Abstract
Loquat (Eriobotrya japonica Lindl.) is a subtropical fruit tree with high medicinal value native to China. Different organs of loquat have been used historically as folk medicines and this has been recorded in Chinese history for thousands of years. Research shows that loquat extracts contain many antioxidants, and different extracts exhibit bioactivity capable of counteracting inflammation, diabetes, cancer, bacterial infection, aging, pain, allergy and other health issues. Bioactive compounds such as phenolics and terpenoids have been isolated and characterized to provide a better understanding of the chemical mechanisms underlying the biological activities of loquat extracts. As the identification of compounds progresses, studies investigating the in vivo metabolism, bioavailability, and structure–activity relationships, as well as potential toxicity of loquat extracts in animal or cell models are receiving more attention. In addition, genetic studies and breeding of loquat germplasms for high contents of health-benefiting compounds may provide new insight for the loquat industry and research. This review is focused on the main medicinal properties reported and the possible pharmaceutically active compounds identified in different loquat extracts.
Keywords: Eriobotrya japonica Lindl., bioactivities, bioactive compounds
1. Introduction
Loquat (Eriobotrya japonica Lindl.) is a subtropical evergreen fruit tree originating in southeastern China. It has been cultivated for more than 2000 years in China and is now commercially cultivated in more than 30 countries worldwide, including Japan, Turkey, Brazil, Spain, India, Pakistan, Israel, and Italy. China is now the largest producer of loquat fruit with a cultivation area of about 170,000 ha and an annual output of about one million tons. The fruit ripen from May through June in the main Chinese production areas such as Zhejiang, Fujian, and Jiangsu provinces, earlier than the majority of other domestically-grown fruits. Therefore, loquat provides an extended dietary source of fresh fruit for Chinese consumers and a high economic return to producers due to the lack of competition within this market niche. There are two main types of loquat fruit, i.e., red-colored and white-colored cultivars, where different carotenoids accumulations cause the fruit color and nutritional differences [1].
Loquat is a plant with high medicinal value and different organs that have been used historically as folk medicines for thousands of years. Loquat extracts have been used for the treatment of cough, chronic bronchitis (CB), inflammation, diabetes, and cancer in Chinese folk medicine. Ancient literature, such as the ‘Compendium of Materia Medica’ [2], described the origin, classification, breeding methods, and medicinal value of the loquat tree, and laid the foundation for the development and cultivation of loquat.
The efficacy of loquat, as used in traditional Chinese medicine, is supported by current scientific evidence regarding the pharmacologically active compounds in plant extracts and their structure–activity relationships. The phytonutritional composition of extracts of different organs varies considerably: loquat leaf and flower are rich in phenolics and triterpenes; fruit is rich in sugars, organic acids, carotenoids, flavonoids, phenolic acids, and vitamins; the kernel is a good source of proteins, starch, tannins, and minerals [3,4,5]. Different loquat extracts have been shown to exhibit a wide range of activities. To provide a comprehensive understanding of the current research on the health-promoting effect of loquat extracts, the reported biological activities as well as the key bioactive compounds are summarized in the present review.
2. Bioactivities of Loquat Extracts
2.1. Anti-Inflammatory Activity
In Chinese folk medicine, loquat leaf has been used since ancient times to treat inflammatory diseases such as cough, CB, and asthma [2]. Modern scientific studies using different experimental models have proved the anti-inflammatory capacity of different loquat tissues such as leaf [6,7,8,9,10], seed [11,12] and fruit [13].
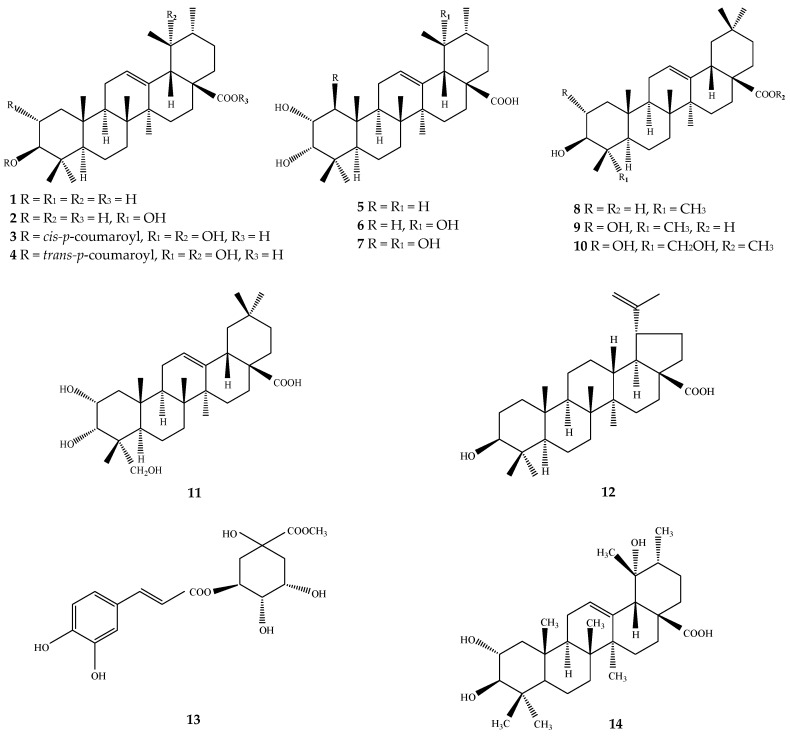
Pulmonary inflammation is a factor in many lung diseases. Lipopolysaccharide (LPS)-induced inflammation is a common experimental model for anti-inflammatory research. Loquat leaf extracts enriched with triterpene acids, especially ursolic acid (1), showed anti-inflammatory effects on alveolar macrophages in rats with LPS-induced CB [7,8,14,15]. Twelve triterpene acids, e.g., seven ursane-type [ursolic acid (1), corosolic acid (2), 3-O-cis-p-coumaroyltormentic acid (3), 3-O-trans-p-coumaroyltormentic acid (4), 3-epicorosolic acid (5), euscaphic acid (6), 1β-hydroxyeuscaphic acid (7)], four oleanane-type [oleanolic acid (8), maslinic acid (9), methyl arjunolate (10), 2α,3α,23-trihydroxyolean-12-en-28-oic acid (11)], and one lupane-type [betulinic acid (12)] isolated from the ethyl acetate-soluble fraction of loquat leaf showed marked anti-inflammatory effects in the inhibition of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear edema of mice, and the 50% inhibitory dose of these twelve compounds ranged from 0.03–0.43 mg per ear (Figure 1) [6]. The mouse paws edema model was also used to assess the anti-inflammatory effect of loquat extract [16,17], and loquat tea extract made from roasted fresh loquat leaves significantly decreased the paw edema of mouse [16].
Figure 1.
Structures of chemical compounds 1–14. 1: ursolic acid; 2: corosolic acid; 3: 3-O-cis-p-coumaroyltormentic acid; 4: 3-O-trans-p-coumaroyltormentic acid; 5: 3-epicorosolic acid; 6: euscaphic acid; 7: 1β-hydroxyeuscaphic acid; 8: oleanolic acid; 9: maslinic acid; 10: methyl arjunolate; 11: 2α,3α,23-trihydroxyolean-12-en-28-oic acid; 12: betulinic acid; 13: methyl chlorogenic acid; 14: tormentic acid.
Loquat seed extracts also showed anti-inflammatory effects in vivo [11,12]. By using a chemotherapy drug (5-fluorouracil)-induced mucositis model in hamsters, the loquat seed extract significantly inhibited the chemotherapy-induced mucositis, and the epithelial injury and bacterial infection were greatly inhibited, together with much lower plasma lipid peroxide level [11]. In another study, by using a dinitrofluorobenzene-induced allergic dermatitis in rat ear as an experimental model, administration of loquat seed extract resulted in significantly inhibited development of allergic dermatitis, where lower ear thickness and serum immunoglobulin E levels as well as improved balance of Th1/Th2 were observed [12].
In addition, loquat juice also showed anti-inflammatory effects. Fruit juice was administrated prophylacticly, postmortemly or concurrently with LPS stimulation, and was found to exhibit a prophylactic effect on LPS-induced inflammation in peritoneal macrophages [13].
