Abstract
Myeloid cells are a unique subset of leukocytes with a diverse array of functions within the central nervous system during health and disease. Advances in understanding of the unique properties of these cells have inspired interest in their use as delivery vehicles for therapeutic genes, proteins, and drugs, or as “assistants” in the clean-up of aggregated proteins and other molecules when existing drainage systems are no longer adequate. The trafficking of myeloid cells from the periphery to the central nervous system is subject to complex cellular and molecular controls with several ‘checkpoints’ from the blood to their destination in the brain parenchyma. As important components of the neurovascular unit, the functional state changes associated with lineage heterogeneity of myeloid cells are increasingly recognized as important for disease progression. In this review, we discuss some of the cellular elements associated with formation and function of the neurovascular unit, and present an update on the impact of myeloid cells on central nervous system (CNS) diseases in the laboratory and the clinic. We then discuss emerging strategies for harnessing the potential of site-directed myeloid cell homing to the CNS, and identify promising avenues for future research, with particular emphasis on the importance of untangling the functional heterogeneity within existing myeloid subsets.
Keywords: myeloid cell, brain, transplantation, microglia
1. Introduction
Diseases of the central nervous system (CNS) are some of the most devastating contributors to disease burden worldwide, and are associated with significant costs to healthcare and productivity [1,2,3]. Despite intensive research, treatment of pathology within the brain remains challenging due to its relative isolation from components of the circulatory system by the blood–brain barrier (BBB). Drug development for CNS disorders has been particularly challenging, as virtually all large molecules and the vast majority of small molecules identified in vitro cannot reach the parenchyma in vivo [4]. Accumulating evidence indicates that, rather than being completely separated from the peripheral immune system, the normal CNS undergoes tightly regulated surveillance by a variety of bone-marrow-derived immune cells, likely through several influx/efflux pathways [5,6,7]. This population of cells also appears to be crucial for neuronal development and function [8]. Also, the influx of leukocytes from the periphery is a hallmark of a number of autoimmune and neurodegenerative conditions, and despite sharing morphological characteristics of CNS-resident microglia, peripheral myeloid cells appear to possess non-redundant functions [6]. Importantly, in many cases the BBB is not simply “leaky”, as dyes and small proteins do not cross from the vasculature into the brain parenchyma [9]. This indicates that, under certain circumstances, leukocytes may in fact be invited guests to the CNS—participants in a coordinated series of interactions with the brain’s endothelial, glial, and epithelial barriers to contribute to brain homeostasis.
In many CNS diseases and in response to irradiation, the key cell type involved in trafficking to the parenchyma is the myeloid cell, a subset of leukocytes central to the innate immune system [10,11]. Myeloid cells are typically released from bone marrow as one of several subsets of monocytes, and following migration to target tissues may differentiate into macrophages or dendritic cells [12]. These cells possess a remarkable diversity of functions, which are dependent on their lineage, gene expression, surface molecules, and interactions with the surrounding environment via messenger molecules such as cytokines and chemokines secreted by other cells. Most notably, they are capable of destroying and clearing pathogens and debris independently via phagocytosis and secretion of proteolytic enzymes, and also of presenting antigens to the adaptive arm of the immune system [13]. This indicates that such cells could be primed for a function of interest via drug administration, genetic modification, or culture with certain molecules. Although transplantation into the CNS can be achieved directly via stereotactic injection, the damage induced by this process precludes it from routine use. Taking advantage of existing mechanisms of selective leukocyte extravasation into the brain parenchyma has emerged as a promising strategy for CNS cell and gene therapy, with tantalizing evidence of success in both preclinical and clinical applications.
The purpose of this review is to highlight the peculiar anatomy of the CNS and the unique mechanisms by which it allows myeloid cells to participate in site-directed homing, immune surveillance, and initiation of neuroprotective or neurotoxic activity. Additionally, promising developments in the use of myeloid-derived cells in the treatment of diseases of the CNS are discussed, and we outline some key targets for future research.
2. Contribution of Myeloid Cells to Central Nervous System (CNS) Homeostasis
2.1. The Resident CNS Myeloid Cell, the Microglia
Despite initial controversy as to their origin, it is now established that microglia are derived from early yolk sac erythromyeloid precursors and migrate to the brain during early embryogenesis [14,15,16], where they remain exclusively throughout life without substantial contribution from bone marrow-derived cells, unless recruited in response to distress signals from the CNS parenchyma and associated endothelial and glial barriers [11,17,18]. In their ramified state, microglia are far from ‘resting’, their processes continuously extend and retract to survey the surrounding parenchyma [19]. In their activated state, microglia- and CNS-infiltrating monocyte-derived macrophages both adapt an amoeboid morphology and share expression of many surface molecules including major histocompatibility class II (MCHII) complexes, which has made identifying the source of such cells in histological sections a challenge [10]. Recent data have established that mouse microglia possess a unique transcriptional and proteomic signature [20,21], including the unique expression of purinergic receptor P2Y12 [20,22] and transmembrane protein 119 [23] on the cell surface, now enabling specific labelling with antibodies. Evidence also indicates there are likely to be several heterogeneous subsets of microglia with region-specific functions, with a recent report highlighting a unique subset of fractalkine receptor (CX3CR1)-positive microglia present in the subventricular zone (SVZ), rostral migratory stream, and olfactory bulb that lacked ‘typical’ microglial markers Iba1, isolectin B4, and CD68, and appeared to be involved in the migration and integration of adult SVZ-derived neural progenitors into the olfactory bulb [24]. Other researchers have also used bone marrow chimeras and promoter-driven transgenic animals to label resident and infiltrating cells separately, and have found that in many conditions, the two subsets of cells vary considerably in their ability to phagocytose pathological deposits of protein, recruit other immune cells via antigen presentation, and contribute to pathology via production of neurotoxic substances [6].
