Abstract
An unusual cAMP signaling system mediates many of the events that prepare spermatozoa to meet the egg. Its components include the atypical, bicarbonate-stimulated, sperm adenylyl cyclase and a cAMP-dependent protein kinase (PKA) with the unique catalytic subunit termed Cα2 or Cs. We generated mice that lack Cα2 to determine its importance in the events downstream of cAMP production. Male Cα2 null mice produce normal numbers of sperm that swim spontaneously in vitro. Thus, Cα2 has no required role in formation of a functional flagellum or the initiation of motility. In contrast, we find that Cα2 is required for bicarbonate to speed the flagellar beat and facilitate Ca2+ entry channels. In addition, Cα2 is needed for the protein tyrosine phosphorylation that occurs late in the sequence of sperm maturation and for a negative feedback control of cAMP production, revealed here. Consistent with these specific defects in several important sperm functions, Cα2 null males are infertile despite normal mating behavior. These results define several crucial roles of PKA in sperm cell biology, bringing together both known and unique PKA-mediated events that are necessary for male fertility.
The terminally differentiated, transcriptionally dormant, and translationally inactive posttesticular spermatozoan has a limited ability to respond to environmental cues encountered as it progresses through the male and female reproductive tracts. During this passage, the bicarbonate anion present in the reproductive fluids (1, 2) has an unexpectedly prominent role in promoting several of the events (3–5), collectively called capacitation, which transform sperm to readiness for fertilization.
Much evidence indicates that bicarbonate directly increases production of cAMP by atypical sperm adenylyl cyclase (sAC) (6, 7), and recent work finds that sAC is required for male fertility and normal sperm motility (8). Although cAMP might open ion channels or activate guanine nucleotide exchange factors, most evidence places cAMP-dependent protein kinase (PKA) as the major downstream effector of cAMP signals in sperm. However, only a small number of sperm proteins have been identified as phospho-substrates of PKA (9, 10). We now apply phenotypic analysis of loss-of-function mutants to examine downstream effects of the PKA subunit Cα2.
Materials and Methods
Targeted Disruption of Cα2. Cα2 null mice were generated from the targeted disruption of exon 1b of the Cα gene by homologous recombination in embryonic stem (ES) cells. The targeting vector was constructed from a 13.2-kb genomic fragment containing exons 1–3 of Cα. A loxP-flanked neomycin phosphotransferase (NEO) cassette and a mutation of the translational initiation codon were inserted into exon 1b. The linearized targeting vector was electroporated into ES cells derived from 129SV/J mice as described (11). Germ-line chimeras were bred to C57BL/6 mice. Pups carrying the NEO were crossed to heterozygous ROSA26-Cre recombinase transgenic mice (from P. Sorriano, Fred Hutchinson Cancer Research Center, Seattle) to remove the NEO. Subsequent backcrossing and selection of ROSA26-Cre-negative mice generated Cα2 heterozygote mutant mice on a 97% pure C57BL/6 background. These mice generated homozygous null, heterozygous, and WT littermates.
Southern and PCR Genotyping Analysis. Genotyping of targeted mice containing the NEO cassette used Southern blot with a PCR-generated (primer pair: 5′-GGAAGAATTCCTGAAAAAATGGGAG and 5′-CTGGGCTGAATTCTGTAGGTAAGG) fragment of intron 2 in the Cα gene to probe EcoRI-digested genomic (“tail-clip”) DNA. The WT allele generated a 10-kb fragment, and the knockout allele generated a 5-kb fragment. Genotyping of the Cre-recombined allele used PCR with two pairs of primers. The first pair (5′-CGAGCCACCGTAATGCTAGT and 5′-TCAGGT T T TCTAGCCCAGGA) probed for the WT allele by amplification of exon 1b. The second pair (5′-TGTTCCCACCCTATCACTCC and 5′-CGGTCTCGACGACGCGCCTCA) probed for the loxP site in the mutant exon 1b.
Sperm Preparation. Mice were killed by CO2 asphyxiation and cervical dislocation. Cauda epididymal sperm were harvested in BSA-fortified (0.5%) HS medium (135 mM NaCl/5 mM KCl/1 mM MgSO4/2 mM CaCl2/1 mM pyruvic acid/20 mM lactic acid/10 mM glucose/20 Hepes, pH 7.4). After 10 min of “swim out” at 37°C, sperm were washed three times and resuspended in BSA-free HS medium.