Increased levels of inducible nitric synthase (iNOS), cyclooxygenase-2 (COX-2), and pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8) have been correlated with inflammation [8,14,18]. Therefore, decreasing pro-inflammatory mediators (such as iNOS, COX-2, TNF-α, IL-6, IL-8, IL-1β) and/or increasing anti-inflammatory cytokine (such as IL-10) secretions are important mechanisms for the anti-inflammation effects of loquat extracts [8,13,14,18,19,20,21]. Such regulation was associated with suppressing the expression and activation of the nuclear factor-κB (NF-κB) [18,19,22,23,24] and/or mitogen-activated protein kinase (MAPK) signaling pathway [15,22,23], which have been suggested as key regulators of the expression of inflammatory mediators in the cellular signaling pathway [25,26].
Treatments such as LPS induce production of iNOS, COX2, TNF-α, IL-1β, and IL-8 in A-549 human lung epithelial cells and loquat leaf extract and its triterpene ursolic acid (1) inhibited the LPS-induced cytokines and the inducible enzyme production via the NF-κB signaling pathway in A-549 cells [18]. The anti-inflammatory effect of loquat leaf extract might result from the inhibition of expression of iNOS and COX-2 through the downregulation of NF-κB activation and MAPK phosphorylation in LPS stimulated RAW264 cells [23] and in the LPS-activated murine peritoneal macrophage model [24]. Similarly, the anti-inflammatory effects of loquat tea extract may rely on the inhibition of the production of iNOS, nitric oxide (NO), IL-6, TNF-α, and on the downregulation of the transforming growth factor-β (TGF-β)-activated kinase-mediated MAPK and NF-κB pathways in mouse macrophage-like RAW 264.7 cells [16]. Mast cells induce the biosynthesis of pro-inflammatory cytokines with immune regulatory properties. Loquat leaf extract inhibited the secretion of TNF-α, IL-6, and IL-8 and attenuated the activation of NF-κB, p38 MAPK and extracellular signal-regulated kinase (ERK) in horbol 12-myristate 13-acetate and calcium ionophore A23187-induced mast cells [22]. More in-depth studies using microarray analysis showed that loquat leaf extracts inhibited the expression of a wide variety of inflammation-related genes in LPS-stimulated human gingival fibroblasts [9].
In animal models, loquat leaf extracts were also found to inhibit the NF-κB activation of alveolar macrophages, which led to the inhibition of the expression of TNF-α, IL-1β, prostaglandin E2 and leukotriene B4 in a dose dependent manner in CB rats [8,14]. In another study, triterpene acids extracts of loquat leaf were found to significantly inhibit the increase in NO concentration and iNOS expression, which may be related to the inhibition of phosphorylation of p38 MAPK and the corresponding signal transduction in alveolar macrophages of CB rats [15]. Administration of fruit juice in the LPS-induced inflammation model also resulted in the increased secretion of anti-inflammatory cytokines, such IL-10, and/or decreased levels of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α in the murine peritoneal macrophage cultures [13].
Antioxidant activity might be another additional molecular mechanism of its anti-inflammatory effects. NF-κB activation is influenced by the cellular oxidative state, and antioxidants such as methyl chlorogenic acid (13) isolated from loquat leaf can inhibit the redox-sensitive NF-κB activation and downregulate NF-κB-dependent gene expression [19]. Treatment with loquat triterpene acids extracts significantly inhibited the methylene dianiline (MDA) level and the expression of heme oxygenase-1, and up-regulated the level of Superoxide dismutase (SOD) expression in cultured alveolar macrophages from CB rats [7]. Tormentic acid (14) from loquat suspension cells decreased paw edema in mice and increased the activities of catalase, SOD, and glutathione peroxidase in liver tissue [17].
2.2. Anti-Diabetic Activity
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia resulting from either a defect in insulin secretion or action. As a traditional folk medicine component, E. japonica also exhibited great anti-diabetes potential. Recent research evidence has shown that loquat leaf or seed extracts are useful in prevention and control of both type-1 and type-2 diabetes [27,28,29,30,31,32,33].
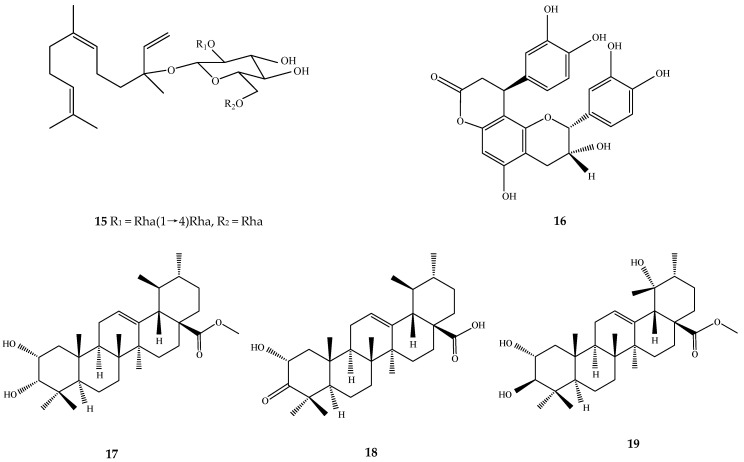
A 70% ethanol extract of Folium Eriobotryae (30 g/kg) showed significant hypoglycemic effect on alloxan-diabetic mice by lowering blood glucose levels [27]. By using the terpenes and flavonoids fraction of loquat leaf, their hypoglycemic potential on alloxan and/or streptozotocin (STZ)-induced diabetic mice was further investigated [27,29,30]. Results showed that the total triterpene acid fraction at 300 mg/kg day caused significant hypoglycemic and hypolipidemic effects on normal, alloxan and STZ-induced diabetic mice [29]. Total sesquiterpenes at 30 g/kg day showed similar hypoglycemic effects on alloxan-diabetic mice [27]. Further isolation of a triterpene acid—euscaphic acid (6)—and a sesquiterpene glycoside—nerolidol-3-O-α-l-rhamnopyranosyl(1,4)-α-l-rhamnopyranosyl(1,2)-[α-l-rhamnopyranosyl(1,6)]-β-d-glucopyranoside (15)—from Folium Eriobotryae showed that both compounds significantly lowered the plasma glucose levels in alloxan-diabetic mice, confirming that these are important active hypoglycemic constituents in loquat leaf (Figure 2) [34,35]. By using STZ-induced diabetic mice model, the flavonoid fraction containing quercetin-3-O-galactosyl-(1,6)-glucoside, quercetin-3-O-sophoroside, quercetin-3-O-rutinoside (rutin), kampferol-3-O-sophoroside, kampferol-3-O-rutinoside, querce-tin-3-O-galactoside (hyperoside), quercetin-3-O-glucoside (isoquercitrin), quercetin-3-O-rhamnoside, and kampferol-3-O-glucoside showed a hypoglycemic effect where a dose of 300 mg/kg significantly decreased the plasma glucose and serum insulin levels [30].
Figure 2.
Structures of chemical compounds 15–19. 15: nerolidol-3-O-α-l-rhamnopyranosyl(1,4)-α-l-rhamnopyranosyl(1,2)-[α-l-rhamnopyranosyl(1,6)]-β-d-glucopyranoside; 16: cinchonain Ib; 17: 3-epicorosolic acid methyl ester; 18: 2-α hydroxy-3-oxo urs-12-en-28-oic acid; 19: tormentic acid methyl ester.