2.2. Non-Microglial Myeloid Cells: Location and Transport in and out of the CNS
In the periphery, immune cells can traffic between lymph nodes and target organs via the blood and lymphatic vessels [25]. However, endothelial cells of CNS blood vessels are connected by tight junctions, which tightly control the passage of nutrients and cells across the vessel walls, and, with the exception of the meninges [26,27], the brain does not appear to have conventional lymphatic vessels, with entry and drainage of interstitial fluid (ISF) and cerebrospinal fluid (CSF) largely along narrow bulk flow channels in basement membranes of parenchymal blood vessels that do not support the passage of cells [28,29,30,31], specifically referred to as paravascular influx of CSF and intramural perivascular ISF drainage by Engelhart and colleagues in [7]. Also, the interfaces between the brain parenchyma, the vasculature, and the CSF-filled subarachnoid space are lined with a layer of astrocytic endfeet, which under normal conditions form an additional barrier to the transport of immune cells into the parenchyma [32].
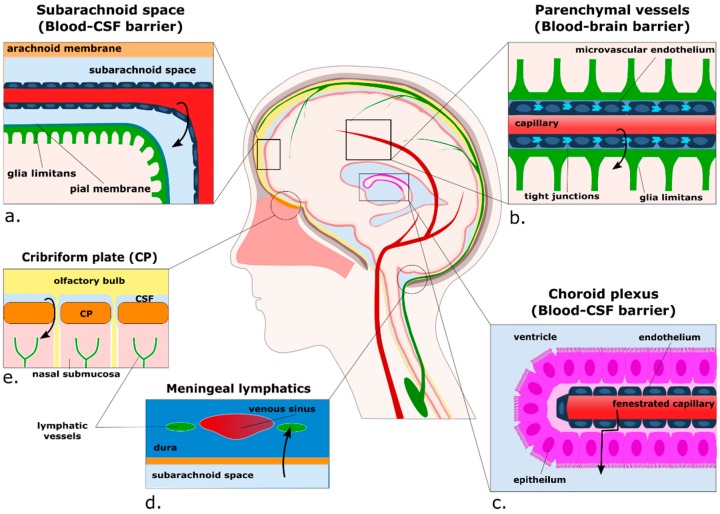
In the healthy CNS, non-microglial myeloid cells are found mostly within the subdural meninges, the choroid plexus, the CSF, and in the perivascular place between microvascular endothelial cells and the glia limitans of CNS blood vessels [28,33] (Figure 1). A recent fate-mapping study has uncovered that, contrary to previous belief, under normal conditions most macrophages in the perivascular and meningeal spaces derive from early fractalkine receptor (CX3CR1)-positive cells that migrate during embryogenesis and, like microglia, remain relatively stable once in their compartments [34]. The choroid plexus, by contrast, houses a population of shorter-lived cells that are gradually replaced in part by blood-borne monocytes. At this stage the functional relevance of these different subsets of macrophages is not known, but putative functions of non-parenchymal CNS myeloid cells as a whole include immune surveillance, regulation of CNS-specific T lymphocyte activity, and phagocytosis of molecules from the CNS parenchyma [5]. Interestingly, two recent studies using two-photon intravital microscopy have identified a small subset of cells residing in the juxtavascular parenchyma expressing either the classical dendritic cell marker CD11c or fractalkine receptor CX3CR1 with processes extended through the glia limitans into the perivascular space [35], or even further into the vascular lumen [36]. These reports extend on early identifications of microglia-like cells embedded in the glia limitans identified by electron microscopy [37]. Another proposed route for brain-to-peripheral communication pathway is the presentation of antigens to peripheral lymph nodes by myeloid cells migrating out of the CNS along with the CSF [7]. These routes include from the subarachnoid space through the cribriform plate into the lymphatics of the nasal submucosa [7,38], and the newly discovered meningeal lymphatic vessels, which are closely associated with venous sinuses in the dura mater and contain populations of immune cells in the steady state [26,27]. The relative contribution of these processes to presentation of antigen to peripheral lymph nodes has yet to be elucidated, but is likely to play an important role in the development of inflammatory disorders such as multiple sclerosis.
Figure 1.
Putative routes for the extravasation and egress of myeloid cells from the brain parenchyma. Cells can traffic from blood to the subarachnoid space in the meninges (a), where they may traffic into the Virchow–Robin spaces at the surface of the brain. Secondly, cells can reach the brain parenchyma via small vessels such as capillaries (b) and post-capillary venules, where they must pass the tight junctions of the microvascular endothelium and breach the glia limitans. Thirdly, cells can reach the cerebrospinal fluid (CSF) through the choroid plexus (c). Cells carrying antigens towards lymph nodes have been proposed to follow at least two routes out of the brain compartment; into lymphatic vessels located in the meninges (d), and with CSF through the cribriform plate alongside olfactory nerves (e). Arrows represent the proposed direction of cell trafficking.