Western Blot Analysis. Sperm pellets or whole testis were homogenized and/or sonicated in 1% Triton X-100 buffer [250 mM sucrose/0.1 mM EDTA/0.5 mM EGTA/10 mM DTT/20 mM Tris, pH 7.6 with protease and phosphatase inhibitors (1 μg/ml leupeptin, 3 μg/ml aprotinin, 40 μg/ml soybean trypsin inhibitor, 0.5 mM 4-[2-aminoethyl]bezenesulfonyl fluoride, 0.1 μM microcystin-LR, 0.2 mM NaF, 0.2 mM orthovanadate)]. Protein (30 μg) in 1× sample buffer [62.5 mM Tris·Cl, pH 6.8/2% (wt/vol) SDS/5% glycerol/0.05% (wt/vol) bromophenol blue] was separated by 10% SDS/PAGE and transferred to a nitrocellulose, blocked (5% BSA in Tris-buffered saline for 30 min), and probed (2 h to overnight) with the primary antibodies for: anti-Cα (a generous gift of S. S. Taylor, University of California, San Diego), anti-RIα (Transduction Laboratories, Lexington, KY), anti-phosphotyrosine (Upstate Biotechnology, Lake Placid, NY), anti-RIIα, anti-Cα2, anti-RI (α and β), or anti-Cβ, as generated and characterized in previous work (11–13). For phosphotyrosine immunoblots, pellets with equal numbers of sperm were lysed in sample buffer, boiled, brought to 5% β-mercaptoethanol, and clarified by centrifugation before loading for SDS/PAGE.
Immunohistochemistry/Confocal Microscopy. Excised testes and caput epidymides were fixed overnight in Bouin's, washed in 70% ethanol, and embedded in paraffin. Sections (8 μm) were dried onto glass slides, washed with xylenes, and stained with hematoxylin/eosin for histological analysis. Alternatively, the tissue was rehydrated in graded washes of decreasing ethanol in water. Suspensions of epididymal sperm were spread onto a glass slide, fixed with methanol, and air-dried.
For immunocytochemistry, testis sections and sperm were treated in Tris-buffered saline (TBS) with 1% SDS for 5 min, rinsed, then blocked in TBS with 5% normal goat serum and 0.2% Triton X-100 for 2 h. Incubation (overnight at 4°C) with primary antibody (Cα 1:500, RIIα 1:500) was followed with secondary antibody (30 min, 37°C; 1:100, goat AlexaFluor anti-rabbit IgG, Molecular Probes). Confocal imaging (Leica TCS SP/MP) was conducted at the Keck Imaging Center of the University of Washington.
Computer-Assisted Semen Analysis. Epididymal sperm were exposed to capacitating conditions (0.5% BSA, 15 mM NaHCO3 in HS medium, 37°C) for 30 min. Percent motility was quantified as described (12) on a Hamilton-Thorn Motility Analyzer. Any movement qualified a sperm as motile.
Waveform Analysis. Individual sperm were examined as described (4, 5). Briefly, stop-motion images were collected at 33-ms intervals for single sperm loosely tethered at the head to a glass chip. A gravity-fed, solenoid-controlled local perfusion device added and removed test solutions. Captured images were processed by using commercial imaging software (metamorph, Universal Imaging, Downingtown, PA) and custom-designed software written in the igorpro (WaveMetrics, Lake Oswego, OR) environment.
Fertility Assessment and in Vitro Fertilization. In three or more trials, male mice (>10 weeks old) were housed with one female (8–15 weeks old) at a time for 1–3 weeks. Delivery of pups was evidence of pregnancy for females. Male mice were scored as fertile if they impregnated at least one female. In vitro fertilization used standard techniques (14–16). Briefly, oocytes from superovulated WT females were treated with hyaluronidase to remove cumulus cells. In some cases, zonae pellucidae were removed by brief treatment in acidic Ringer's solution (137 mM NaCl/26.8 mM KCl/1.6 mM CaCl2/0.5 mM MgSO4/5.5 mM glucose with 4 mg/ml polyvinylpyrrolidone, pH 2.5). Five oocytes were incubated with ≈5 × 105 capacitated sperm in a 50-μl drop of HTF medium (Irvine Scientific) under mineral oil. The rate of successful fertilization was reported as the percent of oocytes that progressed to two-cell embryos after 24 h.
Intracellular Ca2+ Measurements. Depolarization-evoked calcium entry was determined as described (5). Briefly, washed sperm were loaded with indo-1 AM ester and examined by emissionratio photometry. Small clusters (3–5 cells) of loosely tethered sperm were perfused continuously with medium HS alone or supplemented with 15 mM NaHCO3, or with 15 mM NaHCO3 and 200 μM 3-isobutyl-1-methylxanthine (IBMX), except during ≈10-s depolarizing stimuli with medium K8.6 (135 mM KCl/5 mM NaCl/2 mM CaCl2/1 mM MgCl2/30 mM N-Tris[hydroxymethyl]methyl-3-aminopropanesulfonic acid/10 mM glucose/10 mM lactic acid/1 mM pyruvic acid, adjusted to pH 8.6 with NaOH). Rates of depolarizationevoked Ca2+ entry were calculated from the linear portion of the rising phase of response in the background-corrected, calibrated indo-1 ratiometric signal.
cAMP and ATP Content and Phosphodiesterase (PDE) and AC Assays. Sperm were incubated in HS medium, counted, and divided into various treatment groups (none, 200 μM IBMX, 15 mM NaHCO3, or 30 μM H89). Incubations were ended by the addition of 5 vol of ice-cold 10 mM HCl in 100% ethanol. Samples were kept on ice for 15 min, then lyophilized and assayed for cAMP (Assay Designs, Ann Arbor, MI) per the manufacturer's protocol for acetylated samples. Epididymal sperm in HS medium were counted and lysed to determine intracellular ATP by using the ATP Determination Kit (Molecular Probes) according to the manufacturer's instructions. Sperm were sonicated in buffer containing 1% Triton X-100 and assayed as described for PDE (17) and sAC (18) activity.
