Abstract
Objective: We examined whether epidermal growth factor receptor (EGFR) expression in circulating tumor cells (CTCs) can be used to predict survival in a population of bone-metastatic castration-resistant prostate cancer (mCRPC) patients treated with docetaxel chemotherapy. Methods: All patients with mCRPC who had experienced treatment failure with androgen-deprivation therapy and had received docetaxel chemotherapy were eligible. CTCs and EGFR expression in CTCs were enumerated with the CellSearch System in whole blood. This system is a semi-automated system that detects and enriches epithelial cells from whole blood using an EpCAM antibody-based immunomagnetic capture. In addition, the EGFR-positive CTCs were assessed using CellSearch® Tumor Phenotyping Reagent EGFR. Results: The median CTC count at baseline before starting trial treatment was eight CTCs per 7.5 mL of blood (range 0–184). There were 37 patients (61.7%) who had ≥5 CTCs, with median overall survival of 11.5 months compared with 20.0 months for 23 patients (38.3%) with <5 CTCs (p < 0.001). A total of 15 patients (40.5%, 15/37) with five or more CTCs were subjected to automated immunofluorescence staining and cell sorting for EGFR protein. Patients with EGFR-positive CTCs had a shorter overall survival (OS) (5.5 months) than patients with EGFR-negative CTCs (20.0 months). CTCs, EGFR-positive CTCs, and alkaline phosphatase (ALP) were independent predictors of overall survival time (p = 0.002, p < 0.001, and p = 0.017, respectively). Conclusion: CTCs may be an independent predictor of OS in CRPC treated with docetaxel chemotherapy. The EGFR expression detected in CTCs was important for assessing the response to chemotherapy and predicting disease outcome.
Keywords: circulating tumor cells, castration-resistant prostate cancer, biomarker, EGFR
1. Introduction
Castration-resistant prostate cancer (CRPC) is a complex process involving many different signal transduction pathways. The epidermal growth factor receptor (EGFR) has been reported to provide an important mechanism for the progression of CRPC [1,2]. Hyperactivity of EGFR is related to androgen independence of prostate cancer cells. Levels of EGFR immunoreactivity were increased in hormone-independent human prostate cancer cell lines [3,4]. Several investigators demonstrated EGFR expression as high as 90%–100% in tissue from patients with metastatic CRPC [5,6].
Several groups have reported that the number and characteristics of circulating tumor cells (CTCs) in cancer patients are parallel to tumor progression and response to treatment [7,8,9]. CTCs are generally thought to separate from tumors of patients with advanced cancer prior to detection in circulation. The developed CellSearch System (Veridex) was designed to detect CTCs in whole blood. This system was developed using epithelial cell adhesion molecule (EpCAM) and cytokeratin antibody-based immunomagnetic capture and automated staining methodology. With this system, highly reproducible quantitative results from different laboratories can be obtained. Isolation and capture techniques of CTCs have been reported by several groups; however, only CellSearch has been analytically validated and approved by the Food and Drug Administration (FDA) [7,9]. Primary studies established that CTCs can be used in conjunction with other modalities for monitoring patients with different metastatic cancers [8,9]. Recent studies have demonstrated that CTC markers may change over the course of therapy [10,11,12,13].
We therefore examined the surface EGFR expression levels in the prognostic and therapeutic value of CTCs before docetaxel chemotherapy in a population of bone-metastatic castration-resistant prostate cancer (mCRPC) patients at Kyorin University.
2. Results
2.1. CTC Count
The median CTC count at baseline before starting the trial treatment was 8 CTC per 7.5 mL of blood (range 0–184). Overall, 23 patients (38.3%) had a CTC count of <5, while 37 patients (61.7%) had a CTC count of ≥5.
2.2. Baseline CTC Count Correlation with Patient Characteristics
The correlation of CTC count distribution and baseline characteristics is shown in Table 1. Multivariate analysis demonstrated that higher CTC counts were associated with: ALP > UNL (p = 0.002), hemoglobin level of <11.5 g/dL (p = 0.034), PSA of >30 ng/mL (p = 0.042) EOD > 3 (p = 0.003), and a Gleason score >9 (p = 0.021). Patients with bone plus lymph node metastases had a higher median CTC count than patients with only bone metastases (p = 0.014).
Table 1.
