Significance
Successful completion of daily activities relies on the ability to select the relevant features of the environment to pay attention to and remember. Disruptions of these processes can lead to disorders, such as attention-deficit hyperactivity disorder and age-related memory loss. To devise therapeutic strategies, we must understand the neural circuits underlying normal cognition. One important pathway is the signaling of dopamine, a reinforcement-related neurotransmitter, in the hippocampus, a spatial learning and memory center. Surprisingly, the brain region supplying dopamine to the dorsal hippocampus is unclear. This study provides direct evidence that the noradrenergic locus coeruleus coreleases dopamine in the dorsal hippocampus and provides insight into dopamine function in selective attention and spatial learning and memory.
Keywords: dopamine, locus coeruleus, hippocampus, memory, attention
Abstract
Dopamine neurotransmission in the dorsal hippocampus is critical for a range of functions from spatial learning and synaptic plasticity to the deficits underlying psychiatric disorders such as attention-deficit hyperactivity disorder. The ventral tegmental area (VTA) is the presumed source of dopamine in the dorsal hippocampus. However, there is a surprising scarcity of VTA dopamine axons in the dorsal hippocampus despite the dense network of dopamine receptors. We have explored this apparent paradox using optogenetic, biochemical, and behavioral approaches and found that dopaminergic axons and subsequent dopamine release in the dorsal hippocampus originate from neurons of the locus coeruleus (LC). Photostimulation of LC axons produced an increase in dopamine release in the dorsal hippocampus as revealed by high-performance liquid chromatography. Furthermore, optogenetically induced release of dopamine from the LC into the dorsal hippocampus enhanced selective attention and spatial object recognition via the dopamine D1/D5 receptor. These results suggest that spatial learning and memory are energized by the release of dopamine in the dorsal hippocampus from noradrenergic neurons of the LC. The present findings are critical for identifying the neural circuits that enable proper attention selection and successful learning and memory.
Dopamine is a neurotransmitter released throughout the brain to encode salience and facilitate the formation of associative memory (1, 2). When released into the dorsal hippocampus, dopamine binds to D1/D5 receptors to promote attention, episodic memory formation, spatial learning, and synaptic plasticity (3–5). Successful spatial learning requires that hippocampal place cells, location-encoding pyramidal neurons (6), display consistent and stable patterns of neural activity, a process that can be enhanced by selective attention to spatial cues and by dopamine agonists (7, 8). Conversely, dopamine receptor blockade attenuates the ability of spatial attention to stabilize the firing pattern of hippocampal place cells (8). The role of dopamine in driving attentional processes is highlighted by the fact that methylphenidate, one of the most common treatments for attention-deficit hyperactivity disorder (ADHD), improves attention by increasing synaptic availability of dopamine in the hippocampus, as well as in the prefrontal cortex and striatum (9–11). These findings suggest that dopamine is critical for the selective attention underlying spatial learning and memory.
For decades, the ventral tegmental area (VTA) has been the presumed source of hippocampal dopamine. However, in recent years, the source of dopamine in the dorsal hippocampus has become less clear. McNamara et al. (12) argued that a dopaminergic projection from the VTA to the dorsal hippocampus promoted hippocampal reactivation during sleep and stabilized memory. However, only 10% of the sparse projection from the VTA to the hippocampus contains dopamine, raising the question of whether this weak VTA projection could be solely responsible for activating the dense network of dopamine receptors found in the dorsal hippocampus (13–16). Moreover, whereas the ventral aspect of the hippocampus, an area associated with learned fear and anxiety (17), receives significant dopaminergic projections from the VTA (13, 14), the dorsal region of the hippocampus, which houses the place cells required for spatial memory (7), has relatively sparse dopaminergic innervation from the VTA. We therefore set out to identify the neurons that provide dopaminergic tone to the dorsal hippocampus and to assess their involvement in spatial learning and attention.
Dopamine is removed from hippocampal synapses by the norepinephrine transporter (18), which suggests that dopamine may also be released from norepinephrine-containing neurons outside of the VTA. Although indirect action via the VTA had not been ruled out, electrical and pharmacological stimulation of the noradrenergic locus coeruleus (LC) increased dopamine levels in the prefrontal cortex and modulated hippocampal synaptic transmission (19, 20). Also, dopamine-mediated enhancement of excitatory transmission in the hippocampus can be reversed by tyrosine hydroxylase (TH) knockdown in the LC, but not in the VTA (21). Smith and Greene (21) therefore suggested that dopamine may be released from the LC into the dorsal hippocampus. A major caveat of the study is that the synaptic transmission effects are produced in vitro by amphetamine, a drug known to increase extracellular dopamine concentrations (22). The finding provides no insight as to whether dopamine is released from the LC under more physiological, nondrug conditions, a question we test directly in our study.
Taken together, there are some groups who argue that the VTA is the main source of dopamine to the dorsal hippocampus (12–14) and others who support the idea that dopamine is released from LC neurons (19–21). These conflicting hypotheses leave unanswered the question of which brain region supplies the dorsal hippocampus with dopaminergic tone. We combined optogenetic, biochemical, and behavioral methods to identify the major source of dopamine in the dorsal hippocampus and then examined the involvement of this dopaminergic pathway in selective attention and spatial learning and memory.
Results
VTA Dopamine Neurons Project More Widely to the Ventral Versus Dorsal Hippocampus.