Statistics. All results are reported as mean ± SEM.
Results and Discussion
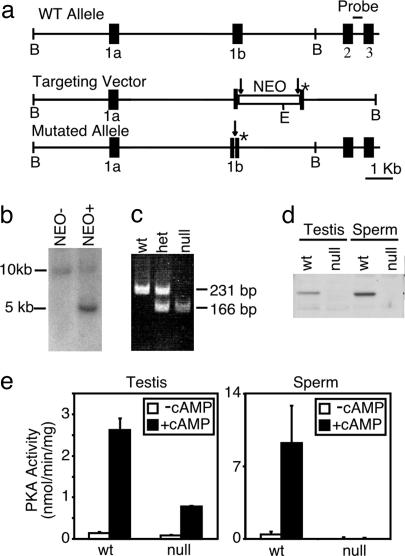
Targeted Disruption of Cα2. We had shown previously that mice lacking both the somatic and male germ-cell isoforms of Cα were severely runted and infertile (12). Their overall poor health and high rate of neonatal mortality made these animals unsuitable for study of sperm-specific roles for PKA. Refining our genetic approach, we took advantage of the endogenous male germ cell-specific expression of Cα2 (19–21) to remove PKA activity specifically from postpachytene spermiogenic cells. Exon 1b of the Cα gene was replaced with a loxP-flanked neomycin resistance gene (NEO) by homologous recombination in embryonic stem cells (Fig. 1a). Heterozygous offspring were identified by Southern blotting (Fig. 1b) and crossed to transgenic mice expressing Cre-recombinase under the ROSA-26 promoter to excise the NEO, leaving behind one loxP site and a mutation of the translation initiation codon (ATG) in every reading frame (Fig. 1a). Mice carrying the recombined, mutated allele were identified by PCR genotyping (Fig. 1c). Western blot analysis confirmed the absence of Cα2 in homogenates of testes and sperm (Fig. 1d). As expected, expression of Cα1 was unchanged in testis and all other tissues examined. The Cβ isoform was not detected in extracts prepared from either WT or Cα2 null sperm (data not shown). For functional confirmation of these results, cAMP-dependent kinase activity was assayed in extracts of testes and epididymal sperm (Fig. 1e). Testes of Cα2 null mice retained ≈25% of the WT PKA activity, reflecting expression of Cα1 in testicular somatic and prepachytene germ-line cells (Fig. 6, which is published as supporting information on the PNAS web site). Sperm of Cα2 null mice lacked both basal and cAMP-stimulated PKA activity. Together, these results confirm that we created a male germ cell-specific disruption of PKA activity.
Fig. 1.
Targeted disruption of Cα2. (a) Schematic representation of the Cα gene, targeting vector, and mutated Cα2 allele. A loxP (→)-flanked NEO cassette and a mutation in the initiation codon (*) were inserted into exon 1b. The probe for Southern blot analysis is indicated between exons 2 and 3. Restriction enzyme sites shown are BamH1 (B) and EcoR1 (E). (b) Southern blot analysis of EcoR1-digested genomic DNA from the offspring of germ-line chimeric mice without (NEO–) or with (NEO+) the targeted NEO-containing allele. (c) PCR genotyping of genomic DNA after Cre-recombinase-mediated removal of the NEO cassette. The WT allele yielded a 231-bp product, and the mutated allele yielded a 166-bp product. (d) Western blot analysis of testis extracts from WT and homozygous null mice using a polyclonal antibody raised against the first 10 aa of Cα2.(e) In vitro kinase assay of testis and sperm extracts measuring basal (–cAMP) or total (+cAMP) PKA activity.
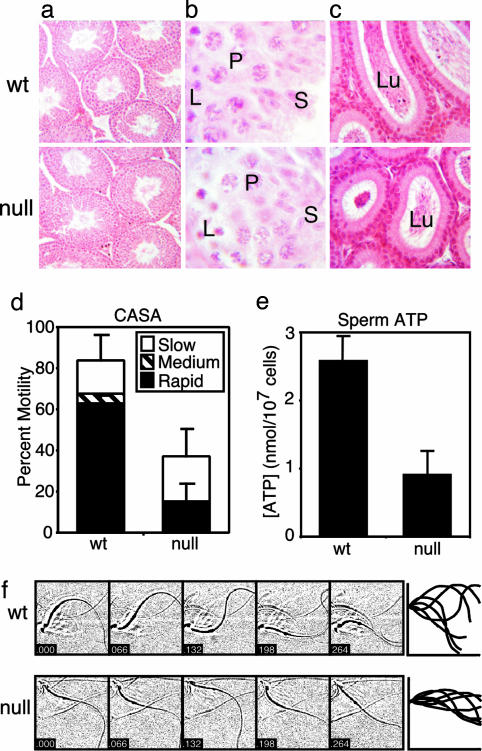
Phenotype of Cα2 Null Mice. All Cα2 null males had normal body weight and testis weight, but were completely infertile (0/9 null males produced litters when bred to at least three females over 3 months) despite normal mounting behavior and production of copulatory plugs. Male and female heterozygote and null female mice were fertile. The histology of the testes of WT and Cα2 null mice was indistinguishable (Fig. 2 a and b), and sections from the caudae epidymides of both the WT and mutant males contained densely packed tubules (Fig. 2c), consistent with the similar numbers of released cauda epididymal sperm (see Fig. 6). Differences were evident in the motility of the epididymal sperm incubated in vitro and analyzed by computer-assisted semen analysis. The proportion of motile Cα2 null sperm was reduced, particularly within the rapidly moving subpopulation (Fig. 2d). A decreased ATP content is associated with, and perhaps contributes to, the reduced motility of the Cα2 null sperm (Fig. 2e). This decreased ATP content also suggests that Cα2 may have a required role in setting the basal rate of energy production, or that the metabolic machinery of the Cα2 null sperm is not fully functional.
