Significance
Life on earth largely depends on the capture of light energy by plants through photosynthesis. Light is essential, but excess light is dangerous. Energy dissipation as heat is a major mechanism induced to protect the photosynthetic machinery. We report that UV-B perception by a specific photoreceptor in the nucleocytosolic compartment leads to protection of the photosynthetic machinery in the chloroplast of a green alga. The underlying mechanism is markedly different from the response to high light. UV-B photoreceptor-mediated signaling activates a safety valve, allowing the release of the excess energy as heat, helping the algae to cope with too much light energy.
Keywords: nonphotochemical quenching, UV-B photoreceptor, PSBS, LHCSR1, photoprotection
Abstract
Life on earth is dependent on the photosynthetic conversion of light energy into chemical energy. However, absorption of excess sunlight can damage the photosynthetic machinery and limit photosynthetic activity, thereby affecting growth and productivity. Photosynthetic light harvesting can be down-regulated by nonphotochemical quenching (NPQ). A major component of NPQ is qE (energy-dependent nonphotochemical quenching), which allows dissipation of light energy as heat. Photodamage peaks in the UV-B part of the spectrum, but whether and how UV-B induces qE are unknown. Plants are responsive to UV-B via the UVR8 photoreceptor. Here, we report in the green alga Chlamydomonas reinhardtii that UVR8 induces accumulation of specific members of the light-harvesting complex (LHC) superfamily that contribute to qE, in particular LHC Stress-Related 1 (LHCSR1) and Photosystem II Subunit S (PSBS). The capacity for qE is strongly induced by UV-B, although the patterns of qE-related proteins accumulating in response to UV-B or to high light are clearly different. The competence for qE induced by acclimation to UV-B markedly contributes to photoprotection upon subsequent exposure to high light. Our study reveals an anterograde link between photoreceptor-mediated signaling in the nucleocytosolic compartment and the photoprotective regulation of photosynthetic activity in the chloroplast.
Light is essential for photosynthesis, but absorption of excess light energy is detrimental. To avoid photodamage, photosynthetic light harvesting is regulated by nonphotochemical quenching (NPQ), which allows dissipation of harmful excess energy as heat through its qE (energy-dependent nonphotochemical quenching) component (1–6). Specialized members of the light harvesting complex (LHC) protein family, such as Photosystem II Subunit S (PSBS) in higher plants or members of the LHC Stress-Related (LHCSR) family in mosses and algae, are central to qE (7–11). Protonation of key residues in these proteins triggers qE in response to the acidification of the thylakoid lumen, which is coupled to photosynthetic electron transport (7, 9). Furthermore, the deepoxidation of violaxanthin to zeaxanthin, which is also activated by the acidification of the thylakoid lumen, enhances qE (12). In response to high levels of visible light, LHCSR3 protein accumulation is of major importance for qE capacity in Chlamydomonas reinhardtii (11). The induction of LHCSR3 expression under high light is thought to involve retrograde signaling, from the chloroplast to nuclear gene expression (13), and recent data show that the response is also dependent on the phototropin (PHOT) blue light photoreceptor (14).
UV-B radiation is intrinsic to sunlight reaching the earth surface and is potentially damaging to living tissues. UV-B stress tolerance is induced through the specific activation of acclimation responses (15–20). Plants sense UV-B radiation via the homodimeric UV-B photoreceptor UV Resistance Locus 8 (UVR8) (21–23) that is mainly localized in the cytosol (24). Absorption of UV-B photons by intrinsic tryptophan residues leads to UVR8 monomerization, interaction with the E3 ubiquitin ligase Constitutively Photomorphogenic 1 (COP1), accumulation in the nucleus, and changes in gene expression (19, 21–29). After photoreception, UVR8 returns to the homodimeric ground state by redimerization (30, 31). The UVR8–COP1 pathway is evolutionarily conserved and induces UV-B acclimation and protection in Chlamydomonas (32).
Photodamage is associated with the UV-B part of the sunlight spectrum (33, 34). In both Arabidopsis and Chlamydomonas, some of the UV-B–induced genes encode chloroplast proteins, and UV-B acclimation allows maintenance of photosynthetic efficiency under elevated levels of UV-B (32, 35). However, a direct mechanistic link between UVR8 photoreceptor signaling and photoprotection of the photosynthetic machinery has remained unknown. Here, we describe a distinct qE response in Chlamydomonas that is based on direct UV-B reception by UVR8, which, together with COP1, initiates anterograde signaling and the chloroplastic accumulation of LHCSR and PSBS proteins and results in the protection of the photosynthetic machinery.
Results and Discussion
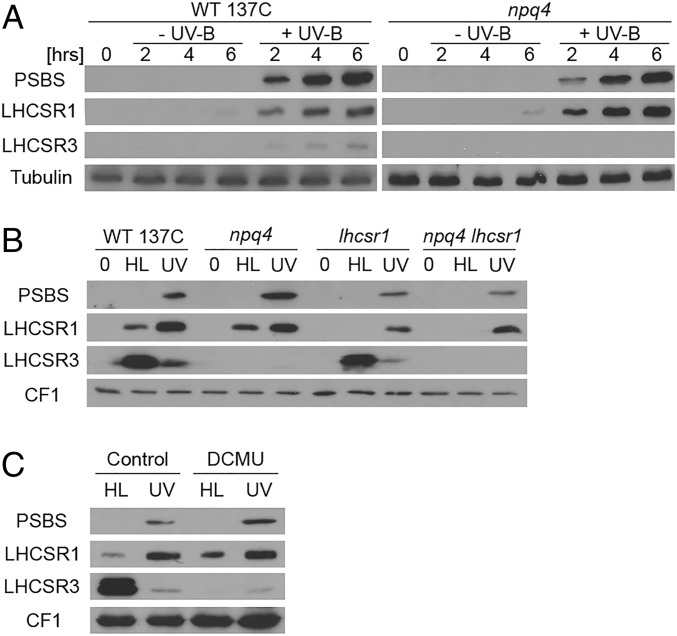
A recent transcriptome analysis revealed that nuclear-encoded PSBS, LHCSR1, and LHCSR3 transcripts accumulate in Chlamydomonas exposed to a low dose of UV-B (32). We thus tested whether the respective proteins accumulate under this condition, which induces UV-B acclimation and tolerance (32). Indeed, we found that UV-B induced a marked accumulation of the PSBS and LHCSR1 proteins and, to a lesser extent, LHCSR3 (Fig. 1A). This pattern was strikingly distinct from the high-light response (350 µmol⋅m−2⋅s−1), when LHCSR3 accumulated strongly, LHCSR1 accumulated less, and PSBS was undetectable (Fig. 1B). At higher light intensity (900 µmol⋅m−2⋅s−1), PSBS expression was detectable (Fig. S1) (36, 37), although at lower levels than under UV-B (Fig. S1). In the npq4 mutant deleted for the LHCSR3 genes LHCSR3.1 and LHCSR3.2 that encode identical proteins (11), UV-B induction of LHCSR1 and PSBS was comparable to the wild type (WT) (Fig. 1A). Interestingly, UV-B–responsive accumulation of LHCSR1 and PSBS proteins was not affected by treatment with the photosystem II (PSII) inhibitor dichlorophenyl-dimethylurea (DCMU), in sharp contrast to LHCSR3 under high light (Fig. 1C). Thus, induction of LHCSR1 and PSBS by UV-B does not depend on photosynthetic electron transfer, unlike LHCSR3 induction under high light (38). We conclude that UV-B and high light induce clearly distinct patterns of expression of qE-related proteins.
