Abstract
Introduction
Mansonella perstans is a human filarial parasite transmitted by biting midges (Diptera: Ceratopogonidae) belonging to the genus Culicoides and it is widely spread in sub-Saharan Africa. While most cases are asymptomatic, mansonelliasis can be associated with angioedema, arthralgia, swellings, pain in the scrotum or in serous cavities such as the pleura, the peritoneum, the pericardium, etc. Mansonelliasis can be really hard to treat, but it has been shown that an intensive treatment using albendazole can clear the parasite.
Case report
Here we describe a case of a 16 months-old malnourished child with pneumonia due to M. perstans in the east of the Democratic Republic of Congo.
Conclusion
Although our investigations confirmed M. perstans infection, this case shows that it is very difficult to come to a conclusive diagnosis.
Keywords: mansonelliasis, pneumonia, filariasis, malnutrition
Introduction
M. perstans is widely distributed in Africa and is endemic for transmission in this continent in a total of 33 countries.1 Infections with M. perstans are highly prevalent in endemic areas, especially in children. Despite this, few studies have been carried out on its epidemiology or associated health outcomes. Hence, no standard protocol for treatment of mansonelliasis is available.1 M. perstans can produce a wide range of signs and symptoms such as: angioedema, arthralgia, swellings, pain in the scrotum or in serous cavities such as the pleura, the peritoneum, the pericardium, etc1. However most cases of M. perstans infection are asymptomatic.2
Here we report the case of a child with malnutrition who developed pneumonia by M. perstans.
Case report
A 16 months-old boy was admitted in the nutritional center of the Provincial General Hospital of Bukavu (Democratic Republic of Congo) for protein-energy malnutrition. The onset of the disease had been 3 months earlier, by anorexia and lower limb edema. At admission, his heart rate was 140 beats per minute, respiratory rate 40 breaths per minute, and temperature 37.5 °C. He had a weight of 6.35 kg, for a height of 65 cm and brachial perimeter of 13.4 cm. Thus, anthropometric indexes were as follow: height-for-age under the 5th percentile, weight-for-height under the 25th percentile and weight-for-age under the 5th percentile. We noted that the patient was coughing, had pruritic subcutaneous lesions, his hairs had lost pigmentation and he had edema of the legs and the face. Chest auscultation revealed crackles in the bases of the two lungs and there was dullness on percussion on the interested fields. The examination of his heart was normal except for slight tachycardia. Palpation of his abdominal right upper quadrant was painful and he had hepatomegaly.
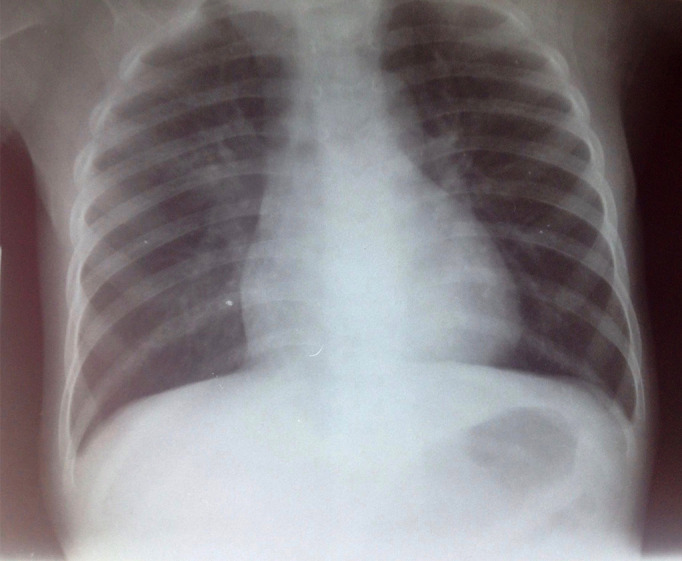
Laboratory tests showed: white blood cells at 12,800 per μL with leukocytes differential of 23% lymphocytes, 62% neutrophils, 7% monocytes, 7% eosinophils, 1% basophils; red blood cells 2,840,000 per μL, hemoglobin 6.9 per μL, hematocrit 21%; and 196,000 platelets per μL. HIV serology was negative, with negative HBsAg, negative anti-HCV, negative Ziehl Neelsen stain and negative M. tuberculosis PCR from gastric aspirate, negative blood film for malaria, negative bacteriological urinalysis, albumin 2.1 mg/dL, negative proteinuria and negative glycosuria. His chest X-ray showed a base reticular opacity on the right field (Figure 1).