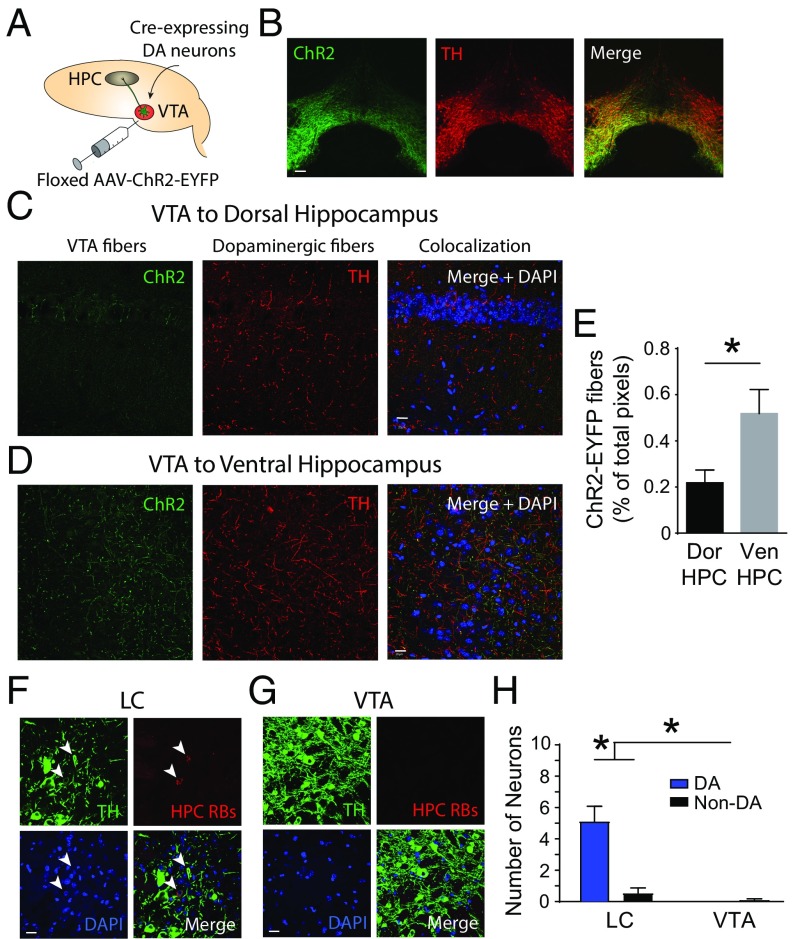
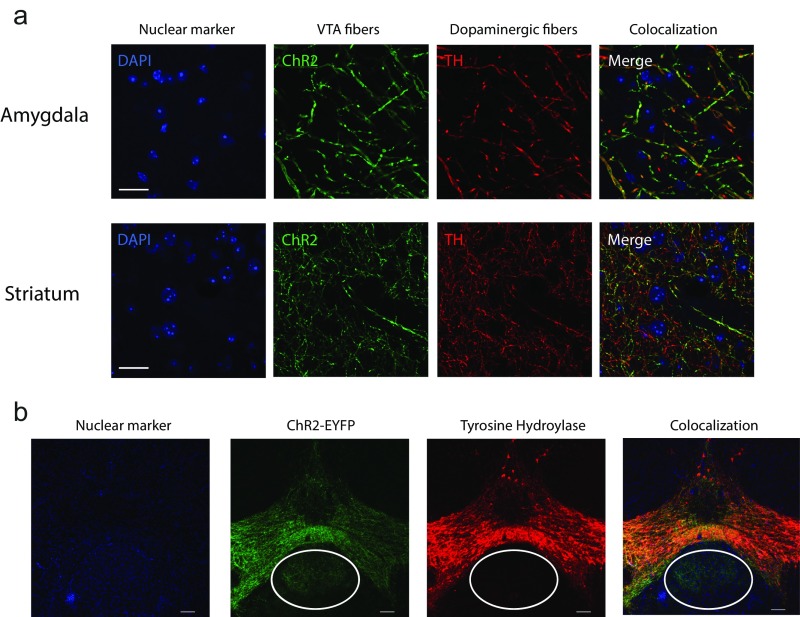
To identify the neurons that release dopamine in the dorsal aspect of the hippocampus, we first selectively labeled and then stimulated dopamine axons in the dorsal hippocampus using the neuronal specificity afforded by optogenetics. Using the Cre-Lox method for gene expression, we used mice that expressed Cre under control of the dopamine transporter with an internal ribosome entry site (IRES-Cre mice). We then transfected dopaminergic cell bodies in the VTA and their axonal projections by injecting a floxed adenoassociated virus coding for Channelrhodopsin-2 (ChR2), a light-sensitive ion channel coupled to enhanced yellow fluorescent protein (EYFP), a fluorescent reporter gene (Fig. 1 A and B). The genetic precision of optogenetics eliminates the possibility of nonspecific binding and injection site spillover that can accompany classical immunohistochemical techniques. To map dopamine axonal projections, we imaged ChR2-EYFP–expressing VTA dopamine axons and immunostained for TH (Fig. 1 C and D), an enzyme in the pathway for dopamine synthesis that highlights catecholaminergic structures (23). Confirming the efficacy of our cell type-specific labeling method, we observed fibers in expected VTA projection sites, including the striatum and amygdala (Fig. S1A), as well as in the ventral hippocampus (Fig. 1D). However, we found very sparse expression of VTA dopamine axons in the cortical area 1 (CA1) region of the dorsal hippocampus (Fig. 1C). We observed a significantly greater number of VTA axons in the ventral versus dorsal hippocampus (Fig. 1E).
Fig. 1.
Dopaminergic projection from the VTA to the CA1 region of the dorsal hippocampus is very sparse in dopamine transporter (DAT)-IRES-Cre mice. (A) Schematic of the experimental approach depicts infection of VTA dopamine (DA) neurons and axons with ChR2-EYFP to allow for axonal tracing. AAV, adenoassociated virus; HPC, hippocampus. (B) ChR2-EYFP expression was restricted to VTA dopamine neurons. ChR2 (green) illustrates ChR2-EYFP expression, TH (red) is a dopamine marker, and Merge indicates combined signals. (Scale bar: 100 μm.) (C) ChR2+ fibers (green) indicate the dearth of dopamine-containing axons in the dorsal hippocampus originating from the VTA. (Scale bar: 20 μm.) (D) ChR2+ VTA dopamine axons are abundant in the ventral hippocampus. DAPI (blue) is a nuclear marker. (Scale bar: 20 μm.) (E) Significantly more ChR2+ fibers are found in the ventral hippocampus (Ven HPC = 0.52 ± 0.10%) versus the dorsal hippocampus (Dor HPC = 0.22 ± 0.05%) (Mann–Whitney test, *P = 0.022). (F) Neurons of the LC project to the dorsal hippocampus as indicated by (red) neurons retrogradely labeled from the dorsal hippocampus. TH (green), retrobeads from the dorsal hippocampus (HPC RBs, red), and DAPI (blue) are shown. (Scale bar: 20 μm.) (G) VTA was devoid of dorsal hippocampus-projecting dopamine neurons. (Scale bar: 20 μm.) (H) Significantly more dopamine-containing neurons in the LC versus the VTA project to the dorsal hippocampus [two-way ANOVA, LC DA = 4.92 ± 0.95, LC non-DA = 0.50 ± 0.34, VTA DA = 0, VTA non-DA = 0.08 ± 0.08; F(1,44) = 19.82; n = 12; *P < 0.001].
Fig. S1.
Optogenetic approaches confirmed the presence of VTA dopamine axon expression in the amygdala and striatum. (A) Dopamine axons arising from the VTA (ChR2+, TH+) innervate the amygdala and striatum in DAT-IRES-Cre mice. DAPI (blue) is a nuclear marker, ChR2 (green) indicates ChR2-EYFP expression, TH (red) is a dopamine marker, and Merge indicates combined signals. (Scale bar: 20 μm.) (B) Expression of ChR2-EYFP in the midbrain of Th-IRES-Cre mice was not restricted to VTA dopamine neurons. (Scale bar: 100 μm.)
We repeated the procedure of tracing the VTA to the dorsal hippocampus projection using Th-IRES-Cre mice and similar to results in DAT-IRES-Cre mice, we found scarce expression of VTA dopamine axons in the dorsal hippocampus. However, upon inspection of the midbrain, we also observed ChR2-EYFP viral expression in neurons outside of VTA dopamine cells, and therefore eliminated the dataset (Fig. S1B). Indeed, it has previously been reported that Cre-induced reporter expression in the midbrain of Th-IRES-Cre and Th-Cre mice is nonspecific for VTA dopamine neurons (24).
Catecholamine Neurons of the LC Project to the Dorsal Hippocampus.
To explore the source of dopamine to the dorsal hippocampus, we used retrograde tracing to locate the cell bodies of catecholamine neurons that project directly to the dorsal hippocampus (Fig. 1 F and G). We injected red retrobeads into the dorsal CA1 region of the hippocampus in naive C57BL/6 mice and did not label dopamine cell bodies in the VTA, providing further evidence that the catecholamine projection from the VTA to the dorsal hippocampus is sparse at best (Fig. 1G). In contrast, the retrobeads fluorescently labeled significantly more catecholamine neurons in the LC (Fig. 1H), another brain region related to attention and arousal (25, 26). Unlike the VTA, which contains neurons that release dopamine, cells of the LC are thought to produce dopamine solely as an intermediate in the synthesis of norepinephrine (27). We now hypothesize that LC neurons corelease dopamine along with norepinephrine in the dorsal hippocampus.