By using the high-fat (HF) diet-induced diabetic C57BL/6J mice model, the hypoglycemic effects of loquat extracts were also investigated [36,37]. Loquat leaf extract containing corosolic acid (2) and maslinic acid (9) significantly ameliorated the hyperglycemia, hyperleptinemia, and hyperinsulinemia in 45% HF diet C57BL/6J mice [36]. Cell suspension cultures of loquat contain a great number of pentacyclic terpenoids including tormentic acid (14), corosolic acid (2), ursolic acid (1), maslinic acid (9), and oleanolic acid (8) [37]. Addition of such cell suspension extract to the HF diet mice resulted in the prevention of the increase in the levels of blood glucose, insulin, leptin and homeostasis model assessment for insulin resistance index in the HF-diet mice [37]. By using the type 2 diabetic Otsuka Long–Evans Tokushima fatty (OLETF) rats and KK-Ay mice as experimental models, the hypoglycemic effects of loquat seeds were studied and results showed that OLETF rats fed a diet with 10% powdered loquat seed resulted in consistently reduced blood glucose concentration and serum insulin level compared to the control group [28]. In addition, ethanol extracts of loquat seed suppressed the increase in blood glucose for four months and improved the glucose tolerance in KK-Ay mice [28]. A new fermented tea product produced by co-fermentation of loquat leaf and summer-harvested green tea leaf (50 mg/kg) showed suppression of blood glucose level and a corresponding reduction in serum insulin secretion in maltose-loaded Sprague–Dawley (SD) rats [38]. Interestingly, such an effect was not observed when sucrose or glucose were administered to SD rats. Loquat leaf extracts led to a significant inhibition of the increase in serum glucose, total cholesterol and triglyceride levels induced in a hypercholesterolemic zebrafish model by feeding a high cholesterol diet [10].
In another study, a water extract of loquat leaf significantly increased the insulin secretion from INS-1 cells and decreased the insulin level for as long as 240 min post-administration in rats [39]. Cinchonain Ib (16) was found to enhance insulin secretion from INS-1 cells in the same study and may have insulinotropic effect for managing type 2 diabetes (Figure 2) [39].
Corosolic acid (2) isolated from loquat leaf promoted 3H-glucose uptake, inhibited the differentiation of preadipocytes into adipocytes, and downregulated the expression of peroxisome proliferator-activated receptor (PPAR)-γ and the CCAAT/enhancer binding protein-α in 3T3-L1 adipocytes [40]. Therefore, corosolic acid (2) might regulate carbohydrate metabolism without increasing adiposity [40]. Glucocorticoids are important regulators of metabolic processes including gluconeogenesis, and elevated glucocorticoids have been associated with hyperglycemia, insulin resistance and type 2 diabetes. Therefore, inhibition of the glucocorticoids-activating enzyme 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1), which catalyzes the conversion of inactive 11-ketoglucocorticoids to active 11β-hydroxyglucocorticoids, is an important therapeutic target of antidiabetic medicines [41,42]. Among six traditional antidiabetic medicinal plants, loquat leaf extracts preferentially inhibited 11β-HSD1 [41]. The bioactivity-guided isolation of bioactive constituents resulted in the identification of corosolic acid (2), 3-epicorosolic acid methyl ester (17), 2-α hydroxy-3-oxo urs-12-en-28-oic acid (18), tormentic acid methyl ester (19), ursolic acid (1) as low micromolar inhibitors of 11β-HSD1 (Figure 2) [42].
2.3. Anti-Cancer Activity
As a traditional folk medicine component, loquat extracts have also displayed chemoprotective properties against various cancer cell lines. Modern science studies have demonstrated at the protein and gene level that loquat extracts can suppress cell carcinogenesis at different progression stages, such as cancer initiation, proliferation, and metastasis [43,44,45,46].
Both water and ethanol extracts of loquat leaf inhibited 7,12-dimethylbenz[α]anthracene (DMBA)-induced breast cancer in rats, and water extracts showed a higher inhibitory activity [46]. Both extracts inhibited the development of breast cancer by significantly suppressing the initiation and proliferation of tumor cells.
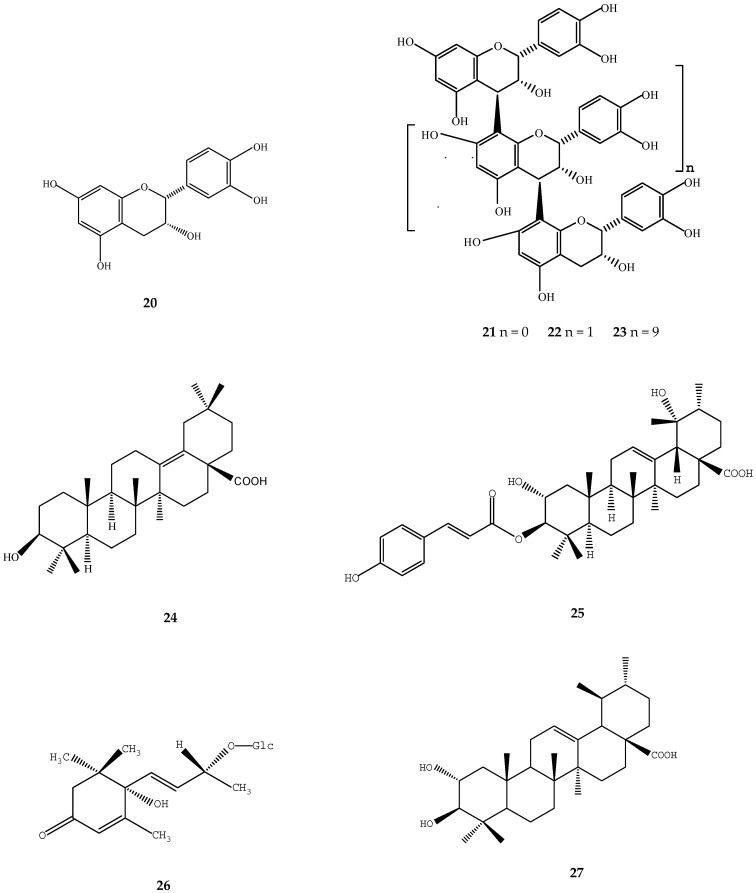
A large number of studies have demonstrated the cytotoxicity of loquat extract on different cancer cell lines. In an evaluation of 14 oriental medicinal herbs for antiproliferative activities, loquat leaf showed strong cytotoxicity in cell lines of estrogen receptor-negative breast cancer (MDA-MB-231), cervix epitheloid (HeLa) and lung (A549) carcinoma [47]. Ursolic acid (1) and oleanolic acid (8) isolated from loquat leaf significantly suppressed the proliferation of human lymphoid Molt 4B cells, which may result from the depletion of polyamines by inhibiting ornithine decarboxylase and S-adenosylmethionine decarboxylase activities [43]. Different procyanidin oligomers from loquat leaf showed selective cytotoxicity against human squamous cell (HSC-2) carcinoma and human salivary gland tumor cell [44]. Epicatechin (20), procyanidin B-2 (21), procyanidin C-1 (22), and procyanidin oligomer (23) showed increased cytotoxic activity against HSC-2 cells as molecular weight increased and such cytotoxic activity may be due to the prooxidant action of these polyphenols (Figure 3) [44]. Four triterpene acids, i.e., δ-oleanolic acid (24), ursolic acid (1), 3-O-(E)-p-coumaroyl tormentic acid (25), and betulinic acid (12) isolated from the methanol extracts of loquat leaf exhibited cytotoxicity against human HL60 cells (EC50 = 5.0–8.1 μM) and they also exhibited potent DNA topoisomerase I inhibition (IC50 = 20.3–36.5 μM) (Figure 3) [48]. Further study showed that 3-O-(E)-p-coumaroyl tormentic acid (25) induced apoptotic cell death in HL60 line mainly via the mitochondrial pathway and would be a promising compound for treatment of human leukemia [48].
Figure 3.
Structures of chemical compounds 20–27. 20: epicatechin; 21: procyanidin B-2; 22: procyanidin C-1; 23: procyanidin oligomer; 24: δ-oleanolic acid; 25: 3-O-(E)-p-coumaroyl tormentic acid; 26: roseoside; 27: 2α-hydroxyursolic acid.
By using a two-stage carcinogenesis assay on mouse skin, roseoside (26) isolated from loquat leaf was found to be the main compound that significantly delayed carcinogenesis induced by peroxynitrite as an initiator and TPA as a promoter (Figure 3) [49]. In another two-stage in vivo carcinogenesis test, euscaphic acid (6) showed significant antitumor promoting effects on mouse tumor induced by 7,12-DMBA as an initiator and TPA as a promoter [6].
Hydrophilic loquat extracts also showed in vivo anticancer activity in Meth-A-fibrosarcoma-bearing mice, operating through immunomodulatory activity, as indicated by factors such as interferon-gamma, interleukin-17, and TGF-β1 [45]. The possible constituents of such immunomodulatory activity require further investigation.