3. Passing the Checkpoints to the Parenchyma: A Focus on the Neurovascular Unit
During certain disease states and in response to radiation-induced disruption of homeostasis, circulating myeloid cells migrate into the brain parenchyma and surrounding spaces, where they may participate in phagocytosis and proteolytic digestion of macromolecules such as amyloid beta [39], or initiate the recruitment of effector cells such as T cells towards sites of pathology [40]. Their entrance to these compartments involves receptor-mediated chemotaxis and extravasation across the blood vessel endothelium, where they may (1) pass through the leptomeningeal vessels and epithelial cells of surrounding choroid plexus into the CSF-containing ventricles, which flow into the subarachnoid space; (2) enter the subarachnoid space directly via the leptomeningeal vessels; or (3) enter the perivascular space of arteries or post-capillary venules bordered by the glia limitans [41,42]. In the case of the latter, infiltrating cells participate in receptor-mediated chemotaxis towards astrocyte-released chemokines such as chemokine ligand 2 (CCL2), and release proteolytic enzymes to facilitate their passage through the basement membranes of the glia limitans [43,44]. The following section will discuss the unique anatomy of the perivascular niche and the sequential steps followed by leukocytes to pass the cellular and molecular checkpoints from the blood into the brain.
3.1. Anatomy of the Perivascular Niche
The perivascular niche is a major site of influx of leukocytes during the induction of experimental autoimmune encephalitis (EAE) [7,40], and is an important site of amyloid-beta deposition in Alzheimer’s disease (AD) and cerebral amyloid angiopathy (CAA) [30,45], indicating this region is a key target for resolution of brain pathology. The perivascular niche is a multi-layered structure composed of numerous interdependent cell types, which varies according to vessel type and location [7]. The post-capillary venules are the main site of leukocyte extravasation into the inflamed CNS and are the primary site of “inflammatory cuffs” observed in models of EAE [40] (Figure 2). Nearby capillaries, by contrast, do not have a “perivascular space” as the basement membranes of endothelial and glial barriers are fused [7].
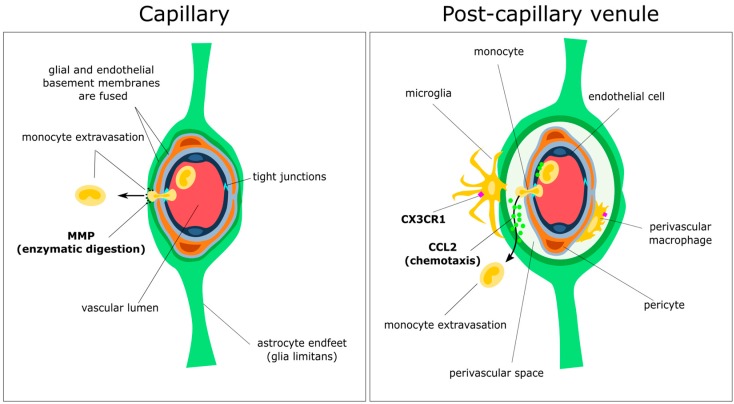
Figure 2.
Simplified schematic drawing of the anatomy and function of the perivascular niche. The blood–brain barrier consists of a layer of microvascular endothelial cells connected by selective tight-junctions that prevent the passage of most large molecules and cells. These junctions rapidly remodel to allow passage of leukocytes such as monocytes through to the glial and endothelial basement membranes. An important distinguishing feature between capillaries and post-capillary venules is the separation of glial and endothelial basement membranes in the latter (right), forming the perivascular space. Also embedded in the endothelial basement membrane are pericytes, a population of mesenchymal cells responsible for maintaining endothelial cell formation and function, and polarising astrocyte endfeet. Perivascular macrophages (labelled, right) may derive from embryonic precursors, or may arise from infiltrating monocytes that have passed through the post-capillary endothelium. To reach the CNS parenchyma, cells must pass through the glia limitans, consisting of astrocyte endfeet attached to a basement membrane (arrows). This is achieved in part by coordinated chemotaxis with chemokines such as chemokine ligand 2 (CCL2; right arrow), and enzymatic digestion of intact basement membrane by matrix metalloproteinases (MMPs; left arrow). Also pictured are rare fractalkine receptor (CX3CR1)-positive parenchymal cells that appear to extend processes through the glia limitans, possibly to sample antigen from the perivascular niche.
Enclosing the lumen of the post-capillary venule is a layer of specialised microvascular endothelial cells attached to a basement membrane connected by tight junctions intermingled with adherens junctions, which serve to regulate paracellular diffusion of ions and maintain cell–cell contacts, respectively [46]. The distinct morphology of the endothelial cells and associated zipper-like junctions serve to tightly regulate the movement of nutrients and cells by forming an extremely selective physicochemical barrier [46,47]. The tight junctions themselves are composed of a network of transmembrane proteins including occludin, claudins, and junctional adhesion molecules [47]. These proteins are organized and linked to the cortical actin cytoskeleton by intracellular scaffolding proteins such as zonula occludens-1. For a review of tight and adherens junction components and their roles in maintenance of the BBB, see [46]. Embedded in the endothelial basement membrane are pericytes, thought to be derived from neural crest cells in the neuroectoderm, and mesenchymal stem cells in the mesoderm [48]. Pericytes are identified based on the expression of a number of molecules including platelet-derived growth factor receptor-β (PDGFR-β) [49], regulator of g-protein signalling 5 (RGS5) [50], and Aminopeptidase N (CD13) [51,52]. More reliably, pericytes can be identified by their ultrastructural localization to the endothelial basement membrane in semi-thin electron microscopy sections [53]. Although a number of studies have suggested that cells expressing typical pericyte markers can migrate and differentiate into antigen-presenting cells in response to ischemia or brain injury [54,55,56,57], there is still the possibility that experimental conditions as such may induce the expression of the aforementioned ‘pericyte’ markers on myeloid cells [53]. Thus uncertainty remains over their lineage and differentiation capacity [55,58]. Of the many roles ascribed to pericytes, there is strong evidence for a crucial role in the formation and function of the BBB, participating in structural support of the mirovasculature, vasodynamic control, basement membrane synthesis and repair, organization of endothelial cells, and the polarization of astrocyte endfeet associated with the glia limitans [48,59,60]. Inside the perivascular space are a population of fractalkine receptor-positive perivascular macrophages derived from early embryonic progenitors [34]. Under certain circumstances, particularly following irradiation or injury, these cells may also be replaced by blood-borne progenitors [61,62,63,64,65]. Enveloping the perivascular space is the glia limitans, a barrier formed by astrocyte endfeet and surrounding basement membrane. This barrier is the final checkpoint for infiltrating immune cells to enter the brain parenchyma, and is a crucial regulator of inflammatory processes and dysfunction of nearby neural tissue. For a review of molecular mediators of astrocyte barrier function, see [66].