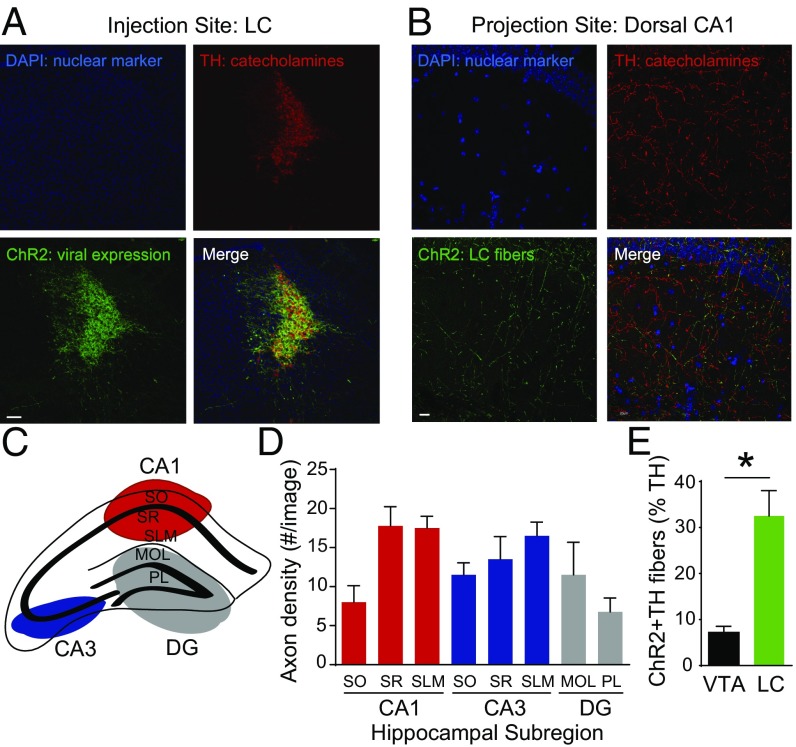
To determine whether the catecholamine terminals of the LC are appropriately positioned to influence hippocampal activity, we next isolated and mapped the catecholamine pathway from the LC to the dorsal hippocampus in Th-IRES-Cre mice. The LC projection to the hippocampus (28), specifically to the ventral hippocampus, has been documented (29); however, the anatomical localization of LC catecholamine terminals within distinct dorsal hippocampal subregions has not been well characterized. Using ChR2-EYFP as a neuronal label, we found that catecholamine neurons in the LC indeed send numerous axons to the dorsal hippocampus (Fig. 2 A and B). Given that place cells are found in the CA1 and CA3 regions in the dorsal hippocampus (6), we quantified LC terminal expression in these hippocampal subregions (schematic in Fig. 2C). We found dense LC catecholamine fiber expression throughout the dorsal CA1 region (Fig. 2D). This finding suggests that LC catecholamine fibers terminate in close proximity to the dendritic branches of hippocampal place cells, neurons that encode position in space and are tuned by selective attention to environmental cues (7, 8). We next compared the density of LC catecholamine axons with the density of VTA dopamine axons in the dorsal CA1 region and found that LC axons are substantially denser than VTA fibers (Fig. 2E).
Fig. 2.
Distribution of LC catecholamine axons in the dorsal hippocampus of Th-IRES-Cre mice. (A) ChR2 successfully infected catecholamine-containing neurons in the LC. (Scale bar: 100 μm.) (B) ChR2+ LC catecholamine axons are found in the dorsal hippocampus. (Scale bar: 20 μm.) (C) Dorsal hippocampal subregions. (D) LC catecholamine fiber density in hippocampal subregions (one-way ANOVA, Kruskal–Wallis test, P = 0.024; n = 4). MOL, molecular layer of the dentate gyrus (DG); PL, polymorphic layer of the DG; SLM, stratum lacunosum-moleculare; SO, stratum oriens; SR, stratum reticulatum. DAPI (blue) is a nuclear marker, ChR2 (green) indicates ChR2+ LC axons, TH (red) indicates catecholamine-containing fibers, and Merge indicates combined signals. (E) LC catecholamine axon density is significantly greater than the density of VTA dopamine axons in the dorsal hippocampus (LC: 32.53 ± 5.48%, VTA: 7.20 ± 1.32%, Mann–Whitney test, *P < 0.001).
Previous studies support the hypothesis that LC neurons are capable of coreleasing dopamine in the dorsal hippocampus. Electrical stimulation of the LC produces increased tissue content and pharmacological action of dopamine in the hippocampus (20, 30). However, these studies did not rule out network action via the VTA and failed to demonstrate release of dopamine from noradrenergic LC axons into the dorsal hippocampus. An increase in dopamine tissue content may suggest intracellular accumulation of norepinephrine synthesis products, but does not provide evidence that these neurons are capable of dopamine release. We attempted to address these unresolved questions using the genetic and anatomical specificity afforded by optogenetics.
Dopamine Is Released from Axons of the LC in the Dorsal Hippocampus.
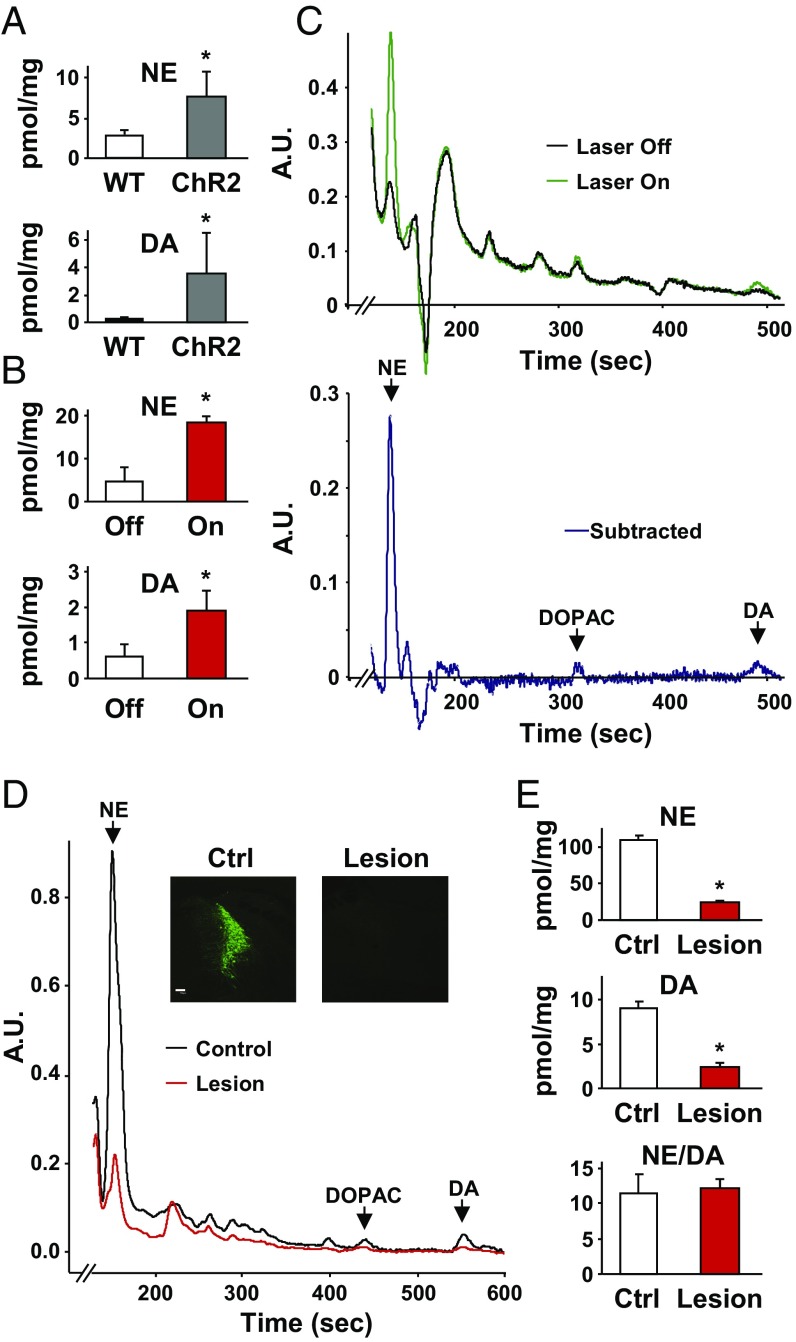
To assess directly whether and to what extent dopamine is released from axons of the LC, we measured extracellular dopamine concentrations in acute hippocampal slices using high-performance liquid chromatography (HPLC). Dorsal hippocampus coronal brain sections (250 μm) were prepared from Th-IRES-Cre mice in which LC catecholamine neurons and fibers were infected with the floxed ChR2-EYFP virus. We then photostimulated LC axons in the hippocampus ex vivo and compared dopamine release in the extracellular fluid of stimulated hippocampal slices with identically handled wild-type controls lacking ChR2 expression. Optical stimulation of LC catecholamine terminals in the dorsal hippocampus evoked significantly higher levels of both dopamine and norepinephrine release compared with controls (Fig. 3A). Similarly, optically stimulated slices (laser on) showed significantly higher extracellular concentrations of dopamine and norepinephrine than nonstimulated slices expressing ChR2-EYFP (laser off; Fig. 3 B and C). These findings confirm that both neurotransmitters were released from LC axons into the dorsal hippocampus.
