Abstract
The geographic distribution of three Misgurnus species, M. anguillicaudatus, M. bipartitus, and M. mohoity, displays a specific pattern in China, coincident with temperature zones. In this study, we sequenced the transcriptomes of these three species and used the sequences to investigate the lineage-specific adaptations within the genus Misgurnus. In total, 51 orphan genes (19 in M. anguillicaudatus, 18 in M. bipartitus, and 14 in M. mohoity) that may contribute to the species-specific adaptations were identified. An analysis of 1392 one-to-one orthologous genes revealed significantly higher ratios of nonsynonymous-to-synonymous substitutions in the M. mohoity lineage than in M. anguillicaudatus. The genes displaying signatures of positive selection and rapid evolution in Misgurnus were involved in four function categories, (1) energy metabolism; (2) signal transduction; (3) membrane; and (4) cell proliferation or apoptosis, implying that these candidate genes play critical roles in the thermal adaptation of the fish to their living environments. We also detected more than five positively selected sites in cldn15lb and isca1, which function as important factors in paracellular Na+ transport and Fe/S cluster assembly, respectively. Overall, our study provides valuable insights into the adaptive evolution of loaches from different temperature zones in China and is a foundation for future studies to clarify the genetic basis of temperature adaptation in fishes.
Keywords: transcriptome, adaptive evolution, temperature zone, Misgurnus
1. Introduction
Environmental heterogeneity is one of the most important factors influencing evolutionary trajectories of species. The climate difference between the south (subtropical and tropical zone) and north (warm temperate and mid-temperate zone) of China (Qinling Mountains-Huaihe River line boundary) affects the distributions and environmental adaptations of species, which respond to the temperature variations. The seasonal temperature variations in the northern area (warm temperate zone and mid-temperate zone) are more obvious and rapid than those in the southern area of China. Importantly, the temperature variations in water bodies have immediate effects on fish because the rate of heat exchange between the animal and the ambient water is high. Temperature affects virtually all biochemical, physiological and life history activities of fish [1], and environmental temperature profoundly impacts both the phenotypes and geographical distributions of fish in the southern and northern parts of China. For instance, Carassius carassius [2] and Cyprinus carpio [3], which can withstand wide seasonal extremes of temperature, overwinter well in ponds in northeast China. In contrast, Oreochromis niloticus, which cannot survive temperatures of less than 10–12 °C for more than a few days, is only distributed in southern China [4,5].
Previous studies have reported the biochemical and physiological responses required to adapt to thermal changes, including in muscle fibres [1] and enzymatic activity [6]. Recent studies have primarily used genomic approaches to study the genetic mechanisms of temperature adaptation in fish, and genes involved in physiological adaptations to temperature stress have been identified in many fish species. Gracey et al. [7] performed a large multi-tissue analysis of the responses to increasing cold in the common carp and demonstrated that one response involved the upregulation of transcripts involved in stress responses, cytoskeletal reconstruction, protein catabolism, lipid metabolism and energy pathways. Similar results were observed by Ju et al. [8], who reported the differential gene expression of Ictalurus punctatus in response to cold acclimation. Smith et al. [9] conducted RNA-seq analysis in Melanotaenia fluviatilis and showed that genes corresponding to critical metabolic pathways were upregulated to ensure temperature tolerance. Although these studies provided important insights into the physiological changes and variability in gene regulation to temperature change, little information is yet available on the evolutionary adaptions of fish species to the seasonal temperature variations in their living environments.
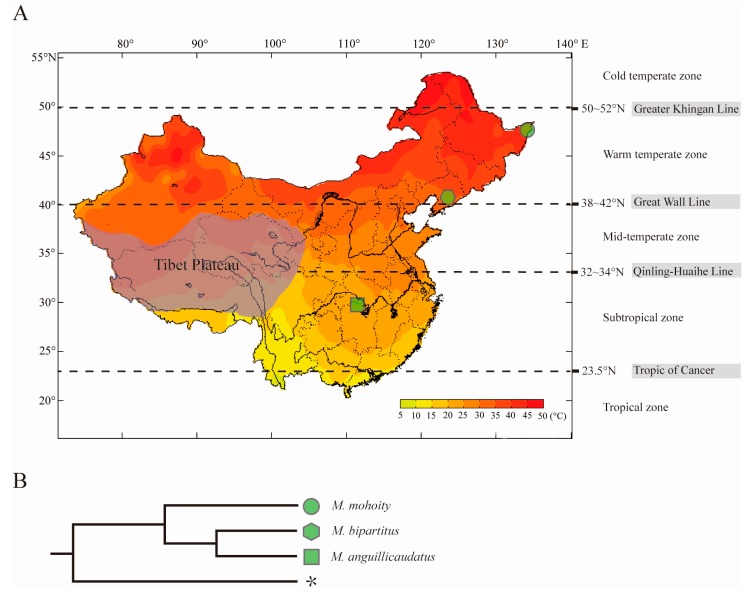
In 1925, Nichols [10] first described and reported the species distribution of the genus Misgurnus in China. Chen and Zhu [11] proposed that the ancestors of genus Misgurnus originated in the Southeast Asia region (north of Wallace’s line), including Kepulauan Sunda Besar, Thailand, Myanmar and south of China (tropical regions), and subsequently radiated into temperate zones via a northern route of expansion, which has been confirmed by Perdices et al. [12,13]. Three species, Misgurnus anguillicaudatus, Misgurnus mohoity, and Misgurnus bipartitus, are distributed throughout China, except on the Tibetan plateau. Misgurnus anguillicaudatus is widely distributed in the middle and lower reaches of the Yangtze River Basin, whereas M. bipartitus is restricted to north of the Yellow River in China, and M. mohoity only occurs in the Amur River Basin in northeast China, Mongolia, and the Far East region of Russia [13,14]. The specific geographical distributions of these three loaches are clearly consistent with the demarcation of temperature zones in China (Figure 1A). Therefore, they provide a rich source of naturally occurring genetic variation within and between species, which can be used to explore biological processes that are important in adaptive evolution. Here, a comprehensive transcriptome analysis of the three species, M. anguillicaudatus, M. bipartitus, and M. mohoity, was undertaken to investigate their evolutionary rates and to identify the genes that may have facilitated the lineage-specific thermal adaptations of these three loach species.
Figure 1.