3.2. Initial Attraction: Monocyte–Endothelium Interactions
The migration of peripheral immune cells through the cerebral microvasculature follows the same general course as in the periphery, starting with selectin and adhesion molecule-mediated tethering and rolling of leukocytes to the endothelium, where they are activated, encounter integrins and immobilized chemokine ligands, and, if recognized by their cognate receptors, will tightly adhere to the endothelium and extend processes through the intercellular junctions to initiate diapedesis [39,67]. Although this remains to be confirmed in vivo, evidence from in vitro experiments indicates that leukocytes may extravasate across the BBB endothelium in a paracellular (between cells) or transcellular (through cells) fashion, with paracellular transport more common for monocytes and T cells, and an apparent preference for tri-cellular junctions over the bi-cellular route [68,69]. By contrast, neutrophils appear to favour the transcellular route through simulated blood-brain and blood-CSF barriers [70,71]. Diapedesis, the passage of cells through an intact endothelial barrier, is associated with rapid remodelling of tight junctions, such that as the leukocyte travels through the junction, the gaps are rapidly replaced with membrane from the endothelial lateral border recycling compartment, and the leukocyte itself forms transient connections with unligated cell adhesion molecules such as PECAM1-1 and CD99 [68]. This serves to preserve BBB integrity and prevents uncontrolled influx of solutes, proteins and other cells from the periphery during the process of extravasation. Thus the leukocyte has passed the first ‘checkpoint’ through to the perivascular space without significantly disrupting homeostasis.
3.3. Gatekeepers: Astrocytes and the Glia Limitans
Once inside the perivascular space, infiltrating immune cells may either remain within the perivascular space, or may go on to breach the glia limitans. This is particularly pertinent in the case of EAE, where activated T lymphocytes and monocyte-derived macrophages are known to breach the glia limitans of the spinal cord through proteolytic cleavage of dystroglycan, laminin, extracellular matrix, and other membrane proteins by matrix metalloproteinases (MMP-) 2, 7, and 9 [44]. Astrocytes also release MMP-7, though to a lesser extent than myeloid cells [72]. Nonetheless, this may indicate that the glia limitans itself is “inviting” the entry of immune cells from the perivascular space.
3.4. Spotlight on Coordinated Chemotaxis: The CCL2/CCR2 Pathway
This concept of “invited” migration to the brain parenchyma during demyelination is even better demonstrated with new insights gained regarding chemokine ligand 2 (CCL2), a crucial chemokine for egress of monocytes from bone marrow, and their transport through both endothelial and glial checkpoints to the brain parenchyma. Although many chemokines are expressed in the neurovascular unit in response to pathological insults, CCL2 has received considerable attention as the ligand and its receptor are essential for migration of the largest populations of leukocytes across the BBB in many mouse models of injury and disease [43,73,74,75,76,77,78], and expression of CCL2 in response to brain pathology is associated with production of many associated metalloproteinases and cytokines such as MMP-10, MMP-12, IL-1, and TNF-α [78]. Many cells in the neurovascular unit can release CCL2 in response to insult or injury. These include neurons [79,80], astrocytes [43,73], microglia [73,81], and microvascular endothelial cells [43]. The coordinated expression of chemokines from these cells guides the extravasation of leukocytes from the vasculature, and towards the site of action. For example, injured neurons in the superficial cervical ganglia express Ccl2 mRNA, but the ligand itself is found localized to nearby microvascular endothelial cells, in which the mRNA expression of Ccl2 is undetectable [79]. This appears to indicate that CCL2 has been released by the neurons and has travelled through the extracellular space to the endothelial cells, where they bind to receptors and are stabilized for presentation to infiltrating leukocytes. Another elegant study illustrates the relative contribution of CCL2 release from astrocytes and endothelial cells in the recruitment of leukocytes in EAE, by comparing astrocyte and endothelial-specific Ccl2-knockout mice [43]. When astrocyte CCL2 is absent, infiltrating cells become trapped in the perivascular space, reducing the severity of the pathology but maintaining the same time of onset. Conversely, if endothelial cell CCL2 is absent, the pathology is delayed but similar in severity, with an apparent stalling of leukocytes in the vascular lumen. In this case it is possible that the smaller chemokine gradients induced by the comparatively distant release of CCL2 by astrocytes (and possibly other cells) was able to preserve leukocyte influx to the parenchyma, albeit less efficiently. In summary, both major checkpoints of the neurovascular unit appear to participate in the coordinated influx of immune cells from the periphery. Whether this is a beneficial or detrimental response depends on the specific pathology, thus, chemokine-mediated leukocyte influx remains a complex, yet promising target for therapeutic intervention.