Figure 1. Chest X-ray at admission. Note a reticular opacity in the base of the right field.
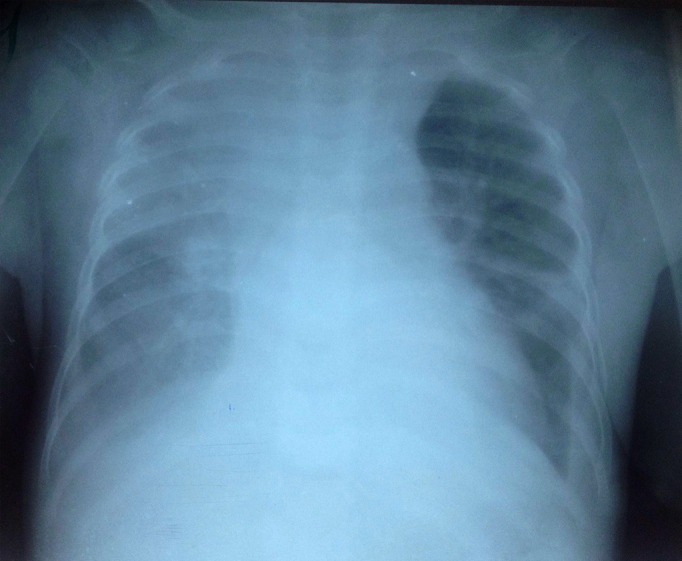
The patient was started on treatment protocol for malnutrition, community acquired pneumonia and cutaneous staphylococcal infection including milk F-75 (formula 75, a supplementary starter milk for feeding malnourished children, contains 75 kilocalories per 100 milliliters) 100 mL every 3 hours, cefotaxime 450 mg every 8 hours, gentamicin 20 mg once per day and cloxacillin 400 mg every 8 hours. However, infection was still present after 7 days of treatment. As the dyspnea worsened and the Keith Edward score for tuberculosis was 9 (the length of illness: more than four weeks, the nutritional status: weight for age under 60%, the malnutrition status had not improved after four weeks of treatment and the changes in temperament: anorexia), the patient began a tuberculosis treatment protocol with RHZ 1 tablet per day before breakfast (rifampicin 60 mg, isoniazid 30 mg, pyrazinamide 150 mg) and ethambutol 60 mg 1 tablet per day before breakfast. We had to discontinue cefotaxime and gentamicin after 8 days of treatment while maintaining cloxacillin for skin infection, and stopping it after 21 days. Ceftriaxone 520 mg daily was added to his treatment from the 30th day of hospitalization for a duration of 8 days. After 38 days of tuberculosis treatment, no improvement was observed while the dyspnea worsened. A new chest radiography showed a pleural effusion (Figure 2).
Figure 2. Chest X-ray after 45 days of admission. The reticular opacity fills both pulmonary fields and there are signs of pleural effusion.