Fig. 1.
UV-B and high light induce distinct patterns of qE-related proteins. (A) Immunoblot analysis of PSBS, LHCSR1, and LHCSR3 protein levels in the WT (WT 137C) and npq4 mutant exposed to UV-B (+UV-B) for 2, 4, and 6 h or not exposed (−UV-B; protected by a UV-B–absorbing long-pass filter). Tubulin levels are shown as loading control. (B) Immunoblot analysis of PSBS, LHCSR1, and LHCSR3 protein levels in WT, npq4, lhcsr1, and npq4 lhcsr1 before treatment (0) and after exposure for 6 h to high light (HL) or to UV-B (UV). (C) Immunoblot analysis of PSBS, LHCSR1, and LHCSR3 protein levels in WT in the presence or absence of the photosynthetic electron transport inhibitor dichlorophenyl-dimethylurea (DCMU; 5 µM). (B and C) ATPase (CF1) levels are shown as loading control.
Fig. S1.
Expression of qE-related proteins under strong high light. Immunoblot analysis of PSBS, LHCSR1, and LHCSR3 protein levels in the WT (WT 137C) and the npq4, lhcsr1, and npq4 lhcsr1 mutants exposed for 6 h to UV-B or to high light (HL; 900 µmol⋅m−2⋅s−1). Tubulin levels are shown as loading control. Note that after the high-light treatment, the maximum quantum yield of PSII (Fv/Fm) drops to 0.309 ± 0.014 (n = 4) in npq4, indicative of photodamage.
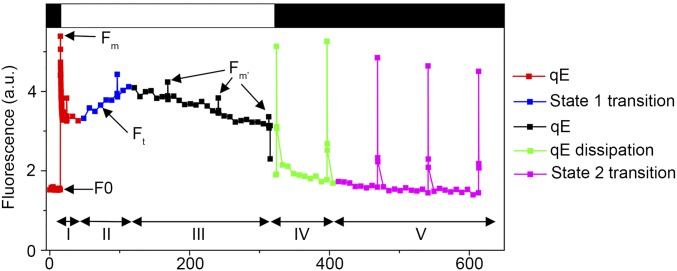
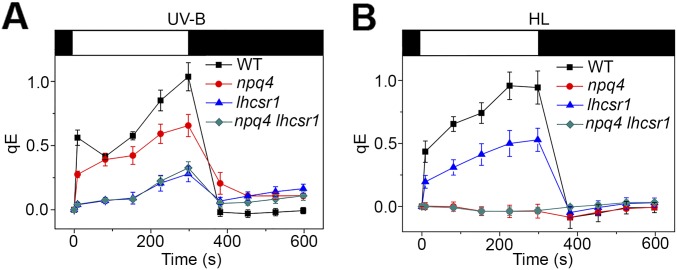
The marked accumulation of PSBS and LHCSR1 prompted us to test whether UV-B indeed increases qE capacity. NPQ includes qE and state transition (qT), which lead to rather complex kinetics of chlorophyll fluorescence upon exposure of Chlamydomonas to high light (Fig. S2) (39). We used nigericin to abolish the proton gradient and specifically quantify the qE component of NPQ (Fig. 2 A–D) (12, 39). Indeed, a clear nigericin-sensitive qE was observed in WT cells preexposed to low levels of UV-B for 6 h (Fig. 2 A and E and Fig. S3A). Another feature characteristic of qE was rapid relaxation upon returning to the dark condition (Fig. S3). qE capacity developed already after 2 h of UV-B exposure, and after 6 h of UV-B, it was similar to qE after 6 h of high-light exposure (Fig. 2E). These data clearly indicate that exposure to UV-B induces qE capacity in Chlamydomonas.
Fig. S2.
Five distinguishable phases in the fluorescence dynamics of Chlamydomonas cells. The kinetics of the WT + UV-B sample of Fig. 2A (without nigericin) is reproduced here as an illustration. Fluorescence dynamics were evaluated by using a chlorophyll fluorescence imaging system (Fluorcam; Photon Systems Instruments). The protocol included a dark preadaptation period of 5 min, a period of strong light (white bar; 5 min; 750 µmol⋅m−2⋅s−1) followed by a period of relaxation in the dark (black bar; 5 min). Maximal chlorophyll fluorescence was measured with saturating flashes (2,500 µmol⋅m−2⋅s−1) at regular 60-s intervals (to measure Fm at the end of the dark preadaptation and Fm′ during light exposure). Chlorophyll fluorescence was also monitored throughout the experiment (Fo after the dark preadaptation, Ft in the light). The approximate time ranges of the successive phases attributed to the qE and qT components of NPQ upon light exposure are indicated at the bottom of the panels with roman numerals I–V as described (33). The predominant component in each of the five phases is also highlighted in color according to the key shown in the box on the right. Upon exposure to strong light, the fast initial quenching of Fm′ is due to qE (phase I), followed by a transient increase due to a counteracting qT transition toward state 1 (phase II). When this state transition saturates, the qE component becomes more prevalent again, and Fm′ decreases (phase III). During the subsequent dark period, a rapid rise of the Fm′ peaks corresponds to the fast relaxation of qE (phase IV), followed by a Fm′ decrease due to a slower qT transition toward state 2 (phase V). a.u., arbitrary units.
Fig. 2.