Fig. 3.
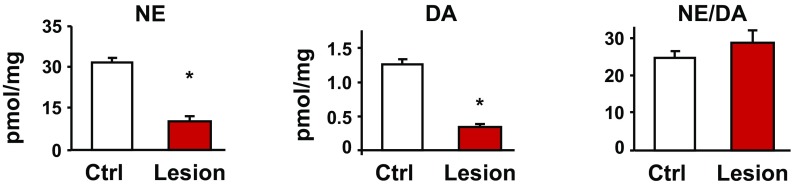
Dopamine is released from LC axons in the hippocampus. (A) Average dopamine and norepinephrine concentrations in the extracellular fluid of photostimulated hippocampal slices from mice expressing ChR2 in LC axons and their non-ChR2–expressing littermates [wild-type (WT): DA = 0.11 ± 0.05, norepinephrine (NE) = 3.05 ± 0.22, n = 3; ChR2: DA = 3.57 ± 1.14, n = 7; Student’s t test, *P = 0.017). (B) Higher dopamine and norepinephrine levels were also measured in the supernatant of ChR2-expressing hippocampal slices that were optically stimulated (On) versus unstimulated (Off) controls (nonstimulated: DA = 0.60 ± 0.18, NE = 4.75 ± 1.65, n = 4; stimulated: DA = 1.88 ± 0.29, NE = 18.25 ± 0.85, n = 4; Student’s t test, *P = 0.029). (C) Representative HPLC traces show clear peaks for norepinephrine, dihydroxyphenylacetic acid (DOPAC), and dopamine in the extracellular fluid of stimulated slices versus nonstimulated controls. A.U., arbitrary units. (D) HPLC trace of hippocampal tissue samples from animals with a unilateral, Caspase-3–induced lesion of catecholamine neurons in the LC. (Insets) TH staining of the LC region. (Scale bar: 100 μm.) (E) Both dopamine and norepinephrine tissue levels in the dorsal hippocampus were nearly eliminated by lesioning LC catecholamine neurons. (Control: DA = 9.06 ± 0.64, NE = 108.96 ± 6.32, n = 5; Lesion: DA = 2.47 ± 0.49, NE = 24.85 ± 1.22, n = 5; Student’s t test, P < 0.001). The ratio of norepinephrine to dopamine was the same in control and lesioned hemispheres (Control: 11.67± 2.49, Lesion: 12.29 ± 1.16; *P > 0.05).
We next lesioned catecholamine neurons of the LC selectively using a floxed Caspase-3 virus (University of North Carolina Vector Core) (31) and measured a 72.7% reduction of dopamine and a 77.2% reduction of norepinephrine tissue levels in the hippocampus (Fig. 3 D and E). The ratio of norepinephrine to dopamine remained the same in lesioned and control samples (Fig. 3E). We then stimulated nonspecific neurotransmitter release into the extracellular fluid by incubating slices in potassium chloride to promote release of dopamine and norepinephrine from all axons in the dorsal hippocampus. A selective lesion of LC catecholamine neurons reduced the extracellular dopamine concentration by 67.9% and norepinephrine by 83.8% (Fig. S2). This finding suggests that the majority of the dopaminergic tone present in dorsal hippocampal tissue and actively released into the extracellular space originated from neurons of the LC. Together, these results provide a direct demonstration that dopamine is released from LC axons into the dorsal hippocampus.
Fig. S2.
Average norepinephrine (NE) and dopamine (DA) concentrations in the extracellular fluid of hippocampal slices from animals with a unilateral, Caspase-3–induced lesion of catecholamine neurons in the LC. The lesioned hemisphere (Lesion) was compared with the nonlesioned hemisphere (Ctrl) of the same mice following stimulation with 40 mM KCl for 5 min. Similar to whole-tissue levels of dopamine and norepinephrine (Fig. 3E), stimulation-dependent release of both neurotransmitters was significantly decreased by lesioning LC catecholamine neurons [Control (n = 5): DA = 1.28 ± 0.07, NE = 31.30 ± 1.59; Lesion (n = 5): DA = 0.36 ± 0.02, NE = 10.49 ± 1.60; t test, *P < 0.001]. In contrast, the ratio of norepinephrine to dopamine remained the same, suggesting decreased release from the same axon terminals (Control = 24.72 ± 1.70, Lesion = 28.66 ± 3.56).
Optogenetic Activation of LC Catecholamine Axons in the Dorsal Hippocampus Promotes Selective Attention and Spatial Learning via the Dopamine D1/D5 Receptor.