Fig. 2.
Testis and sperm analysis for Cα2 null mice. Histological comparison of stained WT and null tissue sections: (a) testis sections at low magnification (×200); (b) higher magnification (×1,000) of a stage IX-X tubule with labeled leptotene spermatocytes (L), pachytene spermatocytes (P), and elongating spermatids (S); and (c) caudae epidymides showing the lumen (Lu) of the tubules (×200). (d) Computer-assisted semen analysis (CASA) of motility of WT and null epididymal sperm examined after 30 min in capacitating conditions. Distributions of progressive motility were categorized as slow, medium, or fast. n = 3 mice per genotype, >200 cells per mouse. (e) ATP content of WT and null sperm. n = 3 mice per genotype. (f Left) Stop-motion images (48 × 48 μm) of WT and null sperm in HS medium (without  ) collected at 66-ms intervals. (Right) Flagellar traces for one complete beat cycle from the cells shown to the left, after rotation to a horizontal axis and alignment to a common origin.
) collected at 66-ms intervals. (Right) Flagellar traces for one complete beat cycle from the cells shown to the left, after rotation to a horizontal axis and alignment to a common origin.
Additional differences were found by using stop-motion imaging (5) to compare flagellar waveforms of WT and null sperm (Fig. 2f). Alignment of the waveform traces from such time series of images revealed rigidity in the proximal 30 μm of the flagellum of the Cα2 null sperm. On average, the beat amplitude measured at 30 μm along the beat axis was reduced from 32 ± 1 to 13 ± 1 μm. In contrast, resting flagellar beat frequency of mutant sperm was slightly elevated, ≈1.5-fold.
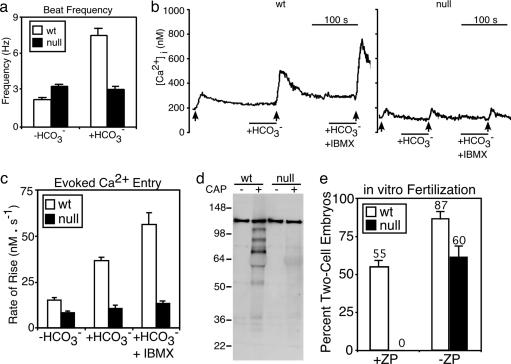
Past work described a rapid (t1/2 ≈10 s), sustained, bicarbonate-mediated speeding of the flagellar beat that defines the early activation of sperm motility when sperm contact the reproductive fluids at mating (5). In vitro, 60 s of bicarbonate treatment increased the beat frequency of WT sperm ≈3-fold but elicited no change in Cα2 null sperm (see Fig. 3a and Movies 1 and 2, which are published as supporting information on the PNAS web site). Hence, Cα2 is required for acceleration of the flagellar beat by bicarbonate, consistent with the hypothesis that activation involves PKA-mediated phosphorylation of flagellar proteins to increase ATP-dependent motor activity. It is possible, but less likely, that some metabolic defect in Cα2 null sperm prevents acceleration of the flagellar motor by bicarbonate.
Fig. 3.
Absence of bicarbonate-stimulated and capacitation-dependent events in Cα2 null sperm. (a) Flagellar beat frequency of WT (n = 8) and null (n = 11) sperm. (b) Representative traces of indo-1 ratio-photometric monitoring of intracellular free [Ca2+] for WT and null sperm perfused with HS medium alone, then supplemented with 15 mM NaHCO3, then with 15 mM NaHCO3 and 200 μM IBMX. The ↑ indicate 10 s of depolarizing stimulus with K8.6 medium. (c) Conditioning with  facilitates the rate of Ca2+ entry determined from the averaged linear rising phase of responses as in b (WT n = 11, null n = 14). (d) Western blot analysis of phosphotyrosine content from WT and null sperm examined before (CAP–) or after (CAP+) a 90-min exposure to capacitating conditions. (e) In vitro fertilization of WT oocytes [+ or – zona pellucida (ZP)] using WT or null sperm. The percentage of two-cell embryos was determined 24 h after addition of sperm. Data represent the means of three independent experiments involving 75–145 oocytes, n = 3–5 male mice for each genotype.
facilitates the rate of Ca2+ entry determined from the averaged linear rising phase of responses as in b (WT n = 11, null n = 14). (d) Western blot analysis of phosphotyrosine content from WT and null sperm examined before (CAP–) or after (CAP+) a 90-min exposure to capacitating conditions. (e) In vitro fertilization of WT oocytes [+ or – zona pellucida (ZP)] using WT or null sperm. The percentage of two-cell embryos was determined 24 h after addition of sperm. Data represent the means of three independent experiments involving 75–145 oocytes, n = 3–5 male mice for each genotype.