The sampling sites (A) and the phylogenetic relationships (B) of three species in genus Misgurnus. The color gradient in (A) indicates annual range of temperature in Mainland China, data from China Meteorological Administration (website: http://data.cma.cn); * in (B) indicates the outgroup Danio rerio.
2. Results
2.1. Transcriptome Assembly and Annotation
In total, 88,842,922, 116,147,180, and 76,502,750 raw reads were generated for M. anguillicaudatus, M. bipartitus and M. mohoity, respectively. After raw data were subjected to quality control to remove and trim the low-quality reads, adapters, polyA tails, and reads containing more than 5% unknown nucleotides, only clean reads were left. With the Trinity de novo assembler, 105,215, 84,887, and 78,707 unigenes were produced for M. anguillicaudatus, M. bipartitus and M. mohoity, respectively. The assembly results are summarized in detail in Table S1. To validate and annotate the assembled unigenes, the unigenes generated with Trinity were subjected to BLASTX searches against public protein databases (NR, Swiss-Prot, KEGG, KOG). The annotation results for the unigenes are summarized in Figure S1. The annotation results for the NR database revealed the majority of the sequences identified with BLAST most closely matched genes from other fish species, as expected, especially Danio rerio (65.86%–67.73%), Astyanax mexicanus (7.35%–8.06%) and Larimichthys crocea (2.83%–3.58%).
2.2. Species-Specific Orphan Genes
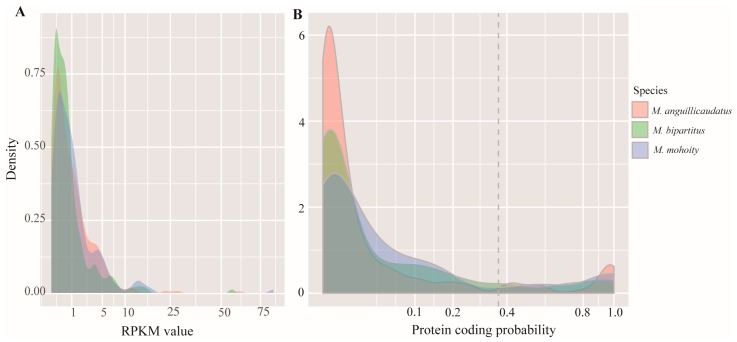
Orphan genes, with no recognizable homology to any sequences in other species, may contribute to the species-specific adaptations [15]. To identify orphan genes, a series of BLASTX searches were performed with the three species using an E value threshold of 1 × 10−5. The pairwise comparisons of D. rerio, C. carpio, O. niloticus proteomes and the three loach species CDS datasets resulted in a number of species-specific genes. In total, 2545, 1170 and 1005 species-specific genes were identified in M. anguillicaudatus, M. bipartitus and M. mohoity, respectively, after all-to-all BLASTX analyses. It is noteworthy that the average expression levels of these novel genes were low (Figure 2A). Because the assembly accuracy is affected by low read counts, we set a cutoff value of reads per kilobase of transcript per million mapped reads (RPKM > 1) to eliminate the assembly error. To exclude any of these genes that had orthologous in other species, we further checked them against the NR, Swiss-Prot and KEGG databases, after which 181, 163, and 104 orphan genes remained for M. anguillicaudatus, M. bipartitus and M. mohoity, respectively. The coding potential of these novel genes, the prediction of coding potential was performed with the Coding Potential Assessment Tool (CPAT, http://lilab.research.bcm.edu/cpat) [16]. According to the authors of CPAT, the optimum cut-off for protein coding probability was set at 0.364 to extract potential coding genes [17]. From the remaining 448 genes, 51 orphan genes (19 in M. anguillicaudatus, 18 in M. bipartitus, and 14 in M. mohoity) were identified in the genus Misgurnus (Figure 2B; Table S2). Because the uniqueness of these genes implies their species specificity, the biological functions of these proteins may encode key determinants of adaptation.
Figure 2.
The distribution of RPKM values in species-specific genes (A) and protein coding probability of orphan genes predicted by CPAT (B) in three loaches. The dotted line in (B) represents the 0.364 cut-off for protein coding probability.
2.3. Orthologs Identification and Phylogenetic Relationship
To evaluate the evolutionary dynamics of Misgurnus in response to their specific geographic environments, putative single-copy orthologues among three loaches were identified. In total, 1392 one-to-one orthologues, ranging in length from 150 to 5175 bp, were obtained and were used in the subsequent evolutionary analysis. The lengths of the orthologues were reduced when the alignments were trimmed with Gblocks (Figure S2). Phylogenetic trees were inferred from the concatenation of orthologues using NJ and ML methods. The consensus tree revealed that M. anguillicaudatus and M. bipartitus grouped into one clade with high bootstrap values (100%), implying a close relationship (Figure 1B). This taxonomic relationship is consistent with the results of a previous study based on the mitochondrial genome [18].
2.4. Accelerated Evolution in the Misgurnus Lineages
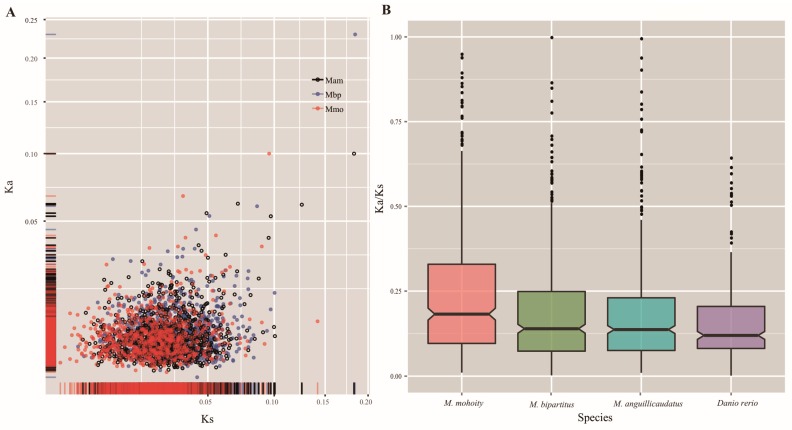
The Ka/Ks ratio is widely recognized as an indicator of selective pressure during evolution [19]. To evaluate the overall differences in the selective constraints at the gene level within the three lineages, the substitution rates (Ka, Ks, and Ka/Ks) for each orthologous gene were calculated using the free ratio model in codeml (Figure 3). The levels of the Ka/Ks ratios showed declining trends in the three lineages, with the M. mohoity lineage displaying higher Ka/Ks ratios than the M. anguillicaudatus or M. bipartitus lineage (Wilcoxon rank sum test, p < 3.39 × 10−7 or p < 9.28 × 10−8). The Ka/Ks ratio for each branch of a concatenated alignment of orthologues was also calculated, and the results also indicated that M. mohoity had higher Ka/Ks ratios than the M. bipartitus and M. anguillicaudatus branches. Overall, 254 genes had higher Ka/Ks ratios in the M. mohoity lineage than in M. anguillicaudatus and M. bipartitus lineages, whereas 146 and 143 genes with higher Ka/Ks ratios were observed in M. anguillicaudatus and M. bipartitus, respectively. To investigate the functions of the lineage-specific genes displaying accelerated evolution (Table S3), the biological functions of the genes with high Ka/Ks ratios were characterized with the KEGG database. Metabolic pathways and the mitogen-activated protein kinase (MAPK) signaling pathway were enriched in all three lineages. The top three functional terms enriched among the genes with high Ka/Ks ratios were “ATP synthase”, “membrane dipeptidase”, and “ribosome biogenesis protein YTM1”, which are associated with energy metabolism and the progress of protein synthesis or hydrolysis in loaches.