Association between circulating tumor cells (CTC) and baseline characteristics.
| Variables | N | CTC/7.5 mL | p-Value | |
|---|---|---|---|---|
| Mean | Range | |||
| CTC count at base | 60 | 8 | 0–184 | 0.042 |
| PSA (ng/mL) | ||||
| <30 | 30 | 2 | 0–3 | 0.021 |
| ≥30 | 30 | 15 | 0–184 | |
| Biopsy Gleason score | ||||
| 7–8 | 29 | 2 | 0–6 | 0.003 |
| 9–10 | 31 | 19 | 0–184 | |
| EOD | ||||
| 1–2 | 39 | 1 | 0–14 | 0.034 |
| 3–4 | 21 | 23 | 0–184 | |
| Hemoglobin (g/dL) | ||||
| <11.5 | 35 | 18 | 0–184 | 0.059 |
| ≥11.5 | 25 | 5 | 0–13 | |
| Serum albumin (g/dL) | ||||
| <3.7 | 28 | 19 | 0–184 | 0.059 |
| ≥3.7 | 22 | 4 | 0–12 | |
| Alkaline phosphatase (IU/L) | ||||
| <313 | 21 | 3 | 0–34 | 0.002 |
| ≥313 | 39 | 18 | 0–184 | |
| Lactate dehydrogenase (IU/L) | ||||
| <226 | 26 | 2 | 0–18 | 0.066 |
| ≥226 | 34 | 19 | 0–184 | |
| Disease involvement | ||||
| Only bone | 42 | 6 | 0–45 | 0.014 |
| Bone plus node | 18 | 15 | 0–184 | |
CTC median and range are expressed as cells per 7.5 mL of blood. The extent of bone metastasis was classified by the extent of disease (EOD) grade.
2.3. Analysis of Epidermal Growth Factor Receptor (EGFR) Protein Expression in CTCs
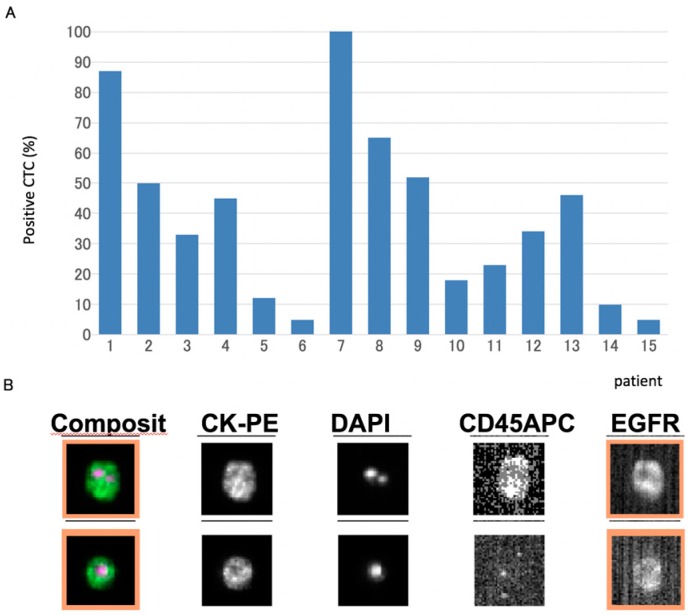
There were 15 patients (40.5%, 15/37) with five or more CTCs subjected to automated immunofluorescence staining and cell sorting for EGFR protein. The percentage of CTCs positive for EGFR ranged from 5% to 100%, with a median of 39%. The distribution of percentages is shown in Figure 1.
Figure 1.
(A) Quantitation of epidermal growth factor receptor (EGFR) expression in CTCs by automated immunofluorescence assay. The percentage of EGFR-positive CTCs relative to total CTCs was analyzed in samples with >5 CTCs and (B) A patient sample shows an individual CTC stained positive for EGFR expression.