PKA Cα2 Requirement for Enhancement of Ca2+ Entry by Bicarbonate. Elevations in intracellular cAMP also rapidly enhance depolarization-evoked entry of calcium into sperm (5, 22). WT and Cα2 null sperm have similar basal rates of evoked calcium entry. Conditioning with bicarbonate increased the rate 2- to 3-fold for WT sperm, but failed to facilitate channel activity of Cα2 null sperm (Fig. 3 b and c). Recent work found that proteins of the CatSper family are expressed exclusively in sperm and have sequence similarity to a six-transmembrane domain repeat of the voltage-dependent calcium channels (16, 23, 24). Although direct evidence of their function as channels is not available, both CatSper1 (4) and CatSper2 (D.F.B., unpublished data) are required for depolarization-evoked calcium entry into sperm. In the simplest interpretation, these proteins comprise all or part of the voltage-gated channels opened by depolarization of sperm membrane potential. Phosphorylation may potentiate the activation of the putative CatSper1/2 channels as it does for other voltage-gated Ca2+ channels (25).
Defects in Late Events of Capacitation for Cα2 Null Sperm. The preparation of sperm for fertilization also involves events that occur on a slower time scale (26, 27). These include increases in protein tyrosine phosphorylation (28). Fig. 3d shows that prolonged incubation under capacitating conditions increased the phosphotyrosine immunoreactivity of extracts of WT but not Cα2 null sperm. This requirement of Cα2 for engagement of the protein phosphorylation cascade of capacitation confirms and consolidates previous proposals that PKA-mediated phosphorylation is a necessary first step to activate sperm protein tyrosine phosphorylation (28).
Late in the capacitation sequence, sperm also hyperactivate; flagellar beat amplitude and asymmetry increase, and the linearity of swimming paths decreases (29). Hyperactivation requires CatSper proteins, which presumably serve as the route of entry for the Ca2+ that operates directly on the flagellar axoneme to increase waveform asymmetry. CatSper-null sperm can penetrate and fertilize zona-free but not zona-intact eggs in vitro, indicating that an inability to initiate or sustain hyperactivation may be the sole or major lesion underlying the male infertile phenotype of CatSper null mutants (4, 30). Cα2 null sperm had a similar ability to fertilize zona-free but not zona-intact eggs (Fig. 3e), suggesting that they also may not hyperactivate. Indeed, after a 1-h incubation in capacitating conditions, visual inspection of the Cα2 null sperm did not detect cells with the characteristic hyperactivated waveform. However, the proportion of motile cells was decreased substantially, indicating that the apparent requirement of Cα2 for hyperactivation should be viewed very cautiously.
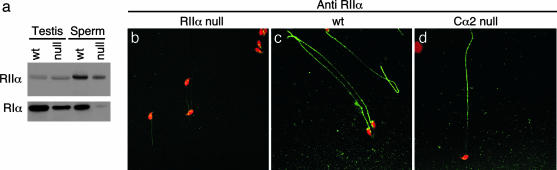
Stabilization and Localization of PKA in Sperm. Past work in somatic cells found that the regulatory (R) and catalytic (C) subunits of the tetrameric PKA holoenzyme complex (R2C2) displayed codependent stability. Decreased expression of either R or C subunits resulted in loss or reduction in the content of the other (11, 12, 31). WT testes and sperm express RIIα and RIα, but not RIIβ and RIβ (data not shown). In Cα2 null sperm, the RIα protein is greatly diminished and the content of RIIα protein is reduced (Fig. 4a). This loss of RIα protein from the germ cells also is reflected by a reduction of RIα in the testis extracts. The RIIα that remains in Cα2 null sperm is most abundant in the principal piece of the flagellum and is greatly reduced in the midpiece and end-piece regions (Fig. 4 c and d). The specificity of the RIIα antibody is demonstrated by the lack of staining in the RIIα null sperm (Fig. 4b).
Fig. 4.
Expression and localization of RI subunits in Cα2 null sperm. (a) Western blot analysis of extracts of WT and Cα2 null testis and sperm, probed for RIIα and RIα protein. (b–d) Immunocytochemical localization of RIIα protein (green) in fixed cauda epididymal sperm from RIIα null mice (negative control) (b), WT mice (c), and Cα2 null mice (d). Sperm heads were stained with propidium iodide (red).
The TAKAP80, AKAP3, and AKAP4 A-kinase anchoring proteins are major proteins of the fibrous sheath that defines the principal piece of the flagellum. They bind RII subunits with higher affinity than RI (32–34). These characteristics provide a reasonable explanation for both the localization and preferential retention of RIIα in the Cα2 null sperm. Furthermore, the localization and stabilization of RIIα protein (expression of which begins postmeiotically and does not overlap with Cα1 expression) suggests that assembly of high-affinity PKA–AKAP complexes is independent of the presence of C subunit.