4. Myeloid Cells: The White Knights of CNS Therapy?
The field of neuro-immunology has undergone a major shift in recent decades, sparked by the recognition that infiltrating leukocytes and resident microglia can have both harmful and trophic effects on diseased neural tissue. Immunotherapies are gaining traction as promising strategies for treating a wide variety of diseases, with many clinical trials underway. However, therapeutic strategies are yet to mature, likely because the true complexity of immune dysfunction within the CNS during disease is not fully understood. In this section we will discuss the progress of research targeting the transplantation of the myeloid family of cells for the resolution of a selection of diseases affecting the CNS.
4.1. Genetic Deficiencies: Lysosomal/Peroxisomal Storage Diseases
Lysosomal storage diseases (LSDs) and peroxisomal storage diseases (PSDs) are rare, typically fatal, multisystem childhood disorders usually resulting from a genetic deficiency of one or more lysosomal/peroxisomal enzymes [82,83,84]. A major component of many LSDs/PSDs is progressive neurodegeneration associated with accumulation of macromolecular substrates and associated neuroimmune activity [85]. Bone marrow transplantation has been used for decades as a treatment for MLD/LSDs; however, outcomes remain suboptimal, with incomplete resolution of symptoms, graft-versus-host disease, and death still commonly reported [86]. In animal models, it is the F4/80+ myeloid cells that migrate into the brain parenchyma and phagocytose aberrant deposits of lipid and other substrates following bone marrow transplantation, indicating that they are likely to be the driver of therapeutic action [83,87,88,89,90]. Evidence from these experiments indicates it may be possible to enhance treatment to prevent the impairments in motor conduction associated with LSDs by transplanting gene-modified hematopoietic stem cells (HSCs) over-expressing the missing gene. Other experiments have shown that this effect is enhanced when gene expression is tied to the CD11b (myeloid-specific) promoter [91], in this case correcting both proteoglycan build-up and open-field mouse behaviour.
Furthermore, the first clinical trial of autologous HSC transplantation with lentivirally transduced, “supertherapeutic” ARSA gene expression in pre-symptomatic children with arylsulfatase A (ARSA) deficiency (the cause of metachromatic leukodystrophy) was recently completed, and appears to have been successful at preventing the demyelination associated with sulfatide accumulation for at least two years post-therapy [92,93]. This promising data indicates myeloid cells are likely to be a key target for gene therapy; however, longer term follow-up and larger studies will be needed to determine whether the strategy successfully prevents the progression of disease. Also of interest would be whether this strategy would be capable of arresting disease progression in patients already affected by the disease.
4.2. Neurodegenerative Diseases
Many neurodegenerative diseases are characterized by the aggregation of proteins and peptide fragments within the brain, and the impaired clearance of these products is hypothesized to underlie the pathogenesis of these diseases [53], although their role as initiators of disease remains controversial [94,95,96]. Several pharmaceutical companies are progressing through clinical trials utilizing targeted immunotherapy against aggregated protein products, either through vaccination or antibody administration, with limited evidence of success. Initial data suggests these therapies are unlikely to be a ‘magic bullet’ for neurodegeneration, as dramatic clinical improvement has yet to be shown in phase III trials, and side effects have been relatively common [97,98]. In this section we discuss the rationale and preclinical evidence behind targeted myeloid-based cell therapy for a selection of neurodegenerative disorders, which may have the potential to enhance the clearance of protein and peptide fragments from the CNS.
4.2.1. Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of dementia worldwide [99]. The hallmarks of AD are progressive loss of neurons and synapses, associated with the presence of amyloid beta (Aβ) plaques throughout the brain parenchyma and around blood vessels, and tau neurofibrillary tangles [100]. In common with other brain pathologies, models of AD are associated with the presence dystrophic microglia with characteristics of an activated state, such as an amoeboid morphology and expression of MHC antigens, but with unique hallmarks of dysfunction including ultrastructural signs of oxidative stress [101,102]. With the suggestion that dystrophic microglia accumulate over the course of ageing and in many cases of neurodegenerative disease, microglial age-related senescence was proposed as a key contributor to neurodegeneration. Microglial dysfunction appears to precede alterations in processing and subsequent aggregation of Aβ, and has even been suggested to underlie the disease itself [102,103,104]. In line with this theory, activation of complement cascade and aberrant pruning of synapses by dysfunctional microglia are early events preceding the appearance of overt pathology in some animal models of familial AD [105], and apparently occurs independently of neuronal protein aggregation [106]. Several animal models of familial AD have nonetheless shown that infiltrating monocyte-derived macrophages may be intimately involved in restricting disease progression, possibly via the phagocytic clearance of Aβ [107,108,109]. Despite the controversy, clearance of aggregated proteins remains a promising strategy for disease modification.