Figure 3.
The Ka, Ks, and Ka/Ks ratios for terminal branches were estimated from each orthologous genes in three loaches. (A) Scatter plot of Ka and Ks in three loaches based on the free-ratio model; (B) The distribution of Ka/Ks ratios for terminal branches. D. rerio was selected as a representative. Mam, Mbp, and Mmo in (A) indicate M. anguillicaudatus, M. bipartitus and M. mohoity, respectively.
2.5. Fast Evolving Genes and Positive Selection Analysis
The fast-evolving genes (FEGs), which displayed significantly higher Ka/Ks ratios in a specific lineage compared with the other lineages, were identified using the branch model of codeml. In total, 205, 192, and 191 FEGs were identified in the M. anguillicaudatus, M. bipartitus, and M. mohoity lineages, respectively (Table S4). The branch-site model was used to determine the positively selected genes (PSGs) and the positive selected sites in codons along particular lineages. We identified 63 PSGs in M. anguillicaudatus, 53 PSGs in M. bipartitus, and 50 PSGs in M. mohoity (Table S5). In the comparison of FEGs and PSGs, a set of overlapping genes (n = 61) in the sets of PSGs and FSGs was identified (Table 1; Table S6). To identify the potential genes directly involved in adaption evolution of three Misgurnus species, we focused on the functions of these overlapping genes. The GO (Gene Ontology) enrichment analysis revealed that these overlapping genes were significantly enriched in “membrane” (p = 0.035) and “integral component of membrane” (p = 0.033). To further investigate the biological function of these genes, we searched for them manually in the UniProt and NR databases. The functions of the overlapping genes were assigned to four categories (Table 2): (1) energy metabolism (e.g., dnajb1a, ndufs3, thrsp, srd5a2a); (2) membrane (e.g., cldn15lb, tm4msf5, tmem101); (3) signal transduction (e.g., stk17b, trim35, rad20); and (4) cell proliferation or regeneration (e.g., diabloa, bmf1, cdc23, ddx41).
Table 1.
The summary of estimated Ks, Ka/Ks, GC3, the number of FEGs and PSGs and number of overlapping genes between classes FEGs and PSGs.
| Species | Ks | Ka/Ks | GC3 | FEGs | PSGs | Overlapping |
|---|---|---|---|---|---|---|
| M. anguillicaudatus | 0.0127 | 0.1827 | 0.5099 | 205 | 63 | 28 |
| M. bipartitus | 0.0111 | 0.1994 | 0.5091 | 193 | 53 | 18 |
| M. mohoity | 0.0092 | 0.2271 | 0.5095 | 191 | 50 | 15 |
Abbreviation: Ks: substitution rates for synonymous sites; Ka/Ks: the average ratio of nonsynonymous to synonymous Substitutions; GC3: overall GC content in third codon positions; FEGs: fast evolving genes; PSGs: positively selected genes.
Table 2.
The four function categories of fast evolving and positively selected genes in three Migurnus fishes.
| Species | Gene | Function Terms | GO Number |
|---|---|---|---|
| Signal transduction | |||
| M. anguillicaudatus (n = 6) | stk17b | Intracellular signal transduction | GO: 0035556 |
| trim35 | Zinc ion binding | GO: 0008270 | |
| gatad1 | Zinc ion binding | GO: 0008270 | |
| oprm1 | G-protein coupled receptor | GO: 0001664 | |
| selenbp1 | Selenium binding | GO: 0008430 | |
| card19 | NF-κB signaling | GO: 0043124 | |
| M. bipartitus (n = 3) | rab20 | GTP binding | GO: 0005525 |
| mhc1laa | Antigen binding | GO: 0006955 | |
| spred1 | Inactivation of MAPK activity | GO: 0000188 | |
| M. mohoity (n = 2) | efcc1 | Calcium ion binding | GO: 0005509 |
| trim63b | Zinc ion binding | GO: 0008270 | |
| Membrane | |||
| M. anguillicaudatus (n = 2) | sgcb | Membrane organization | GO: 0061024 |
| cldn15lb | Tight junction | GO: 0030054 | |
| M. bipartitus (n = 2) | tmem101 | Transmembrane | GO: 0016021 |
| rhbdd2 | Serine-type endopeptidase activity | GO: 0004252 | |
| M. mohoity (n = 4) | tmem88b | Integral component of membrane | GO: 0016021 |
| tmem223 | Integral component of membrane | GO: 0016021 | |
| tm4sf5 | Integral component of membrane | GO: 0016021 | |
| tpbgb | Integral component of membrane | GO: 0016021 | |
| Energy metabolism | |||
| M. anguillicaudatus (n = 6) | ubiad1 | Antioxidant activity | GO: 0016209 |
| ndufb11 | Oxidation-reduction process | GO: 0055114 | |
| dnajc19 | Hsp70 protein binding | GO: 0030544 | |
| alg1 | Protein glycosylation | GO: 0006486 | |
| thrsp | Lipid metabolic process | GO: 0006629 | |
| impad1 | Myo-inositol biosynthesis | GO: 0006021 | |
| M. bipartitus (n = 4) | ndufs3 | Oxidoreductase activity, acting on NAD(P)H | GO: 0004128 |
| crot | Fatty acid beta-oxidation | GO: 0006631 | |
| pdxp | Heat shock protein binding | GO: 0031072 | |
| dnajb1a | Hsp70 protein binding | GO: 0030544 | |
| M. mohoity (n = 3) | slc50a1 | Sugar transmembrane transporter activity | GO: 0051119 |
| srd5a2a | Steroid metabolic process | GO: 0008202 | |
| dnajc1 | ATPase activator activity | GO: 0001671 | |
| Cell proliferation/apoptosis | |||
| M. anguillicaudatus (n = 9) | diabloa | Apoptotic process | GO: 0006915 |