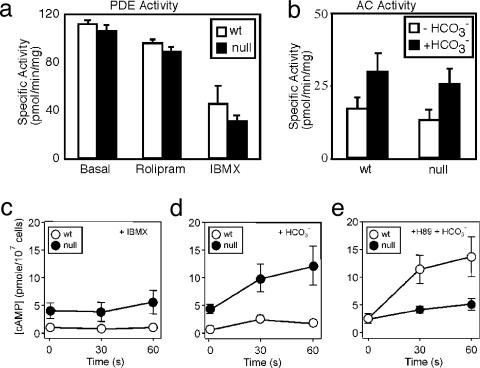
Negative Feedback Control of cAMP Synthesis. Feedback regulation of cAMP content can be achieved in many somatic cells through PKA-dependent activation of PDE activity or inhibition of AC activity (35, 36). Consistent with the presence of a negative feedback pathway, Cα2 null sperm have a 5-fold elevated basal cAMP content (4.2 ± 0.7 vs. 0.9 ± 0.2 pmol per 107 cells). An alternative explanation for the increased cAMP content is that the absence of Cα2 alters the expression or retention of PDE or AC protein during spermiogenesis. We tested this hypothesis by measuring PDE and AC activity in sperm homogenates. The basal PDE activities of WT and Cα2 null sperm were identical and showed the same sensitivity both to the PDE4 inhibitor rolipram and the nonselective PDE inhibitor IBMX (Fig. 5a). The basal AC activities of WT and Cα2 null sperm also were the same and increased ≈2-fold in the presence of  (Fig. 5b). We conclude from these data that C α2 null sperm have no change in the amount of either PDE or AC. To explain the elevated cAMP in the Cα2 null sperm, we postulated that the feedback regulation of cyclase or PDE activity must be lost during the preparation of sperm extracts.
(Fig. 5b). We conclude from these data that C α2 null sperm have no change in the amount of either PDE or AC. To explain the elevated cAMP in the Cα2 null sperm, we postulated that the feedback regulation of cyclase or PDE activity must be lost during the preparation of sperm extracts.
Fig. 5.
PKA-mediated feedback control of sAC. Total basal PDE assayed in extracts of WT and Cα2 null sperm. (a) Sensitivity of PDE activity to rolipram (20 μM) and IBMX (200 μM). (b) AC activity in sperm extracts assayed in the presence and absence of 15 mM NaHCO3.(c–e) cAMP content was monitored over a 1-min time course for WT (○) and Cα2 null (•) sperm in HS medium supplemented at t = 0 with 200 μM IBMX (c), 15 mM NaHCO3 (d), or 15 mM NaHCO3 with 30 μM H89 applied after a 10-min exposure to 30 μM H89 alone (e). n = 3–6 mice per genotype.
To seek further evidence of feedback regulation in the intact cell, we used pharmacological manipulations of the cAMP second messenger system. Inhibition of basal PDE activity with IBMX had little effect on cAMP content of WT sperm during a 1-min incubation, but increased the content in null sperm slightly (Fig. 5a). A 10-min incubation with IBMX increased the cAMP content of WT and mutant sperm 3- and 10-fold, respectively (see Fig. 7, which is published as supporting information on the PNAS web site). These findings suggested that basal AC activity is elevated in the Cα2 null sperm. The kinetics of cAMP accumulation during 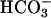 stimulation of the sperm revealed additional differences between the two genotypes. The cAMP content of WT sperm increased ≈3-fold after 30 s in bicarbonate, then fell (Fig. 5d). By 3 min cAMP had returned to near basal levels (data not shown). In contrast, bicarbonate elicited a more robust and sustained rise in cAMP in Cα2 null sperm (Fig. 5d). A similar, but significantly enhanced and prolonged, rise occurred when
stimulation of the sperm revealed additional differences between the two genotypes. The cAMP content of WT sperm increased ≈3-fold after 30 s in bicarbonate, then fell (Fig. 5d). By 3 min cAMP had returned to near basal levels (data not shown). In contrast, bicarbonate elicited a more robust and sustained rise in cAMP in Cα2 null sperm (Fig. 5d). A similar, but significantly enhanced and prolonged, rise occurred when 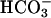 was applied together with IBMX (Fig. 7). Hence, Cα2 limits cAMP accumulation without the involvement of IBMX-sensitive PDE activity.
was applied together with IBMX (Fig. 7). Hence, Cα2 limits cAMP accumulation without the involvement of IBMX-sensitive PDE activity.