UV-B induces the capacity for qE. The qE component of NPQ was determined by chlorophyll fluorescence measurements in the presence (red circles) or absence (black squares) of 10 µM nigericin. Dark-adapted cells (black bar at top) were exposed to strong light for 300 s (750 µmol⋅m−2⋅s−1; white bar) and then returned to the dark (dark bar). Fluorescence (relative units; r.u.) was monitored continuously (open symbols) and during saturating flashes (2,500 µmol⋅m−2⋅s−1 at 60-s intervals; filled symbols). (A and B) WT (WT 137C) (A) and npq4 (B) after exposure for 6 h to UV-B. (C and D) WT (C) and npq4 (D) after exposure for 6 h to high light (HL). (E and F) qE values after 2, 4 or 6 h of exposure to UV-B (+UV-B) or without exposure (−UV-B) and after 6 h of exposure to high light (HL). Means ± SD are shown (n = 3) for qE calculated at the end of the actinic light treatment. (G) qE values after 6-h exposure of the WT and in mutants npq4, lhcsr1 and npq4 lhcsr1 to UV-B or to high light (HL). Means ± SD are shown (n = 4 for HL; n = 6 for UV samples).
Fig. S3.
Time course of qE induction in Chlamydomonas WT 137C, npq4, lhcsr1, and npq4 lhcsr1 cultures that had been exposed for 6 h to UV-B (A) or high light (B). The qE component of NPQ was determined by chlorophyll fluorescence measurements in the presence or absence of nigericin as described in Fig. 2.
The marked accumulation of PSBS and LHCSR1 suggested that qE induced by UV-B is partly independent of LHCSR3. Consistently, a clear UV-B–induced qE component was observed in the LHCSR3-deficient npq4 mutant (Fig. 2 B and F and Fig. S3A), albeit with an amplitude (qE = 0.65 ± 0.04; n = 3) lower than in WT (qE = 1.01 ± 0.06; n = 3) (Fig. 2 E and F). In contrast, npq4 cells exposed to high light for 6 h showed no significant induction of qE (Fig. 2 D and F and Fig. S3B). Thus, we conclude that part of the qE capacity induced by UV-B is not dependent on LHCSR3, which raises the possibility that PSBS or LHCSR1 or both together may contribute to qE.
We then tested induction of qE capacity in the mutant lhscr1 and the double-mutant npq4 lhcsr1, the latter of which is strongly impaired in qE after exposure to high light (7). lhcsr1 carries a point mutation in the coding sequence that substitutes tyrosine-164 for asparagine (Y164N). LHCSR1Y164N accumulated in lower amounts in lhcsr1 than LHCSR1 in the WT after UV-B treatment (Fig. 1B). Nonetheless, qE was much lower in lhcsr1 than in npq4 after UV-B exposure (Fig. 2G). This finding is in support of a major role for LHCSR1 in UV-B–induced qE capacity. Conversely, after exposure to high light, a comparison of npq4 and lhcsr1 showed that LHCSR3 was a main agent of qE, but that LHCSR1 also contributed (Fig. 2G) (40). In stark contrast to the absence of qE in response to high light, qE in the double-mutant npq4 lhcsr1 after UV-B exposure was clearly detectable and similar to lhcsr1 (Fig. 2G and Fig. S3A). This finding may indicate PSBS activity in the absence of LHCSR3 and LHCSR1, although we cannot exclude the possibility that LHCSR1Y164N retains some activity. We conclude that LHCSR1 and possibly PSBS are key effectors after UV-B treatment, whereas LHCSR3 is the major active agent after high-light exposure.
PSBS activity in response to high light has been reported only very recently in Chlamydomonas (36, 37). To investigate a possible contribution of PSBS to qE after UV-B exposure, we tested whether constitutive expression of PSBS in the Chlamydomonas chloroplast enhances qE in the npq4 mutant background (Fig. S4). The overexpression of PSBS increased the amplitude of qE in the npq4 mutant after UV-B exposure (Fig. S4 H and I). Thus, in agreement with two recent reports (36, 37), PSBS is functional in Chlamydomonas and may contribute to qE activity in response to UV-B.
Fig. S4.
Constitutive PSBS expression suppresses the reduced qE phenotype of the npq4 mutant after UV-B exposure. (A) Sequence of the PSBS1 protein with the transit peptide predicted by the PredAlgo algorithm shown in red. It should be noted that the two paralogous genes PSBS.1 and PSBS.2 of C. reinhardtii encode proteins that differ in only one residue (at position 28). (B) Synthetic sequence of the psbS transgene excluding the predicted transit peptide, optimized for the codon use of the C. reinhardtii chloroplast. (C) Map of the transgene inserted in the inverted repeat of the chloroplast genome. Expression of the psbS sequence is driven by the psaA promoter/5′ UTR and by the rbcL 3′ UTR. The selection marker atpA::aadA::rbcL confers resistance to spectinomycin. The primers used for genotyping (AG1, AG2, and AG3) are indicated by arrows below the map. (D) Genotyping of psaA::psbS transformants in the npq4 nuclear background. DNA extracts from eight independent transformants (nos. 1–8) served as template for PCR amplification with the primers AG1/AG2 or AG1/AG3 (the products are loaded in alternate lanes of the agarose gel used for electrophoresis). AG1: 5′-GTC GTG GAG TAT TTA ATA CAG C-3′; AG2: 5′-GAC TTG TTG GTA AAA CTG C-3′; AG3: 5′-GCT TTT GTT CCC TTT AGT G-3′. The PCR of the untransformed npq4 mutant is shown as a control. PCR of an 80-fold dilution of the npq4 template (npq4/80) indicates that even a single copy of the untransformed genome would have been detected had the transformants been heteroplasmic (each Chlamydomonas cell harbors a single chloroplast containing ∼80 copies of the chloroplast genome). This analysis shows that lines 1, 2, 4, 6, and 7 were homoplasmic. (E) Cultures of untransformed npq4 and psaA::psbS transformants in the npq4 background were grown in acetate-containing medium (Tris acetate phosphate) under dim white light (10 µmol⋅m−2⋅s−1). Total proteins were extracted and analyzed by SDS/PAGE and immunoblotting with antisera against PSBS or tubulin as a control. (F and G) Immunodetection of PSBS (F) and LHCSR1 (G) in npq4 and psaA::psbS transformant no. 4 exposed to UV-B for 6 h. Increasing protein amounts of npq4/psaA::psbS (F) or npq4 (G) are loaded to show the linearity of the detection. CF1 (ATPase) is shown as a loading control. (H) qE values in npq4 (n = 6) and npq4/psaA::psbS strains (n = 5) after 6-h UV-B exposure. (I) Time course of qE induction in npq4 (n = 6) and npq4/psaA::psbS (n = 5) after 6 h UV-B exposure. (J) Cultures of npq4 and npq4/psaA::psbS (grown in minimal medium and not exposed to UV-B, because PSBS expression is constitutive in the latter strain) were photographed (t = 0), exposed to high light (1,000 µmol⋅m−2⋅s−1) for 5 h, and photographed again (t = 5 h). Constitutive overexpression of PSBS leads to a delay in photobleaching. Data shown are representative of four independent biological repetitions.