| api5 | Apoptotic process | GO: 0006915 | |
| fam212aa | Cartilage development | GO: 0051216 | |
| yap1 | Cartilage development | GO: 0051216 | |
| thumpd2 | RNA binding | GO: 0003723 | |
| bmf1 | Positive regulation of apoptotic process | GO: 0043065 | |
| lcmt1 | Methyltransferase activity | GO: 0008168 | |
| ankrd1a | Sarcomere | GO: 0030017 | |
| krt222 | Structural molecule activity | GO: 0005198 | |
| M. bipartitus (n = 7) | gins2 | DNA replication | GO: 0006260 |
| cdc23 | Cell division | GO: 0051301 | |
| plekho1b | Regulation of myoblast fusion | GO: 1901739 | |
| cwc25 | Nucleoplasm | GO: 0005654 | |
| ctdspl3 | Protein dephosphorylation | GO: 0006470 | |
| ddx41 | Apoptotic process | GO: 0006915 | |
| ddx52 | rRNA processing | GO: 0006364 | |
| M. mohoity (n = 5) | ogfr | Opioid receptor activity | GO: 0004985 |
| eepd1 | DNA repair | GO: 0006281 | |
| dnph1 | Positive regulation of cell growth | GO: 0030307 | |
| rpz | Fin development | GO: 0033333 | |
| cited4b | Muscle cell differentiation | GO: 0042692 | |
In the M. anguillicaudatus lineage, two genes (sgcb, cldn15lb) were related to “membrane”, and six genes (ubiad1, ndufb11, dnajc19, diabloa, thrsp, alg1) present in both PSGs and FEGs were involved in “energy metabolism”, suggesting that M. anguillicaudatus might have adapted to specific temperature conditions by adjusting its energy metabolism. The thrsp gene, which encodes a protein that is important in the regulation of lipid metabolism [20], is rapidly evolving and under positive selection in the M. anguillicaudatus lineage. Notably, among the three Misgurnus lineages, three genes in the DnaJ (Hsp40) heat shock proteins family were positively selected and fast evolving: dnajc19 in M. anguillicaudatus, dnajb1a in M. bipartitus, and dnajc1 in M. mohoity. The DnaJ (Hsp40) heat shock protein family, which is involved in Hsp40 chaperone systems by stimulating Hsp70s ATPase activity, is an important group of proteins in the thermal stress responses of fish [21,22]. This result indicates that these Misgurnus species induce a group of proteins termed heat shock proteins to adapt to the temperature variations and delay or prevent potential thermal injury [23,24,25].
In summary, (1) at the PSG and FEG levels, the patterns of evolutionary adaptations in the three lineages indicated that M. anguillicaudatus is most strongly adapted, followed by M. bipartitus then M. mohoity; (2) the genes assigned to membrane, signal transduction, energy metabolism and cell proliferation or apoptosis are under positive selection and fast evolving in all three lineages; (3) more genes involved in energy metabolism (e.g., ATP-binding, oxidative phosphorylation, oxidoreductase activity) and signal transduction (e.g., zinc ion binding, G-protein coupled receptor) are enriched in the M. anguillicaudatus lineage than in M. mohoity or M. bipartitus; (4) three DnaJ (Hsp40) genes (dnajc19, dnajb1a, and dnajc1) involved in the response to thermal stress are rapidly evolving and under positive selection in the three lineages, respectively; (5) genes related to the membrane, especially transmembrane protein genes, were positively selected and fast evolving in M. mohoity, implying that biological membranes are exposed to stress in the extremely cold temperature of its environment.
2.6. Lineage-Specific Positively Selected Sites
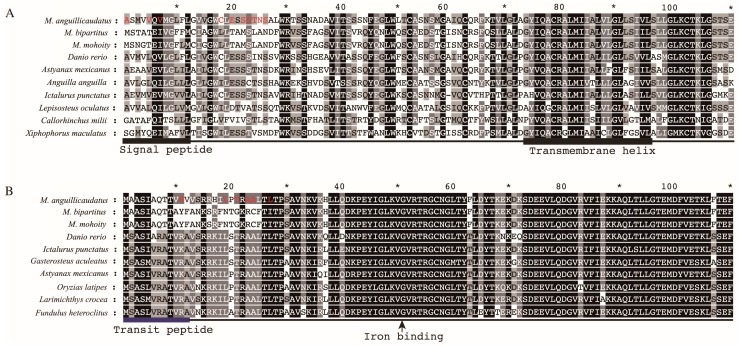
The positively selected sites were identified using LRTs with FDR correction (p < 0.05) and Bayes Empirical Bayes estimates (BEB > 0.95). Twenty, 20, and nine PSGs with sites under positive selection were detected in the M. anguillicaudatus, M. bipartitus, and M. mohoity lineage, respectively (Table S7). The numbers of positively selected sites in the M. anguillicaudatus (44 sites) and M. bipartitus (32 sites) lineages were higher than that in the M. mohoity (13 sites) lineage. Among these positively selective sites, 10 sites in cldn15lb and six sites in isca1, which are involved in paracellular Na+ transport and Fe/S cluster assembly, were detected in the M. anguillicaudatus lineage, implying the biological importance of these two genes in adaptations of M. anguillicaudatus. Given that no suite temples were available for the homology modeling of Cldn15lb and Isca1 in the public protein databases, the functional significance of the positively selected sites was further investigated by locating them within the secondary structures of Cldn15lb and Isca1, which were predicted with homology searching, SignalP [26], and TMHMM [27]. In Cldn15lb, four positively selected sites (1A, 5V, 7V, 18C) occurred in the signal peptide (Figure 4A), whereas another six sites occurred in chain segments. Similarly, protein structure prediction showed that three positively selected sites of isca1 occurred in the transit peptide (Figure 4B) and the remained sites were located in the chain segments.
