using Eq. 2, the average interaction force reads
| [6] |
This force adds to the effective repulsion associated to antialigned self-propulsion forces (Eq. 4), so that the transition between monolayers (blue in Fig. 2) and 3D cell arrangements (red in Fig. 2) is predicted by the condition . This sets a critical cell–cell adhesion energy
| [7] |
above which cells are expected to fully overlap or, alternatively, a critical CIL repolarization rate above which cell extrusion is prevented. Therefore, by opposing cell extrusion, CIL hinders the collapse of cell monolayers into 3D aggregates. Indeed, a sufficiently fast repolarization of cell motility may stabilize cell monolayers even when cell–cell adhesion is stronger than cell–substrate adhesion, (Fig. 2).
In simulations, we characterize the degree of cell overlap in terms of the average distance between contacting cells (Fig. 5D). We then identify the transition when half of the contacting cells are at the critical distance for extrusion, , while the other half are fully spread, . Hence, the transition is defined by (crosses in Fig. 2), in qualitative agreement with the mean-field analytical prediction.
Monolayer instability occurs through a dewetting process whereby holes appear in the cell monolayer, which rapidly evolves into a network structure, as observed in ref. 34. Subsequently, different regions of the network slowly collapse into separate aggregates (Movie S7). In general, the 3D aggregate–monolayer transition can be viewed as a wetting transition of the cell colony (35) enabled by cell insertion or extrusion (36). Thus, our results show how CIL favors tissue wetting by orienting cell motility toward free space.
Discussion and Perspectives
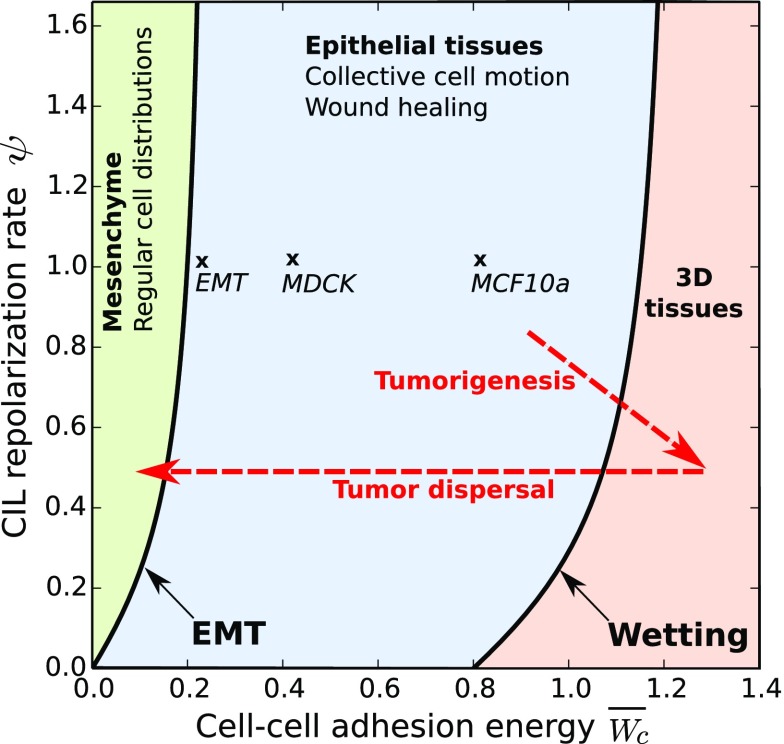
Based on experimental observations, we propose that the different organizations of cell colonies that emerge from our generic model correspond to different well-known tissue phenotypes (Fig. 6). First, the noncohesive phase, in which cells are not in contact, might correspond to mesenchymal tissues. Experiments show that CIL leads to regular distributions of mesenchymal cells during development (24, 25). This observation is consistent with the transition toward an ordered structure of cell clusters by increasing CIL strength (Fig. 2).
Fig. 6.
Proposal for the classification of different tissue phenotypes (boldface type) in terms of the phases of the model (colors). The association is based on the indicated features and supported by the parameter estimates for two epithelial tissues (44) and an EMT (43) (crosses and main text). Speculated trajectories in cellular interaction parameters during cancer progression are also included (dashed arrows).
