Significance
Our knowledge of the diet of early hominins derives mainly from animal skeletal remains found in archaeological sites, leading to a bias toward a protein-based diet. We report on the earliest known archive of food plants found in the superimposed Acheulian sites excavated at Gesher Benot Ya‘aqov, Israel. These remains, some 780,000 y old, comprise 55 taxa, including nuts, fruits, seeds, vegetables, and plants producing underground storage organs. They reflect a varied plant diet, staple plant foods, seasonality, and hominins’ environmental knowledge and use of fire in food processing. Our results change previous notions of paleo diet and shed light on hominin abilities to adjust to new environments and exploit different flora, facilitating population diffusion, survival, and colonization beyond Africa.
Keywords: Acheulian, food plants, paleo diet, use of fire, seasonality
Abstract
Diet is central for understanding hominin evolution, adaptation, and environmental exploitation, but Paleolithic plant remains are scarce. A unique macrobotanical assemblage of 55 food plant taxa from the Acheulian site of Gesher Benot Ya‘aqov, Israel includes seeds, fruits, nuts, vegetables, and plants producing underground storage organs. The food plant remains were part of a diet that also included aquatic and terrestrial fauna. This diverse assemblage, 780,000 y old, reflects a varied plant diet, staple plant foods, environmental knowledge, seasonality, and the use of fire in food processing. It provides insight into the wide spectrum of the diet of mid-Pleistocene hominins, enhancing our understanding of their adaptation from the perspective of subsistence. Our results shed light on hominin abilities to adjust to new environments, facilitating population diffusion and colonization beyond Africa. We reconstruct the major vegetal foodstuffs, while considering the possibility of some detoxification by fire. The site, located in the Levantine Corridor through which several hominin waves dispersed out of Africa, provides a unique opportunity to study mid-Pleistocene vegetal diet and is crucial for understanding subsistence aspects of hominin dispersal and the transition from an African-based to a Eurasian diet.
Diet is central for understanding the evolution, adaptation, environmental exploitation, cognition, technology, and survival of prehistoric hominins. Reconstructions of Acheulian diets are based on skeletal material (1), isotopic signatures (2), ecological models reconstructing African paleoenvironments (3), comparative studies of primate behavior, especially that of chimpanzees and bonobos (4), and the diets of modern hunter-gatherers (5).
Direct data on Paleolithic plant diets are scarce, because plant remains are perishable, and most information is circumstantial (e.g., isotopic ratios reflecting C3/C4 plant taxa use relations) and insufficient for detailed reconstruction (1). Direct but limited evidence for plant consumption is sometimes found, however, in calculus (6). Earlier overemphasis of animal proteins and fats in reconstructions of prehistoric diet because of their better-preserved remains has been somewhat moderated by using ethnographic analogies (7). Recently, marine resources have also been considered (8) but are relevant only for coastal or aquatic-related sites. Overall, understanding the role of plants in early hominin diets has been based on meager direct evidence from only a few sites, such as Kalambo Falls in Africa (9) and Kärlich in Europe (10).
The waterlogged Acheulian site of Gesher Benot Ya‘aqov (GBY) yielded many well-preserved macrobotanical remains, including wood and bark (11); fruits, nuts, and seeds (12, 13); and pollen (14). Many inedible food plant seeds belong to species that have other plant food organs, such as vegetables and underground storage organs (USOs) (13, 15). These plant assemblages, originating in the Levantine Corridor through which several hominin waves dispersed out of Africa, create a special opportunity to study hominin vegetal diet during Early–Middle Pleistocene times. GBY is, thus, a key site for understanding hominin dispersal and colonization out of Africa from the perspective of plant food gathering, nutrition, and seasonality, illuminating the transition from an African-based diet to a Eurasian one. Our focus on plant foods stems from GBY’s exceptionally rich botanical remains as well as comparisons with diets of current hunter-gatherers (16) and wild food plant gathering in traditional Near Eastern societies.
The Hula Valley (196 km2) (17) and the surrounding mountains form a catchment area of ∼1,500 km2, harboring over 300 food plant species (18) (Table S1). The immediate environmental setting of GBY included three different habitats: (i) lake, (ii) terrestrial, and (iii) wetlands that are seasonally flooded in some years, depending on the lake’s water level.
Table S1.
Common edible plants of wet habitats of the Upper Jordan Valley
| Species | Abundance (17) | Edible part | Processing | GBY |
| USOs | ||||
| Alisma plantago-aquatica (18) | Common | Rhizome | Raw (dried), roasted | G |
| Alisma lanceolatum (56) | Common | Rhizome | Raw (dried), roasted | S |
| Arundo donax (18) | Common | Rhizome | Cooked, baked, roasted | — |
| Butomus umbellatus (18) | Common | Rhizome | Cooked, roasted | S |
| Calystegia sepium (18) | Common | Rhizome | Cooked | — |
| Cyperus rotundus (18) | Common | Rhizome | Raw, roasted | G |
| Lycopus europaeus (18) | Common | Rhizome | Raw, cooked | S |
| Lythrum salicaria (18) | Common | Root | Raw | |
| Nuphar lutea (18) | Very common | Rhizome | Cooked, baked | S |
| Phragmites australis (18) | Common | Rhizome | Raw, cooked, roasted | — |
| Potamogeton pectinatus (18) | Common | Rhizome | Raw | S |
| Scirpus maritimus (18, 57) | Common | Rhizome | Raw, cooked, roasted | G |
| Typha domingensis (18) | Common | Young rhizome | Cooked, roasted | G |
| Fruits | ||||
| F. carica (18) | Common | Fruit | Raw | S |
| Rubus sanguineus (18) | Common | Fruit | Raw, cooked | S |
| Seeds | ||||
| Catabrosa aquatica (18) | Common | Seed | Baked? | — |
| Foeniculum vulgare (18) | Common | Seed | Raw, cooked | S |
| Melilotus albus (18) | Common | Seed | Cooked | — |
| Nuphar lutea (18) | Very common | Seed | Raw, cooked, baked | S |
| Plantago lanceolata (18) | Common | Seed | Baked | — |
| Sorghum halepense (18) | Very common | Grain | Raw, cooked, baked | — |
| Salix alba (18) | Common | Inner bark | Raw | G |
| Vegetables | ||||
| Adiantum capillus-veneris (18) | Very common | Leaf | Raw | — |
| Apium nodiflorum (18) | Common | Leaf | Raw | — |
| Calystegia sepium (18) | Common | Shoot | Raw | — |
| Ceratophyllum demersum (18) | Common | Leaf | Raw? | S |
| Echinochloa crus-galli (18) | Very common | Young stem | Raw, cooked | — |
| Foeniculum vulgare (18) | Common | Young stem, leaf, fruit | Raw, cooked | S |
| Helminthotheca echioides (18) | Common | Young leaf | Raw | — |
| Lemna minor (18) | Common | Leaf | Raw | — |
| Lepidium latifolium (18) | Common | Young leaf | Raw, cooked | — |
| Lythrum salicaria (18) | Common | Young leafy shoots | Raw | — |
| Melilotus albus (18) | Common | Young stem, leaf, pod | Raw | — |
| Mentha aquatica (18) | Common | Leaf | Raw, cooked | — |
| Mentha pulegium (18) | Common | Leaf | Raw, cooked | — |
| Nasturtium officinale (18) | Common | Leaf | Raw, cooked | S |
| Nuphar lutea (18) | Very common | Leaf, petiole | Cooked | S |
| Persicaria lapathifolia (18) | Common | Young leaf | Raw, cooked | S |
| Phragmites australis (18) | Common | Young shoot | Cooked, roasted | — |
| Plantago lanceolata (18) | Common | Young leaf | Raw, cooked | — |
| Potamogeton pectinatus (18) | Common | Leaf, stem | Raw? | S |
| Salix alba (18) | Common | Leaf, young shoot | Raw, baked | G |
| Scirpus holoschoenus (58) | Common | Basal parts of stems | Raw | S |
| Sparganium erectum (59) | Common | Stem base | Raw, cooked | S |
| Trifolium repens (18) | Common | Young leaf | Raw | G |
| Typha domingensis (18) | Common | Shoot | Cooked, roasted | G |
| Verbena officinalis (18) | Common | Leaf | Cooked | S |
| Veronica anagallis-aquatica (18) | Common | Leaf | Raw, cooked | — |
The table presents 48 edible vegetal organs that originated from 37 species that currently inhabit wet ecosystems in the Upper Jordan Valley. The list includes wet habitat species that are common in the Upper Jordan Valley today. Fifteen of these species are common to the present day Upper Jordan Valley and GBY. Seven other species have either related species or members of the same genus that were identified in the GBY assemblages. The common food types in the wet habitat are green vegetables (26 items) followed by USOs (13), seeds (7), and fruits (2). Of these vegetal items, 32 (67%) can reportedly be consumed raw (23 green vegetables, 8 USOs, 2 fruits, and 4 seeds), whereas the other 12 items (4 green vegetables, 6 USOs, and 2 seeds) are consumed after processing by fire (roasting or cooking). G, the same genus was identified at GBY; S, the same species is present at GBY.
GBY is located in the southern Hula Valley and assigned to the Lower–Middle Pleistocene (marine isotope stages) (18–20), ∼780,000 y ago (12). Excavations and deep core drillings revealed a thick sedimentary sequence of the paleo-Lake Hula margin (19) deformed by later intensive tectonics (20) and including 26 archaeological layers, among which 15, estimated to represent occupations of about 50 ka, are rich (12, 19). Stone artifacts, fossil animal bones, and well-preserved organic remains provide data on the Acheulian paleoenvironment, ecology, habitat, and cultural realm.