It has become evident that resident microglia and blood-derived macrophages behave differently in the presence of Aβ [45]. Although microglia appear to internalize as much Aβ as peripheral macrophages in vitro, subsequent lysosomal fragmentation is slow, incomplete, and easily overwhelmed by the presence of large amounts of protein [110]. Thus it is possible that infiltration of peripheral myeloid cells is a protective response to amyloid loading. Interestingly, common genetic risk factors indicate that impaired clearance of Aβ by macrophages may be a central part of disease pathogenesis. For example, rare mutations in the triggering receptor expressed on macrophages-2 (TREM2) gene are associated with an increased risk of developing neurodegenerative diseases including AD, and many mouse models of familial Alzheimer’s display alterations in Trem2 expression [111,112]. Although animal models report seemingly conflicting effects of eliminating or overexpressing Trem2 [113,114], and the relative contribution of peripheral and CNS-resident cells is yet to be investigated, most studies agree that the receptor (and by extension, myeloid cells) plays an important role in the response to deposition of Aβ in AD, likely though effects on cell surface transport and phagocytosis [115,116]. The most extensively recognized gene locus for AD risk is the APOE gene, with the APOE4 allele considered risk-inducing, and APOE2 generally considered protective [117]. Macrophages are known producers of apolipoprotein, and in vitro challenge of macrophages from AD transgenic mice expressing the three major isoforms of APOE indicates that the APOE2 allele may confer an enhanced ability to phagocytose Aβ on myeloid cells associated with increased expression of MMP-9 [118]. Additionally, transplantation of APOE3, but not APOE4 expressing bone marrow improves pathology and behavioural changes in double-transgenic AD mice in vivo [119]. Another strategy used in vivo was to disrupt transforming growth factor-beta (TGF-β)-mediated transcription regulation driven by the myeloid-specific CD11c promoter, which resulted in major attenuation of plaque formation (~90%) and improvement of behavioural deficits in Tg2756 AD mice [108]. This was associated with increased infiltration of macrophages around cerebral vessels and amyloid plaques in vivo, and enhanced phagocytosis of Aβ by macrophages in vitro. Overall it appears that enhancing the Aβ clearance capacity of macrophages by increasing lipid binding or proteolytic enzymes, or perhaps replacing senescent microglia with enhanced cells to avoid unwanted bystander damage, might become a strategy for CNS tissue regeneration.
Although current therapeutics approved for use in mild to moderate AD purportedly target neurons directly, several lines of evidence indicate the myeloid lineage of cells may be additional targets of these drugs. For example, acetylcholinesterase inhibitors have been used to enhance cholinergic neurotransmission, and have been modestly successful at delaying cognitive decline [120]. Both blood-borne macrophages and resident microglia possess alpha 7 nicotinic acetylcholinergic receptors, and their activation on microglial cells prevents Aβ-induced oxidative stress and facilitates clearance of Aβ in vitro [121,122]. Thus it is tempting to speculate that the immunomodulatory properties of these drugs may be contributing to their modest success. At present, a popular strategy for eliminating Aβ in clinical trials is targeted immunization involving either an active vaccine against specific Aβ fragments, or intravenous antibody infusion. The first vaccination trials with AN1792 were discontinued due to the induction of meningoencephalitis associated with T-cell infiltration in several patients [123], and treatment only elicited a sufficient antibody response in ~50% of patients [124]. Additionally, cerebral amyloid angiopathy persisted despite clearance of cortical plaques in more than one case [125,126]. Similar rates of response have been observed in trials of a newer vaccine designed to avoid the T-cell specific response observed in the AN1792 trial [127]. Given the limited response rates to active vaccination (potentially due to age-related immune senescence), direct administration of antibodies has been tested, but none of the phase III trials so far have demonstrated significant improvements in symptoms or slowing of cognitive decline [97,98]. Interestingly, both animal models and clinical trials have also demonstrated that CAA can persist after monoclonal antibody-mediated parenchymal clearance of Aβ [128,129]. Although this is likely to depend on the specific target peptide fragments, it does indicate that stimulating an endogenous immune response may not be sufficient to arrest the progression of vascular Aβ deposition once it has already progressed, and perhaps that the neurovascular unit may be a particularly important target for therapy in the future.
Although no transplantation trials appear to be in progress, one phase II trial is currently underway investigating use of Granulocyte-Macrophage Colony-Stimulating Factor (Sargramostim; GM-CSF) for the mobilization of hematopoietic progenitors in patients with AD (NCT01409915). Animal trials have indicated that improvements in amyloid load and cognitive function following this treatment is associated with increased density of Iba-1 positive cells around plaques [130]. Although the authors did not distinguish resident microglia from infiltrating myeloid cells, which also express Iba1, it is possible that increased influx or priming of peripheral myeloid cells is driving this response. Indeed, in vitro evidence suggests GM-CSF treatment of monocytes enhances their capacity to traffic across endothelial barriers resembling the BBB, and promotes the release of reactive oxygen species and interleukin-6 [131].
In summary, although the precise roles of specific myeloid subsets in the promotion or response to neurodegeneration are yet to be fully untangled, accumulating evidence indicates that myeloid cells from both within and outside the CNS have important roles to play in the modulation of neurodegenerative processes. Thus, the myeloid lineage remains a key target for therapeutic strategies, whether used to replace or support senescent microglia, or to facilitate clearance of aggregated proteins.