Under high light, qE is enhanced by the deepoxidation of violaxanthin to zeaxanthin (12), both of which are bound by LHCSR3 (8). After UV-B exposure, the level of violaxanthin increased in the WT (Fig. S5A), but there was no significant difference in the deepoxidation state (DES) of these xanthophylls (Fig. S5B). DES increased within the subsequent 5-min exposure to high light that was used to measure qE. These data indicate that, in parallel to the accumulation of qE-related proteins, acclimation to UV-B induces violaxanthin accumulation, the rapid conversion of which to zeaxanthin upon exposure to high light may enhance qE. A similar accumulation of violaxanthin after exposure to UV-B was also observed in the double mutant npq4 lhcsr1 (Fig. S5C). It is of interest that in the lhcsr1 and npq4 lhcsr1 mutants, qE started to develop upon high-light exposure more slowly than in the WT or npq4 (Fig. S3A). One possible interpretation is that a PSBS-dependent component of qE requires the accumulation of zeaxanthin.
Fig. S5.
UV-B induces violaxanthin accumulation. The xanthophyll pigments were analyzed in WT 137C in growth conditions (0; n = 3), after 6 h high light (HL; n = 2) and in cells exposed to UV-B for 6 h (+UV-B; n = 3) or not exposed (−UV-B; n = 3). An additional sample was collected at the end of the 5-min high-light period used for the measurement of qE after 6-h exposure to UV-B (+ UV-B + 5 min HL). (A) Relative violaxanthin content (normalized to β-carotene). (B) Deepoxidation ratio [DES; evaluated as zeaxanthin/(zeaxanthin + violaxanthin); antheraxanthin levels were negligible]. (C) Relative violaxanthin content, with or without UV-B exposure, in the WT and in two biological replicates (A and B) of the npq4 lhcsr1 double mutant.
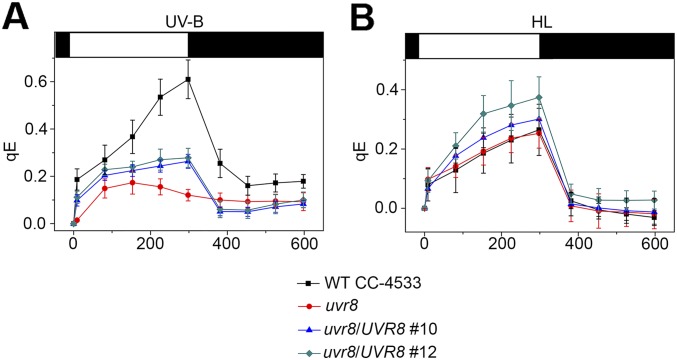
We next addressed the regulation of LHCSR1, LHCSR3, and PSBS expression under UV-B. Similar to Arabidopsis (16, 19, 21–23, 41), Chlamydomonas expresses a homodimeric UVR8 photoreceptor that monomerizes in response to UV-B (Fig. S6) and subsequently interacts with COP1 (32). We obtained a uvr8 insertional mutant (42) of Chlamydomonas containing no detectable UVR8 protein (Fig. 3A and Fig. S6). Expression of UVR8 was partially restored in two independent complementation lines (Fig. 3A). The quantum yield of PSII was affected by UV-B in uvr8 (Fig. 3C), indicating a defect in photoprotection. This increased sensitivity was not observed under high light and was rescued in the complemented lines (Fig. 3C). Thus, UVR8 regulates an acclimatory response in Chlamydomonas specific to UV-B.
Fig. S6.
The UVR8 dimer monomerizes under UV-B. Immunodetection of UVR8 in uvr8 and cop1hit1 and their respective WTs (CC-4533 and CC-124) exposed (+) or not (−) to UV-B for 1 h. Nondenatured protein samples (Upper) or boiled samples (Lower) were loaded for each strain. Stars indicate a nonspecific band.
Fig. 3.
UVR8 is required for the UV-B response leading to enhanced qE. (A) Immunoblot analysis of UVR8 in the WT (WT CC-4533), uvr8, and two independent uvr8/UVR8 complemented lines (nos. 10 and 12). (B) Immunoblot analysis of PSBS, LHCSR1, and LHCSR3 protein levels in the WT, uvr8, and complemented lines in normal growth conditions (0) or after 6-h exposure to UV-B (UV). The ATPase (CF1) levels are shown as loading control. (C) The quantum yield of PSII (Fv/Fm) was monitored in the WT, uvr8, and complemented strains exposed for 6 h to UV-B or to high light (HL). Note that Fv/Fm of untreated uvr8 was comparable to WT: uvr8 = 0.760 ± 0.022; WT = 0.745 ± 0.017; n = 4. (D) Quantitative RT-PCR analysis of PSBS, LHCSR1, and LHCSR3 RNA expression after 1-h UV-B exposure of the WT, uvr8, and uvr8/UVR8 complemented line (no. 10). (E) qE values in the WT, uvr8, and complemented lines (nos. 10 and 12) exposed for 6 h to UV-B or to high light (HL). Note that CC-4533 has a lower qE after HL than the other WT strains used in this work. (F) Immunoblot analysis of PSBS, LHCSR1, and LHCSR3 in WT (CC-4533) and uvr8 in normal growth conditions (0) or after exposure to UV-B (UV) or for 4 h to high light (HL). The ATPase (CF1) levels are shown as loading control.
uvr8 was defective in the UV-B–induced accumulation of the PSBS, LHCSR1, and LHCSR3 transcripts (Fig. 3D). Consistently, uvr8 was also impaired in UV-B–dependent accumulation of the corresponding proteins, but this phenotype was restored in complemented lines (Fig. 3B). We further tested whether the induced capacity for qE in response to UV-B depends on UVR8. After UV-B exposure, qE was impaired in uvr8 and partially restored in the complemented lines (Fig. 3E and Fig. S7A). This involvement of UVR8 was specific for UV-B responsiveness; in contrast, the uvr8 mutant still expressed LHCSR3 and a low level of LHCSR1 protein and showed a normal capacity for qE in response to high light (Fig. 3 E and F and Fig. S7B). That the signaling pathways in response to UV-B or high light were different is consistent with our observation that only the latter seems to require photosynthetic electron transport (Fig. 1C).
Fig. S7.
Time course of qE induction in Chlamydomonas WT CC-4533, uvr8, and rescued lines (uvr8/UVR8 10 and 12) cultures that had been exposed for 6 h to UV-B (A) or high light (B). The qE component of NPQ was determined by chlorophyll fluorescence measurements in the presence or absence of nigericin as described in Fig. S1. Note that in the uvr8 mutant after UV-B (A; red), the residual signal does not show a fast reversibility in the dark, which would be expected for a true qE response as observed in the WT and the rescued lines.