Reconstructions of the paleolake margin and the flora of its diverse adjacent habitats are based on paleobotanical remains (12–14, 18) compared with the current local flora (17), while taking into account differences imposed by recent anthropogenic environmental changes and agricultural activity. Most tree species found at GBY (13) still grow today within a 1-km radius from GBY, an indication of a Mediterranean climate 780,000 y ago.
Results
The botanical remains discussed here have two different sources: archaeological layers and geological layers devoid of archaeological remains (Methods and Table S2). The strata must have been rapidly sealed, because in the Mediterranean climate, deterioration of uncharred plant material exposed to atmospheric conditions is swift.
Table S2.
Archaeological horizons bearing macrobotanical remains discussed in this study arranged by stratigraphic location from youngest to oldest (top to bottom, respectively)
| Area and layer | Level | Area* | Lithic counts | ||
| Volume† | >2 cm‡ | ≤2 cm§ | |||
| C | |||||
| V-5 | 6.39 | 1.59 | 408 | 36,770 | |
| V-6 | 7.04 | 1.97 | 356 | 6,585 | |
| A | |||||
| I-4 | 5.25 | 1.57 | 32 | 6,696 | |
| I-5 | 5.0 | 0.55 | 63 | 15,350 | |
| B | |||||
| II-2/3 | 4.67 | 0.47 | 139 | 7,502 | |
| II-5 | 25.0 | 13.0 | 180 | 3,903 | |
| II-5/6 | 19.14 | 0.38 | 142 | 10,531 | |
| II-6 | L1 | 23.79 | 4.28 | 2,295 | 58,086 |
| II-6 | L2 | 25.62 | 3.07 | 1,412 | 79,670 |
| II-6 | L3 | 17.92 | 2.50 | 1,199 | 96,094 |
| II-6 | L4 | 16.64 | 2.16 | 1,729 | 118,434 |
| II-6 | L4b | 13.69 | 0.82 | 768 | 8,778 |
| II-6 | L5 | 13.39 | 1.20 | 450 | 37,609 |
| II-6 | L6 | 12.62 | 1.38 | 732 | 13,357 |
| II-6 | L7 upper occupation | 12.60 | 1.38 | 1,098 | 25,915 |
| II-6 | L7 northern test pit | 2.75 | 1.51 | 332 | 12,555 |
| II-6 | L7 southern test pit | 4.25 | 2.89 | 104 | 6,874 |
| Total | 215.76 | 40.72 | 11,439 | 544,709 | |
Area is in square meters, and volume is in cubic meters and counts of lithics.
Area represents the spatial extent of the excavated material.
Volume is the excavated area multiplied by the estimated mean of excavated thickness based on cross-sections.
Lithic counts represent the total number of lithic artifacts of all raw materials, including items larger than 2 cm (i.e., macroartifacts: flakes and flake tools, cores and core tools, and bifacial tools).
Lithic counts represent the total number of lithic artifacts of all raw materials, including smaller items (i.e., microartifacts).
We consider plants species to be food plants when they are consumed by recent rural societies, a minimal criterion because the more hardy Paleolithic hominins probably consumed additional plant taxa that are not used today. We assume that most of the food plant remains were brought to the site deliberately by hominins rather than by natural agents. However, an unknown proportion of the food plant remains may have arrived without hominin intervention. We used food plant frequency in archaeological vs. geological layers as evidence for their deliberate collection by the Acheulian inhabitants. Although some plant species could have been used for other purposes (fibers, medicine, fish poisoning, and tool making), we focused on food plants. We paid special attention to food plant taxa that appeared in conspicuously high proportions in at least four archaeological layers and were not common in geological ones.
Macrobotanical remains were discovered in both archaeological and geological layers through the entire 34-m depositional sequence, except for a single lignite layer (II-16). Over 100,000 macrobotanical fragments were studied. Of these fragments, the minimal number of seeds or fruits is 22,714, of which 20,912 were identified to the species/genus level. Poor preservation made 1,802 seeds and fruits unidentifiable. The identified specimens comprise 117 taxa (78 species and 39 genera), including 48 nonfood taxa (Table S3).
Table S3.
List of non-edible plant taxa found at GBY
| Dry Habitat | Wet Habitat |
| Adonis sp. | Aldrovanda vesiculosa |
| Ajuga cf. chamaepites | Ceratophyllum cf. submersum |
| cf. Anagallis | Cladium mariscus |
| Amaranthus sp. | Cyperus cf. articulatus |
| Anthemis sect. Maruta | Glinus lotoides |
| Anthemis cf. pseudocotula | Heliotropium supinum |
| Bifora testiculata | Hypericum cf. hircinum |
| Bupleurum lancifolium | Najas foveolata |
| Chrozophora sp. | Najas minor |
| cf. Crepis | Potamogeton acutifolius/trichoides |
| Erodium gruinum | Potamogeton coloratus/polygonifolius |
| Euphorbia aulacosperma | Ranunculus sceleratus |
| Euphorbia chamaesyce/maculata | Ranunculus subgen. Batrachium |
| Euphorbia helioscopia | Stratiotes intermedius |
| Euphorbia valerianifolia type | Verbena officinalis |
| Fumaria sp. | Verbena supina |
| Heliotropium cf. europaeum | Vitex sp. |
| Hymenocarpos circinnatus | Zannichellia palustris |
| Hypericum cf. triquetrifolium | |
| Mercurialis annua | |
| Ochthodium aegyptiacum | |
| Picris cf. altissima | |
| Ranunculus arvensis | |
| Ranunculus cf. marginatus | |
| Ricinus communis | |
| Solanum villosum | |
| Stipa bromoides | |
| Styrax officinalis | |
| Thymelaea passerina | |
| Valerianella cf. muricata |
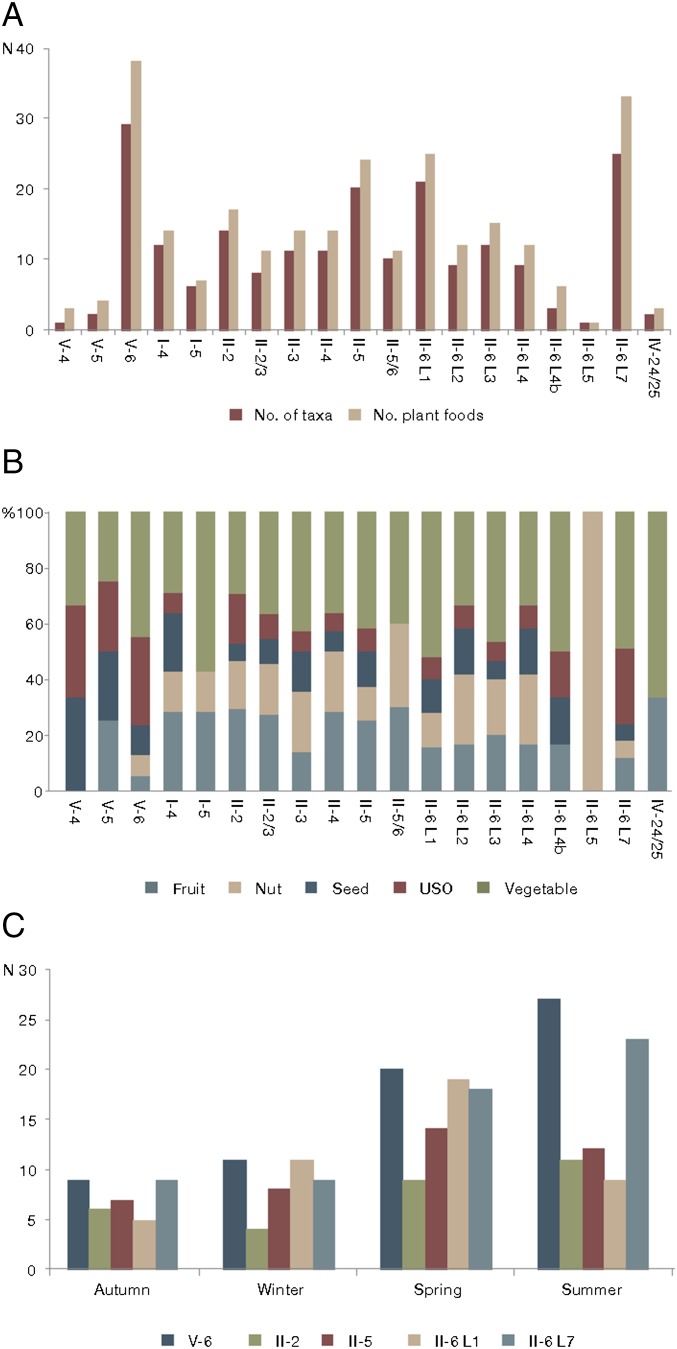
The food plant assemblage comprises 9,148 plant remains (Fig. 1) belonging to at least 55 species. The exact number of species is uncertain, because 11 taxa were identified only to the genus level, and some of their remains belong to several different but unidentified species within these genera. They include nuts, species producing USOs, fruits, seeds, and vegetables (Table S4). In 11 edible species, several organs are eaten (Table S4). Some of the archaeological horizons are significantly richer and more diverse in edible taxa than others (Fig. 2, Dataset S1, and Tables S5 and S6).