We hypothesized that PKA is involved in a feedback inhibitory loop on sAC activity in sperm and attempted to replicate the findings from the genetic model through pharmacological inhibition of PKA by using the ATP-binding site-directed agent, H89. A 10-min incubation with H89 increased the basal cAMP content of WT sperm, suggesting that sAC is tonically inhibited by PKA. Furthermore, bicarbonate stimulation of WT sperm in the presence of H89 (Fig. 5e) produced cAMP accumulation with kinetics similar to the Cα2 null sperm during the first minute of their exposure to 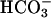 alone (Fig. 5d). Fig. 5e also shows that H89 unexpectedly decreased the
alone (Fig. 5d). Fig. 5e also shows that H89 unexpectedly decreased the 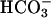 -enhanced accumulation of cAMP in the Cα2 null sperm. This action is not a consequence of direct blockade of sAC activity by H89 because we see no effect of this drug on sAC activity in vitro (Fig. 7). However, H89 is a competitive inhibitor for ATP binding to PKA and is known to inhibit at least three other kinases with similar or greater potency than that for PKA (37). In the mutant sperm that are devoid of the PKA catalytic subunit, H89 has actions on cAMP accumulation that illustrate its potential for nonspecific effects. These nonspecific actions may be exacerbated by the lowered ATP content of the Cα2 null sperm. Regardless of its actions on the Cα2 null sperm, H89 treatment of WT sperm mimics the enhanced early (<1 min) accumulation of cAMP by the Cα2 null sperm, consistent with the proposed PKA-dependent feedback inhibition on sAC activity.
-enhanced accumulation of cAMP in the Cα2 null sperm. This action is not a consequence of direct blockade of sAC activity by H89 because we see no effect of this drug on sAC activity in vitro (Fig. 7). However, H89 is a competitive inhibitor for ATP binding to PKA and is known to inhibit at least three other kinases with similar or greater potency than that for PKA (37). In the mutant sperm that are devoid of the PKA catalytic subunit, H89 has actions on cAMP accumulation that illustrate its potential for nonspecific effects. These nonspecific actions may be exacerbated by the lowered ATP content of the Cα2 null sperm. Regardless of its actions on the Cα2 null sperm, H89 treatment of WT sperm mimics the enhanced early (<1 min) accumulation of cAMP by the Cα2 null sperm, consistent with the proposed PKA-dependent feedback inhibition on sAC activity.
Conclusions
The reproductive success of mammalian species requires precise orchestration of multiple posttesticular events in sperm. The work presented here shows that a highly specialized, bicarbonate-responsive cAMP-PKA axis coordinates many of the steps that lead to fertilization competency for sperm. In particular, we have identified required roles for the sperm-specific PKA Cα2 in control of the unique CatSper-dependent entry of calcium and the atypical sAC-dependent production of cAMP. We have yet to determine whether these roles for PKA are through direct phosphorylation of CatSper or sAC. However, it is clear that PKA not only directs the majority of cAMP-mediated signaling in sperm, but also is required for the attenuation of the cAMP signal itself.
Supplementary Material
Acknowledgments
We thank Bertil Hille for helpful discussions, support, and use of his laboratory. This work was supported through agreement U54-HD12629 of the Specialized Cooperative Centers Program in Reproduction Research of the National Institutes of Health, National Institutes of Health Grant GM 32875 (to G.S.M.), and Deutsche Forschungsgemeinschaft Grant WE 2344/4-1 (to G.W.). M.A.N. was supported by National Institutes of Health Training Grant T32 GM 07270.
Abbreviations: PDE, phosphodiesterase; AC, adenylyl cyclase; sAC, atypical sperm AC; PKA, cAMP-dependent protein kinase; NEO, neomycin phosphotransferase; IBMX, 3-isobutyl-1-methylxanthine.
References
- 1.Boatman, D. E. & Robbins, R. S. (1991) Biol. Reprod. 44, 806–813. [DOI] [PubMed] [Google Scholar]
- 2.Okamura, N., Tajima, Y., Soejima, A., Masuda, H. & Sugita, Y. (1985) J. Biol. Chem. 260, 9699–9705. [PubMed] [Google Scholar]
- 3.Gadella, B. M. & Harrison, R. A. (2000) Development (Cambridge, U.K.) 127, 2407–2420. [DOI] [PubMed] [Google Scholar]