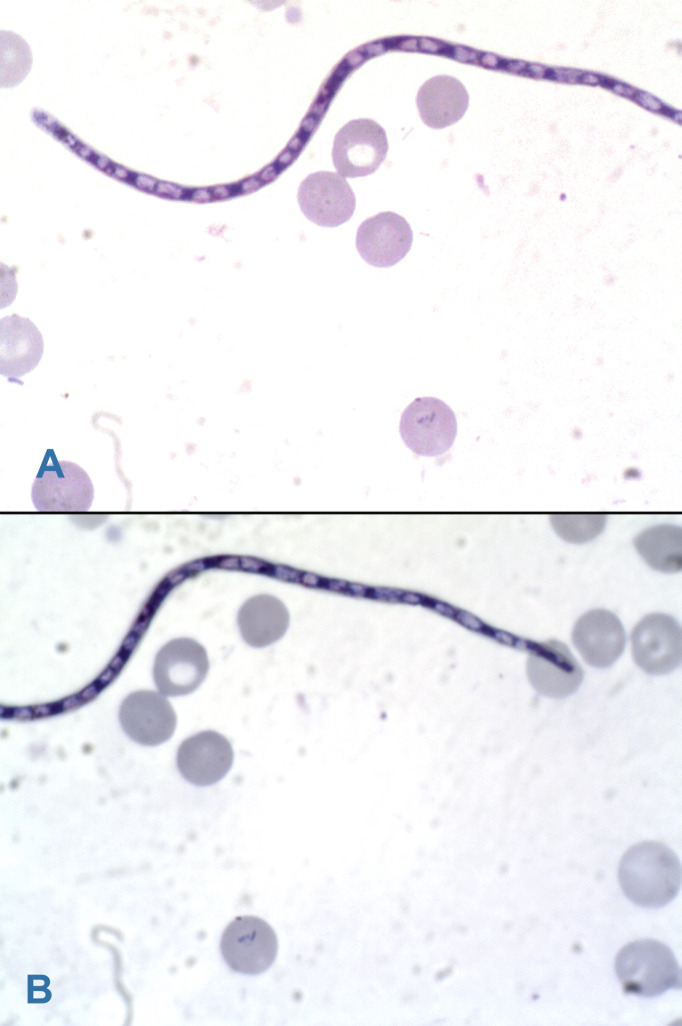
We then did a pleural tap, which drained out a chylous fluid. Microscopic examination of the fluid only showed lymphocytes and the fluid culture was negative and so was the blood culture. The M. tuberculosis PCR performed from this liquid was negative and so was that performed from the gastric aspirate. Clindamycin 700 mg every 8 hours and penicillin G 150,000 IU every 6 hours were then added to his treatment from the 45th day of hospitalization, for 8 days. After 51 days of hospitalization, we carefully examined microscopically a thick blood film in Giemsa staining, which then revealed the presence of M. perstans (Figures 3A and 3B) in a low density.
Figure 3. A and B. Microfilaria of M. perstans: an unsheathed microfilaria with blunt tails and nuclei extending to the end of the tail (Giemsa staining, light microscopy).
A 3 weeks treatment protocol with 400 mg albendazole twice daily was started, while RHEZ, clindamycin and penicillin G were discontinued. Two weeks later, the cutaneous lesions healed, the dyspnea improved and the pleural effusion resorbed as well as the edema. After albendazole treatment, the patient gained two kg and microfilaria could no longer be detected in the blood smear.
Discussion
M. perstans is responsible for a filariasis present in Africa and equatorial America. At least part of the pathogenesis of this disease may be induced by the immune response to the presence of the parasite, and eosinophilia is generally present as well.2,3
In this case, the patient had a misleading malnutrition with edema of the legs, pruritic cutaneous lesions, pneumonia and plural effusion. Peripheral blood stain showed M. perstans microfilaria. As pneumonia healed and pleural effusion resorbed after albendazole treatment, the pulmonary localization of M. perstans was confirmed as diagnosis.
Indeed, M. perstans associated to pleural effusion is not common but has been previously reported;4 Kahn JB has reported back in 1983 a pleural effusion associated to M. perstans.5
However, other uncommon localizations of the parasite have been reported, for example Sanata Bamba et al. reported vaginal localization, Cohen et al. described ocular localization, Baird et al. on the conjunctiva; and Mateu et al. in the salivary gland.3,6–8
M. perstans microfilaria (the larva of filaria) move in the bloodstream while the adult filaria appear to live mainly in the serous body cavities.1 However, those uncommon localizations appear sometimes without the presence of M. perstans microfilaria in the bloodstream.