2.4. Multivariate Analyses Indicate that CTCs at Baseline Are an Independent Predictor of Overall Survival
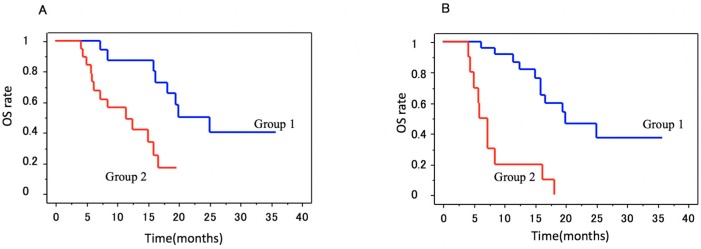
The survival rates were calculated from the time of the baseline blood sampling. The patient charts were examined to determine OS, which ranged from 5.0 to 37.0 months (mean 13.7 ± 9.2, median 15.3). Multivariate analysis demonstrated that patients with a CTC count of ≥5 at baseline had a shorter OS (11.5 months) than patients with a CTC count of <5 (20.0 months) (Figure 2A). Multivariate analysis demonstrated that patients with EGFR-positive CTCs had a shorter OS (5.5 months) than patients with EGFR-negative CTCs (20.0 months) (Figure 2B). CTC count of ≥5, EGFR-positive CTCs, and ALP > UNL were independently correlated with a poor OS (Table 2).
Figure 2.
(A) Kaplan—Meier analysis of baseline CTCs to predict overall survival time in patients with mCRPC. The overall survival time was significantly shorter in patients with ≥5 CTCs. A total of 23 patients (38.3%) with <5 CTCs/7.5 mL of blood (group 1) had a median overall survival time of 20.0 months compared with 11.5 months in 37 patients (61.7%) with ≥5 CTCs (group 2) (p = 0.0017, log-rank test) and (B) Kaplan—Meier analysis of baseline CTC-EGFR to predict overall survival time in patients with mCRPC. The overall survival time was significantly shorter in patients with EGFR-positive CTCs. There were 45 (75.0%) with EGFR-negative CTCs (group 1) that had a median overall survival time of 20.0 months compared with 5.5 months in 15 patients (25.0%) with EGFR-positive CTCs (group 2) (p < 0.001, log-rank test).
Table 2.
Baseline prognosis factors for overall survival.
| Variables | No. | Univariate Analysis | Multivariate Analysis | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|
| CTC count | |||||
| CTC < 5 | 23 | 0.001 | 0.002 | 3.24 | 1.2–4.3 |
| CTC ≥ 5 | 37 | ||||
| CTC-EGFR | |||||
| (−) | 45 | <0.001 | <0.001 | 4.01 | 1.1–6.8 |
| (+) | 15 | ||||
| PSA (ng/mL) | |||||
| <30 | 30 | 0.015 | 0.053 | 4.01 | 1.1–6.8 |
| ≥30 | 30 | ||||
| Biopsy Gleason score | |||||
| 7–8 | 29 | 0.011 | 0.42 | 1.02 | 0.6–2.9 |
| 9–10 | 31 | ||||
| EOD | |||||
| 1–2 | 39 | 0.012 | 0.285 | 1.49 | 0.5–6.8 |
| 3–4 | 21 | ||||
| Hemoglobin (g/dL) | |||||
| <11.5 | 35 | 0.028 | 0.247 | 1.11 | 0.6–5.6 |
| ≥11.5 | 25 | ||||
| Serum albumin (g/dL) | |||||
| <3.7 | 38 | 0.029 | 0.312 | 1.08 | 0.7–5.9 |
| ≥3.7 | 22 | ||||
| Alkaline phosphatase (IU/L) | |||||
| <313 | 21 | 0.003 | 0.017 | 2.46 | 1.2-5.3 |
| ≥313 | 39 | ||||
| Lactate dehydrogenase (IU/L) | |||||
| <226 | 26 | 0.008 | 0.121 | 1.9 | 0.5–2.9 |
| ≥226 | 34 | ||||
| Disease involvement | |||||
| Only bone | 42 | 0.071 | 0.226 | 1.17 | 0.6–2.4 |
| Bone plus node | 18 | ||||
The extent of bone metastasis was classified by the extent of disease (EOD) grade.
3. Discussion
Recently, CTCs have been widely recognized as a prognostic biomarker for prostate cancer patients using the FDA-cleared CellSearch System. Numerous studies have demonstrated the association between CTC baseline levels and clinical outcomes in metastatic patients. Scher et al. have reported that the combination of CTC number and LDH level was a surrogate for survival at the individual-patient level in the COU-AA-301 trial comparing abiraterone acetate plus prednisone versus prednisone alone for patients with metastatic CRPC [13].