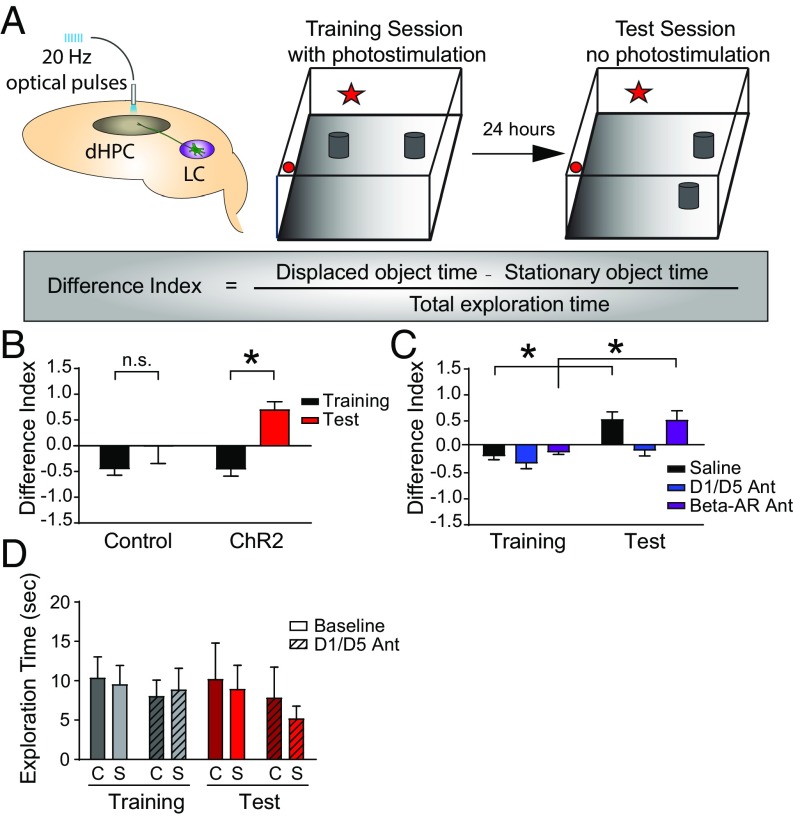
To assess the behavioral significance of the dopamine pathway from the LC to the hippocampus, we examined mice in a spatial object recognition task while activating ChR2-EYFP+ LC axons in the dorsal hippocampus (Fig. 4A). We allowed mice to explore a square arena with distinct walls containing two identical objects during a 5-min training session. One day later, we challenged these mice to explore the arena during a 5-min test session in which the least preferred object was moved to another position. During the training session for this task, both ChR2-EYFP–expressing Th-IRES-Cre animals and their wild-type controls received intrahippocampal optical stimulation. We then assessed performance by determining the difference index, a normalized measure indicating the relative amount of time spent exploring the displaced object. Increased attention to the displaced object during the test session generates a higher difference index and correlates positively with the degree of successful spatial learning and memory. In ChR2-EYFP–expressing animals, optical activation of hippocampal LC catecholamine terminals during training significantly increased the average difference index in the test session. This enhancement of spatial learning only occurred in optically stimulated animals, and not in wild-type controls (Fig. 4B). These results demonstrate that photostimulation of the LC-to-hippocampus catecholamine pathway promoted spatial learning and converted nonlearners into learners.
Fig. 4.
Dopamine release from the LC to the dorsal hippocampus improved spatial object recognition via dopamine D1/D5 receptors. (A) Experimental paradigm. dHPC, dorsal hippocampus. (B) ChR2-stimulated animals (heterozygous Th-IRES-Cre, n = 10) show significantly enhanced spatial learning compared with control animals (wild-type littermates, n = 6). Difference index: mean ± SEM; ChR2 (n = 10): Training = −0.45 ± 0.14, Test = 0.70 ± 0.15; Control (n = 6): Training = −0.45 ± 0.12, Test = −0.01 ± 0.34; two-way ANOVA, F(1,14) = 16.78; *P = 0.001, Sidak’s multiple comparison test. n.s., not significant. (C) Intrahippocampal injection of SCH23390, a dopamine D1/D5 receptor antagonist, but not propranolol, a beta-adrenergic receptor antagonist (AR Ant), reversed optically stimulated LC-hippocampus–induced enhancement of performance versus saline controls. D1/D5 Ant (n = 6): Training = −0.35 ± 0.10, Test = −0.11 ± 0.10; Saline (n = 7): Training = −0.21 ± 0.08, Test = 0.48 ± 0.15; Beta-AR Ant (n = 4): Training = −0.13 ± 0.05, Test = 0.47 ± 0.18; two-way ANOVA, treatment factor F(2,38) = 6.840; *P = 0.004, Sidak’s multiple comparison test. (D) Total object exploration time during 5 min of training did not change significantly in the presence of the D1/D5 antagonist. C, Control; S, ChR2 optical stimulation (P > 0.05).
The LC-to-hippocampus projection was previously thought to be strictly noradrenergic (28); therefore, the function of dopamine released from LC fibers into the dorsal hippocampus was not known. Given the dearth of catecholamine innervation from the VTA, we now asked whether the LC actively releases behaviorally relevant dopamine concentrations into the dorsal hippocampus. To address this question, before the optical stimulation training session, we infused into the dorsal hippocampus one of three compounds: (i) saline; (ii) SCH23390, a dopamine D1/D5 receptor antagonist we have previously used to block the effects of attention on place cell stability (8); or (iii) propranolol, a beta-adrenergic receptor antagonist. Site-specific delivery (Fig. S3) of the dopamine D1/D5 receptor antagonist blocked the memory-enhancing effects of LC-to-hippocampus photostimulation, consistent with the hypothesis that LC neurons do, in fact, release dopamine into the dorsal hippocampus to promote spatial learning and memory (Fig. 4C). The norepinephrine antagonist had no significant effect on the LC-to-hippocampus optical stimulation-induced learning enhancement (Fig. 4C). Given that the difference index is a normalized measure of activity and that total exploration times in antagonist experiments were not significantly different from baseline experiments, it is highly unlikely that this effect was due to alterations in locomotion (Fig. 4D).
Fig. S3.
Examples of LC viral expression and dorsal hippocampal cannula placement. (A) Cannula placements aimed at the dorsal hippocampus. (B) Nuclear stain (DAPI, blue) showing the placement of a representative cannula above the CA1 region of the dorsal hippocampus. (Scale bar: 200 μm.) (C) Coronal section of a representative ChR2-EYFP-transfected (green) LC (TH, red). (Scale bar: 100 μm.)
Optogenetic Activation of LC Catecholamine Axons in the Dorsal Hippocampus Increases the Rate of Spatial Learning and Memory.
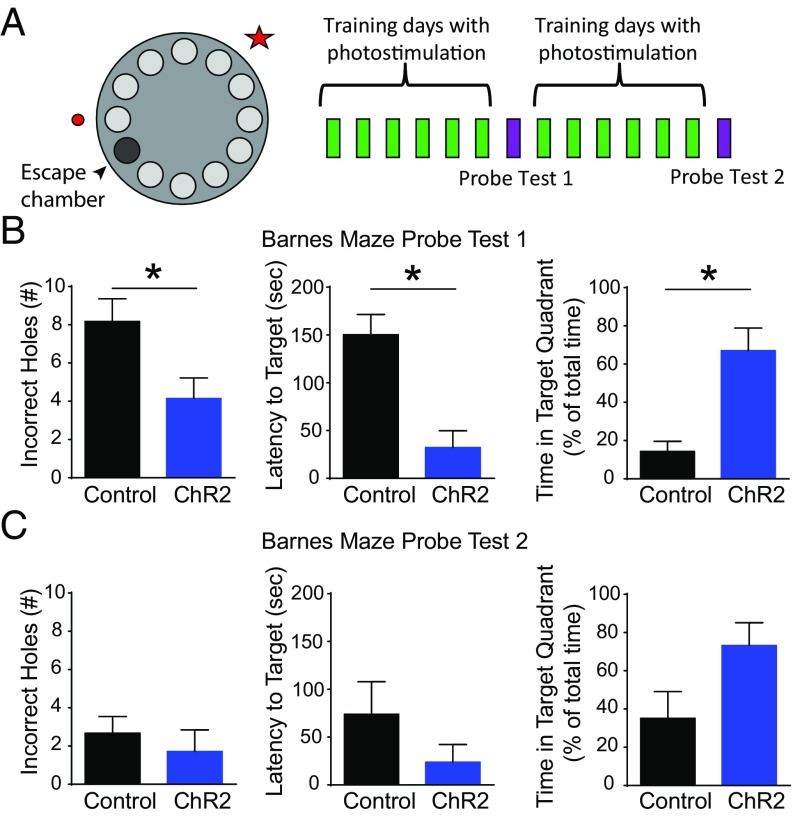
To explore the role of the catecholaminergic projection from the LC to the dorsal hippocampus in spatial learning and memory further, we also performed the Barnes Maze task. In this hippocampus-dependent paradigm, animals explore a circular platform with equidistant holes surrounding the periphery (Fig. 5A). One hole leads to an escape box, which, when entered, turns off the aversive bright lights. Animals received optical stimulation of LC axons in the dorsal hippocampus during each of the daily training sessions. After 6 d of training, the escape box was removed and optical stimulation was discontinued to test the memory retention for the target hole. Optical stimulation resumed for the next 6 d of training to the same target hole, followed by a second probe day. Animals were connected to a fiber optic cable that did not deliver photostimulation on probe days. LC-to-hippocampus–stimulated animals learned this task significantly better than controls during probe test 1, as indicated by a fewer number of errors, shorter latency to the target hole, and more time spent in the target quadrant (Fig. 5B). By probe test 2, control animals had sufficiently learned the task, yielding no significant difference between the two groups (Fig. 5C). These findings demonstrate that in a task learned by control animals, activation of LC axons in the dorsal hippocampus of ChR2-expressing mice significantly increased the rate of learning. These results reinforce earlier findings and support the idea that dopamine release from the LC to the dorsal hippocampus is a significant component of the spatial learning and memory circuit.