The cohesive phase, in which cells maintain contact, can correspond to epithelial tissues. In the active polar liquid state, CIL induces cells to spontaneously invade empty spaces within the tissue, similarly to wound healing processes characteristic of epithelia. Indeed, simulations of prepared wounds reproduce the closure dynamics observed in experiments (37) (SI Appendix). In the absence of CIL, healing is severely impaired (SI Appendix), in agreement with experiments upon inhibition of Rac1 (38), a key protein for CIL behavior (39).
In addition, the parameters of our phase diagram can be estimated from experiments for two epithelial cell lines. By fitting the MSD of a SPP with rotational diffusion (28), , to experimental data for Michigan Cancer Foundation 10a (MCF10a) cells (SI Appendix), we estimate a self-propulsion velocity m/min and a diffusion coefficient min−1. This gives a Péclet number , too low to produce motility-induced phase separation (32, 40). In turn, the duration of cell–cell contact during CIL events allows the estimation of the rate of repolarization of cell motility. For two mesenchymal cell types, hemocytes (21) and fibroblasts (41), this gives min−1. The same estimate is obtained for epithelial Madin Darby canine kidney (MDCK) cells from the time that a wound needs to start closing (42). Then, assuming these parameter values are similar for MCF10a and MDCK cells, we estimate for both cell lines. Self-propulsion forces can be estimated from traction force measurements, which yield nN and nN in MCF10a and MDCK tissues, respectively (43). Finally, cell–cell and cell–substrate adhesion energies can be related to an effective elastic modulus of an expanding monolayer and to the total cellular strain at which the expansion stops (44). From Eq. 2, . In turn, corresponds to the cell–cell distance at mechanical equilibrium, , namely at which the total cell–cell force vanishes:
| [8] |
Then, using m and the values of and reported in ref. 44, we infer and for the MCF10a tissue and and for the MDCK tissue. The transition to 3D structures would then occur at for the MCF10a tissue. Thus, this tissue type falls well within the polar liquid state, in which cells form a collectively migrating continuous monolayer as experimentally observed. In contrast, the MDCK tissue is closer to the wetting transition, which we estimate at . Thus, although the MDCK tissue also falls within the polar liquid state, it may form 3D structures more easily, in line with experimental observations (12).
Now, the transition from cohesive to noncohesive phases should correspond to the epithelial–mesenchymal transition (EMT), which is associated to down-regulation of cell–cell adhesion proteins (3, 4). Our prediction sheds light on the role of CIL in the EMT (Fig. 6). As above, we can estimate the parameters for an EMT in an expanding MCF10a monolayer. Upon a knockdown of cell–cell adhesion proteins, the epithelial tissue disaggregates at an intercellular stress Pa (43). This translates into the critical cell–cell adhesion for the loss of cohesiveness by , with m the height of the monolayer. Hence, we estimate , consistent with the prediction at .
A tissue may also undergo an EMT by increasing cell traction forces, such as upon treatment with hepatocyte growth factor (44, 45). In our diagram, an increased self-propulsion force yields a lower dimensionless cell–cell adhesion energy whereas its critical value depends only on CIL (Eq. 5), hence causing the EMT.
In conclusion, the estimates and observations support the association of epithelial tissues to the cohesive phase. Nevertheless, some mesenchymal cells can also migrate collectively as a consequence of CIL (6, 13, 23) or of increased cell–cell adhesion (46). Therefore, these specific phenotypes might also correspond to the active polar liquid state. However, whether the features of collective mesenchymal cell migration (47) fully agree with our results deserves further exploration.
Finally, in our model, the overlapped phase corresponds to 3D tissues. Their structure is not captured by our 2D model, which predicts only the onset of their appearance. In experiments, the transition from a cell monolayer to a 3D aggregate can be induced in many ways (48), such as by increasing the density of cell–cell adhesion proteins (35, 49). Alternatively, one can reduce the density of cell–substrate proteins (49, 50), which, in our diagram, entails a decrease of the critical cell–cell adhesion for the wetting transition, Eq. 7. Three-dimensional aggregates also form when the substrate is softened (51), which simultaneously decreases cell tractions and cell–substrate adhesion . This increases while keeping , and hence , constant.