We found CTCs in 61.7% of CRPC patients with pre-docetaxel using a cutoff of five cells per 7.5 mL of blood. A threshold of ≥5 CTCs per 7.5 mL of blood was used to evaluate the suitability of CTCs to predict survival using FDA-cleared this system. We examined the usefulness of CTCs for predicting survival in 60 CRPC patients treated with docetaxel chemotherapy. Patients with <5 CTCs per 7.5 mL of blood had a median OS time of 20.0 months compared with 11.5 months in patients with ≥5 CTCs (p < 0.001). The results demonstrated that the evaluation of CTC levels accurately and reproducibly predicted clinical outcome, as previously reported [14]. Apart from a CTC count of ≥5, ALP > UNL was also independently correlated with a poor OS. Goldkorn et al. reported the correlation with CTCs by analyzing CTCs in 161 patients with CRPC treated with first-line TXT-based therapy in SWOG S0421 [12]. The authors demonstrated that baseline CTC counts were correlated with recognized prognostic markers, including PSA, alkaline phosphatase, hemoglobin, liver disease, and bone pain. Median OS was 26 months for the group of CTCs of <5 versus 13 months for those patients with CTCs ≥5 per 7.5 mL at baseline. Unfortunately, the relationship with LDH was not assessed, as in this study. Taken together, CTCs at baseline are a strong, independent prognostic biomarker pre-docetaxel.
We identified the surface EGFR expression in CTCs before docetaxel chemotherapy in the CRPC. EGFR plays an important role in cell proliferation, migration, motility, invasion, and survival in malignant cells. EGFR is often overexpressed and is associated with aberrant signaling leading to aggressive malignancies and poor patient survival rate [15]. It has been reported that nearly 30% of PCa cases overexpress EGFR and that deregulation of EGFR-mediated signaling pathways is associated with poor clinical outcomes [16,17]. Theil et al. detected tumor-associated transcripts of EGFR in patients with metastatic PCa in 42.8% using CellCollector [18]. Shaffer et al. detected EGFR expression in 18/20 (90%) of CTCs in patients with metastatic PCa using the CellSearch system [19]. In other cancers, EGFR expression in CTCs is associated with poor prognosis and overall survival. Vila et al. reported a noticeable positive correlation between the number of EGFR-positive CTCs and worse prognosis, and overall survival in prostate cancer, breast cancer and lung cancer [20]. Serrano et al. reported that EGFR-positive CTCs indicate vulnerability to visceral disease [21]. Among our analyzed CRPC blood samples with a CTC count of ≥5 (n = 37), 40.5% (n = 15) were positive for EGFR in CTCs. Patients with EGFR-positive CTCs had a shorter OS (5.5 months) than patients with EGFR-negative CTCs (20.0 months). The tumor cells isolated from the blood may provide a better overall reflection of the biological heterogeneity of the disease than tumors from a specific site. Shaffer et al. reported that the percentage of EGFR-positive CTCs in the 20 samples analyzed ranged from 0% to 100%, as in this study [19]. These results suggest progression of cancer and may be a valuable tool in the near future. We need to characterize CTCs in PCa as well as the overall number of CTCs in order to gain a better understanding.
Several groups reported the clinical importance of androgen receptor in CTCs as a marker. Androgen receptor splice variant 7 (AR-V7), the most commonly expressed AR-V in CTCs, has been shown to provide a mechanism for development and progression of CRPC. Antonarakis et al. reported AR-V7-positive patients were associated with resistance to treatment with abiraterone and enzalutamide [22]. In addition, AR-V7 in CTCs from patients with metastatic CRPC is not associated with primary resistance to docetaxel chemotherapy. In AR-V7-positive patients, docetaxel chemotherapy appears to be more efficacious than enzalutamide or abiraterone therapy [23,24]. Analyses of tumor heterogeneity, as in this study, are very important for identifying which CTCs in CRPC have aggressive or dormant behaviors.
4. Materials and Methods
4.1. Patient Characteristics
The clinical characteristics of the patients are shown in Table 3. There were 60 mCRPC patients treated at Kyorin University Hospital between April 2012 and March 2014 who were enrolled. All patients with mCRPC who had experienced treatment failure due to androgen-deprivation therapy and had received docetaxel-based chemotherapy were eligible. All patients received 4 mg zoledronic acid every four weeks in addition to androgen-deprivation therapy. Disease progression was defined as documented PSA progression according to the Prostate-Specific Antigen Working Group 2 criteria and a PSA of >5 ng/mL, or as objective progression by Response Evaluation Criteria in Solid Tumors (RECIST) criteria for patients with measurable disease. The ethics committee of the university approved the study protocol according to the Declaration of Helsinki (number: H26-005-03 and 31-05-2014). All patients provided written consent.