Figure 4.
The locations of positively selected sites in Cldn15lb (A) and Isca1 (B) in M. anguillicaudatus. The amino acids colored red represent the positively selected sites. The asterisk above the sequences represents length scale of amino acid sequence.
3. Discussion
Since the development of next-generation sequencing technology, RNA-seq has been widely used as an efficient and accessible approach to determine the evolutionary histories of non-model organisms when no genome information is available [28,29,30]. Here, we sequenced and assembled the transcriptomes of three closely related Misgurnus species, M. anguillicaudatus, M. bipartitus, and M. mohoity, to identify the genetic mechanisms in their adaptation to the temperature variations in their environments and provide valuable information for further studies to understand the genetic makeup of other fishes in thermal adaptations. According to the fishery resources survey undertaken by our laboratory [18] and previous studies [10,13,14,31,32], the geographic distributions of these three Misgurnus species show specific patterns, which are coincident with the temperature zones in China. Misgurnus mohoity only occurs in Heilongjiang Province (annual range of temperature 50 °C), and M. bipartitus is restricted to north of the Yellow River (annual range of temperature 25 °C). The main distribution of M. anguillicaudatus is the middle and lower reaches of the Yangtze River Basin (south of Qinling Mountains–Huaihe River Line). Thus, these species present an ideal model in which to investigate the genetic adaptations of fish to different temperature zones.
Orphan genes reflect important evolutionary processes that contribute to evolutionary innovations [33]. In this study, we utilized transcriptome data to identify genes that are present in the three Misgurnus species without detectable sequence similarity in the genomes of other organisms. Using strict cutoffs for RPKM and protein coding probability, we identified a total of 51 orphan genes, (19 in M. anguillicaudatus, 18 in M. bipartitus, and 14 in M. mohoity) in the genus Misgurnus. The orphan genes may have evolved novel functional roles that contribute to species-specific environmental adaptations [15]. For instance, previous studies reported that the orphan genes in Daphnia pulex are specifically activated in response to environmental stimuli [34], and the orphan genes in Arabidopsis thaliana are enriched in response to a wide range of abiotic stresses [35]. The orphan genes identified in Misgurnus species should be important targets in further studies of the developmental and environmental adaptations of these three closely related species.
In addition to orphan genes, a total of 1392 one-to-one orthologous genes were identified to investigate the adaptive evolution of the three Misgurnus lineages. The overall evolutionary rates indicated that the Misgurnus lineages exhibit accelerated evolution compared with that of the zebrafish. Among the three Misgurnus lineages, the evolutionary rates of M. mohoity were highest within the Misgurnus lineage, followed by M. bipartitus and M. anguillicaudatus, suggesting that M. mohoity has adaptively accelerated to better adapt to its living conditions. Previous studies have proposed that accelerated evolution can be caused by the relaxation of functional constraints [36], and this requires further investigation with population genomic analyses. To investigate the potential genes directly involved in the adaptive responses to different temperatures, we focused on the overlapping genes of FEGs and PSGs in the three Misgurnus lineages. After function annotation of the overlapping genes, they were assigned to four categories: signal transduction, energy metabolism, membrane, and cell proliferation/regeneration. (1) Signal transduction: The transduction of signals indicating abiotic or biotic stress is critical for the adaption and survival of fish under various environment conditions [37]. For example, oprm1, a fast-evolving and positively selected gene in M. anguillicaudatus, encodes a G-protein coupled receptor that mediates an array of downstream cellular responses to transduce the stimulus signal [38]. Similarly, rab20 induces small GTPase-mediated signal transduction and promotes the cellular responses to stimulus [39]. Some of these genes related to zinc ion binding, i.e., trim35, gatad1, trim63b, play key roles in detecting and conveying outside signals into cells, allowing them to respond to their living environments in specific ways [40]. Therefore, we infer that zinc ion might be an important molecule in Misgurnus in the transmission of temperature stress signaling from the environment; (2) Energy metabolism: Under temperature stress, genes involved in glycolysis (e.g., alg1), lipid and steroid metabolism (e.g., thrsp, srd5a2a), and oxidation–reduction process (e.g., ubiad1, ndufb11) were enriched, suggesting that fish resist temperature changes with energy metabolism. Previous studies reported that lipid metabolism is associated with cold stress tolerance in common carp [7] and flounder [41] by increasing the level of unsaturated fatty acids and stabilizing lipid fluidity. This indicates that the genes associated with lipid and steroid metabolism mediate the fluidity and flexibility of the cell membranes and are critical for the cellular membrane response to cold stress in loaches; (3) Membrane: Membranes act as selectively protective barriers in organisms and provide a fluid matrix for signal sensing and transduction, molecular transport, and energy metabolism. Transmembrane proteins function as channels and gateways for the transport of specific substances, and temperature is a key factor in the maintenance of the membrane fluidity. To adapt to different environmental temperatures, genes related to transmembrane proteins are fast evolving and positively selected in M. bipartitus (tmem101) and M. mohoity (tmem88b, tmem223, and te4sf5); (4) Cell proliferation or apoptosis: Notably, the cell initiates intracellular apoptotic signaling in response to stress, which may bring about cell suicide. Previous studies proposed that heat, radiation, nutrient deprivation, viral infection, hypoxia, and increased intracellular calcium concentrations can trigger cell apoptosis [42,43,44,45]. The apoptotic process, initiated through the intrinsic pathway or extrinsic pathway, is activated by the intracellular signals generated when cells are stressed. It is noteworthy that genes involved in cell apoptosis were enriched in M. mohoity, suggesting that apoptosis has played an important role in the adaptation to low temperatures. As well as the fast-evolving and positively selected genes we identified here, other genes are also important, and will offer further insights into our understanding of the adaptive evolution of the genus Misgurnus.
4. Materials and Methods
4.1. Ethics Statement
All animals and experiments were conducted in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China; No. [2006]398, 30 September 2006). All efforts were made to minimize suffering.