Fig. 5.
Optogenetic activation of LC catecholamine axons in the dorsal hippocampus increased the rate of learning in the Barnes Maze task. (A) A schematic of the Barnes Maze and training protocol is shown. Small light gray circles represent empty holes, and the dark gray circle indicates the position of the hole leading to a goal box in which the animal can escape to turn the lights off. Memory retention was tested by probe sessions during which the escape box and optical stimulation were removed. (B) On probe day 1, optically stimulated animals learned the task significantly better than wild-type controls [Control (n = 6), ChR2 (n = 7): incorrect holes visited before reaching target hole: *P = 0.030, latency to reach target hole from trial start: *P = 0.005, time spent in target quadrant: *P = 0.015]. (C) By probe day 2, both controls and optically stimulated animals learned the task well, as indicated by no significant difference between the two groups (P > 0.05).
Optogenetic Activation of LC Catecholamine Axons in the Dorsal Hippocampus Had No Effect on a Modified Conditioned Place Preference Task.
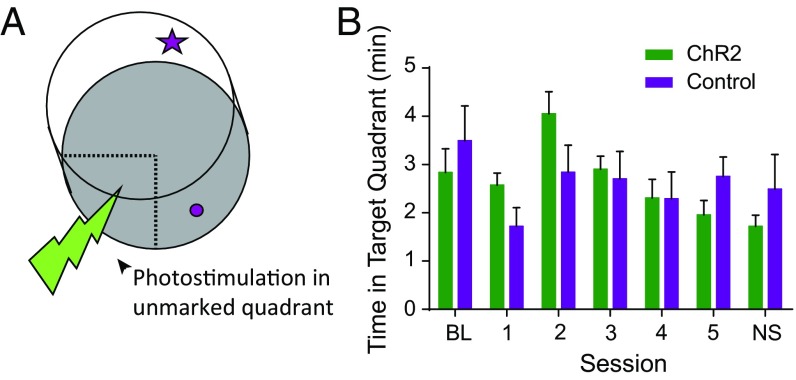
To assess whether photostimulation of the LC-to-hippocampus catecholamine pathway is inherently reinforcing, we performed a conditioned place preference task modified to increase involvement of hippocampus-dependent spatial learning circuits. Animals explored a circular chamber with cues surrounding the walls for spatial orientation. We delivered photostimulation each time the animal explored an unmarked target quadrant of the arena (Fig. 6A). This spatial version of conditioned place preference was designed to assay whether the LC-to-hippocampus pathway provided reinforcement in the absence of selective attention to a specific object or goal. Optically stimulated animals did not display a significant change in the amount of time spent in the unmarked target region versus wild-type controls (Fig. 6B). This finding is consistent with the argument that LC-to-hippocampus stimulation enhances selective attention to specific stimuli, thereby energizing spatial learning and memory. This result demonstrates that dopamine signaling in the dorsal hippocampus did not produce an inherently rewarding or reinforcing signal but, instead, promoted selective attention to salient environmental cues.
Fig. 6.
Optogenetic activation of LC catecholamine axons in the dorsal hippocampus had no effect on a modified conditioned place preference task. (A) Schematic of the circular chamber in which animals received photostimulation of the catecholaminergic pathway from the LC to the dorsal hippocampus when traversing through the unmarked target quadrant. (B) ChR2-stimulated animals (n = 10) did not spend significantly more time in the target quadrant than wild-type controls (n = 6), two-way ANOVA, F(1,14) = 0.00, P > 0.05. The numbers 1–5 represent 5 d of exploration with optical stimulation in the target region. BL, baseline session with no optical stimulation; NS, no stimulation day.
Discussion
These findings provide direct evidence that efferents from the LC are not purely noradrenergic, but corelease dopamine in the dorsal hippocampus. The LC dopamine signal drives the selective attention underlying spatial learning and memory. Numerous reports suggest that the VTA is the main source of dopamine to the dorsal hippocampus (12–14), whereas others suggest that the LC supplies the hippocampus with dopamine (19–21). The present results reconcile the discrepancy between dense dopamine receptor expression and sparse VTA dopamine axon networks in the dorsal hippocampus and support the hypothesis that dopaminergic tone in the dorsal hippocampus arises from neurons of the LC.
Previous work by McNamara et al. (12) demonstrated that activation of VTA dopamine axons in the dorsal hippocampus promoted spatial memory for a reward location. Our work adds to these findings by suggesting that dopamine release from LC neurons energizes the attention component of the spatial environment in the absence of reward and supports the acquisition of spatial memory. Given that catecholamine neurons of the LC contribute 73% of all measurable dopaminergic tone in the dorsal hippocampus, we argue that dopamine release from the LC may have more significant influence over dorsal hippocampus neuronal activity than the VTA.
Our findings that LC neurons corelease dopamine in the dorsal hippocampus are consistent with the earlier finding that LC neurons also corelease dopamine in the prefrontal cortex and drive dopamine-dependent activity in the hippocampus (19, 21). Moreover, the current findings provide anatomical support for the documented functional distinctions between the dorsal and ventral hippocampus (17). The density of VTA dopamine terminals in the ventral hippocampus, but sparse expression in the dorsal hippocampus, suggests that hippocampal subregion-dependent behaviors are driven by different neuromodulatory pathways. Specifically, LC catecholamine release may be the primary source of synaptic modulation responsible for tuning the space-encoding place cells found in the dorsal hippocampus. In contrast, VTA dopamine innervation in the ventral hippocampus may provide the incentive salience and motivational drive underlying emotion-based learning.
The present findings also expand the list of dopamine-releasing brain regions to include the LC. Numerous studies postulate dopamine release from the LC into the hippocampus, but rely on pharmacological blockade of the dopamine D1/D5 receptor to infer the presence of dopamine. However, the possibility exists that the D1/D5 receptor is activated by a ligand other than dopamine or undergoes receptor–receptor interactions. Norepinephrine has a Kd of 50 μM at the dopamine D1 receptor (32), and the possibility exists that norepinephrine action at the dopamine D1 receptor is responsible for the effects reported by Smith and Greene (21). In fact, norepinephrine promotes cAMP accumulation in embryonic retinas via the dopamine D1/D5 receptor (33), and norepinephrine-induced increases in adenyl cyclase activity in retinal homogenates can be reversed by the dopamine D1/D5 receptor antagonist (34). In the present study, we use biochemical approaches to directly demonstrate dopamine corelease from LC axons in the dorsal hippocampus.