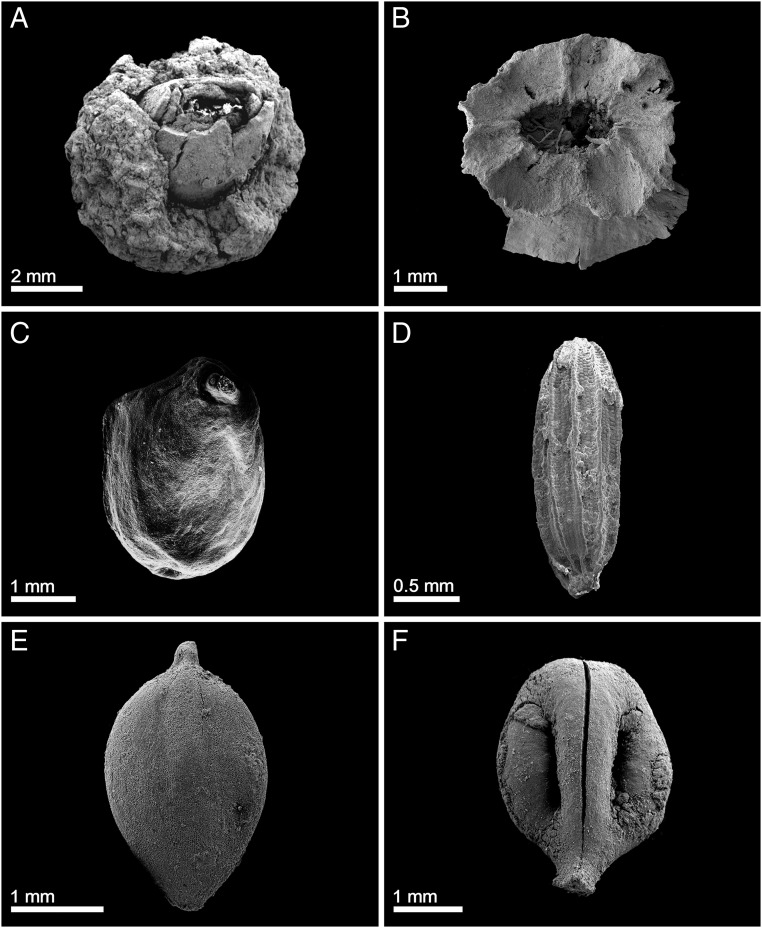
Fig. 1.
Food plant remains from GBY. (A) Quercus sp., young cupule (layer II-6 L1); (B) T. natans, upper tip of nut (layer III-7); (C) Nuphar luteum, seed (layer II-7); (D) B. umbellatus, seed (layer III-4); (E) Scirpus lacustris, seed (layer II-9); and (F) Vitis sylvestris, pip (layer IV-7).
Table S4.
Edible species found at GBY and their present frequency in the Hula Valley: edible organs, total amount found at the site by frequency of appearance, seasonality, and edible parts
| Species | Edible organ | Remnant in GBY | Finds in GBY (n organs/n layers) | Presence in the Hula Valley | Main season of exploitation |
| Nuts | |||||
| E. ferox | Seed* (21) | Seed | 641/21 | Ex | End of summer (21) |
| Pistacia atlantica | Nutlet* (18) | Nutlet | 1/1 | HF | Autumn (18) |
| Quercus calliprinos/ithaburensis | Nut* (60) | Acorn, cupule | 88/17 | F | Autumn–winter (18) |
| Trapa natans | Nut* (18) | Nut (calyx) | 872/38 | F | Autumn (18) |
| Species producing USOs | |||||
| Alisma lanceolatum | Rhizome† (18) | Seed | 120/16 | F | Summer (18) |
| Butomus umbellatus | Rhizome† (60) | Seed | 43/12 | F | Summer (18) |
| Damasonium alisma | Rhizome (18) | Seed | 16/8 | LF | Summer (18) |
| Lycopus europaeus | Rhizome* (18) | Mericarp | 776/22 | F | Summer (18) |
| Myriophyllum spicatum | Rhizome* (18) | Nutlet | 130/14 | LF | Summer (18) |
| Nuphar lutea | Rhizome† (18) | Seed | 880/22 | F | Throughout the year‡ |
| Nymphaea alba | Rhizome† (18) | Seed | 192/13 | F | Throughout the year‡ |
| Potamogeton crispus | Rhizome (18) | Nutlet | 20/3 | — | Summer (18) |
| Potamogeton pectinatus | Rhizome (18) | Nutlet | 5/4 | — | Summer (18) |
| Sagittaria sagittifolia | Rhizome† (18) | Seed | 116/19 | Ex | Summer–autumn (18) |
| Scirpus lacustris | Rhizome* (18) | Nutlet | 2243/18 | F | Summer (18) |
| Silybum marianum | Root* (18) | Achene | 42/13 | HF | Winter–spring (18) |
| Sparganium erectum | Rhizome† (59) | Nutlet | 3/2 | F | Summer‡ |
| Typha cf domingensis | Rhizome (60) | Nutlet | 75/14 | F | Throughout the year (18) |
| Fruits | |||||
| Capparis cf spinosa | Young fruit (60) | Seed | 49/16 | HF | Spring–summer (58) |
| F. carica | Fruit (18) | Seed | 79/12 | F | End of summer (61) |
| Olea europaea | Fruit (18) | Stone | 388/14 | F | Autumn (60) |
| Rubus cf sanguineus | Fruit (60) | Nutlet | 313/25 | F | Summer–autumn (18) |
| Sambucus sp. | Fruit (18) | Seed | 2/1 | — | End of summer–autumn (58) |
| V. sylvestris | Fruit (18) | Pip | 395/41 | LF | End of summer–autumn (18) |
| Ziziphus spina-christi | Fruit (60) | Stone | 10/8 | HF | Autumn/spring (62) |
| Seeds | |||||
| Aegilops cf geniculata | Grain* (18) | Grain | 2/2 | F | Spring–beginning of summer (18) |
| Avena sp. | Grain* (18) | Grain | 2/2 | HF | Spring–beginning of summer (18) |
| Carthamus sp. | Seed‡ | Achene | 7/5 | F | Summer‡ |
| Chenopodium sp. | Seed† (60) | Seed | 69/13 | F | Summer (18) |
| Hordeum spontaneum | Grain* (18) | Grain | 1/1 | F | Spring–summer (18) |
| Malva sp. | Seed* (60) | Mericarp | 3/2 | F | Winter–spring (18) |
| Nuphar lutea | Seed* (18) | Seed | 880/22 | F | Summer (18) |
| Onobrychis sp. | Seed† (60) | Pod | 21/11 | F | Spring–summer‡ |
| Silybum marianum | Seed† (60) | Achene | 42/13 | HF | Spring–beginning of summer (18) |
| Species producing organs eaten as vegetables | |||||
| Alcea sp. | Stem, bud (60) | Seed | 21/12 | F | Spring (18) |
| Allium cf neapolitanum | Bulb* (18) | Seed | 1/1 | F | Winter–spring (18) |
| Beta vulgaris | Leaf* (60) | Nutlet | 29/9 | F | Winter–spring (18) |
| Ceratophyllum demersum | Leaf (18) | Nutlet | 318/30 | F | Spring–summer (18) |
| Chenopodium sp. | Leaf* (18) | Seed | 69/13 | F | Spring–summer (18) |
| Chrysanthemum coronarium | Stem, inflorescence bud* (60) | Achene | 2/2 | F | Spring (18) |
| Foeniculum vulgare | Leaf, stem (60) | Achene | 50/13 | HF | Spring–summer (18) |
| Hippuris vulgaris | Leaf* (18) | Nutlet | 40/4 | Ex | Summer–autumn (51, 63) |
| Lomelosia cf prolifera | Stem, head (60) | Calyx | 190/34 | F | Spring (18) |
| Malva sp. | Leaf,* stem† (60) | Mericarp | 3/2 | F | Winter–spring (18) |
| Medicago coronata | Leaf, stem (18) | Pod | 6/4 | F | Winter–spring (18) |
| Medicago sp. a | Leaf, stem (18) | Pod | 45/12 | F | Winter–spring (18) |
| Medicago sp. b | Leaf, stem (18) | Pod | 18/6 | F | Winter–spring (18) |
| Medicago sp. c | Leaf, stem (18) | Pod | 9/4 | F | Winter–spring (18) |
| Medicago sp. d | Leaf, stem (18) | Pod | 2/2 | F | Winter–spring (18) |
| Medicago sp. | Leaf, stem (18) | Pod | 79/23 | F | Winter–spring (18) |
| Montia minor | Leaf, stem (58) | Seed | 5/5 | Ex | Spring (58) |
| Najas delilei | Leaf, stem (18) | Nutlet | 454/10 | LF | Spring–summer (18) |
| Nasturtium/Roripa | Leaf, stem (60) | Seed | 1/1 | F/LF | Spring–autumn (18) |
| Nymphaea alba | Fruit (58) | Seed | 192/13 | F | Summer (58) |
| Nymphoides peltata | Leaf, stem (18) | Seed | 72/17 | Ex | Summer (18) |
| Persicaria lapathifolia | Leaf (18) | Nutlet | 47/5 | F | Spring–summer (18) |
| Potamogeton crispus | Leaf† (18) | Nutlet | 20/3 | — | Summer (18) |
| Potamogeton distinctus | Leaf (18), | Nutlet | 116/16 | Ex | Summer (18) |
| Potamogeton pectinatus | Leaf, stem (18) | Nutlet | 5/4 | — | Summer (18) |
| Raphanus cf raphanistrum | Young leaf stem and inflorescence (58, 64) | Silique | 55/15 | LF | Winter–spring (18) |
| Scirpus holoschoenus | Basal parts of stems (58) | Nutlet | 27/11 | F | Spring (58) |
| Scirpus lacustris | Young shoot,* base of stem* (65) | Nutlet | 2,243/18 | F | Spring–summer (18) |
| Silybum marianum | Leaf,* young stem* (18) | Achene | 42/13 | HF | Winter–spring (18) |
| Sinapis arvensis | Leaf, stem (18) | Seed | 2/1 | F | Spring (18) |
| Sparganium erectum | Stem†‡ (58) | Nutlet | 3/2 | F | Winter–summer‡ |
| Typha cf domingensis | Shoot* (18) | Nutlet | 75/14 | F | Throughout the year‡ |
| V. sylvestris | Leaf† (18) | Pip | 395/41 | LF | Spring–summer (18) |
Ex, extinct in the Hula Valley; F, frequent; HF, high frequency; LF, low frequency (after 17).