COP1 acts together with UVR8 in UV-B signaling in plants (16, 19, 32, 43). UVR8 monomerization under UV-B was not affected in the Chlamydomonas cop1hit1 mutant (Fig. S6). However, like uvr8, the cop1hit1 mutant (44) was also compromised in the UV-B–responsive accumulation of PSBS, LHCSR1, and LHCSR3 proteins (Fig. S8A), as well as in the induction of the corresponding transcripts (Fig. S8B) (32). Likewise, qE was lower in the cop1hit1 mutant than in WT, but was restored in the complemented cop1hit1/COP1 strains (Fig. S8C). The data clearly show that qE capacity under UV-B requires the UVR8 photoreceptor and its downstream signaling partner COP1.
Fig. S8.
COP1 is required for the UV-B response leading to enhanced qE. (A) Immunoblot analysis of PSBS, LHCSR1, and LHCSR3 in WT (WT CC-124), cop1hit1, and cop1hit1/COP1 complemented strains (nos. 157 and 166) exposed for 6 h to UV-B. Tubulin levels are shown as loading control. (B) Quantitative RT-PCR analysis of PSBS, LHCSR1, and LHCSR3 RNA expression after 1 h of UV-B exposure in the WT, cop1hit1, and cop1hit1/COP1 complemented strain (no. 166). Data are normalized to levels in the WT in growth conditions (0). CNRQ, calibrated normalized relative quantities. (C) qE values after 6-h UV-B exposure in the WT, cop1hit1, and cop1hit1/COP1 complemented strains (nos. 157 and 166).
We next addressed the physiological relevance of UV-B–enhanced qE capacity for photoprotection against high-light stress. We first compared the extent of photoinhibition under high light of cells that had been acclimated to a low dose of UV-B for 16 h compared with untreated controls. After 60 min of high-light stress treatment, the quantum yield of PSII was 1.13-fold higher in UV-B–acclimated WT cells (Fv/Fm +UV) than in the nonacclimated controls (Fv/Fm –UV) (Fig. 4A and Tables S1 and S2). Upon prolonged exposure to high light, the UV-B–acclimated WT culture remained green, whereas the nonacclimated control culture bleached (Fig. 4B). In contrast to the WT, the uvr8 mutant pretreated with UV-B did not show reduced photoinhibition or protection from high-light-induced bleaching (Fig. 4A, B). We further tested whether this UVR8-mediated photoprotection from high-light stress can be directly linked to the enhanced qE capacity. Indeed, npq4 and lhcsr1 showed less UV-B–induced photoprotection than WT (Fig. 4C), and the mutant cultures showed less UV-B acclimation than the WT culture (Fig. 4D). Furthermore, the double mutant npq4 lhcsr1 was even more strongly affected in these acclimation responses (Fig. 4 C and D). To investigate whether PSBS may also play a role in photoprotection, we compared the npq4 mutant overexpressing PSBS in the chloroplast with the parental npq4 strain. Because expression of PSBS in the transformants is constitutive, these experiments were performed without prior exposure to UV-B. Overexpression of PSBS led to a delay in bleaching of the culture under high light and thus contributed to photoprotection (Fig. S4J). We conclude that the UVR8-mediated UV-B induction of LHCSR1, LHCSR3, and possibly PSBS markedly contributes to photoprotection under high light.
Fig. 4.
UV-B acclimation promotes photoprotection. (A) The maximum quantum yield of PSII was monitored in cell cultures of WT (WT CC-4533), uvr8, and uvr8/UVR8 complemented line 10 that had been previously exposed for 16 h to UV-B (Fv/Fm +UV) or were left untreated (Fv/Fm –UV). The effect of acclimation is expressed as the ratio (Fv/Fm +UV)/(Fv/Fm –UV) (error bars represent the SEM; n = 5) measured at the end of acclimation (0) and after a subsequent high-light treatment (1 h, 700 µmol⋅m−2⋅s−1). (B) Cell cultures that had been exposed for 16 h to UV-B (+UV) or untreated controls (−UV) were photographed (t = 0) and then exposed to high light (1,000 µmol⋅m−2⋅s−1) for 5 h and photographed again (t = 5 h). Data shown are representative of three independent biological repetitions. (C and D) As A and B, but for the WT (WT 137C) and mutants npq4, lhcsr1, and npq4 lhcsr1. (C) Error bars represent the SEM; n = 3. (D) Data shown are representative of three independent biological repetitions. (E) Scheme of photoreceptor-mediated photoprotection of the photosynthetic machinery after UV-B exposure compared with high light.
Table S1.
Data used to calculate the results in Fig. 4A
| WT CC-4533 | uvr8 | uvr8/UVR8 no. 10 | ||||
| −UV | +UV | −UV | +UV | −UV | +UV | |
| 0 | 0.745 ± 0.003 | 0.751 ± 0.003 | 0.760 ± 0.002 | 0.746 ± 0.002 | 0.763 ± 0.002 | 0.760 ± 0.002 |
| 1 h | 0.241 ± 0.007 | 0.275 ± 0.007 | 0.273 ± 0.008 | 0.265 ± 0.010 | 0.307 ± 0.008 | 0.350 ± 0.008 |
Values are average ± SEM; n = 5.
Table S2.
Data used to calculate the results in Fig. 4C
| WT 137C | npq4 | lhcsr1 | npq4 lhcsr1 | |||||
| −UV | + UV | −UV | + UV | −UV | + UV | −UV | + UV | |
| 0 | 0.766 ± 0.006 | 0.759 ± 0.005 | 0.752 ± 0.007 | 0.748 ± 0.006 | 0.734 ± 0.003 | 0.742 ± 0.005 | 0.746 ± 0.008 | 0.742 ± 0.004 |
| 1 h | 0.320 ± 0.030 | 0.420 ± 0.043 | 0.241 ± 0.010 | 0.276 ± 0.013 | 0.239 ± 0.005 | 0.273 ± 0.007 | 0.245 ± 0.014 | 0.253 ± 0.011 |
Values are average ± SEM; n = 3.
Conclusion
In Chlamydomonas, perception of UV-B photons induces an acclimation response that reduces photodamage to the photosynthesis machinery. This response involves increased accumulation of violaxanthin and also expression of qE-related proteins, mainly LHCSR1 and PSBS, in contrast to LHCSR3, which is induced most in high light (Fig. 4E). The UV-B signal appears to act as a proxy for high light, priming the cells for photoprotection. Exposure to high light then rapidly triggers the development of qE and the conversion of violaxanthin to zeaxanthin. The action spectrum of photodamage to the photosynthetic electron transfer chain peaks in UV-B, at wavelengths that are most detrimental to PSII and the manganese cluster involved in water oxidation (33, 34). Thus, predominant expression of LHCSR1 and PSBS under UV-B vs. LHCSR3 under high light may indicate an evolutionary divergence of the signaling pathways, potentially coupled to differences in the activities of these proteins.