Table 3.
Patients’ clinical characteristics per study group.
| No. of Patients | n = 60 |
|---|---|
| Mean age | 71 (57–82) |
| median PSA (ng/mL) | 33.7 (9.4–3241.7) |
| ≤10.0 | 2 (3.3%) |
| 10.1–20 | 12 (20.0%) |
| 20.1–30 | 16 (26.7%) |
| 30.1–40 | 7 (11.7%) |
| 40.1–50 | 7 (11.7%) |
| 50.1–100 | 11 (18.3%) |
| ≥100.1 | 5 (8.3%) |
| Gleason score | |
| 7 | 8 (13.3%) |
| 8 | 21 (35.0%) |
| 9 | 19 (31.7%) |
| 10 | 12 (20.0%) |
| EOD | |
| 1 | 20 (33.3%) |
| 2 | 19 (31.7%) |
| 3 | 13 (21.7%) |
| 4 | 8 (13.3%) |
| Disease involvement | |
| Only bone | 32 (53.3%) |
| Bone plus node | 28 (46.7%) |
| WHO perfomance score | |
| 0 | 56 (93.3%) |
| 1 | 4 (6.7%) |
| Median Hemoglobin (g/dL) | 10.6 (9.1–12.9) |
| Median serum albumin (g/dL) | 3.4 (2.9–4.1) |
| Median alkaline phosphatase (IU/L) | 342 (178–561) |
| Median lactate dehydrogenase (IU/L) | 265 (189–438) |
Number (and percentage) of patients shown unless otherwise indicated. The extent of bone metastasis was classified by the extent of disease (EOD).
4.2. Drug Administration
Docetaxel (70–75 mg/m2) and dexamethasone (8 mg/body) were given by intravenous infusion every three to four weeks. Subjects were simultaneously treated with hormonal therapy with an luteinizing hormone-releasing hormone analogue and daily oral dexamethasone (0.5–1.0 mg/day).
There were 28 (44%) patients who had received cabazitaxel after they were treated with docetaxel. Cabazitaxel was given at a dose of 25 mg/m2 intravenously every three to four weeks. A total of 32 (56%) patients received best care support.
4.3. Samples
Blood samples of patients diagnosed with mCRPC and treated with docetaxel chemotherapy were drawn into CellSave® Preservative Tubes (Immunicon, Huntingdon Valley, PA, USA) or an ethylene-diamine-tetra-acetic acid (EDTA) Vacutainer®, an evacuated blood drawtube containing EDTA as an anticoagulant and a cellular preservative. All samples were maintained at ambient temperature, with those in EDTA tubes processed within 6 h of collection and those in CellSave Preservative Tubes processed within 72 h of collection.
4.4. Isolation and Enumeration of Circulating Tumor Cells (CTCs—CellSearch System)
The FDA-cleared CellSearch System (Veridex LLC, Warren, NJ, USA) was used for the isolation and enumeration of CTCs. This system was described previously [25]. In brief, 10 mL samples of blood were drawn into a CellSave Preservative Tube. The Cell Search Epithelial Cell Kit (Veridex LLC, Warren, NJ, USA) consists of EpCAM antibody-covered ferroparticles, and processed on a CellTracks Autoprep (Immunicon). Enriched epithelial cells were identified by immunofluoresence staining with Cell Track Analyzer II (Immunicon). Cells were scored as CTCs when 4’ 6-diamidino-2-phenylindole (DAPI)-stained nucleated cells expressed cytokeratin 8, 18 and 19, excluding White Blood Cell contamination by negative selection with CD-45 staining. Automatically selected images were reviewed by the operator for identification. In addition, the EGFR-positive CTCs were assessed using CellSearch® Tumor Phenotyping Reagent EGFR [19]. The results of CTCs and EGFR expression in the CTCs are always expressed as the number of cells per 7.5 mL of blood. We identified the surface EGFR expression in CTCs before docetaxel chemotherapy in the CRPC.