Better when cooked.
Must be cooked.
Lev-Yadun S, Melamed Y authors' field experience.
Fig. 2.
Count, frequency, types, and seasonality of food plants found at GBY arranged from youngest layer to oldest layer (left to right, respectively) (Table S5). (A) Number of taxa and plant foods in the archaeological layers. (B) Frequency of food organ types in the archaeological layers. (C) Frequency of edible organ types according to seasonality in the richest archaeological layers.
Table S5.
Frequency of edible organ types and seasonality in the archaeological layers arranged from youngest to oldest (left to right, respectively)
| Type of plant food | V-4 | V-5 | V-6 | I-4 | I-5 | II-2 | II-2/3 | II-3 | II-4 | II-5 | II-5/6 | II-6 L1 | II-6 L2 | II-6 L3 | II-6 L4 | II-6 L4b | II-6 L5 | II-6 L7 | IV-24/25 | Total |
| Autumn | — | 2 | 9 | 5 | 3 | 6 | 3 | 3 | 5 | 7 | 4 | 5 | 4 | 5 | 4 | 1 | — | 9 | 1 | 11 |
| Winter | 2 | 1 | 11 | 2 | 2 | 4 | 3 | 2 | 4 | 8 | 3 | 11 | 4 | 6 | 4 | 3 | — | 9 | — | 17 |
| Spring | 3 | 2 | 20 | 7 | 5 | 9 | 7 | 8 | 8 | 14 | 6 | 19 | 7 | 9 | 7 | 5 | — | 18 | 2 | 32 |
| Summer (+ end of summer) | 1 | 4 | 27 | 9 | 2 | 11 | 5 | 6 | 6 | 12 | 4 | 9 | 4 | 5 | 4 | 3 | 1 | 23 | 2 | 32 |
| Fruit | — | 1 | 2 | 4 | 2 | 5 | 3 | 2 | 4 | 6 | 3 | 4 | 2 | 3 | 2 | 1 | — | 4 | 1 | 6 |
| Nut | — | — | 3 | 2 | 1 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | — | 1 | 2 | — | 3 |
| Seed | 1 | 1 | 4 | 3 | — | 1 | 1 | 2 | 1 | 3 | — | 3 | 2 | 1 | 2 | 1 | — | 2 | — | 5 |
| USO | 1 | 1 | 12 | 1 | — | 3 | 1 | 1 | 1 | 2 | — | 2 | 1 | 1 | 1 | 1 | — | 9 | — | 13 |
| Vegetable | 1 | 1 | 17 | 4 | 4 | 5 | 4 | 6 | 5 | 10 | 4 | 13 | 4 | 7 | 4 | 3 | — | 16 | 2 | 26 |
| No. of taxa | 1 | 2 | 29 | 12 | 6 | 14 | 8 | 11 | 11 | 20 | 10 | 21 | 9 | 12 | 9 | 3 | 1 | 25 | 2 | 43 |
| No. of plant foods | 3 | 4 | 38 | 14 | 7 | 17 | 11 | 14 | 14 | 24 | 11 | 25 | 12 | 15 | 12 | 6 | 1 | 33 | 3 | 53 |
Table S6.
Variability in edible organ remains between archaeological layers arranged from young to old (left to right, respectively)
| Taxon | Edible organ | V-4 | V-5 | V-6 | I-4 | I-5 | II-2 | II-2/3 | II-3 | II-4 | II-5 | II-5/6 | II-6 L1 | II-6 L2 | II-6 L3 | II-6 L4 | II-6 L4b | II-6 L5 | II-6 L7 | IV-24/25 | Total |
| Carthamus sp. | Achene | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Chenopodium sp. | Seed | — | — | 3 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 3 |
| E. ferox | Seed | — | — | 1 | 1 | — | 46 | 17 | 3 | 4 | 222 | 25 | 204 | 45 | 8 | 5 | — | 1 | 18 | — | 593 |
| F. carica | Fruit | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | 1 |
| Lomelosia cf prolifera | Head | — | — | 1 | 5 | 2 | 2 | — | 3 | 1 | 4 | 1 | 26 | 2 | 1 | 1 | — | — | 4 | 2 | 51 |
| Nuphar lutea | Seed | — | 1 | 67 | 2 | — | — | — | — | — | 2 | — | — | — | — | — | — | — | 1 | — | 73 |
| Nymphaea alba | Seed | — | — | 2 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | 3 |
| Olea europaea | Fruit | — | — | — | 3 | — | 8 | 2 | — | 7 | 105 | 9 | 182 | 39 | 11 | 10 | — | — | 2 | — | 371 |
| Onobrychis sp. | Seed | — | — | 1 | 2 | — | — | — | — | — | 1 | — | 1 | — | — | — | — | — | — | — | 5 |
| Quercus calliprinos/ithaburensis | Acorn | — | — | 4 | — | — | 1 | — | 3 | 2 | 13 | 4 | 21 | 7 | 2 | 3 | — | — | — | — | 55 |
| Silybum marianum | Achene | 1 | — | 2 | — | — | 1 | 1 | — | 1 | 2 | — | 3 | 13 | 4 | 3 | 1 | — | 3 | — | 34 |
| Trapa natans | Fruit | — | — | 50 | 43 | 8 | 36 | 20 | 6 | 5 | 238 | 19 | 161 | 25 | 1 | 6 | — | — | 4 | — | 611 |
| V. sylvestris | Fruit | — | 1 | 29 | 22 | 8 | 17 | 12 | 1 | 6 | 34 | 5 | 54 | 12 | 3 | 7 | 1 | — | 8 | 4 | 217 |
| Ziziphus spina-christi | Fruit | — | — | — | — | 1 | 3 | — | — | 1 | 1 | — | 1 | — | 1 | — | — | — | — | — | 7 |
| No. of taxa with edible remains | 1 | 2 | 10 | 7 | 4 | 8 | 5 | 7 | 8 | 11 | 6 | 9 | 7 | 8 | 7 | 2 | 1 | 8 | 2 | 13 | |
| Sum of edible remains | 1 | 2 | 160 | 78 | 19 | 114 | 52 | 18 | 27 | 623 | 63 | 653 | 143 | 31 | 35 | 2 | 1 | 41 | 6 | 2,025 |
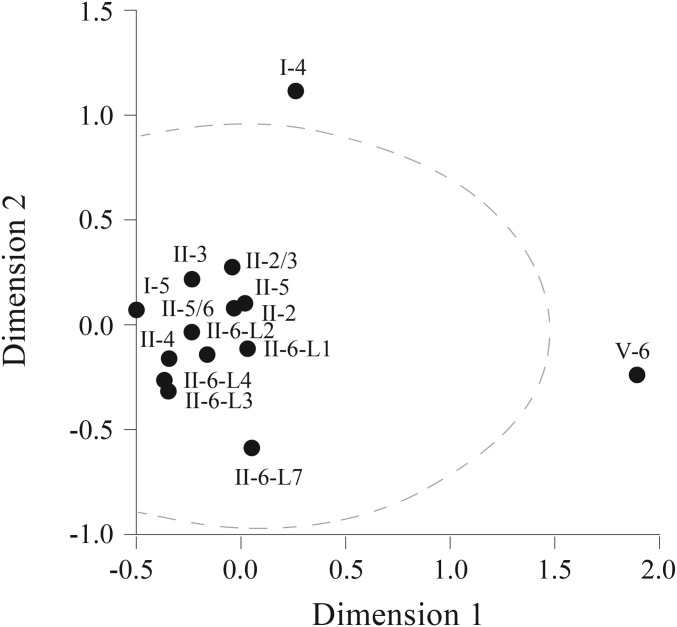
An obvious question is whether the proportion of food plant taxa differs significantly between archaeological and geological layers. Our statistical analysis [multidimensional scaling (MDS)] (Methods, Statistical Analysis) showed that most horizons are fairly similar with respect to plant taxa composition (Fig. S1); only layers I-4 and V-6 were outside the boundaries of the 95% ellipse, implying a significantly different plant composition in these two layers relative to all others. This MDS model fitted well the observed distance matrix (99% of the dispersion was accounted for, and the stress value was 0.0098).
Fig. S1.
MDS (PROXCAL) plot of 14 archaeological layers based on the Chebychev distance calculated from the proportion of 36 plant species remains. Dispersion accounted for (i.e., variance explained) =0.99, and the stress (i.e., the degree of correspondence between the distances among points calculated by the MDS map and the input matrix) =0.0098. The dashed line denotes the 95% confidence ellipse.
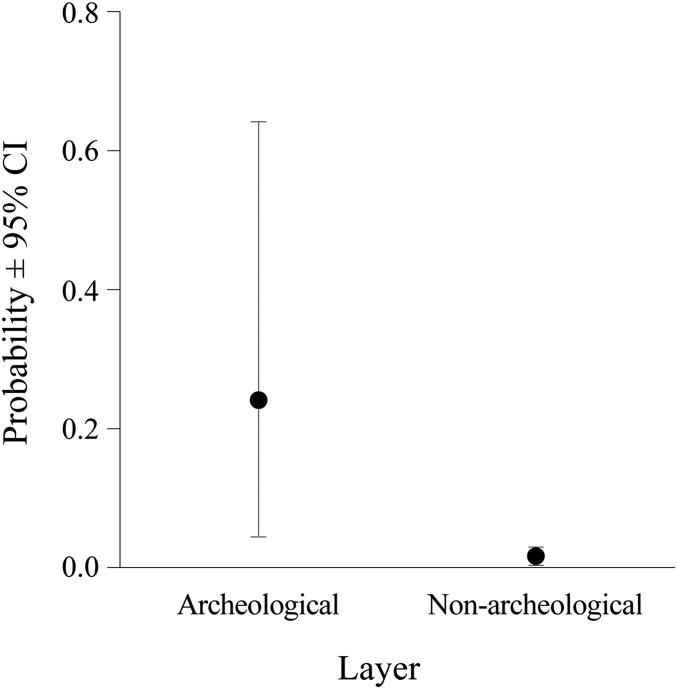
Although the probability of finding food plant remains did not differ between archaeological and geological layers when the frequency of total items or number of plant taxa was considered [likelihood ratio (LR) ≤ 0.7, P ≥ 0.389], the probability of finding items relating to key food plants (staples) [Methods, Key (Staple) Food Plants] was significantly greater in archaeological layers than in geological layers (LR = 5.1, P = 0.024) (Fig. 3). In other words, the mean probability of finding key food plants was an order of magnitude higher in archaeological layers than in nonarchaeological layers (Fig. 3).