4.2.2. Amyotrophic Lateral Sclerosis (ALS)
ALS is a neurodegenerative disease characterized by the progressive loss of motor neurons in the cerebral cortex, brain and spinal cord, resulting in paralysis and eventual death [132,133]. Many gene mutations have been identified in familial cases, which has enabled the generation of transgenic models to investigate pathogenesis and treatment options [134]. Evidence from superoxide dismutase-1 (SOD1) mutant mice suggests that non-neuronal cells contribute substantially to the neurodegenerative process, and that myeloid cells in particular play an important role [135,136]. Although there appears to be some disagreement over the role of monocyte infiltration in ALS progression, it appears the phenotype of immune cells interacting with motor neurons changes throughout the course of disease, indicating they could be a useful target for therapy [132,137]. Initial evidence for this line of enquiry has come from a recent study, which has identified major alterations in composition and function of peripheral monocytes in the blood of 10 ALS patients compared to controls [138]. Researchers found blood from patients displayed an increase of classical, “inflammatory” CD14++CD16− monocytes relative to non-classical CD14+CD16++ cells. In vitro, the CD16− monocytes from these patients were significantly less efficient at phagocytosis of yeast-derived particles, and more inclined to adhere to collagen-coated chamber walls under shear stress. Post-mortem investigations also indicated an increase in migration of monocyte derived cells into the spinal cord of ALS patients. This raises the possibility that in ALS, neurodegeneration is accompanied by an inefficient innate immune response, which could be modulated by selective targeting of myeloid cells.
Clinical success for ALS has been limited. The drug most commonly used to address ALS, Riluzole, appears to prolong patient survival by several months on average, with only small improvements in motor function [139]. Riluzole is purported to reduce excitotoxicity associated with ALS via the inhibition of neuronal glutamate release, in part through its actions on voltage-dependent sodium channels [140]. Interestingly, a recent study has demonstrated that in vitro, Riluzole is capable of modulating responses of rat microglia to inflammatory stimuli, possibly via activation of two calcium-activated potassium channels, SK3 (KCa2.3) and SK4 (KCa3.1) [141]. Specifically, administration of the drug attenuated “pro-inflammatory” cytokine mRNA expression in response to lipopolysaccharide challenge, and enhanced expression of brain-derived neurotrophic factor (BDNF) mRNA following stimulation with interleukin-4. Although further study is required to identify the sites of action and therapeutic relevance of this effect, this study has highlighted a previously under-recognized molecular pathway for neuroprotection.
Although therapeutic strategies targeting the myeloid lineage are yet to gain significant attention in the clinic, several lines of evidence suggest it may gain traction in the future. For example, SOD1 mutant mice transplanted with bone marrow stimulated by granulocyte colony stimulating factor (G-CSF) were partially protected from motor neuron loss in the ventral horn of the spinal cord [142]. This was accompanied by an influx of myeloid cells with a “microglial” phenotype, and increased expression of neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and angiogenin. Similar results were obtained in double-transgenic, immune cell-deficient mSOD1G93A/PU.1−/− mice, in which transplantation with wild-type bone marrow was associated with a slowing of disease progression compared to controls transplanted with marrow from mSOD1G93A mice [136]. Also, a phase 1 trial using NP001 (sodium chlorite) to downregulate inflammatory pathways in myeloid cells was conducted recently based on promising preclinical results, with preliminary results suggesting it may slow disease progression [143]. If successful, this approach underscores the potential for myeloid cell-based therapies for ALS.
4.3. Autoimmune Diseases: Multiple Sclerosis
Multiple sclerosis (MS) is a heterogeneous, chronic demyelinating disease affecting the CNS, with unknown aetiology [144]. MS pathogenesis is characterized by early infiltration of leukocytes into the brain and spinal cord, leading to discrete lesions and loss of associated axons [47]. Although T cells play a central role in the progression of MS and its classical animal model experimental autoimmune encephalomyelitis (EAE), innate immune activity is critical for the progression and resolution of demyelination [145]. This involves marked but temporary monocyte extravasation across CNS gateways to the parenchyma [146], and secretion of cytotoxic factors such as inflammatory cytokines and reactive oxygen/nitrogen species by infiltrating inflammatory monocyte-derived macrophages during the initiation of demyelination (the effector phase) [147,148]. Factors important for the remyelination process include microglia/macrophage phagocytosis of debris and stimulation of oligodendrocyte progenitor cell differentiation, via clearance of axonal debris and release of neurotrophic molecules such as Activin A [147,149,150]. Also of note is the role of antigen presentation to primed T cells by CD11c+ (primarily dendritic) cells located at the meninges and perivascular spaces during the effector phase of EAE [151,152]. Historically, the distinction between resident and infiltrating myeloid cells has been complicated by overlapping phenotypic characteristics of reactive microglia and infiltrating monocyte-derived macrophages, as well as confounding effects associated with conditioning for transplantation of labelled blood-derived cells [153]. Recently, a study employing targeted expression of CCR2/CX3CR1 fluorescent reporters has uncovered distinct patterns of behaviour and associated gene expression of CCR2+ monocyte-derived macrophages and resident CX3CR1+ microglia during the induction phase of EAE [154]. Blood-derived cells were found in direct contact with nodes of Ranvier and adopted a distinctly pro-inflammatory gene expression profile, whereas microglia underwent marked downregulation of metabolic genes, made direct contact with macrophages but not with axons, and appeared to be taking up myelin debris left by the infiltrating cells. This raises the possibility that microglia may be particularly suited towards neuroprotection during the initiation of MS, and indicates their gene expression profile may uncover some targets that could be modified in blood-derived cells for therapeutic purposes. At this stage the contributions of myeloid subsets to the resolution of demyelination are less clear, although both resident and infiltrating cells appear to play a role in oligodendrocyte differentiation, and evidence points towards ‘M2’ polarization as beneficial [153]. The heterogeneous phenotypes of macrophages along the ‘M1-M2’ spectrum might be one of the reasons for such contradictory results in animal models of MS [155]. This complexity is reflected in a study of tissue from multiple sclerosis patients, in which a significant number of myeloid cells within chronic lesions expressed markers typical of both M1 and M2 states, namely CD40 and mannose receptor [156]. It could be argued that these descriptors of polarization states as M1/M2, or pro/anti-inflammatory are insufficient to properly characterize the parenchymal myeloid cells response to stimuli [157]. It is also possible that this heterogeneity reflects the developmental specialization of specific myeloid subsets, which has not yet been fully characterized.