One implication of our finding is that the peripheral signals that carry information about arousal, novelty, stress, and sleep-wake states may drive the release of dopamine in the dorsal hippocampus as well as other LC projection sites, such as the prefrontal cortex and amygdala. The current results further suggest that LC dopamine release may play a role in energizing spatial learning and memory by increasing attention to salient features of the environment. Activation of the LC-to-hippocampus pathway did not produce place preference in the absence of salient environmental objects, suggesting that photostimulation is not inherently reinforcing but may promote selective attention to relevant cues in the given setting. Finally, insofar as this work relates to attention and memory it may prove helpful for identifying therapeutic targets of dopamine- and norepinephrine-related disorders, such as ADHD.
Materials and Methods
Details on mice and procedures regarding optogenetics, behavioral paradigms, immunohistochemistry, and HPLC are detailed in SI Materials and Methods. Animal experiments were carried out following the guidelines of the Institutional Animal Care and Use Committee of Columbia University.
SI Materials and Methods
Animals.
Th-IRES-Cre and dopamine transporter (DAT)-IRES-Cre male mice on a C57BL/6 background and naive C57BL/6 male mice were used for this study. All animal care and use followed the Institutional Animal Care and Use Committee guidelines set forth by the New York State Psychiatric Institute and Columbia University. Th-IRES-Cre mice were obtained from the laboratory of Eric Huang at the University of California, San Francisco, and were rederived before use at Columbia University. DAT-IRES-Cre and naive C57BL/6 mice were obtained from The Jackson Laboratory (www.jax.org). Heterozygous males were crossed with wild-type females to produce the heterozygous and wild-type adult animals used in this study. Females were also used, and they display the same optically induced enhancement in spatial object recognition as reported here in males.
Optogenetics.
We selectively expressed the light-sensitive ChR2 in catecholamine neurons using the Cre-Lox method of gene expression. Transgenic mice expressed Cre under control of the TH or DAT promoter. An adenoassociated virus [AAV2/5.EF1a.DIO.hChR2(H134R)-EYFP.WPRE.hGH obtained from Penn Vector] coding for a reversed copy of the ChR2-EYFP genes, flanked on either side by a Lox site, delivered genetic material to all neurons at the injection site. Only in those neurons expressing Cre were the genes cleaved and inverted, allowing transcription of a functional copy of ChR2 and EYFP in catecholamine neurons. This process renders dopamine-containing cell bodies and terminals light-sensitive and fluorescent (35, 36). During stereotactic brain surgery, we delivered 0.3 μL of virus to the VTA [anteroposterior (AP): −3.2, mediolateral (ML): 0.5, dorsoventral (DV): −5.0] or 1.0 μL to the LC (AP: −1.5, ML: 3.0, DV: −4.0) unilaterally at a rate of 0.2 μL/min, waiting 10 min before retracting the microinjector. For baseline experiments, a ceramic ferrule attached to a fiber optic cable was implanted above the CA1 region of the dorsal hippocampus (AP: −1.8, ML: 1.6, DV: −1.2) and secured using dental cement. For antagonist experiments, a guide cannula was aimed at the dorsal hippocampus (1 mm dorsal to the brain region; Fig. S3), allowing insertion of either a microinjector or fiber optic cable. Animals recovered for at least 4 wk before behavioral experiments. A DPSS laser (473 nm; Laserglow Technologies) provided the blue light source for optogenetic stimulation. Fiber optic power output remained within the range of 4–10 mW as confirmed by measurements before each training session.
Behavioral Paradigms.
Spatial object recognition.
Th-IRES-Cre mice (heterozygous experimental animals and their wild-type controls) explored an 18 × 18-inch square chamber with two cues on opposite walls for orientation during five 10-min habituation sessions over 3 d. After habituation, mice explored the arena during a 5-min training session in which two identical cylinders were positioned in the back corners of the arena, 10 cm from each wall. Th-IRES-Cre heterozygous animals expressing ChR2 in catecholamine-containing LC neurons and their wild-type controls received intrahippocampal optical stimulation throughout the training session (20 Hz, ten 5-ms pulses, every 30 s). Twenty-four hours later, animals were returned to the same environment and allowed to explore the arena during a 5-min test session in which the least preferred object was displaced. Sessions were recorded via Neuralynx image capture software as well as by video camera (Canon). Two LEDs attached to the tether allowed for automated tracking of the mouse position. Time spent exploring each object consisted of nose touches to the object or orientation toward the object within 1 cm of touching. Exploration was hand-scored because automated measurements falsely included inattentive moments of walking past the object. Behavioral experiments were executed and analyzed blinded to the experimental group. The optically stimulated spatial object recognition task was replicated twice, yielding the same results each time:
For pharmacological experiments, animals received dorsal hippocampus saline (0.5 μL) or antagonist infusions in their home cage: SCH 23390 (3.47 mM) or propranolol (1.35 mM) over 2.5 min, 10 min before the training session. Animals that received failed infusions (as determined by lack of movement of a fluid indicator through the clear tubing) were eliminated from the study before decoding control versus experimental (five animals) groups. Two-way ANOVAs with Sidak’s multiple comparison test were used to determine significant differences between training and test sessions. Data represented as mean ± SEM.
Barnes Maze.
Animals explored an elevated circular platform with equidistant holes surrounding the periphery. One hole led to an escape chamber, which, when entered, turned the lights off for 1 min. Before the first day of training, animals were placed in the escape box with the lights off for 3 min. For the subsequent 6 d of training, animals were given one 3-min session per day to explore the platform. If the animals did not enter the chamber spontaneously, they were gently guided to the target and remained in the safety box with lights off for 1 min. Optical stimulation of the LC-to-hippocampal pathway was delivered throughout the training session (20 Hz, 5-ms pulses for 5 s every 15 s; stimulation was delivered more frequently than in the spatial object recognition task to ensure that the number of experienced stimulation bouts was similar). After 6 d of training, a probe test session was administered in which the escape box and optical stimulation were removed from the apparatus. This procedure allowed for testing the memory for the position of the target hole. Errors were counted as the number of incorrect holes visited before exploring the target hole. Latency to travel to the target was measured from the start of the session. Time spent in target quadrant was calculated as a percentage of the total session time. The protocol was repeated for the next 7 d, with the target in the same position as the first training set. Mann–Whitney tests were used to analyze data. Nonparametric tests were used for smaller sample sizes.
Spatial conditioned place preference.
Animals freely explored a circular chamber featuring visual cues on the wall. Each time the animal entered and explored an unmarked target quadrant, an automated system delivered 20 Hz of optical stimulation (5-ms pulses) continuously. Animal movement was tracked via an overhead camera recording the LEDs mounted on the animal’s head. Experiments and analysis were conducted blinded to the experimental group.
Immunohistochemistry.
Coronal sections (40–50 µm) of mouse brains fixed in 4% (wt/vol) paraformaldehyde were prepared via a vibratome or cryostat as described previously (7). Using indirect immunofluorescence methods, sections were incubated in primary antibodies against rabbit TH (AB152, 1:200; Millipore) and chicken anti-GFP (AB13970, 1:10,000; Abcam) for VTA fiber quantification. After overnight incubation in secondary antibodies against rabbit and chicken (Alexa 594 or Alexa 488, 1:1,000), slides were cover-slipped with Vectashield mounting medium with DAPI to visualize ChR2-EYFP, TH, and nuclei. Red retrobeads were obtained from Lumafluor, Inc., and 1.0 μL was infused into the right dorsal hippocampus at a rate of 0.2 μL/min.
HPLC.