Fig. 3.
The probability (±95% confidence intervals) of detecting the remains of a key food plant in archaeological and geological layers. This probability was significantly different between archaeological and geological layers (LR = 5.1, P = 0.024).
Discussion
There is extensive variability in the frequency of food plant taxa and organs between the different archaeological layers (Fig. 2, Dataset S1, and Tables S5 and S6): four archaeological assemblages (layers V-6, II-5, II-6 level 1, and II-6 level 7) are richer than others in plant foods (Fig. 2, Dataset S1, and Table S5). This high variability between the archaeological layers is not related to the sedimentary environment (Methods, Sampling and Sorting of Plant Remains), and after calculation of the number of potential types of edible organs, vegetables were seen to be the most frequent followed by USOs (Fig. 2 and Table S5). However, the actual number of plant remains shows that the most nutritious nuts and USO-producing species were the most common followed by fruits (Table S7), explaining their abundance in layer II-5 and layer II-6 level 1.
Table S7.
GBY: number of edible taxa and count of their remains (seeds, nuts, fruits, vegetables, and USOs) in the archaeological horizons from youngest to oldest (left to right, respectively)
| Type of edible organ | V-4 | V-5 | V-6 | I-4 | I-5 | II-2 | II-2/3 | II-3 | II-4 | II-5 | II-5/6 | II-6 L1 | II-6 L2 | II-6 L3 | II-6 L4 | II-6 L4b | II-6 L5 | II-6 L7 | IV-24/25 | Total |
| Fruit | ||||||||||||||||||||
| No. of edible taxa | — | — | 1 | 3 | 1 | 4 | 2 | 1 | 3 | 5 | 2 | 3 | 1 | 2 | 1 | — | — | 3 | — | 5 |
| Count | — | — | 12 | 5 | 1 | 18 | 4 | 1 | 10 | 115 | 13 | 208 | 39 | 12 | 10 | — | — | 7 | — | 455 |
| Fruit/vegetable | ||||||||||||||||||||
| No. of edible taxa | — | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | — | 1 | 1 | 1 |
| Count | — | 1 | 29 | 22 | 8 | 17 | 12 | 1 | 6 | 34 | 5 | 54 | 12 | 3 | 7 | 1 | — | 8 | 4 | 224 |
| Nut | ||||||||||||||||||||
| No. of edible taxa | — | — | 3 | 2 | 1 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | — | 1 | 2 | — | 3 |
| Count | — | — | 55 | 44 | 8 | 83 | 37 | 12 | 11 | 473 | 48 | 386 | 77 | 11 | 14 | — | 1 | 22 | — | 1,282 |
| Seed | ||||||||||||||||||||
| No. of edible taxa | — | — | 1 | 2 | — | — | — | 1 | — | 1 | — | 2 | 1 | — | 1 | — | — | — | — | 2 |
| Count | — | — | 1 | 3 | — | — | — | 1 | — | 1 | — | 4 | 1 | — | 1 | — | — | — | — | 12 |
| Seed/USO | ||||||||||||||||||||
| No. of edible taxa | — | 1 | 1 | 1 | — | — | — | — | — | 1 | — | — | — | — | — | — | — | 1 | — | 1 |
| Count | — | 1 | 67 | 2 | — | — | — | — | — | 2 | — | — | — | — | — | — | — | 1 | — | 73 |
| Seed/USO/vegetable | ||||||||||||||||||||
| No. of edible taxa | 1 | — | 1 | — | — | 1 | 1 | — | 1 | 1 | — | 1 | 1 | 1 | 1 | 1 | — | 1 | — | 1 |
| Count | 1 | — | 2 | — | — | 1 | 1 | — | 1 | 2 | — | 3 | 13 | 4 | 3 | 1 | — | 3 | — | 35 |
| Seed/vegetable | ||||||||||||||||||||
| No. of edible taxa | — | — | 1 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Count | — | — | 3 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 4 |
| USO | ||||||||||||||||||||
| No. of edible taxa | — | — | 6 | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 3 | — | 6 |
| Count | — | — | 124 | — | — | 4 | — | — | — | — | — | — | — | — | — | — | — | 32 | — | 160 |
| USO/vegetable | ||||||||||||||||||||
| No. of edible taxa | — | — | 4 | — | — | 1 | — | — | — | — | — | 1 | — | — | — | — | — | 4 | — | 5 |
| Count | — | — | 8 | — | — | 1 | — | — | — | — | — | 2 | — | — | — | — | — | 8 | — | 19 |
| Vegetable | ||||||||||||||||||||
| No. of edible taxa | — | — | 10 | 3 | 3 | 3 | 2 | 4 | 3 | 8 | 4 | 10 | 2 | 5 | 2 | 1 | — | 10 | 1 | 18 |
| Count | — | — | 96 | 10 | 5 | 4 | 2 | 7 | 3 | 23 | 4 | 56 | 5 | 6 | 2 | 1 | — | 29 | 2 | 255 |
| Total no. of taxa | 1 | 2 | 29 | 12 | 6 | 14 | 8 | 11 | 11 | 20 | 10 | 21 | 9 | 12 | 9 | 3 | 1 | 25 | 2 | 43 |
| Total no. of remains | 1 | 2 | 397 | 86 | 22 | 128 | 56 | 23 | 31 | 650 | 70 | 713 | 147 | 36 | 37 | 3 | 1 | 110 | 6 | 2,519 |
The GBY plant foods include a high diversity of plant organs that could have furnished hominins with rich year-round nutrition. Although the edible plant taxa found are only about 20% of the current Upper Jordan Valley food plant taxa (Table S1), they include six locally extinct species that existed there during the Early–Middle Pleistocene (SI Text, Extinct Species) (15). Of these species, Euryale ferox, Sagittaria sagittifolia, and Trapa natans could have been used as staple foods, because they are known to grow in dense patches in shallow water and are extant crops in East Asia (refs. 21, 22, p. 518, and 23).
The nutritional values of the food plant organs show that nuts were the most efficient food source at GBY. Two of them, Gorgon nuts (E. ferox) and water chestnut (T. natans), are highly nutritious and rich in starch and proteins. Popped seeds of Gorgon nuts contain 77% (wt/wt) carbohydrates, 9.7% (wt/wt) protein, and 0.1% fat (per 100 g) (24). The seeds of water chestnut are composed of 52% (dry wt/dry wt) starch, 15% (wt/wt) protein, and 7.5% (wt/wt) fat (23). When oak acorns are added as a starch source, combined with Olea fruits and Silybum seeds as oil sources, a picture of diverse plant-based nutrition emerges, enriched by dozens of other nonstaple food plant taxa.
Conclusions
Hominin consumption of plant foods necessitated knowledge of their growing localities, seasonality, toxicity, and availability (25). Plant phenology in the Hula Valley is mainly dictated by the Mediterranean climate (hot, dry summers but rainy winters). The seasonal or perennial lake water-level fluctuations either flooded or exposed significant areas, causing plants to respond with either growth or lack of growth. Winter and spring are rich in green vegetables (e.g., Beta vulgaris and Malva nicaeensis), whereas in late spring/early summer, many edible seeds and fruits are especially available (e.g., Silybum marianum and Ziziphus spina-christi).
Nut and fruit availability is greater in summer and autumn in the contemporary Upper Jordan Valley. When the data of all of the archaeological layers at GBY are combined, the highest productivity and availability of food plant species are in spring (32 species) and summer (32 species) (Fig. 2 and Table S5). However, each of the archaeological layers contains food plant species of all seasons, indicating year-round occupations (SI Text, Seasonality).
Two factors influenced seasonality and accessibility to food plants at GBY. First, there is a considerable altitudinal difference between the Hula Valley, the eastern Galilee Mountains, and the Golan Heights (a maximal elevation of 800 m over a minimal distance of 2.5 km), resulting in a difference of several weeks between ripening of the same species in the valley vs. the mountains (26). Second, moderate lakeshore slopes and oscillations of water level may have prolonged or shortened the seasonal availability of aquatic and bank food plants, prolonging, for instance, the summer cropping season of Scirpus rhizomes, which start growing when water retreats. The duration of palatability differs among species: some have a short season, whereas hard nuts have a long one. Acorns, for instance, are available for several months, but their seasonality is also affected by competition with animals (e.g., wild boar, rodents, and birds) that consume large amounts of acorns (27).
The GBY inhabitants had access to 14 USO-producing species (Table S4), many of which could be eaten year round. However, the palatability of USOs changes, with highly palatable young storage roots/rhizomes gradually becoming fibrous/woody and hence, less palatable. Low palatability of USOs can, in certain taxa, be handled by roasting/grinding/leaching to deactivate toxins or extract starch from fibrous tissues. There is ample evidence for the important role of fire at GBY, with its control and repeated use shown by burned lithics and charred wood, bark, grains, and fruits (28, 29). Fire was instrumental in enriching the value of the diet and adding resources that are unpalatable without heating (30). Roasting could have enabled the addition to the diet of important plant foods: Nuphar lutea rhizomes, Butomus umbellatus, and Sparganium erectum rhizomes (Table S1). Roasting improved the taste/digestibility of foods, such as oak acorns, rhizomes, and young Phragmites australis shoots, common plants that could provide considerable amounts of staple foods. Roasting also enabled popping of E. ferox (31), and fire could prolong the palatability season of tubers of Cyperus rotundus and Scirpus maritimus, which are eaten either raw or roasted (32).