In addition, loquat leaf and seed extracts also showed significant anti-metastatic properties by inhibition of the migration and invasion of MDA-MB-231 human breast cancer cells and B16F10 melanoma cells, which was partially through the inhibition of matrix metalloproteinase-2 (MMP-2) and MMP-9 [22,24]. Ursolic acid (1) and 2α-hydroxyursolic acid (27) isolated from loquat extracts were indicated as key active compounds since both of them also significantly suppressed MMP-2 and MMP-9 activities (Figure 3) [24].
2.4. Antioxidant Activity
By using multiple antioxidant assay methods, diverse studies have demonstrated the strong antioxidant capacity of loquat extracts in vitro and in vivo. Both phenolic compounds and triterpene acids may contribute to such activity in different tissues of loquat.
The frequently reported antioxidant assay methods include Trolox equivalent antioxidant capacity (TEAC), 1,1-diphenyl-2-picrylhydrazyl radical (DPPH·) scavenging capacity, 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assays, ferric reducing antioxidant power assay (FRAP), total antioxidant capacity, and extracts of loquat leaf, flower, fruit, and seed exhibited strong antioxidant capacity based on different assays [46,50,51,52,53,54]. Among 56 selected Chinese medicinal plants, loquat leaf showed higher antioxidant capacities than 54 other medicinal plants based on TEAC and FRAP assays [55]. The ABTS+ scavenging capacity of loquat flower was highly correlated with phenolics and flavonoids, and the correlation coefficients were 0.973 and 0.886, respectively [53]. Loquat fruit of different cultivars growing in different countries such as Turkey and China also showed significantly different antioxidant capacities, indicating the influence of both genetic background and growth environment on the accumulation of antioxidants [56,57].
High correlation between the antioxidant capacity and the total phenolic content were observed in loquat fruit of different cultivars grown in Turkey [58] or in China [5]. By dividing loquat fruit extract intro hydrophilic and lipophilic fractions, phenolic content and antioxidant activity of loquat fruit from 24 cultivars grown in China were investigated and the results showed that phenolic compounds are the major contributor to the hydrophilic antioxidant activity, while carotenoids were associated with the lipophilic antioxidant activity [52]. Loquat seed contained much higher content of polyphenolic compounds and showed stronger DPPH· scavenger activity than peel and pulp extracts [51].
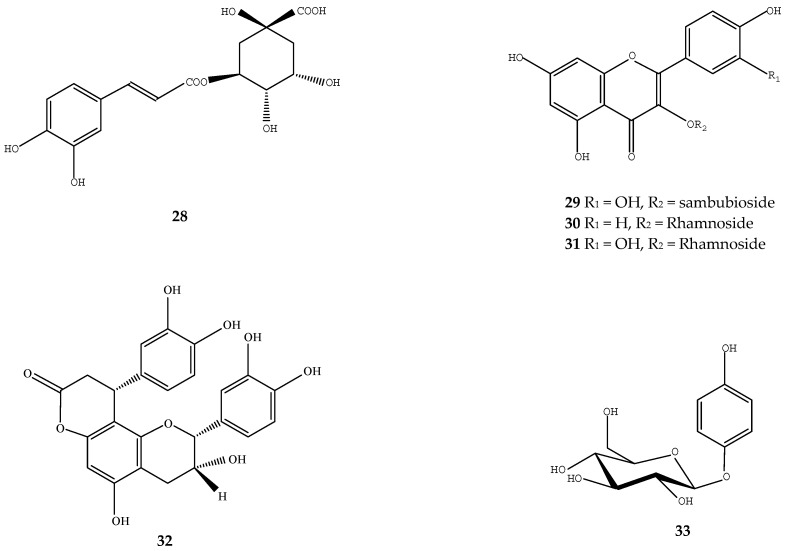
Chlorogenic acid (28) and quercetin-3-sambubioside (29), methyl chlorogenate (13), kaempferol-3-rhamnoside (30), quercetin-3-rhamnoside (31) isolated from loquat leaf extract all showed prominent inhibitory activity against free radical generation using the dichlorofluorescein method (Figure 4) [59]. The n-butanol, methanol and water fractions of loquat seed extract contained abundant polyphenols and showed high radical scavenging activity and inhibitory activity on lipid peroxidation, while the low-polar n-hexane and ethyl acetate fractions, which contained β-sitosterol, showed high lipid peroxidation inhibition activity [60]. In addition, ethanol extracts of loquat seeds were also effective in suppressing the oxidation of linoleic acid and the 2,20-azobis(4-methoxy-2,4-dimethylvaleronitrile)-induced low density lipoprotein oxidation [51]. In loquat leaf, cinchonain Ib (16), cinchonain Ia (32), epicatechin (20), quercetin-3-O-α-l-rhamnoside (31), and arbutin (33) have been identified as the important antioxidants exhibiting high antioxidant activity based on DPPH and FRAP assays (Figure 4) [61].
Figure 4.
Structures of chemical compounds 28–33. 28: chlorogenic acid; 29: quercetin-3-sambubioside; 30: kaempferol-3-rhamnoside; 31: quercetin-3-rhamnoside; 32: cinchonain Ia; 33: arbutin.
By using different cell models, different loquat fruit and leaf extracts showed protective effects against intracellular reactive oxygen species (ROS) [46,62,63]. Loquat fruit extract significantly inhibited the formation of ROS and NO in leukocytes and erythrocytes induced by the antibiotic chloramphenicol [63]. Ethanol extracts of loquat leaf showed hepatoprotective effects against ethanol-induced toxicity in HepG2 cells and a decrease in intracellular ROS formation, and increase in hepatic antioxidant activity, as well as increased cellular viability were observed [62]. Loquat leaf extract significantly increased antioxidant enzyme activities of SOD, catalase, glutathione-S-transferase, glutathione peroxidase, glutathione reductase, reduced glutathione in HepG2 cells [62]. In another cell model using β-amyloid-induced oxidative stress in neuronal PC12 cells, treatment of loquat leaf efficiently suppressed the formation of intracellular ROS formation by Aβ1-42 peptide and inhibited neuronal cell death [46]. Ursolic acid (1) in loquat leaf was reported to increase catalase activities in mouse liver [64].
By using animal models, loquat seed extracts were found to significantly reduce oxidative stress in adriamycin-induced nephropathy in rats by increasing the reduced glutathione levels in renal tissue and lowering the lipid peroxide levels in plasma and renal tissue [65]. In addition, loquat seed extract enhanced antioxidant enzyme activity and reduced lipid peroxidation in liver tissue of rats with non-alcoholic steatohepatitis [66].
2.5. Other Bioactivities
Loquat extracts have shown other bioactivities such as improvement of liver function [66,67], lung [68], renal [65] and neuronal cells [46], and also loquat extracts showed anti-obesity and hypolipidemic activity [36,37,69], anti-thrombotic potential [20], antiaging effects [70], anti-allergic [22], and antinociceptive activities [24], etc.
Both the 70% ethanol and methanol extracts of loquat seed showed significant inhibition of the development of liver fibrosis in the dimethylnitrosamine-induced hepatopathic rats and the loquat extracts significantly decreased the level of l-asparate aminotransferase (AST), l-alanine aminotransferase (ALT), and hydroxyproline levels and increased the retinoid levels in hepatopathic rats [67]. The unsaturated fatty acids linolenic and linoleic acids and the sterol β-sitosterol within the extracts may contribute to such hepatoprotective functions [67]. Long-term heavy consumption of alcohol may result in the development of alcohol related liver disease, where the induction of cytochrome P-450 2E1 (CYP2E1) by ethanol could result in a state of oxidative stress. Loquat leaf extracts showed hepatoprotective effects in HepG2 cells overexpressing CYP2E1 by improving the hepatic antioxidant activity and decreasing the formation of intracellular ROS [62]. As a result, loquat leaf extract increased HepG2 cell viability in a concentration-dependent manner and showed protective activity against ethanol-induced toxicity in HepG2 cells [62]. In addition, loquat seed extracts also showed protective activity against non-alcoholic steatohepatitis. The 70% ethanol extract of loquat seed significantly inhibited the increases in ALT and AST levels and the formation of fatty droplets in the liver in rats [66]. Such effects of inhibition of fatty liver and fibrosis may result from the elevated antioxidant enzyme activity that may alleviate oxidative stress in rats with non-alcoholic steatohepatitis [66].