The monolayer–spheroid transition has been put forward as an in vitro model for tumor formation and spreading (48). In this context, our predictions may contribute to appreciating the role of CIL in cancer progression (52) (Fig. 6). Indeed, down-regulation of cell–cell adhesion and enhanced traction forces promote metastasis, which may proceed through many steps involving collective cell migration, dissemination of cell clusters, and a final EMT (1–4, 53).
Conclusions
In summary, we studied the organization of cell colonies by means of SPP simulations. The interactions capture specific cellular behaviors such as CIL and give rise to several structures and collective dynamics (Fig. 2). Our results show how CIL leads to regular cell arrangements and hinders the formation of cohesive tissues, as well as their extrusion-mediated collapse into 3D aggregates. Self-organized collective cell motion, with tensile intercellular stresses, also emerges from CIL interactions.
In addition, we have analytically derived an effective CIL-induced cellular repulsion force, which yields explicit predictions for transitions between noncohesive, cohesive, and 3D colonies. Based on experimental observations and parameter estimates, we associate these phases to mesenchymal, epithelial, and 3D tissue phenotypes, respectively. Thus, our predictions may have implications for processes in development and disease that modify the tissue phenotype. In general, our active soft matter approach paves the way toward a physical understanding of multicellular organization and collective cell behavior.
Methods
We performed simulations of SPP in an overdamped system. Velocities are computed by solving . Positions are updated using an explicit Euler scheme and the orientations using the Euler–Maruyama method, with . We simulate rectangular domains of – cells, enclosed by means of a stiff repulsive potential. To avoid boundary effects, cells close to the border are excluded from the analysis. A full description of the methods is given in SI Appendix.
Supplementary Material
Acknowledgments
We thank E. Bazelières and X. Trepat for experimental help and T. Odenthal and S. Vanmaercke for help with implementation and proofreading. H.R. and B.S. acknowledge support from the Agency for Innovation by Science and Technology in Flanders (no. 111504) and KU Leuven Research Fund (PF/2010/07). R.A. acknowledges support from Fundació “La Caixa,” Ministry of Economy, Industry and Competitiveness (MINECO) (FIS2013-41144-P), and Departament d’Universitats, Recerca i Societat de la Informació (DURSI) (2014-SGR-878). I.P. acknowledges support from MINECO (FIS 2011-22603), DURSI (2014SGR-922), and Generalitat de Catalunya under Program Icrea Acadèmia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521151113/-/DCSupplemental.
References
- 1.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3(5):362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10(7):445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(6):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Nieto MA. Epithelial plasticity: A common theme in embryonic and cancer cells. Science. 2013;342:708. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 5.Méhes E, Vicsek T. Collective motion of cells: From experiments to models. Integr Biol. 2014;6:831–854. doi: 10.1039/c4ib00115j. [DOI] [PubMed] [Google Scholar]
- 6.Szabó A, Mayor R. Modelling collective cell migration of neural crest. Curr Opin Cell Biol. 2016;42:22–28. doi: 10.1016/j.ceb.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabó B, et al. Phase transition in the collective migration of tissue cells: Experiment and model. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74(6):061908. doi: 10.1103/PhysRevE.74.061908. [DOI] [PubMed] [Google Scholar]
- 8.Belmonte JM, Thomas GL, Brunnet LG, de Almeida RMC, Chaté H. Self-propelled particle model for cell-sorting phenomena. Phys Rev Lett. 2008;100:248702. doi: 10.1103/PhysRevLett.100.248702. [DOI] [PubMed] [Google Scholar]
- 9.Henkes S, Fily Y, Marchetti MC. Active jamming: Self-propelled soft particles at high density. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;84:040301. doi: 10.1103/PhysRevE.84.040301. [DOI] [PubMed] [Google Scholar]