The macrobotanical assemblage of GBY has a surprisingly rich vegetal dietary potential that includes dozens of edible species. It has provided unprecedented data on Acheulian plant use, illustrating the diversity of the exploited habitats that provided a large quantity and wide variety of edible plant organs found in association with a rich material culture. The seasonality of the edible plants clearly indicates that the GBY hominins could have occupied the site year round (Fig. 2 and Table S5).
We have considered a variety of possible hypotheses to explain the abundance of edible plants in the archaeological layers at GBY. Among these hypotheses were issues of taphonomy, availability, and the possible impact of paleoclimatic changes. Based on the results of this study and our past multidisciplinary studies on this site (11, 19, 28, 29, 33–38), we suggest that the abundance of food plant remains in the archaeological layers is a result of deliberate hominin behavior (Dataset S1). The remains of the key food plants are 10 times more abundant in the archaeological layers than in the geological ones (Fig. 3). This remarkable difference is evident despite the extent of interarchaeological layer variability, which also characterizes other components of the archaeological horizons (expressed by extensive differences in the frequencies of lithic artifacts, fauna, and flora) (11, 29, 39).
The food plant remains were part of a much more diverse diet that probably included additional food plant species, fish (33), amphibians, reptiles, birds, mammals, such as fallow deer (40), elephants (34), and various aquatic and terrestrial invertebrates. The remains of many food plants and animals testify to an extremely broad spectrum diet that characterized the site’s many archaeological layers. The GBY hominins applied an African typotechnology to local raw materials to make lithic artifacts that illustrate their African cultural origin but in terms of diet, adapted to plant and animal foods that only partly overlap with the African ones. The continuous presence of the lake and its fluctuating margin habitats together with the surrounding terrestrial habitats provided the GBY hominins with a wealth of food resources, enabling repeated occupations of the same locale during tens of millennia. These prolonged occupations enabled cultural adaptations to the eastern Mediterranean environment. The exploitation of new non-African animal and plant taxa testifies to Acheulian cognitive/cultural flexibility and adaptability. We propose that a wide spectrum of food plants was a permanent aspect of the preagricultural hominin economy and that its infrequent manifestation in archaeobotanical finds probably reflects taphonomic rather than cultural issues.
Located in the Levantine Corridor and reflecting a Mediterranean environment and ecology, the food plants of GBY provide insight into the resources that enabled hominins’ adaptation and survival beyond African habitats, shedding light on the mechanisms that enabled their further dispersal into Eurasia.
Methods
Plant Context, Taphonomy, and Sampling.
Various multidisciplinary studies of the record of GBY have shown that the strata were sealed rapidly and hence, preserved the components (remains of hominin activity and others) and their original spatial organization. These studies include, among others, taphonomic studies of malacology (35), crustaceans (41), fish (29), and mammals (36). In addition, a wealth of taphonomic information originating from the analyses of stone artifacts provides additional insight into the integrity of the assemblages that form the content of the archaeological horizons. It shows that there was no winnowing and hence, microartifacts retained their original spatial locations; that burned microartifacts were found clustered and thus, preserved the original location of hearths; and that some bones from several archaeological horizons were successfully conjoined, providing additional proof of their taphonomic integrity (29, 36, 42, 43). The presence of perishable organic material, such as wood, bark, fruits, and seeds (11, 13, 31), throughout the depositional sequence of GBY further testifies to rapid sealing of the horizons and hence, the integrity and depositional originality of the edible botanical remains.
Sampling and sorting of plant remains.
The excavation area and associated geological trenches are located on the left bank of the Jordan River. The trenches were dug into the Early and Middle Pleistocene deposits perpendicularly to the strike of the layers, and hence, their cross-sections provided a precise record of their thickness. The Early–Middle Pleistocene deposits were not initially visible on the surface of the bank selected for the locations of trenching or excavation.
The trenching revealed a 34-m-thick depositional sequence built of layers both containing archaeological remains (archaeological horizons) and devoid of them (geological layers) (SI Text, Archaeological and Geological Layers). Thus, the discovery of the archaeological horizons was fortuitous and moreover, revealed exceptional concentrations of finds (lithics, fossil bones, and organic materials). The interlayer differences in frequency of finds are indicative of where in the site (and the paleolandscape) the trench or excavation was placed—in the center or on the margins. Considering the above information, it is possible that layers devoid of stone lithic artifacts and consequently, designated geological may, in fact, represent the edges of archaeological layers; the case of a single microartifact (smaller than 2 cm) that was found adjacent to a wooden log in layer II-6 level 14 is an example. Theoretically, enlarging the extent of the excavation could have resulted in the discovery of finds in the layers presently designated geological. It is evident from the stone, bone, and plant finds that the GBY hominins operated beyond the areas in which stone artifacts and bones were found.
Sampling of geological layers (devoid of archaeological finds).
Samples of 0.5–5 kg sediment were obtained from different layers that were exposed in the walls of the geological trenches. The samples were placed in sealed nylon bags to keep them wet. In the laboratory, the sediments were divided into four fractions (0.3–1, 1–2, 2–4, and 4–10 mm) by wet sieving. Items larger than 1 cm were separated by hand or with large tweezers, and those smaller than 0.3 mm were lost through the lower sieve. The botanical remains from each fraction were separated and sorted by spreading the sediment on trays with water and picking them up individually with soft tweezers under a stereoscopic (binocular) microscope at a magnification of up to 25×.
Sampling of archaeological layers.
The entire volume of sediment excavated from the archaeological horizons was wet-sieved during fieldwork by a 2-mm sieve, and hence, the remains are limited to items larger than 2 mm. The wet-sieved sediments were then dried and bagged with their recorded information and transported to the Institute of Archaeology for additional analysis. Sorting of the sieved sediments yielded rich and varied assemblages, such as fruits, seeds, grains, mammalian bones and teeth, fish bones, crab skeletons, and specks of charcoal. Many of the seeds and fruits studied here (4,199 of 25,835) were retrieved by this procedure. The small-seeded species (e.g., Alisma lanceulatum, Chenopodium sp., and Lycopus europaeus) are underrepresented in these samples, because they were retrieved only when they were stuck or buried in large (>2 mm) lumps of mud. Because the wet-sieved sediments were transported from the field with their recorded location, these seeds and fruits could be located within the sediment with a precision of 0.5 × 0.5 × 0.5 m.
Photography.
Seeds were photographed to add a visual illustration, serve as a basis for future comparison of ancient Levantine flora, and rarely, obtain greater confidence in the identifications. Special emphasis was placed on seeds of exotic species. Photography was carried out with a scanning electron microscope (JEOL model JMS-840) of 10×–100,000× magnification and a stereoscopic microscope (Olympus model SZX12) of up to 90× magnification.
Seeds were cleaned by immersion in water using paintbrushes and needles to prepare them for photography. Seeds prepared for SEM photography were pasted on a stab and coated with gold for 10–20 min (depending on their size, shape, and texture). Waterlogged seeds are difficult to dry without destruction of shape and therefore, were fixed by substituting the water with organic materials (critical point drying method). SEM photography was performed at the Faculty of Life Sciences, Bar-Ilan University with the help of Yakov Langsam. The microscope digital photographs were processed by image-editing software (Paint Shop Pro-7). In cases of specimens larger than 1 cm (the maximal size of the SEM chamber used), two parts of the seed were photographed successively and later combined. In cases where it was impossible to achieve the same focus for the two pictures, the determination of the size of the object was slightly affected. In such cases, a small space was left between the two photographs. Tiny pieces of carbon glue strips were attached to the side of the specimen or filled spaces between specimens to overcome difficulties in SEM photography resulting from the height of large specimens.
Taxonomic Identification.
Seed and fruit identification and calculation of the number of specimens.
Waterlogged seeds are extremely sensitive to dryness and can lose their shape easily. Consequently, their processing requires gentle handling during identification and preparation for photography. Furthermore, many of the seeds were found broken, making them even more difficult to identify. The identification process relies on experience and familiarity with the morphology and anatomy of the seeds, fruits, and other plant parts of the local flora. This experience is based on the study of the morphological characteristics of the seeds known for the taxonomic group or groups. Identification within the groups is based on examination of the seeds and fruits with the aid of a reference collection of the plants of Israel and the Middle East (Faculty of Life Sciences, Bar-Ilan University) and publications including illustrated atlases of the plants of Israel and flora of the region (44–48) and worldwide (49–51) and was complemented at times by target-oriented field work in the vicinity of the site.
The taxa classified as vegetable-producing species were identified by their seeds. Therefore and because in some of them, more than one organ can be used as food, we did not have a direct method for counting the number of specimens for statistics.
Plant remains that were identified only to the family or tribe level were defined as unidentified and were not considered to be a component of the number of taxa, which is limited to remains identified to the genus or species level. When two remains were identified, one, because of its bad preservation, to the genus level and the other to a specific species of the same genus, they were considered as two different taxa. When two known variants of a species were identified, we considered them as one taxon. Aegilops geniculata/peregrina and Aegilops cf geniculate as well as Ziziphus lotus/spina-christi and Z. spina-christi are pairs of related species that provide the same edible organ in the same season. Therefore, when counting the number of edible plants, we considered each pair as one taxon.
Here, we consider leaves and young shoots as vegetables, nuts as either very large seeds with a hard coat or fruits with a hard shell, and Gramineae seeds as grains. We determine seasonality according to the phenology of plants under Mediterranean conditions (some plant foods can be gathered in more than one season).