Regulatory loops are known to operate in the chloroplast that adjust photosynthetic activity. The status of the photosynthetic chain, sensed for example through the redox poise of key electron carriers or the pH of the thylakoid lumen, influences target photosynthetic proteins by phosphorylation, the reduction of disulfide bridges, or the protonation of regulatory residues (3, 45). We now demonstrate that the photoreceptor UVR8 and its partner COP1 initiate a signaling pathway under UV-B that induces nuclear gene expression of proteins that are then targeted to the chloroplast, where they are involved in the regulation of photosynthesis. Our findings, together with the recent description of a complementary role for PHOT in high-light activation of LHCSR3 expression (14), add a tier of regulation through anterograde signaling to the better-known feedback mechanisms operating within the chloroplast. In Arabidopsis, photoperception of UV-B by UVR8 is also important for the maintenance of photosynthetic competence, but the underlying mechanism in higher plants is not clear (35). It will be crucial for agricultural productivity and the biotechnological exploitation of photosynthetic processes to better understand the molecular mechanisms leading to photoprotection and reduced photoinhibition under sunlight and its intrinsic UV-B fraction.
Materials and Methods
Algal Material.
The C. reinhardtii mutant strains cop1hit1 (44), uvr8 (LMJ.RY0402.156289) (42), npq4 (11), lhcsr1, and npq4 lhcsr1 (7) were used in this work, together with their respective WT background strains, namely, WT137C (mt+) for npq4, lhcsr1, and npq4 lhcsr1; CC-124 (137c mt−) for cop1hit1; and CC-4533 (cw15 mt−) for uvr8. The cop1hit1/COP1 complementation strains have been described (32).
The UVR8 coding sequence was cloned between the psaD promoter and terminator in a Gateway-compatible derivative of pSL18 (46). The uvr8 mutant was transformed as described (32).
Growth Conditions, UV-B, and High-Light Treatment.
Cells were cultivated in Tris acetate phosphate medium under dim light (5–10 µmol⋅m−2⋅s−1 from fluorescent tubes) at 25 °C. In all experiments, cells were harvested during the exponential phase (1.5–2.5 × 106 cells per mL), washed, and resuspended at 2 × 107 cells per mL in minimum medium [high-salt medium (HSM)] to favor induction of the capacity for qE (11, 13, 39). They were then acclimated to the new conditions under dim light for 1 h before starting the UV-B or high-light treatments. UV-B treatment (0.2 mW⋅cm−2) was provided by Philips TL20W/01RS narrowband UV-B tubes under a filter of the WG series (Schott Glaswerke) with half-maximal transmission at 311 nm (21, 32). For the control samples without UV-B treatment (−UV-B), a 360-nm filter was used to block UV-B (<0.001 mW⋅cm−2). In both cases (+ or – UV-B), cells were concomitantly exposed to dim white light (5 µmol⋅m−2⋅s−1; Osram L18W/30 tubes). It should be noted that the low-level UV-B treatment used here causes only minor damage to PSII, as indicated by the maximal quantum yield of PSII in WT 137C, which was 0.76 ± 0.01 (n = 5) after 6-h treatment compared with 0.80 ± 0.01 (n = 5) in the untreated control. For high-light treatment, cells were exposed under 350 µmol⋅m−2⋅s−1 white light (fluorescent tubes Osram Dulux L) for 6 h, but only 4 h for WT CC-4533, uvr8, and its complemented lines, which are more sensitive.
To determine the effect of UV-B acclimation on subsequent photoprotection, cells (1.5 × 106 mL−1) were treated for 16 h under UV-B (0.07 mW⋅cm−2) as described above. They were then exposed to high light (1,000 µmol⋅m−2⋅s−1) with agitation in glass flasks immersed in a water bath at 25 °C.
Protein Extraction and Immunoblot Analysis.
Protein extraction and immunoblot analysis were performed as described (32). Anti-CrUVR8 (32), anti-LHCSR3 (47), anti-LHCSR1 (AS14 2819;Agrisera), anti-tubulin (gift from Donald Weeks, University of Nebraska, Lincoln, NE), and anti-CF1 (48) were used. Rabbit polyclonal anti-PSBS antibodies were raised and affinity-purified against the peptide C+AINEGSGKFVDEESA (CrPSBS231–245) (Eurogentec), and their specificity was validated by peptide competition assays (Fig. S9); moreover, PSBS was specifically detected in the constitutive PSBS expression lines, but not in the nontransformed control (Fig. S4E).
Fig. S9.
Specificity of the anti-PSBS antibodies assayed by peptide competition. Immunoblot analysis of protein extracts from WT cells treated with UV-B for 6 h (+UV) to induce the expression of endogenous PSBS or left untreated (−UV). The blots were probed with anti-PSBS antibodies only (−) or in competition with the peptide used for immunization (Pep 93: C+AINEGSGKFVDEESA, corresponding to CrPSBS231–245) or of a control peptide (Pep 94: C+DTISERPAGPLQDPR, corresponding to CrPSBS140–154) (both 7 μg⋅mL−1). Pep 93, but not the Pep 94 control, efficiently prevents binding of the antibodies to PSBS.
All bands migrate at the position expected for their size: PSBS, 22 kDa; UVR8, 48 kDa (monomer); LHCSR3, 28 kDa (phosphorylated LHCSR3, ∼30 kDa); and LHCSR1, 27 kDa.
Quantitative Real-Time PCR.
Chlamydomonas RNA was extracted, reverse-transcribed, and analyzed in a 7900HT Real-Time PCR System (Applied Biosystems) as described (32). Expression was normalized to the Cre06.g6364 reference gene (32) and measured in triplicate. The following primers were used: PSBS (5′-CCG CCA TCA ACG GCA AGC AG-3′ and 5′-CCA CCA TGG CCA GGC GAC C-3′), LHCSR1 (5′-AAG ACC CTG CCC GGT GTT AC-3′ and 5′-TGG GTG ATC TCA GAC TCG CGC-3′), LHCSR3 (5′-GGC CGT CAA GTC CGT GTC T-3′ and 5′-GGG AAG GTT CTT CGT GTA TGC G-3′), and Cre06.g6364 (5′-CTT CTC GCC CAT GAC CAC-3′ and 5′-CCC ACC AGG TTG TTC TTC AG-3′).
qE and Photoinhibition Measurements.