- 10.Basan M, Elgeti J, Hannezo E, Rappel W-J, Levine H. Alignment of cellular motility forces with tissue flow as a mechanism for efficient wound healing. Proc Natl Acad Sci USA. 2013;110:2452–2459. doi: 10.1073/pnas.1219937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepúlveda N, et al. Collective cell motion in an epithelial sheet can be quantitatively described by a stochastic interacting particle model. PLoS Comput Biol. 2013;9:e1002944. doi: 10.1371/journal.pcbi.1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deforet M, Hakim V, Yevick HG, Duclos G, Silberzan P. Emergence of collective modes and tridimensional structures from epithelial confinement. Nat Commun. 2014;5:3747. doi: 10.1038/ncomms4747. [DOI] [PubMed] [Google Scholar]
- 13.Woods ML, et al. Directional collective cell migration emerges as a property of cell interactions. PLoS One. 2014;9:e104969. doi: 10.1371/journal.pone.0104969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarle V, Ravasio A, Hakim V, Gov NS. Modeling the finger instability in an expanding cell monolayer. Integr Biol. 2015;7:1218–1227. doi: 10.1039/c5ib00092k. [DOI] [PubMed] [Google Scholar]
- 15.Mones E, Czirók A, Vicsek T. Anomalous segregation dynamics of self-propelled particles. New J Phys. 2015;17:063013. doi: 10.1088/1367-2630/17/6/063013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia S, et al. Physics of active jamming during collective cellular motion in a monolayer. Proc Natl Acad Sci USA. 2015;112(50):15314–15319. doi: 10.1073/pnas.1510973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann J, Camley BA, Rappel W-J, Levine H. Contact inhibition of locomotion determines cell-cell and cell-substrate forces in tissues. Proc Natl Acad Sci USA. 2016;113(10):2660–2665. doi: 10.1073/pnas.1522330113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camley BA, Zimmermann J, Levine H, Rappel W-J. Emergent collective chemotaxis without single-cell gradient sensing. Phys Rev Lett. 2016;116:098101. doi: 10.1103/PhysRevLett.116.098101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abercrombie M, Heaysman JEM. Observations on the social behavior of cells in tissue culture. Exp Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- 20.Abercrombie M, Ambrose EJ. Interference microscope studies of cell contacts in tissue culture. Exp Cell Res. 1958;15:332–345. doi: 10.1016/0014-4827(58)90034-x. [DOI] [PubMed] [Google Scholar]
- 21.Davis JR, et al. Inter-cellular forces orchestrate contact inhibition of locomotion. Cell. 2015;161:361–373. doi: 10.1016/j.cell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai RA, Gopal SB, Chen S, Chen CS. Contact inhibition of locomotion probabilities drive solitary versus collective cell migration. J R Soc Interface. 2013;10:20130717. doi: 10.1098/rsif.2013.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmona-Fontaine C. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villar-Cerviño V, et al. Contact repulsion controls the dispersion and final distribution of Cajal-Retzius cells. Neuron. 2013;77:457–471. doi: 10.1016/j.neuron.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis JR, et al. Emergence of embryonic pattern through contact inhibition of locomotion. Development. 2012;139:4555–4560. doi: 10.1242/dev.082248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fily Y, Marchetti MC. Athermal phase separation of self-propelled particles with no alignment. Phys Rev Lett. 2012;108:235702. doi: 10.1103/PhysRevLett.108.235702. [DOI] [PubMed] [Google Scholar]
- 27.Fily Y, Henkes S, Marchetti MC. Freezing and phase separation of self-propelled disks. Soft Matter. 2014;10:2132–2140. doi: 10.1039/c3sm52469h. [DOI] [PubMed] [Google Scholar]
- 28.Coffey WT, Kalmykov YP, Waldron JT. The Langevin Equation. 2nd Ed World Scientific; Singapore: 2004. [Google Scholar]
- 29.Mladek BM, Gottwald D, Kahl G, Neumann M, Likos CN. Formation of polymorphic cluster phases for a class of models of purely repulsive soft spheres. Phys Rev Lett. 2006;96:045701. doi: 10.1103/PhysRevLett.96.045701. [DOI] [PubMed] [Google Scholar]
- 30.Levis D, Berthier L. Clustering and heterogeneous dynamics in a kinetic Monte-Carlo model of self-propelled hard disks. Phys Rev E Stat Nonlin Soft Matter Phys. 2014;89:062301. doi: 10.1103/PhysRevE.89.062301. [DOI] [PubMed] [Google Scholar]