4.2. Sample Collection
Three loach species, including M. bipartitus sampled from the Hun River in Liaoning province (41°17′ N, 125°23′ E), M. anguillicaudatus sampled from the Xiliang Lake in Hubei province (29°58′ N, 114°01′ E), and M. mohoity sampled from the Amur River in Heilongjiang province (48°21′ N, 135°20′ E), were collected in our study (Figure 1A). All specimens of each species were collected on the same day and under the same conditions. The fishes were euthanized immediately in well-aerated water containing the 100 mg·L−1 concentration of tricaine methanesulfonate (MS-222) before tissue collection. Six adult individuals (three males, three females) of each species were selected, and the tissue samples including dorsal muscle, skin, liver, spleen, intestine and brain were immediately collected to extract total RNA. The samples were snap-frozen in liquid nitrogen and stored at −80 °C.
4.3. Sequencing, Assembly and Annotation
Total RNA was isolated from tissues using RNAiso Plus Reagent (TaKaRa, Dalian, China) according to the manufacturer’s protocol. RNA quality and quantity was measured using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA). All the samples were standardized to 500 ng·μL−1, and equal volumes of the same tissues from different individuals were combined into one pool. The mRNA was enriched using beads with oligo (dT) and fragmented using fragmentation buffer. Three libraries were sequenced with 125 bp pair end using Illumina HiSeq™ 2500. Raw reads produced from sequencing machines was checked and visualized with FastQC Version 0.11.4 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Transcriptome de novo assembly was performed with Trinity [46]. The CD-HIT-EST [47] was used to remove redundant unigenes with parameter “c = 0.95” and “n = 10”. Subsequently, BLASTX alignment (E value ≤ 1 × 10−5) to the protein databases (nonredundant [NR], Swiss-Prot, Kyoto Encyclopedia of Genes and Genomes [KEGG], and euKaryotic Orthologous Groups [KOG]) was performed, and the best aligning results are used to determine the direction of unigenes and amino acid sequences. When a unigene happens to be unaligned to none of the above databases, ESTScan software [48] will be introduced to predict its coding region and sequence direction.
4.4. Identification of Orthologous Genes
Annotations of coding sequences (CDSs) and proteins of two Cypriniformes and one Perciformes species, including C. carpio, Danio rerio, and O. niloticus, were downloaded from Ensembl database (Release 84). The OrthoMCL algorithm [49] was used to generate core-orthologs for the three whole proteomes datasets. Subsequently, all the putative proteins of the three loach species and core-orthologs were aligned (all against all) using BLASTP, and a score for each pair of proteins with significant matches was assigned (E value ≤ 1 × 10−7). Based on the scores, orthologous groups of orthologous genes from different species were identified using OrthoMCL with the default parameters. If more than one unigene were assigned to the same group, the longest unigene was chosen to subsequent analysis. Among the identified groups, only the groups with one to one orthologous relationships were considered for the alignment. PRANK program [50] was used to produce codon alignments for the orthologous sequences with parameter “-codon”. The poor alignment regions were trimmed using Gblocks [51] with the parameter “–t = c”. Alignments with <150 bp (50 codons) were excluded from the dataset. The orphan genes were identified using homology searches method described in previous studies [15,36].
4.5. Phylogenetic Analysis and Substitution Rate Estimation
All trimmed nucleotide sequence alignments were concatenated into superalignments for the phylogenetic analyses, with the SCaFoS program [52]. The phylogenetic trees were inferred from the concatenation of 1392 one-to-one orthologous genes (OGs) using the maximum likelihood (ML) and neighbor joining (NJ) methods with 1000 bootstraps, respectively. The ML analysis was performed with RAxML [53] using GTR-GAMMA substitution model, and the NJ tree was inferred with the TreeBeST program (http://treesoft.sourceforge.net/treebest.shtml). The consensus phylogenetic tree was used as the guide tree in the subsequent analysis.
The codeml program in PAML4.5 [54] with the free ratio model (model = 1) was used to estimate the evolutionary rates of the taxa for each orthologous gene and the concatenation of the orthologous genes. The orthologous genes were discarded if N × dN or S × dS < 1 or dS > 1, according to the criterion proposed in a previous studies [28,55]. The evolutionary rates in each lineage were compared using the Wilcoxon rank sum test in R (http://www.R-project.org/). Fast-evolving genes (FEGs) in a specific lineage were identified with the codeml program using the branch model, as described in previous studies [30,36]. The significance of likelihood ratio tests (LRTs) was evaluated to discriminate between alternative models. Multiple testing was corrected with the false discovery rate method (FDR), implemented in R [56]. If the p value was < 0.05, and a higher Ka/Ks (the ratio of nonsynonymous to synonymous Substitutions) value was obtained than the foreground branch than the background branches, the gene was considered to be a fast-evolving gene. To identify genes under positive selection, the branch-site model in the codeml program was used, with the null model assuming that all branches have been evolving at the same rate and the alternative model allowing foreground branch to evolve under a different rate [29,30]. As before, LRTs were used to discriminate between alternative models for each gene in the gene set, and p value was computed by comparing LRT to the χ2 distribution, with the degree of freedom estimated as the difference of parameters between models. We also corrected for multiple testing using the FDR method. When FDR-adjusted p value was significant (FDR < 0.05), the Bayes Empirical Bayes (BEB) estimates from each model were then used to identify amino acid sites under positive selection. Gene ontology (GO) enrichment analyses were performed using DAVID Functional Annotation Tool.
5. Conclusions
The geographic distribution of three Misgurnus species coincident with temperature zones. The orphan genes identified in Misgurnus species should be important targets in further studies of the developmental and environmental adaptations of these three closely related species. Meanwhile, the genes displaying signatures of positive selection and rapid evolution in Misgurnus were involved in four function categories, including energy metabolism, signal transduction, membrane, and cell proliferation or apoptosis, implying that these candidate genes play critical roles in the thermal adaptation of the fish to their living environments. This result is a prerequisite to revealing the protein function in thermal adaptation. The present study provides some novel insights into the adaptive evolution of loaches from different temperature zones in China and is a foundation for future studies to clarify the genetic basis of temperature adaptation in fishes.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31372180); and the Fundament Research Funds for the Central Universities (No. 2662015PY019). Data Accessibility: Raw sequence data were deposited into the NCBI Short Read Archive with accession number SRR3744972 (M. anguillicaudatus), SRR3744973 (M. bipartitus), SRR3744974 (M. mohoity).
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/17/11/1943/s1.