NPQ was measured with a video-imaging system (Fluorcam; Photon Systems Instruments). After UV-B or high-light treatment, cells were first briefly adapted to the dark (5 min) and then exposed for 5 min to strong actinic light (750 µmol⋅m−2⋅s−1) to monitor the induction of NPQ, and finally returned to the dark to follow its relaxation (Fig. 2 and Fig. S2). Saturating flashes (2,500 µmol⋅m−2⋅s−1) at regular intervals allowed a first measurement of maximal chlorophyll fluorescence (Fm) in the initial dark-adapted state and then measurements of maximum fluorescence (Fm′) during the exposure to strong light, and subsequent relaxation in the dark (Fig. 2 A–D, black peaks; and Fig. S2). The fluorescence in the intervals between the peaks (Ft) was also measured continuously, although this value was not used for the quantitative evaluation of qE.
In Chlamydomonas, the qT component (state transition) of NPQ is induced more slowly than the faster qE component. This process results in a complex time course for the maximum fluorescence Fm′ peaks (Fig. 2 A–D and Fig. S2) (39). The qE component of NPQ, but not the qT component, is entirely dependent on the light-induced acidification of the thylakoid lumen driven by photosynthetic electron flow (12). Therefore, treatment immediately before the fluorescence measurement with the ionophore nigericin (10 µM), which collapses the proton gradient across the thylakoid membrane, inhibits qE (phase I and III) without significantly affecting qT (phase II). Hence, the Fm′ time course in the presence of nigericin shows only the qT component: a fluorescence increase toward state 1 in the strong-light phase and a fluorescence decrease toward state 2 in the dark (Fig. S2 and Fig. 2A, red curve). The nigericin-sensitive qE component of NPQ can be evaluated at the end of the exposure to strong light by first normalizing the curves to the value of Fm in the absence of nigericin and then subtracting the Fm′ peak obtained in the untreated control from that obtained in the presence of nigericin, and normalizing to Fm′ [(Fm′nig − Fm′)/Fm′] (39).
To measure the effect of UV-B acclimation on subsequent photoinhibition, cells (1.5 × 106 mL−1) were treated for 16 h under UV-B (0.02 mW⋅cm−2) as above and then exposed to high light for 1 h (700 µmol⋅m−2⋅s−1). To monitor the maximum quantum yield of PSII (using a Plant Efficiency Analyzer from Hansatech), cells were incubated in the dark for 5 min, the fluorescence was measured before (Fo) and during (Fm) a saturating light pulse, and Fv was calculated as Fm – Fo.
Transgenic Expression of PSBS from the Chloroplast Genome.
The PSBS1 gene was synthesized by Genscript after chloroplast codon use optimization (Fig. S4 A and B). The synthetic PSBS1 gene was cloned into the IR-int vector (49) under the control of the psaA promotor (Fig. S4C), and the construct was integrated into the npq4 strain by helium gun bombardment. Transformants were selected for their spectinomycin resistance, and homoplasmicity of the insertions was screened by PCR (Fig. S4D).
Pigment Analysis.
Carotenoids were analyzed and quantified as described (39, 50).
Acknowledgments
We thank Martin Jonikas and the Chlamydomonas stock center for providing the uvr8 mutant; Michael Hippler and Dimitris Petroutsos for the anti-LHCSR3 antibody; Donald Weeks for the anti-tubulin antibody; Sabeeha Merchant for the anti-CF1 antibody; Sylvain Loubéry for help with statistical analyses; and Giovanni Finazzi, Michael Hothorn, Dimitris Petroutsos, and Jean-David Rochaix for helpful comments on the manuscript. This work was supported by Swiss National Science Foundation Grants 31003A_153475 (to R.U.) and 31003A_146300 (to M.G.-C.); the European Research Council under the European Union’s Seventh Framework Programme (Grant 310539 to R.U.); and the University of Geneva. Construction of the npq4 lhcsr1 mutant was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division under Field Work Proposal 449B. K.K.N. is an investigator for the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation (through Grant GBMF3070).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607695114/-/DCSupplemental.
References
- 1.Li Z, Wakao S, Fischer BB, Niyogi KK. Sensing and responding to excess light. Annu Rev Plant Biol. 2009;60:239–260. doi: 10.1146/annurev.arplant.58.032806.103844. [DOI] [PubMed] [Google Scholar]
- 2.Erickson E, Wakao S, Niyogi KK. Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 2015;82(3):449–465. doi: 10.1111/tpj.12825. [DOI] [PubMed] [Google Scholar]
- 3.Minagawa J, Tokutsu R. Dynamic regulation of photosynthesis in Chlamydomonas reinhardtii. Plant J. 2015;82(3):413–428. doi: 10.1111/tpj.12805. [DOI] [PubMed] [Google Scholar]
- 4.Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol. 2013;16(3):307–314. doi: 10.1016/j.pbi.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Wobbe L, Bassi R, Kruse O. Multi-level light capture control in plants and green algae. Trends Plant Sci. 2016;21(1):55–68. doi: 10.1016/j.tplants.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Ruban AV. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016;170(4):1903–1916. doi: 10.1104/pp.15.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballottari M, et al. Identification of pH-sensing sites in the Light Harvesting Complex Stress-Related 3 protein essential for triggering non-photochemical quenching in Chlamydomonas reinhardtii. J Biol Chem. 2016;291(14):7334–7346. doi: 10.1074/jbc.M115.704601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonente G, et al. Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol. 2011;9(1):e1000577. doi: 10.1371/journal.pbio.1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liguori N, Roy LM, Opacic M, Durand G, Croce R. Regulation of light harvesting in the green alga Chlamydomonas reinhardtii: The C-terminus of LHCSR is the knob of a dimmer switch. J Am Chem Soc. 2013;135(49):18339–18342. doi: 10.1021/ja4107463. [DOI] [PubMed] [Google Scholar]
- 10.Li XP, et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403(6768):391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 11.Peers G, et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature. 2009;462(7272):518–521. doi: 10.1038/nature08587. [DOI] [PubMed] [Google Scholar]
- 12.Niyogi KK, Bjorkman O, Grossman AR. Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell. 1997;9(8):1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petroutsos D, et al. The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii. Plant Cell. 2011;23(8):2950–2963. doi: 10.1105/tpc.111.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petroutsos D, et al. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature. 2016;537(7621):563–566. doi: 10.1038/nature19358. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins GI. Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol. 2009;60:407–431. doi: 10.1146/annurev.arplant.59.032607.092953. [DOI] [PubMed] [Google Scholar]
- 16.Heijde M, Ulm R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012;17(4):230–237. doi: 10.1016/j.tplants.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Jansen MAK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998;3(4):131–135. [Google Scholar]