- 31.Moreno AJ, Likos CN. Diffusion and relaxation dynamics in cluster crystals. Phys Rev Lett. 2007;99:107801. doi: 10.1103/PhysRevLett.99.107801. [DOI] [PubMed] [Google Scholar]
- 32.Redner GS, Baskaran A, Hagan MF. Reentrant phase behavior in active colloids with attraction. Phys Rev E Stat Nonlin Soft Matter Phys. 2013;88(1):012305. doi: 10.1103/PhysRevE.88.012305. [DOI] [PubMed] [Google Scholar]
- 33.Bray AJ. Theory of phase-ordering kinetics. Adv Phys. 1994;43:357–459. [Google Scholar]
- 34.Douezan S, Brochard-Wyart F. Dewetting of cellular monolayers. Eur Phys J E Soft Matter. 2012;35:34. doi: 10.1140/epje/i2012-12034-9. [DOI] [PubMed] [Google Scholar]
- 35.Douezan S, et al. Spreading dynamics and wetting transition of cellular aggregates. Proc Natl Acad Sci USA. 2011;108(18):7315–7320. doi: 10.1073/pnas.1018057108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaune G, et al. How cells flow in the spreading of cellular aggregates. Proc Natl Acad Sci USA. 2014;111(22):8055–8060. doi: 10.1073/pnas.1323788111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cochet-Escartin O, et al. Border forces and friction control epithelial closure dynamics. Biophys J. 2014;106(1):65–73. doi: 10.1016/j.bpj.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anon E, et al. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc Natl Acad Sci USA. 2012;109(27):10891–10896. doi: 10.1073/pnas.1117814109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roycroft A, Mayor R. Molecular basis of contact inhibition of locomotion. Cell Mol Life Sci. 2016;73(6):1119–1130. doi: 10.1007/s00018-015-2090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redner GS, Hagan MF, Baskaran A. Structure and dynamics of a phase-separating active colloidal fluid. Phys Rev Lett. 2013;110(5):055701. doi: 10.1103/PhysRevLett.110.055701. [DOI] [PubMed] [Google Scholar]
- 41.Kadir S, Astin JW, Tahtamouni L, Martin P, Nobes CD. Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J Cell Sci. 2011;124:2642–2653. doi: 10.1242/jcs.087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brugues A, et al. Forces driving epithelial wound healing. Nat Phys. 2014;10:683–690. doi: 10.1038/nphys3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bazellières E, et al. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincent R, et al. Active tensile modulus of an epithelial monolayer. Phys Rev Lett. 2015;115:248103. doi: 10.1103/PhysRevLett.115.248103. [DOI] [PubMed] [Google Scholar]
- 45.Maruthamuthu V, Gardel ML. Protrusive activity guides changes in cell-cell tension during epithelial cell scattering. Biophys J. 2014;107(3):555–563. doi: 10.1016/j.bpj.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plutoni C, et al. P-cadherin promotes collective cell migration via a Cdc42-mediated increase in mechanical forces. J Cell Biol. 2016;212(2):199–217. doi: 10.1083/jcb.201505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theveneau E, Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci. 2013;70:3481–3492. doi: 10.1007/s00018-012-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez-Rodriguez D, Guevorkian K, Douezan S, Brochard-Wyart F. Soft matter models of developing tissues and tumors. Science. 2012;388(6109):910–917. doi: 10.1126/science.1226418. [DOI] [PubMed] [Google Scholar]
- 49.Ryan PL, Foty RA, Kohn J, Steinberg MS. Tissue spreading on implantable substrates is a competitive outcome of cell-cell vs. cell-substratum adhesivity. Proc Natl Acad Sci USA. 2001;98(8):4323–4327. doi: 10.1073/pnas.071615398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravasio A, et al. Regulation of epithelial cell organization by tuning cell-substrate adhesion. Integr Biol. 2015;7:1228–1241. doi: 10.1039/c5ib00196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douezan S, Dumond J, Brochard-Wyart F. Wetting transitions of cellular aggregates induced by substrate rigidity. Soft Matter. 2012;8(17):4578–4583. [Google Scholar]
- 52.Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281:259–262. [Google Scholar]
- 53.Cheung KJ, Ewald AJ. A collective route to metastasis: Seeding by tumor cell clusters. Science. 2016;352(6282):167–169. doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.