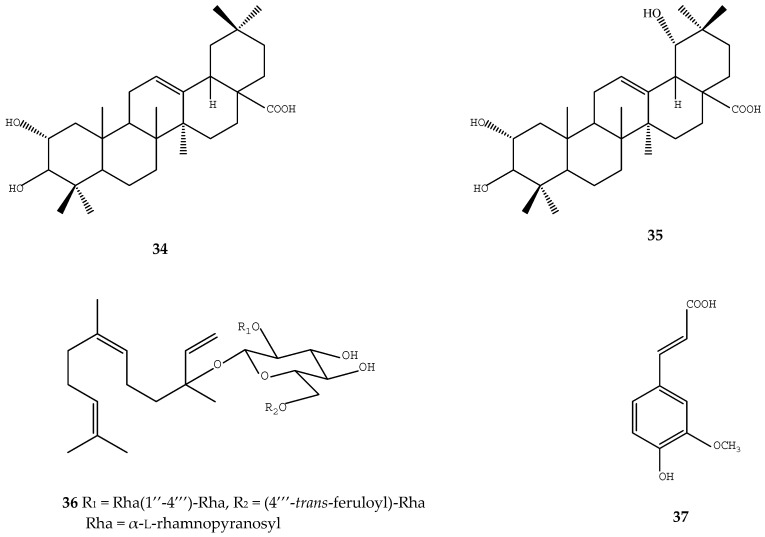
By using a rat model of bleomycin-induced pulmonary fibrosis, triterpene acids of loquat leaf showed antifibrosis effects by ameliorating the lung structure and alleviating fibrosis in the rat, where reduced expression of TNF-α and TGF-β1 both at the protein and mRNA levels were observed in the alveolar macrophage of pulmonary fibrosis rats [68]. The triterpene acids present in such loquat extracts included oleanolic acid (8), α-hydroxyoleanolic acid (34), arjunic acid (35), euscaphic acid (6), and ursolic acid (1), etc. (Figure 5) [68].
Figure 5.
Structures of chemical compounds 34–37. 34: α-hydroxyoleanolic acid; 35: arjunic acid; 36: 3-O-α-l-rhamnopyranosyl-(l→4)-α-l-rhamnopyranosyl-(1→2)-[α-l-(4-trans-feruloyl)-rhamnopyranosyl-(1→6)]-β-d-glucopyranosyl nerolidol; 37: ferulic acid.
Anticancer agents such as adriamycin cause adverse effects such as renal and liver disorders. Studies showed that loquat seed extracts could improve renal function disorder caused by adriamycin in rats and such anti-nephropathy activity may result from the alleviated oxidative stress caused by the antioxidant properties of loquat extracts [65]. Treatment with loquat extract significantly increased the level of reduced glutathione in renal tissue and lowered the level of lipid peroxide in plasma and renal tissue of adriamycin-induced nephropathic rats [65].
Oxidative stress is believed to be involved in the pathogenesis of neurodegenerative disorders. Loquat extract showed neuroprotective effects against Aβ peptide-induced oxidative stress and inhibited Aβ1–42-mediated neuronal cell death in vitro, restored the alternation behavior and reversed the Aβ1–42-induced memory impairment in mice [46]. The loquat extract substantially inhibited lipid peroxidation and restored SOD activity and cognitive deficit induced by Aβ peptide [46].
The pentacyclic terpenoids extracts of cell suspension culture of loquat showed anti-hyperlipidemic effects in HF diet mice since it reduced body weight gain, weights of white adipose tissue (WAT) (including epididymal, perirenal, mesenteric WAT and visceral fat), size of adipocytes in the visceral depots and hepatic triacylglycerol content [36,37]. Such anti-hyperlipidemic effects were correlated with increased protein phosphorylation of AMPK-α (Thr172) both in liver and adipose tissue, increased the adipose PPARγ and hepatic PPARα mRNA levels, and decreased gene expressions of fatty acid synthesis, including acyl-coenzyme A: diacylglycerol acyltransferase 2 [36,37]. Fermented tea product from a combination of loquat leaf and Camellia sinensis green tea leaf exhibited hypotriacylglycerolemic and antiobesity properties through suppression of fatty acid synthesis in liver and postprandial hypertriacylglycerolemia by inhibition of pancreatic lipase [69]. Loquat leaf showed anti-atherosclerotic activity in cellular assays and in a hypercholesterolemic zebrafish model [10].
The membrane glycoprotein thromboplastin is a tissue factor that accelerates blood clotting. Based on a bioassay-directed chromatographic separation technique, a sesquiterpene glycoside, namely 3-O-α-l-rhamnopyranosyl-(l,4)-α-l-rhamnopyranosyl-(1,2)-[α-l-(4-trans-feruloyl)-rhamnopyranosyl-(1,6)]-β-d-glucopyranosyl nerolidol (36) and ferulic acid (37) were identified from the loquat leaf as active compounds that can inhibit 50% of the tissue factor at concentration of 2 and 369 µmol/L, respectively, and thus loquat extracts may have great anti-thrombotic potential (Figure 5) [20].
Loquat seed extract also showed antiaging activity by ameliorating the cellular aging in cultured rat fibroblasts [70]. Treatment of loquat extract for seven days rendered bradykinin (BK)-induced Ca2+ dynamics in senescence cells similar to those in young cells, and retarded and/or protected against cellular aging [70]. The antiosteoporosis effect of loquat leaf was also reported in an ovariectomized mice model [71]. In another study, loquat leaf extract at 50, 125, 250, and 500 μg/mL showed inhibition of the differentiation of osteoclasts and ursolic acid (1) was isolated as the key compound that inhibited osteoclast, based on a bioactivity-guided fractionation [71].
By using the mast cell-mediated anaphylactic reaction as an experimental model, loquat leaf extract was found to inhibit systemic anaphylactic reactions and histamine release from mast cells in mice [22]. It inhibited the production of TNF-α in phorbol 12-myristate 13-acetate and A23187-stimulated human mast cells and was therefore considered as a good candidate as an anti-allergic resource [22]. The n-butyl alcoholic fraction of loquat leaf also demonstrated excellent antinociceptive activity in a dose-dependent manner in experimental pain models and it may act as a weak opioid agonist [24].
3. Data Collection
Our research included a thorough investigation of published literature in order to collect a significant amount of information related to loquat and its biological activities. The main keywords we used were loquat, fruit, seed, leaf, health, biological activity, inflammatory, diabetes, cancer, antioxidant, phenolics, terpenoids, chemical structure, etc. The databases searched included Pubmed and Web of Science.
4. Conclusions
A range of bioactivities has been reported for different loquat extracts, a number of bioactive compounds have been identified, and active research is continuing. Studies investigating in vivo metabolism and bioavailability, synergies and competitive effects, and potential toxicity of loquat extracts in animal or cell models are receiving more attention. Since many important compounds such as ursolic acid (1), chlorogenic acid (28), quercetin glycosides (29,31), and its derivatives have been well studied for their bioactivities as pure chemical compounds, irrespective of the source, eating raw loquat fruit or its processed food products may have similar health-benefiting effects. Furthermore, new applications for different loquat organs as ingredients for functional foods or as a source of therapeutics are anticipated. Extensive studies should be carried out on structure–activity relationships for different bioactive compounds. In addition, breeding and genetic studies of loquat to increase the accumulation of pharmaceutically active compounds for human health may provide a new focus for loquat research and industry.
Acknowledgments
We thank Donald Grierson from the University of Nottingham (UK) for his kind suggestions and efforts in language editing. The work was supported by the National Science Foundation of China (31471922), the Program of International Science and Technology Cooperation (2014DFE30050) and the Fundamental Research Funds for the Central Universities.