- 4.Carlson, A. E., Westenbroek, R. E., Quill, T., Ren, D., Clapham, D. E., Hille, B., Garbers, D. L. & Babcock, D. F. (2003) Proc. Natl. Acad. Sci. USA 100, 14864–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wennemuth, G., Carlson, A. E., Harper, A. J. & Babcock, D. F. (2003) Development (Cambridge, U.K.) 130, 1317–1326. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., Cann, M. J., Litvin, T. N., Iourgenko, V., Sinclair, M. L., Levin, L. R. & Buck, J. (2000) Science 289, 625–628. [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal, B. S. & Conti, M. (2003) Proc. Natl. Acad. Sci. USA 100, 10676–10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito, G., Jaiswal, B. S., Xie, F., Krajnc-Franken, M. A., Robben, T. J., Strik, A. M., Kuil, C., Philipsen, R. L., van Duin, M., Conti, M. & Gossen, J. A. (2004) Proc. Natl. Acad. Sci. USA 101, 2993–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison, R. A. (2004) Mol. Reprod. Dev. 67, 337–352. [DOI] [PubMed] [Google Scholar]
- 10.Ficarro, S., Chertihin, O., Westbrook, V. A., White, F., Jayes, F., Kalab, P., Marto, J. A., Shabanowitz, J., Herr, J. C., Hunt, D. F. & Visconti, P. E. (2003) J. Biol. Chem. 278, 11579–11589. [DOI] [PubMed] [Google Scholar]
- 11.Burton, K., Treash-Osio, B., Muller, C., Dunphy, E. & McKnight, G. (1999) J. Biol. Chem. 274, 24131–24136. [DOI] [PubMed] [Google Scholar]
- 12.Skalhegg, B., Huang, Y., Su, T., Idzerda, R., McKnight, G. & Burton, K. (2002) Mol. Endocrinol. 16, 630–639. [DOI] [PubMed] [Google Scholar]
- 13.Cummings, D. E., Brandon, E. P., Planas, J. V., Motamed, K., Idzerda, R. L. & McKnight, G. S. (1996) Nature 382, 622–626. [DOI] [PubMed] [Google Scholar]
- 14.Naito, K., Toyoda, Y. & Yanagimachi, R. (1992) Hum. Reprod. 7, 281–285. [DOI] [PubMed] [Google Scholar]
- 15.Perez, G. I., Robles, R., Knudson, C. M., Flaws, J. A., Korsmeyer, S. J. & Tilly, J. L. (1999) Nat. Genet 21, 200–203. [DOI] [PubMed] [Google Scholar]
- 16.Ren, D., Navarro, B., Perez, G., Jackson, A. C., Hsu, S., Shi, Q., Tilly, J. L. & Clapham, D. E. (2001) Nature 413, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins, T. J., Mumby, M. C. & Beavo, J. A. (1982) J. Biol. Chem. 257, 1973–1979. [PubMed] [Google Scholar]
- 18.Jaiswal, B. S. & Conti, M. (2001) J. Biol. Chem. 276, 31698–31708. [DOI] [PubMed] [Google Scholar]
- 19.Desseyn, J., Burton, K. & McKnight, G. (2000) Proc. Natl. Acad. Sci. USA 97, 6433–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SanAgustin, J. & Witman, G. (2001) Biol. Reprod. 65, 151–164. [DOI] [PubMed] [Google Scholar]
- 21.SanAgustin, J., Wilkerson, C. & Witman, G. (2000) Mol. Biol. Cell 11, 3031–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wennemuth, G., Westenbroek, R. E., Xu, T., Hille, B. & Babcock, D. F. (2000) J. Biol. Chem. 275, 21210–21217. [DOI] [PubMed] [Google Scholar]
- 23.Quill, T. A., Ren, D., Clapham, D. E. & Garbers, D. L. (2001) Proc. Natl. Acad. Sci. USA 98, 12527–12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobley, A., Pierron, V., Reynolds, L., Allen, L. & Michalovich, D. (2003) Reprod. Biol. Endocrinol. 1, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald, T. F., Pelzer, S., Trautwein, W. & Pelzer, D. J. (1994) Physiol. Rev. 74, 365–507. [DOI] [PubMed] [Google Scholar]
- 26.Visconti, P. E., Westbrook, V. A., Chertihin, O., Demarco, I., Sleight, S. & Diekman, A. B. (2002) J. Reprod. Immunol. 53, 133–150. [DOI] [PubMed] [Google Scholar]
- 27.Yanagimachi, R. (1989) J. Reprod. Fertil. Suppl., 38, 27–33. [PubMed] [Google Scholar]
- 28.Visconti, P. E., Moore, G. D., Bailey, J. L., Leclerc, P., Connors, S. A., Pan, D., Olds-Clarke, P. & Kopf, G. S. (1995) Development (Cambridge, U.K.) 121, 1139–1150. [DOI] [PubMed] [Google Scholar]
- 29.Suarez, S. S. & Ho, H. C. (2003) Cell Mol. Biol. 49, 351–356. [PubMed] [Google Scholar]
- 30.Quill, T. A., Sugden, S. A., Rossi, K. L., Doolittle, L. K., Hammer, R. E. & Garbers, D. L. (2003) Proc. Natl. Acad. Sci. USA 100, 14869–14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amieux, P. S., Cummings, D. E., Motamed, K., Brandon, E. P., Wailes, L. A., Le, K., Idzerda, R. L. & McKnight, G. S. (1997) J. Biol. Chem. 272, 3993–3998. [DOI] [PubMed] [Google Scholar]
- 32.Miki, K. & Eddy, E. (1998) J. Biol. Chem. 273, 34384–34390. [DOI] [PubMed] [Google Scholar]
- 33.Michel, J. J. & Scott, J. D. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 235–257. [DOI] [PubMed] [Google Scholar]
- 34.Tasken, K. & Aandahl, E. M. (2004) Physiol. Rev. 84, 137–167. [DOI] [PubMed] [Google Scholar]
- 35.Sunahara, R. K., Dessauer, C. W. & Gilman, A. G. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 461–480. [DOI] [PubMed] [Google Scholar]
- 36.Macphee, C. H., Reifsnyder, D. H., Moore, T. A., Lerea, K. M. & Beavo, J. A. (1988) J. Biol. Chem. 263, 10353–10358. [PubMed] [Google Scholar]
- 37.Davies, S. P., Reddy, H., Caivano, M. & Cohen, P. (2000) Biochem. J. 351, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.