Author Contributions
Shaokui Yi and Weimin Wang designed the study. Shaokui Yi performed the majority of the laboratory work, statistics and data analysis, and drafted the manuscript. Sai Wang and Shaokui Yi collected the samples. Jia Zhong performed the experiments. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Guderley H. Metabolic responses to low temperature in fish muscle. Biol. Rev. 2004;79:409–427. doi: 10.1017/S1464793103006328. [DOI] [PubMed] [Google Scholar]
- 2.Holopainen I.J., Tonn W.M., Paszkowski C.A. Tales of two fish: The dichotomous biology of crucian carp (Carassius carassius (L.)) in northern Europe. Ann. Zool. Fenn. 1997;34:1–22. [Google Scholar]
- 3.Tanck M.W.T., Booms G.H.R., Eding E.H., Wendellar Bonga S.E., Komen J. Cold shocks: A stressor for common carp. J. Fish Biol. 2000;57:881–894. doi: 10.1111/j.1095-8649.2000.tb02199.x. [DOI] [Google Scholar]
- 4.Atwood H.L., Tomasso J.R., Webb K., Gatlin D.M. Low-temperature tolerance of Nile tilapia, Oreochromis niloticus: Effects of environmental and dietary factors. Aquac. Res. 2003;34:241–251. doi: 10.1046/j.1365-2109.2003.00811.x. [DOI] [Google Scholar]
- 5.Sifa L., Chenhong L., Dey M., Gagalac F., Dunham R. Cold tolerance of three strains of Nile tilapia, Oreochromis niloticus, in China. Aquaculture. 2002;213:123–129. doi: 10.1016/S0044-8486(02)00068-6. [DOI] [Google Scholar]
- 6.Shaklee J.B., Christiansen J.A., Sidell B.D., Prosser C.L., Whitt G.S. Molecular aspects of temperature acclimation in fish: Contributions of changes in enzyme activities and isozyme patterns to metabolic reorganization in the green sunfish. J. Exp. Zool. 1977;201:1–20. doi: 10.1002/jez.1402010102. [DOI] [PubMed] [Google Scholar]
- 7.Gracey A.Y., Fraser E.J., Li W.Z., Fang Y.X., Taylor R.R., Rogers J., Brass A., Cossins A.R. Coping with cold: An integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc. Natl. Acad. Sci. USA. 2004;101:16970–16975. doi: 10.1073/pnas.0403627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju Z., Dunham R.A., Liu Z. Differential gene expression in the brain of channel catfish (Ictalurus punctatus) in response to cold acclimation. Mol. Genet. Genom. 2002;268:87–95. doi: 10.1007/s00438-002-0727-9. [DOI] [PubMed] [Google Scholar]
- 9.Smith S., Bernatchez L., Beheregaray L.B. RNA-seq analysis reveals extensive transcriptional plasticity to temperature stress in a freshwater fish species. BMC Genom. 2013;14:1. doi: 10.1186/1471-2164-14-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols J.T. An analysis of Chinese loaches of the genus Misgurnus. Am. Mus. Novit. 1925;169:1–7. [Google Scholar]
- 11.Chen J.X., Zhu S.Q. Phylogenetic relationships of the subfamily in the loach family Cobitidae (Pisces) Acta Zootax. Sin. 1984;9:201–207. [Google Scholar]
- 12.Perdices A., Bohlen J., Slechtova V., Doadrio I. Molecular evidence for multiple origins of the European spined loaches (Teleostei, Cobitidae) PLoS ONE. 2016;11:e0151228.:1. doi: 10.1371/journal.pone.0144628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perdices A., Vasilev V., Vasil'eva E. Molecular phylogeny and intraspecific structure of loaches (genera Cobitis and Misgurnus) from the Far East region of Russia and some conclusions on their systematics. Ichthyol. Res. 2012;59:113–123. doi: 10.1007/s10228-011-0259-6. [DOI] [Google Scholar]
- 14.Vasil’eva E.D., Vasil’ev V.P., Skomorokhov M.O. Loaches (Misgurnus, Cobitidae) of Russian Asia: II. Morphology, synonymy, diagnoses, karyology, biology, and distribution. J. Ichthyol. 2003;7:501. [Google Scholar]
- 15.Sun W., Zhao X.W., Zhang Z. Identification and evolution of the orphan genes in the domestic silkworm, Bombyx mori. FEBS Lett. 2015;589:2731–2738. doi: 10.1016/j.febslet.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Park H.J., Dasari S., Wang S.Q., Kocher J.P., Li W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013;41:e74. doi: 10.1093/nar/gkt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weikard R., Hadlich F., Kuehn C. Identification of novel transcripts and noncoding RNAs in bovine skin by deep next generation sequencing. BMC Genom. 2013;14:1. doi: 10.1186/1471-2164-14-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi S., Zhong J., Wang S., Huang S., Wang W. Mitochondrial DNA reveals evolutionary status and population genetics of two closely related fish ( Misgurnus bipartitus and Misgurnus mohoity ) in northeast China. Biochem. Syst. Ecol. 2016;68:192–199. doi: 10.1016/j.bse.2016.07.018. [DOI] [Google Scholar]
- 19.Yang Z., Nielsen R. Synonymous and nonsynonymous rate variation in nuclear genes of mammals. J. Mol. Evol. 1998;46:409–418. doi: 10.1007/PL00006320. [DOI] [PubMed] [Google Scholar]
- 20.Breuker C., Moreau A., Lakhal L., Tamasi V., Parmentier Y., Meyer U., Maurel P., Lumbroso S., Vilarem M.J., Pascussi J.M. Hepatic expression of thyroid hormone-responsive spot 14 protein is regulated by constitutive androstane receptor (NR1I3) Endocrinology. 2010;151:1653–1661. doi: 10.1210/en.2009-1435. [DOI] [PubMed] [Google Scholar]
- 21.Qiu X.-B., Shao Y.-M., Miao S., Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders B.M. Stress proteins in aquatic organisms: An environmental perspective. Crit. Rev. Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- 23.Currie S. Encyclopedia of Fish Physiology. Academic Press; San Diego, CA, USA: 2011. Heat Shock Proteins and Temperature; pp. 1732–1737. [Google Scholar]
- 24.Wojtasik B., Kuczynska-Wisnik D. Temperature shock tolerance and heat shock proteins in Arctic freshwater ostracod Candona rectangulata—Preliminary results. Pol. Polar Res. 2012;33:199–206. doi: 10.2478/v10183-012-0011-6. [DOI] [Google Scholar]