Abbreviations
| ABTS | 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulphonic acid) |
| ALT | l-Alanine aminotransferase |
| AST | l-Asparate aminotransferase |
| CB | Chronic bronchitis |
| COX-2 | Cyclooxygenase-2 |
| CYP2E1 | Cytochrome P-450 2E1 |
| DMBA | Dimethylbenz[α]anthracene |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| ERK | Extracellular signal-regulated kinase |
| FRAP | Ferric reducing antioxidant power |
| HF | High-fat |
| HSC | Human squamous cell |
| 11β-HSD1 | 11β-Hydroxysteroid dehydrogenase 1 |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| iNOS | Inducible nitric synthase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MDA | Methylene dianiline |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor-κB |
| NO | Nitric oxide |
| OLETF | Otsuka Long–Evans Tokushima fatty |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| SD | Sprague–Dawley |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| TEAC | Trolox equivalent antioxidant capacity |
| TGF-β1 | Transforming growth factor-beta 1 |
| TNF-α | Tumor necrosis factor-α |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| WAT | White adipose tissue |
Author Contributions
Yilong Liu, Wenna Zhang, Changjie Xu and Xian Li composed the review paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhou C.H., Xu C.J., Sun C.D., Li X., Chen K.S. Carotenoids in white- and red-fleshed loquat fruits. J. Agric. Food Chem. 2007;55:7822–7830. doi: 10.1021/jf071273h. [DOI] [PubMed] [Google Scholar]
- 2.Li S.Z. Compendium of Materia Medica. People’s Medical Publishing House; Beijing, China: 1578. (In Chinese) [Google Scholar]
- 3.Zhou C.H., Chen K.S., Sun C.D., Chen Q.J., Zhang W.S., Li X. Determination of oleanolic acid, ursolic acid and amygdalin in the flower of Eriobotrya japonica Lindl. by HPLC. Biomed. Chromatogr. 2007;21:755–761. doi: 10.1002/bmc.817. [DOI] [PubMed] [Google Scholar]
- 4.Fu X.M., Kong W.B., Peng G., Zhou J.Y., Azam M., Xu C.J., Grierson D., Chen K.S. Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits. J. Exp. Bot. 2012;63:341–354. doi: 10.1093/jxb/err284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W.N., Zhao X.Y., Sun C.D., Li X., Chen K.S. Phenolic composition from different loquat (Eriobotrya japonica Lindl.) cultivars grown in China and their antioxidant properties. Molecules. 2015;20:542–555. doi: 10.3390/molecules20010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banno N., Akihisa T., Tokuda H., Yasukawa K., Taguchi Y., Akazawa H., Ukiya M., Kimura Y., Suzuki T., Nishino H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol. Pharm. Bull. 2005;28:1995–1999. doi: 10.1248/bpb.28.1995. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y., Li J., Cao Q., Yu S.C., Lv X.W., Jin Y., Zhang L., Zou Y.H., Ge J.F. Anti-oxidative effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf in chronic bronchitis rats. Life Sci. 2006;78:2749–2757. doi: 10.1016/j.lfs.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Ge J.F., Wang T.Y., Zhao B., Lv X.W., Jin Y., Peng L., Yu S.C., Li J. Anti-inflammatory effect of triterpenoic acids of Eriobotrya japonica (Thunb.) Lindl. leaf on rat model of chronic bronchitis. Am. J. Chin. Med. 2009;37:309–321. doi: 10.1142/S0192415X09006862. [DOI] [PubMed] [Google Scholar]
- 9.Choi Y.G., Seok Y.H., Yeo S., Jeong M.Y., Lim S. Protective changes of inflammation-related gene expression by the leaves of Eriobotrya japonica in the LPS-stimulated human gingival fibroblast: Microarray analysis. J. Ethnopharmacol. 2011;135:636–645. doi: 10.1016/j.jep.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 10.Kim J.Y., Hong J.H., Jung H.K., Jeong Y.S., Cho K.H. Grape skin and loquat leaf extracts and acai puree have potent anti-atherosclerotic and anti-diabetic activity in vitro and in vivo in hypercholesterolemic zebrafish. Int. J. Mol. Med. 2012;30:606–614. doi: 10.3892/ijmm.2012.1045. [DOI] [PubMed] [Google Scholar]
- 11.Takuma D., Guangchen S., Yokota J., Hamada A., Onogawa M., Yoshioka S., Kusunose M., Miyamura M., Kyotani S., Nishioka Y. Effect of Eriobotrya japonica seed extract on 5-fluorouracil-induced mucositis in hamsters. Biol. Pharm. Bull. 2008;31:250–254. doi: 10.1248/bpb.31.250. [DOI] [PubMed] [Google Scholar]
- 12.Sun G., Liu Y., Zhu J., Iguchi M., Yoshioka S., Miyamura M., Kyotani S. Immunomodulatory effect of Eriobotrya japonica seed extract on allergic dermatitis rats. J. Nutr. Sci. Vitaminol. 2010;56:145–149. doi: 10.3177/jnsv.56.145. [DOI] [PubMed] [Google Scholar]
- 13.Lin J.Y., Tang C.Y. Strawberry, loquat, mulberry, and bitter melon juices exhibit prophylactic effects on LPS-induced inflammation using murine peritoneal macrophages. Food Chem. 2008;107:1587–1596. doi: 10.1016/j.foodchem.2007.10.025. [DOI] [Google Scholar]
- 14.Huang Y., Li J., Wang R., Wu Q., Li Y.H., Yu S.C., Cheng W.M., Wang Y.Y. Effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf on inflammatory cytokine and mediator induction from alveolar macrophages of chronic bronchitic rats. Inflamm. Res. 2007;56:76–82. doi: 10.1007/s00011-006-5185-0. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y., Li J., Meng X.M., Jiang G.L., Li H., Cao Q., Yu S.C., Lv X.W., Cheng W.M. Effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf and MAPK signal transduction pathway on inducible nitric oxide synthase expression in alveolar macrophage of chronic bronchitis rats. Am. J. Chin. Med. 2009;37:1099–1111. doi: 10.1142/S0192415X09007521. [DOI] [PubMed] [Google Scholar]
- 16.Zar P.P.K., Morishita A., Hashimoto F., Sakao K., Fujii M., Wada K., Hou D.X. Anti-inflammatory effects and molecular mechanisms of loquat (Eriobotrya japonica) tea. J. Funct. Foods. 2014;6:523–533. doi: 10.1016/j.jff.2013.11.019. [DOI] [Google Scholar]
- 17.Chang C.T., Huang S.S., Lin S.S., Amagaya S., Ho H.Y., Hou W.C., Shie P.H., Wu J.B., Huang G.J. Anti-inflammatory activities of tormentic acid from suspension cells of Eriobotrya Japonica ex vivo and in vivo. Food Chem. 2011;127:1131–1137. doi: 10.1016/j.foodchem.2011.01.114. [DOI] [PubMed] [Google Scholar]
- 18.Lee C.H., Wu S.L., Chen J.C., Li C.C., Lo H.Y., Cheng W.Y., Lin J.G., Chang Y.H., Hsiang C.Y., Ho T.Y. Eriobotrya japonica leaf and its triterpenes inhibited lipopolysaccharide-induced cytokines and inducible enzyme production via the nuclear factor-κB signaling pathway in lung epithelial cells. Am. J. Chin. Med. 2008;36:1185–1198. doi: 10.1142/S0192415X0800651X. [DOI] [PubMed] [Google Scholar]
- 19.Kwon H.J., Kang M.J., Kim H.J., Choi J.S., Paik K.J., Chung H.Y. Inhibition of NFκB by methyl chlorogenate from Eriobotrya japonica. Mol. Cells. 2000;10:241–246. [PubMed] [Google Scholar]
- 20.Lee M.H., Son Y.K., Han Y.N. Tissue factor inhibitory sesquiterpene glycoside from Eriobotrya japonica. Arch. Pharm. Res. 2004;27:619–623. doi: 10.1007/BF02980160. [DOI] [PubMed] [Google Scholar]