Hippocampal slices (250 μm) were prepared using a Leica 1200VT vibratome after dissecting the brain from Th-IRES-Cre heterozygous and wild-type males that all received infusions of the floxed ChR2-EYFP virus into the LC. Slices of the hippocampus were prepared and incubated at 37 °C for 1 h before stimulation. Each slice was transferred to an epitube containing 10 μL of Tyrode’s solution and was optically stimulated with 20-Hz trains of blue light (5-ms pulses) for 5 min at room temperature via a fiber optic cable coupled to a 473-nm laser (15 mW). For lesion experiments, animals were injected with a floxed Caspase-3 virus (1.0 μL) to induce cell death in catecholamine neurons of the right LC (31). Hippocampal slices were then stimulated with a KCl solution (40 mM in Tyrode’s solution). After stimulation, the supernatant was extracted and mixed 1:1 with 0.1 M perchloric acid and immediately frozen at −80 °C until running the HPLC analysis. The remaining hippocampal tissue was mixed with 30 μL of 0.1 M perchloric acid, homogenized with a hand sonicator, incubated for 5 min on ice, and centrifuged at 6,000 × g for 10 min at 4 °C. The supernatant was transferred to a new tube and frozen at −80 °C for HPLC analysis. The pellet was resolubilized by NaOH, and the protein concentration in the sample was measured by a bicinchoninic acid (BCA) protein assay kit.
Dopamine, norepinephrine, and dihydroxyphenylacetic acid levels were determined by HPLC with electrochemical detection as described previously (37, 38). Molar amounts of metabolites were calculated from areas under HPLC peaks using calibration curves and normalized to protein concentrations in each sample. Averaged traces were generated and subtracted using Igor software.
Acknowledgments
We thank Edmund A. Griffin, Steven A. Siegelbaum, Laurence Abbott, and Joseph Rayman for critical discussions and comments on the manuscript; J. Melanie Philips, Elaine Espinal, and Antonella Perez for assistance with immunohistochemistry; and Elias Pavlopoulous and Luana Fioriti for help with behavioral paradigms. This work was supported by the Burroughs Wellcome Fund Postdoctoral Enrichment Program (K.A.K.), Translational Neuroscience Training Grant T32 MH015174-37 (to K.A.K.), National Institute of Neurological Disorders and Stroke-NIH Grant R01 NS075222 (to E.V.M.), The Jeffry M. and Barbara Picower Foundation (D.S.), the Parkinson’s Disease Foundation (D.S.), Grant R01 DA07418 (to D.S.), Grant R01 DA010154 (to D.S.), the Institute for the Study of Affective Neuroscience (E.R.K.), the Henry M. Jackson Foundation for the Advancement of Military Medicine (E.R.K.), and the Howard Hughes Medical Institute (E.R.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616515114/-/DCSupplemental.
References
- 1.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67(1):145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 2.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 3.Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92(7):2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing B, et al. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience. 2010;169(4):1511–1519. doi: 10.1016/j.neuroscience.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 5.da Silva WC, Köhler CC, Radiske A, Cammarota M. D1/D5 dopamine receptors modulate spatial memory formation. Neurobiol Learn Mem. 2012;97(2):271–275. doi: 10.1016/j.nlm.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 6.O’Keefe J. Do hippocampal pyramidal cells signal non-spatial as well as spatial information? Hippocampus. 1999;9(4):352–364. doi: 10.1002/(SICI)1098-1063(1999)9:4<352::AID-HIPO3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Muzzio IA, et al. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biol. 2009;7(6):e1000140. doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42(2):283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmack SA, Howell KK, Rasaei K, Reas ET, Anagnostaras SG. Animal model of methylphenidate’s long-term memory-enhancing effects. Learn Mem. 2014;21(2):82–89. doi: 10.1101/lm.033613.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17(12):1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668(1-2):71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- 14.Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(1):1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- 15.Lazarov NE, Schmidt U, Wanner I, Pilgrim C. Mapping of D1 dopamine receptor mRNA by non-radioactive in situ hybridization. Histochem Cell Biol. 1998;109(3):271–279. doi: 10.1007/s004180050227. [DOI] [PubMed] [Google Scholar]
- 16.Khan ZU, et al. Dopamine D5 receptors of rat and human brain. Neuroscience. 2000;100(4):689–699. doi: 10.1016/s0306-4522(00)00274-8. [DOI] [PubMed] [Google Scholar]
- 17.Kheirbek MA, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgkvist A, Malmlöf T, Feltmann K, Lindskog M, Schilström B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol. 2012;15(4):531–540. doi: 10.1017/S1461145711000812. [DOI] [PubMed] [Google Scholar]
- 19.Devoto P, Flore G. On the origin of cortical dopamine: Is it a co-transmitter in noradrenergic neurons? Curr Neuropharmacol. 2006;4(2):115–125. doi: 10.2174/157015906776359559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex. 2012;22(9):2131–2138. doi: 10.1093/cercor/bhr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 2012;32(18):6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Köhler C, Goldstein M. Golgi-like immunoperoxidase staining of dopamine neurons in the reticular formation of the rat brainstem using antibody to tyrosine-hydroxylase. J Comp Neurol. 1984;223(2):302–311. doi: 10.1002/cne.902230210. [DOI] [PubMed] [Google Scholar]
- 24.Lammel S, et al. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85(2):429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46(9):1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 26.Howells FM, Stein DJ, Russell VA. Synergistic tonic and phasic activity of the locus coeruleus norepinephrine (LC-NE) arousal system is required for optimal attentional performance. Metab Brain Dis. 2012;27(3):267–274. doi: 10.1007/s11011-012-9287-9. [DOI] [PubMed] [Google Scholar]
- 27.Chandler DJ, Waterhouse BD, Gao WJ. New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Front Neural Circuits. 2014;8:53. doi: 10.3389/fncir.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189(4):699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- 29.Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 1989;9(11):3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scatton B, Dennis T, Curet O. Increase in dopamine and DOPAC levels in noradrenergic terminals after electrical stimulation of the ascending noradrenergic pathways. Brain Res. 1984;298(1):193–196. doi: 10.1016/0006-8993(84)91169-7. [DOI] [PubMed] [Google Scholar]
- 31.Yang CF, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153(4):896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brusniak MYK, Pearlman RS, Neve KA, Wilcox RE. Comparative molecular field analysis-based prediction of drug affinities at recombinant D1A dopamine receptors. J Med Chem. 1996;39(4):850–859. doi: 10.1021/jm950447w. [DOI] [PubMed] [Google Scholar]
- 33.Kubrusly RC, et al. Norepinephrine acts as D1-dopaminergic agonist in the embryonic avian retina: Late expression of beta1-adrenergic receptor shifts norepinephrine specificity in the adult tissue. Neurochem Int. 2007;50(1):211–218. doi: 10.1016/j.neuint.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Vanderheyden P, Ebinger G, Kanarek L, Vauquelin G. Epinephrine and norepinephrine stimulation of adenylate cyclase in bovine retina homogenate: Evidence for interaction with the dopamine D1 receptor. Life Sci. 1986;38(13):1221–1227. doi: 10.1016/0024-3205(86)90177-3. [DOI] [PubMed] [Google Scholar]
- 35.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci. 2015;18(12):1763–1771. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feigin A, et al. Metabolic correlates of levodopa response in Parkinson’s disease. Neurology. 2001;57(11):2083–2088. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- 38.Choi SJ, et al. Changes in neuronal dopamine homeostasis following 1-methyl-4-phenylpyridinium (MPP+) exposure. J Biol Chem. 2015;290(11):6799–6809. doi: 10.1074/jbc.M114.631556. [DOI] [PMC free article] [PubMed] [Google Scholar]