4.5. Statistical Analysis
The time to death was defined as the elapsed time between the date on which blood was drawn and the date of death or last follow-up. Wilcoxon’s rank sum test or Fisher’s exact test was used to test for significant differences in the proportion of patients with CTCs greater than a particular threshold among the various patient characteristics. A threshold of ≥5 CTCs per 7.5 mL, which has been shown to be prognostic in a number of prostate cancer trials, was used for overall survival (OS) analysis. Patients with five or more CTCs were subjected to automated immunofluorescence staining and cell sorting for EGFR protein.
The median survival of patients with values greater than or equal to several PSA thresholds was evaluated to establish a PSA threshold (cut-off: 30 ng/mL) to stratify the patients into two groups. The extent of bone metastasis was classified by the extent of disease (EOD) grade according to the method of Soloway et al. [26]. The criteria for anemia and development of bone metastases were modified to hemoglobin (Hb) of <11.5 g/dL and alkaline phosphatase (ALP) above the upper normal limit (UNL) at our hospital. Median OS was determined for patients with ≥5 CTCs per 7.5 mL of blood at baseline and specified intervals. The patient charts were examined retrospectively to determine the OS time. The correlation of CTCs with OS on Kaplan-Meier survival curves was examined using the log-rank test. Cox logistic regression analysis was performed with nine categorical variables: PSA, Gleason score, EOD, Hb, ALP, lactate dehydrogenase (LDH), albumin, and CTCs.
5. Conclusions
The clinical significance of circulating tumor cell (CTC) detection has been demonstrated in pre-docetaxel chemotherapy, but further study is needed for the molecular characterization of CTCs. Epidermal growth factor receptor (EGFR) expression in CTCs is associated with poor clinical outcomes. The analysis of CTCs has the potential to become one of the most promising tests in oncology.
Acknowledgments
This study was funded by JSPS KAKENHI grant numbers 15K10308 (TO).
Author Contributions
Takatsugu Okegawa was critically involved in project conception, design, experimental procedures and data analysis. Naoshi Itaya, Hidehiko Hara and Mitsuhiro Tambo were involved in project design and patient recruitment. Kikuo Nutahara was central in project conception, design, and coordination. All authors contributed intellectually to the manuscript and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shah R.B., Ghosh D., Elder J.T. Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: Correlation with androgen independence. Prostate. 2006;66:1437–1444. doi: 10.1002/pros.20460. [DOI] [PubMed] [Google Scholar]
- 2.Culig Z., Hobisch A., Cronauer M.V., Radmayr C., Hittmair A., Zhang J., Thurnher M., Bartsch G., Klocker H. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald A., Habib F.K. Divergent responses to epidermal growth factor in hormone sensitive and insensitive human prostate cancer cell lines. Br. J. Cancer. 1992;65:177–182. doi: 10.1038/bjc.1992.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwood E.R., van Dongen J.L., Wood C.G., Liao S., Kozlowski J.M., Lee C. Epidermal growth factor receptor activation in androgen-independent but not androgen-stimulated growth of human prostatic carcinoma cells. Br. J. Cancer. 1998;77:855–861. doi: 10.1038/bjc.1998.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher H.I., Sarkis A., Reuter V., Cohen D., Netto G., Petrylak D., Lianes P., Fuks Z., Mendelsohn J., Cordon-Cardo C. Changing pattern of expression of the epidermal growth factor receptor and transforming growth factor alpha in the progression of prostatic neoplasms. Clin. Cancer Res. 1995;1:545–550. [PubMed] [Google Scholar]
- 6.Di Lorenzo G., Tortora G., D’Armiento F.P., de Rosa G., Staibano S., Autorino R., D’Armiento M., de Laurentiis M., de Placido S., Catalano G., et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- 7.Scher H.I., Jia X., de Bono J.S., Fleisher M., Pienta K.J., Raghavan D., Heller G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G., Uhr J.W., Terstappen L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 9.De Bono J.S., Scher H.I., Montgomery R.B., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K.J., Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 10.Olmos D., Arkenau H.T., Ang J.E., Ledaki I., Attard G., Carden C.P., Reid A.H., A‘Hern R., Fong P.C., Oomen N.B., et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): A single-centre experience. Ann. Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 11.Okegawa T., Nutahara K., Higashihara E. Prognostic significance of circulating tumor cells in patients with hormone refractory prostate cancer. J. Urol. 2009;181:1091–1097. doi: 10.1016/j.juro.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Goldkorn A., Ely B., Quinn D.I., Tangen C.M., Fink L.M., Xu T., Twardowski P., van Veldhuizen P.J., Agarwal N., Carducci M.A., et al. Circulating Tumor Cell Counts Are Prognostic of Overall Survival in SWOG S0421: A Phase III Trial of Docetaxel With or Without Atrasentan for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2014;32:1136–1142. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher H.I., Heller G., Molina A., Attard G., Danila D.C., Jia X., Peng W., Sandhu S.K., Olmos D., Riisnaes R., et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2015;33:1348–1355. doi: 10.1200/JCO.2014.55.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okegawa T., Itaya N., Hara H., Tambo M., Nutahara K. Circulating tumor cells as a biomarker predictive of sensitivity to docetaxel chemotherapy in patients with castration-resistant prostate cancer. Anti-Cancer Res. 2014;34:6705–6710. [PubMed] [Google Scholar]
- 15.Hynes N.E., Lane H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 16.Baek K.H., Hong M.E., Jung Y.Y., Lee C.H., Lee T.J., Park E.S., Kim M.K., Yoo J.H., Lee S.W. Correlation of AR, EGFR, and HER2 Expression Levels in Prostate Cancer: Immunohistochemical Analysis and Chromogenic In situ Hybridization. Cancer Res. Treat. 2012;44:50–56. doi: 10.4143/crt.2012.44.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leotoing L., Manin M., Monte D., Baron S., Communal Y., Lours C., Veyssière G., Morel L., Beaudoin C. Crosstalk between androgen receptor and epidermal growth factor receptor-signalling pathways: A molecular switch for epithelial cell differentiation. J. Mol. Endocrinol. 2007;39:151–162. doi: 10.1677/JME-07-0021. [DOI] [PubMed] [Google Scholar]
- 18.Theil G., Fischer K., Weber E., Medek R., Hoda R., Lücke K., Fornara P. The Use of a New CellCollector to Isolate Circulating Tumor Cells from the Blood of Patients with Different Stages of Prostate Cancer and Clinical Outcomes—A Proof-of-Concept Study. PLoS ONE. 2016;11:e0158354. doi: 10.1371/journal.pone.0158354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaffer D.R., Leversha M.A., Danila D.C., Lin O., Gonzalez-Espinoza R., Gu B., Anand A., Smith K., Maslak P., Doyle G.V., et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin. Cancer Res. 2007;13:2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 20.Vila A., Abal M., Muinelo-Romay L., Rodriguez-Abreu C., Rivas J., López-López R., Costa C. EGFR-Based Immunoisolation as a Recovery Target for Low-EpCAM CTC Subpopulation. PLoS ONE. 2016;11:e0163705. doi: 10.1371/journal.pone.0163705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serrano M.J., Ortega F.G., Alvarez-Cubero M.J., Nadal R., Sanchez-Rovira P., Salido M., Rodríguez M., García-Puche J.L., Delgado-Rodriguez M., Solé F., et al. EMT and EGFR in CTCs cytokeratin negative non-metastatic breast cancer. Oncotarget. 2014;5:7486–7497. doi: 10.18632/oncotarget.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonarakis E.S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J.C., Chen Y., Mohammad T.A., Chen Y., Fedor H.L., et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonarakis E.S., Lu C., Luber B., Wang H., Chen Y., Nakazawa M., Nadal R., Paller C.J., Denmeade S.R., Carducci M.A., et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryce A.H., Antonarakis E.S. Androgen receptor splice variant 7 in castration-resistant prostate cancer: Clinical considerations. Int. J. Urol. 2016;23:646–653. doi: 10.1111/iju.13134. [DOI] [PubMed] [Google Scholar]
- 25.Okegawa T., Nutahara K., Higashihara E. Immunomagnetic quantification of circulating tumors cell as a prognostic factor of androgen-deprivation responsiveness in hormone-naive metastatic prostate cancer patients. J. Urol. 2008;180:1342–1347. doi: 10.1016/j.juro.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Soloway M.S., Hardeman S.W., Hickey D., Raymond J., Todd B., Soloway S., Moinuddin M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1998;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::AID-CNCR2820610133>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]