It appears that M. perstans is very difficult to treat and it is less susceptible to classic microfilaria drugs.1,9
Many treatment regimens have been proposed, associating various results, and monotherapy seems to be less effective than drug combination,1,2,9 with divergent data regarding the benefit of diethylcarbamazine.5 Mebendazole helps to reduce the blood load of M. perstans microfilaria.10 Albendazole, as well as ivermectin are not effective if given at a single dose.9 But more intensive regimens with these drugs have shown their efficiency in reducing and even clearing microfilaria from the blood.11,12 That is why we prescribed to our patient albendazole in the posology of 400 mg twice daily for 3 weeks, with effectiveness after 2 weeks of treatment.
Conclusion
In Bukavu City, we report the first laboratory confirmed case of M. perstans infection in a 16 months-old malnourished patient who developed pneumonia and pleural effusion. He was treated efficiently with albendazole. This first diagnosed case of M. perstans infection in our hospital should raise the interest of searching for the parasite in all malnourished children with pneumonia before the beginning of an eventual treatment for suspected but unconfirmed tuberculosis.
Footnotes
Consent: Written consent was obtained from the parents/legal guardians prior to publication.
Authors’ contributions statement: LK drafted the manuscript. All authors revised and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
References
- 1.Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. 2011;120(Suppl 1):S109–20. doi: 10.1016/j.actatropica.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Bregani ER, Tantardini F, Rovellini A. Mansonella perstans filariasis. Parassitologia. 2007;49:23–6. [PubMed] [Google Scholar]
- 3.Cohen JM, Ribeiro JA, Martins M. Ocular manifestations in mansonelliasis. Arq Bras Oftalmol. 2008;71:167–71. doi: 10.1590/s0004-27492008000200007. [DOI] [PubMed] [Google Scholar]
- 4.Roberts PP. Parasitic infections of the pleural space. Semin Respir Infect. 1988;3:362–82. [PubMed] [Google Scholar]
- 5.Kahn JB. Pleural effusion associated with Dipetalonema perstans (Acanthocheilonema perstans) J Infect Dis. 1983;147:166. doi: 10.1093/infdis/147.1.166. [DOI] [PubMed] [Google Scholar]
- 6.Bamba S, Barro-Traoré F, Liance M, et al. Vaginal localisation of Mansonella perstans: report of a case at the University Hospital of Bobo-Dioulasso, Burkina Faso. Pan Afr Med J. 2012;12:47. [PMC free article] [PubMed] [Google Scholar]
- 7.Baird JK, Neafie RC, Connor DH. Nodules in the conjunctiva, bung-eye, and bulge-eye in Africa caused by Mansonella perstans. Am J Trop Med Hyg. 1988;38:553–7. doi: 10.4269/ajtmh.1988.38.553. [DOI] [PubMed] [Google Scholar]
- 8.Mateu L, Sopena N, Giménez M, Valerio L. [Mansonella perstans isolated on aspiration puncture of a salivary gland] Acta Otorrinolaringol Esp. 2008;59:145–7. doi: 10.1016/s0001-6519(08)73283-7. [DOI] [PubMed] [Google Scholar]
- 9.Asio SM, Simonsen PE, Onapa AW. Mansonella perstans: safety and efficacy of ivermectin alone, albendazole alone and the two drugs in combination. Ann Trop Med Parasitol. 2009;103:31–7. doi: 10.1179/136485909X384929. [DOI] [PubMed] [Google Scholar]
- 10.Coulibaly YI, Dembele B, Diallo AA, et al. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med. 2009;361:1448–58. doi: 10.1056/NEJMoa0900863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipani F, Caramello P, Biglino A, Sacchi C. Albendazole for the treatment of Mansonella perstans filariasis. Trans R Soc Trop Med Hyg. 1997;91:221. doi: 10.1016/s0035-9203(97)90232-7. [DOI] [PubMed] [Google Scholar]
- 12.Duong TH, Kombila M, Ferrer A, Nguiri C, Richard-Lenoble D. Decrease in Mansonella perstans microfilaraemia after albendazole treatment. Trans R Soc Trop Med Hyg. 1998;92:459. doi: 10.1016/s0035-9203(98)91093-8. [DOI] [PubMed] [Google Scholar]