- 21.Matalka K.Z., Ali D., Khawad A.E., Qa’dan F. The differential effect of Eriobotrya japonica hydrophilic leaf extract on cytokines production and modulation. Cytokine. 2007;40:235–240. doi: 10.1016/j.cyto.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.H., Shin T.Y. Anti-inflammatory effect of leaves of Eriobotrya japonica correlating with attenuation of p38 MAPK, ERK, and NF-κB activation in mast cells. Toxicol. In Vitro. 2009;23:1215–1219. doi: 10.1016/j.tiv.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Uto T., Suangkaew N., Morinaga O., Kariyazono H., Oiso S., Shoyama Y. Eriobotryae folium extract suppresses LPS-induced iNOS and COX-2 expression by inhibition of NF-κB and MAPK activation in murine macrophages. Am. J. Chin. Med. 2010;38:985–994. doi: 10.1142/S0192415X10008408. [DOI] [PubMed] [Google Scholar]
- 24.Cha D.S., Eun J.S., Jeon H. Anti-inflammatory and antinociceptive properties of the leaves of Eriobotrya japonica. J. Ethnopharmacol. 2011;134:305–312. doi: 10.1016/j.jep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Laskin D.L., Laskin J.D. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology. 2001;160:111–118. doi: 10.1016/S0300-483X(00)00437-6. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.H., Lee S.Y., Son D.J., Lee H., Yoo H.S., Song S., Oh K.W., Han D.C., Kwon B.M., Hong J.T. Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem. Pharmacol. 2005;69:791–799. doi: 10.1016/j.bcp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Li W.L., Wu J.L., Ren B.R., Chen J., Lu C.G. Pharmacological studies on anti-hyperglycemic effect of Folium Eriobotryae. Am. J. Chin. Med. 2007;35:705–711. doi: 10.1142/S0192415X07005193. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K., Nishizono S., Makino N., Tamaru S., Terai O., Ikeda I. Hypoglycemic activity of Eriobotrya japonica seeds in type 2 diabetic rats and mice. Biosci. Biotechnol. Biochem. 2008;72:686–693. doi: 10.1271/bbb.70411. [DOI] [PubMed] [Google Scholar]
- 29.Lü H., Chen J., Li W.L., Ren B.R., Wu J.L., Kang H.Y., Zhang H.Q., Adams A., de Kimpe N. Hypoglycemic and hypolipidemic effects of the total triterpene acid fraction from Folium Eriobotryae. J. Ethnopharmacol. 2009;122:486–491. doi: 10.1016/j.jep.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Lü H., Chen J., Li W.L., Ren B.R., Wu J.L., Zhang H.Q. Hypoglycemic effect of the total flavonoid fraction from Folium eriobotryae. Phytomedicine. 2009;16:967–971. doi: 10.1016/j.phymed.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Ludvik B., Mahdjoobian K., Waldhaeusl W., Hofer A., Prager R., Kautzky-Willer A., Pacini G. The effect of Ipomoea batatas (Caiapo) on glucose metabolism and serum cholesterol in patients with type 2 diabetes: A randomized study. Diabetes Care. 2002;25:239–240. doi: 10.2337/diacare.25.1.239. [DOI] [PubMed] [Google Scholar]
- 32.Ludvik B., Waldhäusl W., Prager R., Kaulzky-Willer A., Pacini G. Mode of action of Ipomoea batatas (Caiapo) in type 2 diabetic patients. Metabolism. 2003;52:875–880. doi: 10.1016/S0026-0495(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 33.Sakuramata Y., Oe H., Kusano S., Aki O. Effects of combination of Caiapo with other plant-derived substance on anti-diabetic efficacy in KK-Ay mice. Biofactors. 2004;22:149–152. doi: 10.1002/biof.5520220129. [DOI] [PubMed] [Google Scholar]
- 34.Chen J., Li W.L., Wu J.L., Ren B.R., Zhang H.Q. Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.) Lindl.) Phytomedicine. 2008;15:98–102. doi: 10.1016/j.phymed.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Chen J., Li W.L., Wu J.L., Ren B.R., Zhang H.Q. Euscaphic acid, a new hypoglycemic natural product from Folium eriobotryae. Pharmazie. 2008;63:765–767. [PubMed] [Google Scholar]
- 36.Shih C.C., Lin C.H., Wu J.B. Eriobotrya japonica improves hyperlipidemia and reverses insulin resistance in high-fat-fed mice. Phytother. Res. 2010;24:1769–1780. doi: 10.1002/ptr.3143. [DOI] [PubMed] [Google Scholar]
- 37.Shih C.C., Ciou J.L., Lin C.H., Wu J.B., Ho H.Y. Cell suspension culture of Eriobotrya japonica regulates the diabetic and hyperlipidemic signs of high-fat-fed mice. Molecules. 2013;18:2726–2753. doi: 10.3390/molecules18032726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamaya K., Matsui T., Toshima A., Noguchi M., Ju Q., Miyata Y., Tanaka T., Tanaka K. Suppression of blood glucose level by a new fermented tea obtained by tea-rolling processing of loquat (Eriobotrya japonica) and green tea leaves in disaccharide-loaded Sprague–Dawley rats. J. Sci. Food Agric. 2010;90:779–783. doi: 10.1002/jsfa.3883. [DOI] [PubMed] [Google Scholar]
- 39.Qa’dan F., Verspohl E.J., Nahrstedt A., Petereit F., Matalka K.Z. Cinchonain Ib isolated from Eriobotrya japonica induces insulin secretion in vitro and in vivo. J. Ethnopharmacol. 2009;124:224–227. doi: 10.1016/j.jep.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Zong W., Zhao G. Corosolic acid isolation from the leaves of Eriobotrta japonica showing the effects on carbohydrate metabolism and differentiation of 3T3-L1 adipocytes. Asia Pac. J. Clin. Nutr. 2007;16:346–352. [PubMed] [Google Scholar]
- 41.Gumy C., Thurnbichler C., Aubry E.M., Balazs Z., Pfisterer P., Baumgartner L., Stuppner H., Odermatt A., Rollinger J.M. Inhibition of 11β-hydroxysteroid dehydrogenase type 1 by plant extracts used as traditional antidiabetic medicines. Fitoterapia. 2009;80:200–205. doi: 10.1016/j.fitote.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Rollinger J.M., Kratschmar D.V., Schuster D., Pfisterer P.H., Gumyb C., Aubry E.M., Brandstötter S., Stuppner H., Wolber G., Odermatt A. 11β-Hydroxysteroid dehydrogenase 1 inhibiting constituents from Eriobotrya japonica revealed by bioactivity-guided isolation and computational approaches. Bioorg. Med. Chem. 2010;18:1507–1515. doi: 10.1016/j.bmc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Komiya T., Achiwa Y., Katsuzaki H., Imai K., Sakurai S., Urakawa K., Ohnishl K., Adachi T., Yamada T., Hibasami H. Effect of oleanolic and ursolic acids isolated from Loquat (Eriobotrya) on the growth of human lymphoid leukemia cells. Food Sci. Technol. Int. 1998;4:282–284. doi: 10.3136/fsti9596t9798.4.282. [DOI] [Google Scholar]
- 44.Ito H., Kobayashi E., Takamatsu Y., Li S.H., Hatano T., Sakagami H., Kusama K., Satoh K., Sugita D., Shimura S., et al. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem. Pharm. Bull. 2000;48:687–693. doi: 10.1248/cpb.48.687. [DOI] [PubMed] [Google Scholar]
- 45.Alshaker H.A., Qinna N.A., Qadan F., Bustami M., Matalka K.Z. Eriobotrya japonica hydrophilic extract modulates cytokines in normal tissues, in the tumor of Meth-A-fibrosarcoma bearing mice, and enhances their survival time. BMC Complement. Altern. Med. 2011;11:9. doi: 10.1186/1472-6882-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim M., You M., Rhyu D., Jeong K., Kim Y., Baek H., Kim H. Oral administration of loquat suppresses DMBA-induced breast cancer in rats. Food Sci. Biotechnol. 2011;20:491–497. doi: 10.1007/s10068-011-0068-8. [DOI] [Google Scholar]