- 25.Feder M.E., Hofmann G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 2003;61:243. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 26.Petersen T.N., Brunak S., von Heijne G., Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 27.Krogh A., Larsson B., von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 28.Ma X.H., Dai W., Kang J.L., Yang L.D., He S.P. Comprehensive Transcriptome Analysis of Six Catfish Species from an Altitude Gradient Reveals Adaptive Evolution in Tibetan Fishes. G3-Genes Genom. Genet. 2016;6:141–148. doi: 10.1534/g3.115.024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Yang L.D., Zhou K., Zhang Y.P., Song Z.B., He S.P. Evidence for Adaptation to the Tibetan Plateau Inferred from Tibetan Loach Transcriptomes. Genome Biol. Evol. 2015;7:2970–2982. doi: 10.1093/gbe/evv192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backstrom N., Zhang Q., Edwards S.V. Evidence from a House Finch (Haemorhous mexicanus) Spleen Transcriptome for Adaptive Evolution and Biased Gene Conversion in Passerine Birds. Mol. Biol. Evol. 2013;30:1046–1050. doi: 10.1093/molbev/mst033. [DOI] [PubMed] [Google Scholar]
- 31.Vasilev V.P., Vasileva E.D. Comparative karyological analysis of mud loach and spined loach species (genera Misgurnus and Cobitis) from the Far East region of Russia. Folia Zool. 2008;57:51–59. [Google Scholar]
- 32.Li Y.J., Zhang M.Z., Zhuo Y.U., Pang Y.M., Qian C., Katsutoshi A. Morphological variations in amur weatherfish Misgurnus mohoity, northern weatherfish Misgurnus bipartitus and oriental weatherfish Misgurnus anguillicaudatus in China. J. Dalian Ocean Univ. 2010;25:397–401. [Google Scholar]
- 33.Grandaubert J., Bhattacharyya A., Stukenbrock E.H. RNA-seq-Based Gene Annotation and Comparative Genomics of Four Fungal Grass Pathogens in the Genus Zymoseptoria Identify Novel Orphan Genes and Species-Specific Invasions of Transposable Elements. G3-Genes Genom. Genet. 2015;5:1323–1333. doi: 10.1534/g3.115.017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colbourne J.K., Pfrender M.E., Gilbert D., Thomas W.K., Tucker A., Oakley T.H., Tokishita S., Aerts A., Arnold G.J., Basu M.K., et al. The Ecoresponsive Genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donoghue M.T.A., Keshavaiah C., Swamidatta S.H., Spillane C. Evolutionary origins of Brassicaceae specific genes in Arabidopsis thaliana. BMC Evol. Biol. 2011;11:1. doi: 10.1186/1471-2148-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L.D., Wang Y., Zhang Z.L., He S.P. Comprehensive Transcriptome Analysis Reveals Accelerated Genic Evolution in a Tibet Fish, Gymnodiptychus pachycheilus. Genome Biol. Evol. 2015;7:251–261. doi: 10.1093/gbe/evu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long Y., Song G.L., Yan J.J., He X.Z., Li Q., Cui Z.B. Transcriptomic characterization of cold acclimation in larval zebrafish. BMC Genom. 2013;14:1. doi: 10.1186/1471-2164-14-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., Ferguson S.S.G., Barak L.S., Bodduluri S.R., Laporte S.A., Caron M.G. Role for G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proc. Natl. Acad. Sci. USA. 1998;95:7157. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egami Y. Molecular imaging analysis of Rab GTPases in the regulation of phagocytosis and macropinocytosis. Anat. Sci. Int. 2016;91:35–42. doi: 10.1007/s12565-015-0313-y. [DOI] [PubMed] [Google Scholar]
- 40.Samet J.M., Dewar B.J., Wu W., Graves L.M. Mechanisms of Zn2+-induced signal initiation through the epidermal growth factor receptor. Toxicol. Appl. Pharm. 2003;191:86–93. doi: 10.1016/S0041-008X(03)00219-9. [DOI] [PubMed] [Google Scholar]
- 41.Hu J.W., You F., Wang Q., Weng S.D., Liu H., Wang L.J., Zhang P.J., Tan X.G. Transcriptional Responses of Olive Flounder (Paralichthys olivaceus) to Low Temperature. PLoS ONE. 2014;9:e108582. doi: 10.1371/journal.pone.0108582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee C.H., Wu S.B., Hong C.H., Yu H.S., Wei Y.H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013;14:6414–6435. doi: 10.3390/ijms14036414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon K., Zhou S.Y., Lee S.H., Owyang C., DiMagno M.J. Hsp70 and XIAP are potential key molecular mechanisms causing impaired apoptosis and severe acute pancreatitis (AP) in CF mice. Pancreas. 2007;35:417. doi: 10.1097/01.mpa.0000297750.50175.d9. [DOI] [Google Scholar]
- 44.Zucchini N., de Sousa G., Pizzol J., Rahmani R. Molecular mechanisms underlying the hepatotoxicity of lindane in rat hepatocytes: Apoptosis regulation and oxidative stress. Drug Metab. Rev. 2005;37:23. [Google Scholar]
- 45.Gorman A.M., Healy S.J.M., Jager R., Samali A. Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q.D., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W.Z., Godzik A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 48.Iseli C., Jongeneel C.V., Bucher P. ESTScan: A program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1999;99:138–148. [PubMed] [Google Scholar]
- 49.Li L., Stoeckert C.J., Roos D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loytynoja A., Goldman N. An algorithm for progressive multiple alignment of sequences with insertions. Proc. Natl. Acad. Sci. USA. 2005;102:10557–10562. doi: 10.1073/pnas.0409137102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 52.Roure B., Rodriguez-Ezpeleta N., Philippe H. SCaFoS: A tool for selection, concatenation and fusion of sequences for phylogenomics. BMC Evol. Biol. 2007;7:1. doi: 10.1186/1471-2148-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Z.H. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 55.Goodman M., Sterner K.N., Islam M., Uddin M., Sherwood C.C., Hof P.R., Hou Z.C., Lipovich L., Jia H., Grossman L.I., et al. Phylogenomic analyses reveal convergent patterns of adaptive evolution in elephant and human ancestries. Proc. Natl. Acad. Sci. USA. 2009;106:20824–20829. doi: 10.1073/pnas.0911239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.