Key (staple) food plants.
Key food plants include the following taxa: E. ferox, Quercus acorns, S. marianum, Olea europea, T. natans, S. sagittifolia, and Typha. These taxa were selected because of their high nutritional value and the possibility of easily gathering large amounts of their edible organs.
Statistical Analysis.
We used MDS (PROXCAL) (52) to cluster layers based on the relative abundance of plant remains. MDS is a robust approach for visualizing the pattern of proximities (i.e., similarities or distances) among a set of objects. To accommodate for differences in sediment volume examined from each layer, we calculated the proportion of items recovered from each plant species from the total items recovered in each layer. The relative occurrence of the remains of 36 identified plant species was used for calculating the Chebychev distance between each pair of layers. Stress (i.e., the degree of correspondence between the distances among points calculated by the MDS map and the input matrix) and Shepard diagram (i.e., a scatterplot of the input against the output proximities) were used as the measure of fit between the observed and calculated distance matrices. To cluster layers into groups, we used 95% confidence ellipse.
We used plant edibility (i.e., edible or not for humans) as a binary dependent variable in a logistic regression under the framework of generalized linear models. Model distribution was set as binomial, and the link function was set as logit. Each pair of rows in our data stored the identified plant information for one layer: the top for food plants and the next for nonfood plants. Soil type, classification of the layer (i.e., archaeological or geological), and chronological order of the layer were used as independent variables in our models. The frequency of food items in each layer was corrected for the volume of sediment examined from each layer. LR χ2 was used for evaluation of the effect of the above variables on the probability of finding plant food items. Calculations were performed using SPSS (version 22; SPSS Inc.) and JMP (version 12; SAS Inc.).
The type of sediment had a significant effect on the probability of recovering food plant remains (all plant items: LR = 398.1, P < 0.001; item of key food plants: LR = 49.2, P < 0.001). The probability of recovering food plant remains was significantly higher (0.54–0.64) in the storm beach and BC (a contact between black mud and coquina) sediments compared with all other sediment types (0.01–0.03). However, this trend was observed only in the geological layers (all plant items: LR = 396.4, P < 0.001; items of key food plants: LR = 44.1, P < 0.001). In archaeological layers, the probability of recovering food plant remains was independent of soil type (all plant items: LR = 1.2, P = 0.757; items of key food plants: LR = 1.4, P = 0.737).
SI Text
Archaeological and Geological Layers.
The preservation of the macrobotanical remains of GBY is excellent throughout the stratigraphic sequence because of the waterlogged nature of the sediments. The studied sedimentological package, 34-m thick, was divided into archaeological layers, which include archaeological materials (stone artifacts and faunal remains with evidence of carcass exploitation) and geological layers, which are devoid of stone artifacts and mammal remains. From a sedimentological perspective, the geological layers are characterized by black muds, gray muds, coquinas, and rarely, paleosols. The sediments of the archaeological layers include gravels (with redeposited artifacts and mammal bones), coquinas, sands, and rarely, paleosols (12, 19, 53). In the study area (excavated between 1989 and 1997), the richest archaeological layers are bedded in mainly coquinas and more specifically, storm beach layers represented by layer II-6. In addition, archaeological layers are sometimes located at the contact between two different sedimentary units (53). The entire sedimentological package of GBY attests to a lake and lake margin environmental setting and is analogous to the recent Lake Hula, which was drained in the early 1950s (54). The cyclic pattern of the sediments (19, 53) indicates that the deposition of the two mud types (black and gray) took place when the water stand of paleo-Lake Hula was high, and therefore, hominin occupation could not have taken place at such times in the study area. Only when regressive phases of the lakes occurred, a phenomenon assigned to global climatic changes (12, 38), was the landscape suitable for occupation (in close proximity to the water edge). Clearly, some of the lake margin zones were not occupied even when sedimentologically they could have hosted hominin groups. The oscillating paleo-Lake Hula provided a particular wet habitat niche that enabled the growth of edible water plants, both submerged and terrestrial (18, 55).
Extinct Species.
The antiquity of the seed remains that we studied poses yet another difficulty, because they may include extinct species. For example, the regionally extinct water lily Euryale ferox, one of the plants found at GBY, grows today only in Southeast Asia, but its fossil remains have been discovered in Europe. Preliminary identification of its seeds was carried out after drawing of similar seeds from quaternary sediments in Russia (49). Identification of such findings requires us to use data sources of greater variety and from distant geographical areas.
The identification of waterlogged plant macrofossils is often complicated, because the outer layer of the coat usually softens in a humid environment and rots or separates from the seed. Thus, it was often necessary to remove the outer layer of the seed coat in the reference collection to enable comparisons.
Seasonality.
In autumn, acorns, olives, raspberries, and the fruits of Christ’s thorn jujube are abundant; the latter also ripen during May. In summer, fruits of fig (Ficus carica), Syrian pear (Pyrus syriaca), bear’s plum (Prunus ursina), wild almond (Amygdalus korschinskii) in the rare nontoxic individuals, and wild grape vine (Vitis sylvestris) are available.
Supplementary Material
Acknowledgments
We thank the GBY Hebrew University of Jerusalem team, G. Hivroni for computerized graphics, Dr. Y. Langsam and Dr. O. Simchoni for the SEM photography, and S. Gorodetsky for her editorial work. We also thank three reviewers for their constructive comments. The GBY botanical remains are stored at the Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, with the macrobotanical remains stored at the National Natural History Collection (seeds). The German–Israeli Foundation for Scientific Research and Development and three grants from the Hebrew University of Jerusalem supported the various stages of research. This research was also conducted in the framework of a Center of Excellence supported by Israel Science Foundation Grant 300/06 and three previous Israel Science Foundation grants (to M.E.K. and N.G.-I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607872113/-/DCSupplemental.
References
- 1.Ungar PS, Grine FE, Teaford MF. Diet in early Homo: A review of the evidence and a new model of adaptive versatility. Annu Rev Anthropol. 2006;35:209–228. [Google Scholar]
- 2.Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283(5400):368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 3.Sthal AB. Hominid dietary selection before fire. Curr Anthropol. 1984;25(2):151–168. [Google Scholar]
- 4.McGrew WC. Savanna chimpanzees dig for food. Proc Natl Acad Sci USA. 2007;104(49):19167–19168. doi: 10.1073/pnas.0710330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connel JF, Hawkes K, Jones NGB. Hadza scavenging: Implications for Plio-Pleistocene hominid subsistence. Curr Anthropol. 1988;29(2):356–363. [Google Scholar]
- 6.Henry AG, Brooks AS, Piperno DR. Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium) Proc Natl Acad Sci USA. 2011;108(2):486–491. doi: 10.1073/pnas.1016868108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordain L, et al. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71(3):682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- 8.Marean CW, et al. Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature. 2007;449(7164):905–908. doi: 10.1038/nature06204. [DOI] [PubMed] [Google Scholar]
- 9.White F. Identification of fruits and seeds from Site B, Kalambo Falls. In: Clark JD, editor. Kalambo Falls Prehistoric Site. Vol I. Cambridge Univ Press; Cambridge, UK: 1969. pp. 216–217. [Google Scholar]
- 10.Gaudzinski S, Bittmann F, Boenigk W, Frechen M, Kolfschoten TV. Palaeoecology and archaeology of the Kärlich-Seeufer open-air site (Middle Pleistocene) in the central Rhineland, Germany. Q Res. 1996;46(3):319–334. [Google Scholar]
- 11.Goren-Inbar N, Werker E, Feibel CS. The Acheulian Site of Gesher Benot Ya’aqov: The Wood Assemblage. Oxbow Books; Oxford: 2002. [Google Scholar]
- 12.Goren-Inbar N, et al. Pleistocene milestones on the out-of-Africa corridor at Gesher Benot Ya’aqov, israel. Science. 2000;289(5481):944–947. doi: 10.1126/science.289.5481.944. [DOI] [PubMed] [Google Scholar]
- 13.Goren-Inbar N, Sharon G, Melamed Y, Kislev M. Nuts, nut cracking, and pitted stones at Gesher Benot Ya’aqov, Israel. Proc Natl Acad Sci USA. 2002;99(4):2455–2460. doi: 10.1073/pnas.032570499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Zeist W, Bottema S. A palynological study of the Acheulian site of Gesher Benot Ya’aqov, Israel. Veg Hist Archaeobot. 2009;18(2):105–121. [Google Scholar]