- 47.Kang S.C., Lee C.M., Choi H., Lee J.H., Oh J.S., Kwak J.H., Zee O.P. Evaluation of oriental medicinal herbs for estrogenic and antiproliferative activities. Phytother. Res. 2006;20:1017–1019. doi: 10.1002/ptr.1987. [DOI] [PubMed] [Google Scholar]
- 48.Kikuchi T., Akazawa H., Tabata K., Manosroi A., Manosroi J., Suzuki T., Akihisa T. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem. Pharm. Bull. 2011;59:378–381. doi: 10.1248/cpb.59.378. [DOI] [PubMed] [Google Scholar]
- 49.Ito H., Kobayashi E., Li S.H., Hatano T., Sugita D., Kubo N., Shimura S., Itoh Y., Tokuda H., Nishino H., et al. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agric. Food Chem. 2002;50:2400–2403. doi: 10.1021/jf011083l. [DOI] [PubMed] [Google Scholar]
- 50.Ferreres F., Gomes D., Valentão P., Gonçalves R., Pio R., Chagas E.A., Seabra R.M., Andrade P.B. Improved loquat (Eriobotrya japonica Lindl.) cultivars: Variation of phenolics and antioxidative potential. Food Chem. 2009;114:1019–1027. doi: 10.1016/j.foodchem.2008.10.065. [DOI] [Google Scholar]
- 51.Koba K., Matsuoka A., Osada K., Huang Y.S. Effect of loquat (Eriobotrya japonica) extracts on LDL oxidation. Food Chem. 2007;104:308–316. doi: 10.1016/j.foodchem.2006.11.043. [DOI] [Google Scholar]
- 52.Zhou C.H., Li X., Xu C.J., Sun C.D., Chen K.S. Hydrophilic and lipophilic antioxidant activity of loquat fruits. J. Food Biochem. 2012;36:621–626. doi: 10.1111/j.1745-4514.2011.00574.x. [DOI] [Google Scholar]
- 53.Zhou C.H., Sun C.D., Chen K.S., Li X. Flavonoids, phenolics, and antioxidant capacity in the flower of Eriobotrya japonica Lindl. Int. J. Mol. Sci. 2011;12:2935–2945. doi: 10.3390/ijms12052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou W.C., Lin R.D., Cheng K.T., Hung Y.T., Cho C.H., Chen C.H., Hwang S.Y., Lee M.H. Free radical-scavenging activity of Taiwanese native plants. Phytomedicine. 2003;10:170–175. doi: 10.1078/094471103321659898. [DOI] [PubMed] [Google Scholar]
- 55.Song F.L., Gan R.Y., Zhang Y., Xiao Q., Kuang L., Li H.B. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int. J. Mol. Sci. 2010;11:2362–2372. doi: 10.3390/ijms11062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polat A., Caliskan O., Serce S., Saracoglu O., Kaya C. Determining total phenolic content and total antioxidant capacity of loquat cultivars grown in Hatay. Pharmacogn. Mag. 2010;6:5–8. doi: 10.4103/0973-1296.59959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu H.X., Chen J.W. Commercial quality, major bioactive compound content and antioxidant capacity of 12 cultivars of loquat (Eriobotrya japonica Lindl.) fruits. J. Sci. Food Agric. 2011;91:1057–1063. doi: 10.1002/jsfa.4282. [DOI] [PubMed] [Google Scholar]
- 58.Ercisli S., Gozlekci S., Sengul M., Hegedus A., Tepe S. Some physicochemical characteristics, bioactive content and antioxidant capacity of loquat (Eriobotrya japonica (Thunb.) Lindl.) fruits from Turkey. Sci. Hortic. 2012;148:185–189. doi: 10.1016/j.scienta.2012.10.001. [DOI] [Google Scholar]
- 59.Jung H.A., Park J.C., Chung H.Y., Kim J., Choi J.S. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arch. Pharm. Res. 1999;22:213–218. doi: 10.1007/BF02976549. [DOI] [PubMed] [Google Scholar]
- 60.Yokota J., Takuma D., Hamada A., Onogawa M., Yoshioka S., Kusunose M., Miyamura M., Kyotani S., Nishioka Y. Scavenging of reactive oxygen species by Eriobotrya japonica seed extract. Biol. Pharm. Bull. 2006;29:467–471. doi: 10.1248/bpb.29.467. [DOI] [PubMed] [Google Scholar]
- 61.Hong Y.P., Qiao Y.C., Lin S.Q., Jiang Y.M., Chen F. Characterization of antioxidant compounds in Eriobotrya fragrans Champ leaf. Sci. Hortic. 2008;118:288–292. doi: 10.1016/j.scienta.2008.06.018. [DOI] [Google Scholar]
- 62.Bae D., You Y., Yoon H.G., Kim K., Lee Y.H., Kim Y., Baek H., Kim S., Lee J., Jun W. Protective effects of loquat (Eriobotrya japonica) leaves against ethanol-induced toxicity in HepG2 cells transfected with CYP2E1. Food Sci. Biotechnol. 2010;19:1093–1096. doi: 10.1007/s10068-010-0154-3. [DOI] [Google Scholar]
- 63.Eraso A.J., Albesa I. Eriobotrya japonica counteracts reactive oxygen species and nitric oxide stimulated by chloramphenicol. Am. J. Chin. Med. 2007;35:875–885. doi: 10.1142/S0192415X0700534X. [DOI] [PubMed] [Google Scholar]
- 64.Kitani K., Kanai S., Ivy G.O., Carrillo M.C. Pharmacological modifications of endogenous antioxidant enzymes with special reference to the effects of deprenyl: A possible antioxidant strategy. Mech. Ageing Dev. 1999;111:211–221. doi: 10.1016/S0047-6374(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 65.Hamada A., Yoshioka S., Takuma D., Yokota J., Cui T., Kusunose M., Miyamura M., Kyotani S., Nishioka Y. The effect of Eriobotrya japonica seed extract on oxidative stress in adriamycin-induced nephropathy in rats. Biol. Pharm. Bull. 2004;27:1961–1964. doi: 10.1248/bpb.27.1961. [DOI] [PubMed] [Google Scholar]
- 66.Yoshioka S., Hamada A., Jobu K., Yokota J., Onogawa M., Kyotani S., Miyamura M., Saibara T., Onishi S., Nishioka Y. Effects of Eriobotrya japonica seed extract on oxidative stress in rats with non-alcoholic steatohepatitis. J. Pharm. Pharmacol. 2010;62:241–246. doi: 10.1211/jpp.62.02.0012. [DOI] [PubMed] [Google Scholar]
- 67.Nishioka Y., Yoshioka S., Kusunose M., Cui T., Hamada A., Ono M., Miyamura M., Kyotani S. Effects of extract derived from Eriobotrya japonica on liver function improvement in rats. Biol. Pharm. Bull. 2002;25:1053–1057. doi: 10.1248/bpb.25.1053. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y., Huang Y., Huang C., Lv X.W., Liu L., Wang Y.Y., Li J. Antifibrosis effects of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf in a rat model of bleomycin-induced pulmonary fibrosis. J. Pharm. Pharmacol. 2012;64:1751–1760. doi: 10.1111/j.2042-7158.2012.01550.x. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka K., Tamaru S., Nishizono S., Miyata Y., Tamaya K., Matsui T., Tanaka T., Echizen Y., Ikeda I. Hypotriacylglycerolemic and antiobesity properties of a new fermented tea product obtained by tea-rolling processing of third-crop green tea (Camellia sinensis) leaves and loquat (Eriobotrya japonica) leaves. Biosci. Biotechnol. Biochem. 2010;74:1606–1612. doi: 10.1271/bbb.100197. [DOI] [PubMed] [Google Scholar]
- 70.Muramoto K., Quan R.D., Namba T., Kyotani S., Miyamura M., Nishioka Y., Tonosaki K., Doi Y.L., Kaba H. Ameliorative effects of Eriobotrya japonica seed extract on cellular aging in cultured rat fibroblasts. J. Nat. Med. 2011;65:254–261. doi: 10.1007/s11418-010-0481-y. [DOI] [PubMed] [Google Scholar]
- 71.Tan H., Furuta S., Nagata T., Ohnuki K., Akasaka T., Shirouchi B., Sato M., Kondo R., Shimizu K. Inhibitory effects of the leaves of loquat (Eriobotrya japonica) on bone mineral density loss in ovariectomized mice and osteoclast differentiation. J. Agric. Food Chem. 2014;62:836–841. doi: 10.1021/jf402735u. [DOI] [PubMed] [Google Scholar]