- 18.Kliebenstein DJ, Lim JE, Landry LG, Last RL. Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol. 2002;130(1):234–243. doi: 10.1104/pp.005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favory JJ, et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 2009;28(5):591–601. doi: 10.1038/emboj.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González Besteiro MA, Bartels S, Albert A, Ulm R. Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 2011;68(4):727–737. doi: 10.1111/j.1365-313X.2011.04725.x. [DOI] [PubMed] [Google Scholar]
- 21.Rizzini L, et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011;332(6025):103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- 22.Christie JM, et al. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science. 2012;335(6075):1492–1496. doi: 10.1126/science.1218091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, et al. Structural basis of ultraviolet-B perception by UVR8. Nature. 2012;484(7393):214–219. doi: 10.1038/nature10931. [DOI] [PubMed] [Google Scholar]
- 24.Kaiserli E, Jenkins GI. UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell. 2007;19(8):2662–2673. doi: 10.1105/tpc.107.053330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin R, Arongaus AB, Binkert M, Ulm R. Two distinct domains of the UVR8 photoreceptor interact with COP1 to initiate UV-B signaling in Arabidopsis. Plant Cell. 2015;27(1):202–213. doi: 10.1105/tpc.114.133868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown BA, et al. A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci USA. 2005;102(50):18225–18230. doi: 10.1073/pnas.0507187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binkert M, et al. UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell. 2014;26(10):4200–4213. doi: 10.1105/tpc.114.130716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin R, Skvortsova MY, Loubéry S, Ulm R. COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor. Proc Natl Acad Sci USA. 2016;113(30):E4415–E4422. doi: 10.1073/pnas.1607074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X, et al. Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc Natl Acad Sci USA. 2013;110(41):16669–16674. doi: 10.1073/pnas.1316622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heijde M, Ulm R. Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc Natl Acad Sci USA. 2013;110(3):1113–1118. doi: 10.1073/pnas.1214237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heilmann M, Jenkins GI. Rapid reversion from monomer to dimer regenerates the ultraviolet-B photoreceptor UV RESISTANCE LOCUS8 in intact Arabidopsis plants. Plant Physiol. 2013;161(1):547–555. doi: 10.1104/pp.112.206805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilbrook K, et al. UV-B perception and acclimation in Chlamydomonas reinhardtii. Plant Cell. 2016;28(4):966–983. doi: 10.1105/tpc.15.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi S, et al. The solar action spectrum of photosystem II damage. Plant Physiol. 2010;153(3):988–993. doi: 10.1104/pp.110.155747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zavafer A, Chow WS, Cheah MH. The action spectrum of photosystem II photoinactivation in visible light. J Photochem Photobiol B. 2015;152(Pt B):247–260. doi: 10.1016/j.jphotobiol.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Davey MP, et al. The UV-B photoreceptor UVR8 promotes photosynthetic efficiency in Arabidopsis thaliana exposed to elevated levels of UV-B. Photosynth Res. 2012;114(2):121–131. doi: 10.1007/s11120-012-9785-y. [DOI] [PubMed] [Google Scholar]
- 36.Tibiletti T, Auroy P, Peltier G, Caffarri S. Chlamydomonas reinhardtii PsbS protein is functional and accumulates rapidly and transiently under high light. Plant Physiol. 2016;171(4):2717–2730. doi: 10.1104/pp.16.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correa-Galvis V, et al. Photosystem II subunit PsbS is involved in the induction of LHCSR protein-dependent energy dissipation in Chlamydomonas reinhardtii. J Biol Chem. 2016;291(33):17478–17487. doi: 10.1074/jbc.M116.737312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maruyama S, Tokutsu R, Minagawa J. Transcriptional regulation of the stress-responsive light harvesting complex genes in Chlamydomonas reinhardtii. Plant Cell Physiol. 2014;55(7):1304–1310. doi: 10.1093/pcp/pcu068. [DOI] [PubMed] [Google Scholar]
- 39.Allorent G, et al. A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell. 2013;25(2):545–557. doi: 10.1105/tpc.112.108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinc E, et al. LHCSR1 induces a fast and reversible pH-dependent fluorescence quenching in LHCII in Chlamydomonas reinhardtii cells. Proc Natl Acad Sci USA. 2016;113(27):7673–7678. doi: 10.1073/pnas.1605380113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins GI. Structure and function of the UV-B photoreceptor UVR8. Curr Opin Struct Biol. 2014;29:52–57. doi: 10.1016/j.sbi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Li X, et al. An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell. 2016;28(2):367–387. doi: 10.1105/tpc.15.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oravecz A, et al. CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell. 2006;18(8):1975–1990. doi: 10.1105/tpc.105.040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schierenbeck L, et al. Fast forward genetics to identify mutations causing a high light tolerant phenotype in Chlamydomonas reinhardtii by whole-genome-sequencing. BMC Genomics. 2015;16(1):57. doi: 10.1186/s12864-015-1232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serrato AJ, Fernández-Trijueque J, Barajas-López JD, Chueca A, Sahrawy M. Plastid thioredoxins: A “one-for-all” redox-signaling system in plants. Front Plant Sci. 2013;4:463. doi: 10.3389/fpls.2013.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Depège N, Bellafiore S, Rochaix JD. Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science. 2003;299(5612):1572–1575. doi: 10.1126/science.1081397. [DOI] [PubMed] [Google Scholar]
- 47.Naumann B, et al. Comparative quantitative proteomics to investigate the remodeling of bioenergetic pathways under iron deficiency in Chlamydomonas reinhardtii. Proteomics. 2007;7(21):3964–3979. doi: 10.1002/pmic.200700407. [DOI] [PubMed] [Google Scholar]
- 48.Hamel PP, Dreyfuss BW, Xie Z, Gabilly ST, Merchant S. Essential histidine and tryptophan residues in CcsA, a system II polytopic cytochrome c biogenesis protein. J Biol Chem. 2003;278(4):2593–2603. doi: 10.1074/jbc.M208651200. [DOI] [PubMed] [Google Scholar]
- 49.Michelet L, Lefebvre-Legendre L, Burr SE, Rochaix JD, Goldschmidt-Clermont M. Enhanced chloroplast transgene expression in a nuclear mutant of Chlamydomonas. Plant Biotechnol J. 2011;9(5):565–574. doi: 10.1111/j.1467-7652.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 50.Fraser PD, Pinto ME, Holloway DE, Bramley PM. Technical advance: Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2000;24(4):551–558. doi: 10.1046/j.1365-313x.2000.00896.x. [DOI] [PubMed] [Google Scholar]