- 15.Melamed Y, Kislev M, Weiss E, Simchoni O. Extinction of water plants in the Hula Valley: Evidence for climate change. J Hum Evol. 2011;60(4):320–327. doi: 10.1016/j.jhevol.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Marlowe FW. The Hadza: Hunter-Gatherers of Tanzania. Univ of California Press; Berkeley, CA: 2010. [Google Scholar]
- 17.Danin A. Distribution Atlas of Plants in the Flora Palaestina Area. Israel Academy of Sciences and Humanities; Jerusalem: 2004. [Google Scholar]
- 18.Melamed Y. 2003. Reconstruction of the Hula Valley vegetation and the hominid vegetarian diet by the lower Palaeolithic botanical remains from Gesher Benot Ya’aqov. PhD thesis (Bar-Ilan University, Ramat Gan, Israel)
- 19.Feibel CS. Quaternary lake margins of the Levant Rift Valley. In: Goren-Inbar N, Speth JD, editors. Human Paleoecology in the Levantine Corridor. Oxbow Books; Oxford, UK: 2004. pp. 21–36. [Google Scholar]
- 20.Belitzky S. The structure and morphotectonics of the Gesher Benot Ya’aqov area, Northern Dead Sea Rift, Israel. Q Res. 2002;58(3):372–380. [Google Scholar]
- 21.Jha V, et al. Utilization and conservation of Euryale ferox Salisbury in Mithila (North Bihar), India. Aquat Bot. 1991;39(3-4):295–314. [Google Scholar]
- 22.Hedrick UP. Sturtevant’s Edible Plants of the World. Dover; New York: 1972. [Google Scholar]
- 23.Vasil’ev VN. Hydrocaryaceae. In: Shishkin BK, Bobrov EG, editors. Flora of the U.S.S.R. Vol XV. Smithsonian Institution; Washington, DC: 1949. pp. 477–495. [Google Scholar]
- 24.Jha SN. Physical and hygroscopic properties of Makhana. J Agric Eng Res. 1999;72(2):145–150. [Google Scholar]
- 25.Cotton CM. Ethnobotany Principles and Applications; (1996) (Wiley, Chichester, UK)
- 26.Zohary M. Plant Life of Palestine, Israel and Jordan. Ronald; New York: 1962. [Google Scholar]
- 27.Kaplan D. The enigma of the establishment of Quercus ithaburensis park forest in northern Israel: Co-evolution of wild boar and men? Wildl Biol Pract. 2005;1(2):95–107. [Google Scholar]
- 28.Goren-Inbar N, et al. Evidence of hominin control of fire at Gesher Benot Ya’aqov, Israel. Science. 2004;304(5671):725–727. doi: 10.1126/science.1095443. [DOI] [PubMed] [Google Scholar]
- 29.Alperson-Afil N, et al. Spatial organization of hominin activities at Gesher Benot Ya’aqov, Israel. Science. 2009;326(5960):1677–1680. doi: 10.1126/science.1180695. [DOI] [PubMed] [Google Scholar]
- 30.Wrangham RW. Catching Fire: How Cooking Made Us Human. Basic Books; New York: 2009. [Google Scholar]
- 31.Goren-Inbar N, Melamed Y, Zohar I, Akhilesh K, Pappu S. Beneath still waters – multistage aquatic exploitation of Euryale ferox (Salisb.) during the Acheulian. Internet Archaeol. 2014 doi: 10.11141/ia.37.1. [DOI] [Google Scholar]
- 32.Hillman G, Madeyska E, Hather J. Wild plant foods and diet at Late Paleolithic Wadi Kubbaniya: The evidence from charred remains. In: Close AE, editor. The Prehistory of Wadi Kubbaniya: Stratigraphy, Paleoecology, and Environment. Vol 2. Southern Methodist Univ Press; Dallas: 1989. pp. 162–242. [Google Scholar]
- 33.Zohar I, Biton R. Land, lake, and fish: Investigation of fish remains from Gesher Benot Ya’aqov (paleo-Lake Hula) J Hum Evol. 2011;60(4):343–356. doi: 10.1016/j.jhevol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovich R, Biton R. The Early-Middle Pleistocene faunal assemblages of Gesher Benot Ya’aqov: Inter-site variability. J Hum Evol. 2011;60(4):357–374. doi: 10.1016/j.jhevol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Ashkenazi S, Klass K, Mienis HK, Spiro B, Abel R. Fossil embryos and adult Viviparidae from the Early–Middle Pleistocene of Gesher Benot Ya’aqov, Israel: Ecology, longevity and fecundity. Lathaia. 2009;43(1):116–127. [Google Scholar]
- 36.Rabinovich R, Gaudzinski-Windheuser S, Kindler L, Goren-Inbar N. The Acheulian Site of Gesher Benot Ya’aqov: Mammalian Taphonomy – The Assemblages of Layers V-5 and V-6. Springer; Dordrecht, The Netherlands: 2012. [Google Scholar]
- 37.Mischke S, Ashkenazi S, Almogi-Labin A, Goren-Inbar N. Ostracod evidence for the Acheulian environment of the ancient Hula Lake (Levant) during the early-mid Pleistocene transition. Palaeogeogr Palaeoclimatol Palaeoecol. 2014;412:148–159. [Google Scholar]
- 38.Spiro B, Ashkenazi S, Starinsky A, Katz A. Strontium isotopes in Melanopsis sp. as indicators of variation in hydrology and climate in the Upper Jordan Valley during the Early-Middle Pleistocene, and wider implications. J Hum Evol. 2011;60(4):407–416. doi: 10.1016/j.jhevol.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Goren-Inbar N, Sharon G. Invisible handaxes and visible Acheulian biface technology at Gesher Benot Ya’aqov, Israel. In: Goren-Inbar N, Sharon G, editors. Axe Age: Acheulian Tool-Making from Quarry to Discard. Equinox; London: 2006. pp. 111–135. [Google Scholar]
- 40.Rabinovich R, Gaudzinski-Windheuser S, Goren-Inbar N. Systematic butchering of fallow deer (Dama) at the early middle Pleistocene Acheulian site of Gesher Benot Ya’aqov (Israel) J Hum Evol. 2008;54(1):134–149. doi: 10.1016/j.jhevol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Ashkenazi S, Motro U, Goren-Inbar N, Biton R, Rabinovich R. New morphometric parameters for assessment of body size and population structure in freshwater fossil crab assemblage from the Pleistocene site of Gesher Benot Ya’aqov (GBY), Israel. J Archaeol Sci. 2005;32(5):675–689. [Google Scholar]
- 42.Alperson-Afil N, Goren-Inbar N. The Acheulian Site of Gesher Benot Ya’aqov: Ancient Flames and Controlled Use of Fire. Springer; Dordrecht, The Netherlands: 2010. [Google Scholar]
- 43.Goren-Inbar N, Lister A, Werker E, Chech M. A butchered elephant skull and associated artifacts from the Acheulian site of Gesher Benot Ya’aqov, Israel. Paléorient. 1994;20(1):99–112. [Google Scholar]
- 44.Davis PH. Flora of Turkey. Vol 9 Edinburgh Univ Press; Edinburgh: 1985. [Google Scholar]
- 45.Feinbrun-Dothan N. Flora Palaestina. Vol III The Israel Academy of Sciences and Humanities; Jerusalem: 1978. [Google Scholar]
- 46.Feinbrun-Dothan N. Flora Palaestina. Vol IV The Israel Academy of Sciences and Humanities; Jerusalem: 1986. [Google Scholar]
- 47.Zohary M. Flora Palaestina. Vol I The Israel Academy of Sciences and Humanities; Jerusalem: 1966. [Google Scholar]
- 48.Zohary M. Flora Palaestina. Vol II The Israel Academy of Sciences and Humanities; Jerusalem: 1972. [Google Scholar]
- 49.Katz NJ, Katz SV, Kipiani MG, editors. Atlas and Keys of Fruits and Seeds Occurring in the Quaternary Deposits of the USSR. Nauka; Moscow: 1965. [Google Scholar]
- 50.Szilárd S. Magismert II. Academiai Kiado; Budapest: 1967. [Google Scholar]
- 51.Anderson JP. Plants used by the Eskimo of the Northern Bering Sea and arctic regions of Alaska. Am J Bot. 1939;26(11):714–716. [Google Scholar]
- 52.Commandeur JFF, Heiser WJ. Mathematical Derivations in the Proximity Scaling (PROXSCAL) of Symmetric Data Matrices. Department of Data Theory, Univ of Leiden Press; Leiden, The Netherlands: 1993. [Google Scholar]
- 53.Feibel SC. Archaeological sediments in lake margin environments. In: Stein JK, Farrand WR, editors. Sediments in Archaeological Contexts. Univ of Utah Press; Salt Lake City: 2001. pp. 127–148. [Google Scholar]
- 54.Dimentman C, Bromley HJ, Por FD. Lake Hula – Reconstruction of the Fauna and Hydrobiology of a Lost Lake. The Israel Academy of Sciences and Humanities; Jerusalem: 1992. [Google Scholar]
- 55.Mienis HK, Ashkenazi S. Lentic Basommatophora molluscs and hygrophilous land snails as indicators of habitat and climate in the Early-Middle Pleistocene (0.78 Ma) at the site of Gesher Benot Ya’aqov (GBY), Israel. J Hum Evol. 2011;60(4):328–340. doi: 10.1016/j.jhevol.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Yuzepchuk SV. Alismataceae. In: Komarov VL, editor. Flora of U.S.S.R. Vol 1. U.S.S.R. Academy of Sciences; Leningrad, U.S.S.R.: 1934. pp. 218–228. [Google Scholar]
- 57.Rea AM. Gila river Pima dietary reconstruction. Arid Lands Newsletter. 1991;31:3–10. [Google Scholar]
- 58.Tardío J, Pardo-de-Santayana M, Morale R. Ethnobotanical review of wild edible plants in Spain. Bot J Linn Soc. 2006;152(1):27–71. [Google Scholar]
- 59.Fritz D, Ortmeier H, Habegger R. Aquatic vegetables to be cultivated in ponds and marshes. Acta Hortic. 1992;318(22):179–186. [Google Scholar]
- 60.Dafni A. Edible Wild Plants. The Society for the Protection of Nature in Israel; Tel Aviv: 1984. [Google Scholar]
- 61.Shmida A, Aronson A. Fleshy fruits in the flora of Israel and their adaptation to dispersal by animals. Rotem. 1983;10:5–44. [Google Scholar]
- 62.Danin A. Ziziphus spina-christi. In: Alon A, editor. Plants and Animals of the Land of Israel. Vol 10. Association of Nature Protection; Tel Aviv: 1982. pp. 219–220. [Google Scholar]
- 63.Stickney A. Coastal Ecology and Wild Resource Use in the Central Bering Sea Area: Hooper Bay and Kwigillingok. Alaska Department of Fish and Game, Division of Subsistence; Anchorage, AK: 1984. [Google Scholar]
- 64.Dogan Y, Baslar S, Ay G, Mert HH. The use of wild edible plants in western and central Anatolia. Econ Bot. 2004;58(4):684–690. [Google Scholar]
- 65.Moerman D. Native American Ethnobotany. Timber; Portland, OR: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.