Currently, the α4 integrin antibody natalizumab is used for management of relapsing-remitting and progressive multiple sclerosis, with its therapeutic mode of action purported to involve its inhibitory effect on T cell adhesion to vascular endothelium and subsequent extravasation into the brain parenchyma [158,159]. Monocytes also employ α4 integrins to cross the blood–brain barrier [160], indicating they would be affected in a similar manner to therapy. Unfortunately, natalizumab therapy carries a risk of potentially fatal progressive multifocal leukoencephalopathy caused by the reactivation of latent John Cunningham (JC) virus, making it unsuitable for patients with latent infection [161]. Interferon beta, an important immunomodulatory cytokine, has also been used to prevent or reduce the number of MS relapses [162]. Although its many mechanisms of action are not fully understood [163], in a rat model of EAE, interferon beta treatment was associated with a marked reduction of monocyte infiltration to the perivascular space and concurrent downregulation of adhesion molecule expression [164]. Studies have also shown that interferon beta can downregulate antigen presentation in cultured peripheral and CNS-resident APCs via reductions in MCHII expression and alterations in cytokine signalling [165], indicating its therapeutic activity may occur in the meninges, choroid plexus and perivascular spaces, which are crucial locations for antigen presentation to T lymphocytes in EAE [166]. Additionally, interferon beta appears to upregulate microglial clearance of myelin and axonal debris [167,168]. Although moderately successful, therapy with interferons is associated with numerous side effects, including a significantly increased risk of depression and suicidal behaviour [162,169]. This is likely due to the broad range of effects of supraphysiological systemic doses of an endogenous cytokine.
A number of clinical trials are currently underway utilizing the existing capabilities of immune cells to modulate immune response in MS. Firstly, a trial of autologous hematopoietic stem cell transplantation after non-myeloablative chemotherapy was recently conducted [170], with the aim to eliminate autoreactive T cells and re-establish self-tolerance. Early reports indicate improvements in symptoms and reduced frequency of relapses; however, this will need to be followed longer term to determine whether the effect persists well beyond the usual course of disease. Particularly of interest is whether ‘resetting’ the patient’s immune systems in this way is sufficient to halt reactivation of T lymphocytes, or whether the transplanted cells may require enhancement to resist influence from dysfunctional signalling outside of the hematopoietic compartment. A more targeted therapeutic approach currently under investigation involves the use of tolerogenic dendritic cells (tolDCs) for the suppression of cytotoxic T cell responses [171]. Although dendritic cells have long been recognized as potent antigen-presenting cells, it has only recently been discovered that they can also regulate autoreactive T cells via the induction of apoptosis, or by modulating their phenotype towards a T-regulatory (Treg) cell profile [172]. Despite challenges with inducing a stable tolerogenic phenotype in vitro, one laboratory has developed a method of inducing human and mouse tolDCs [173,174], and has successfully applied MOG-loaded tolDCs to a mouse model of EAE with promising results [174,175]. Further research, in particular the upcoming phase I clinical trial (NCT02618902), is anticipated with interest.
4.4. Brain Tumours: Glioma
Malignant gliomas are aggressive tumours arising from cells of astrocyte, oligodendrocyte or ependymal origin, comprising the most common form of malignant tumours on the CNS [176]. Prognosis is poor and recurrence is common, with chemotherapy, radiation, and surgical resection only modestly extending average survival times [177,178]. Gliomas evade detection by inducing robust local and systemic immunosuppression, and secreting immunosuppressive chemokines, prostaglandins, and other ligands to protect themselves against adaptive and innate immune responses [179]. This is associated with increases in circulating T regulatory cells (Tregs) [180] and myeloid-derived suppressor cells (MDSCs) [181], which are recruited to tumours by chemokines such as CCL2 released by tumour-associated microglia and macrophages [182]. Tumour-associated myeloid cells contribute significantly to the tumour mass, and have been proposed to promote tumour growth, survival, and invasion of surrounding tissues by producing growth factors, cytokines, and proteolytic enzymes [179,183]. Alkylating agents such as temozolomide (TMZ) are the standard therapy for glioblastoma, and commonly result in profound myelosuppression [184].
Although myeloid cells undergo a distinct phenotypic shift on contact with the tumour microenvironment, evidence suggests their activity may be required for protection against tumour progression. A number of studies have demonstrated beneficial effects of macrophage/microglial activation strategies such as Stat3 inhibitors [185] and Amphotericin B on protective responses to glioma. Another study demonstrated that ganciclovir-mediated reduction in CD11b+ macrophages led to increases in tumour volume in a syngeneic model of glioma, associated with reductions in TNF-expressing cells and lymphocytes [186]. It could be speculated that incomplete removal of myeloid cells in this study has spared particular subsets with especially detrimental effects on glioma in isolation. In support of this, two recent studies have investigated the role of brain resident CD11b+ cells in the contribution to glioma progression, and have shown that resident rather than infiltrating myeloid cells are the primary contributors to tumour growth and angiogenesis. In these studies, head-shielded irradiation with or without selective ablation of CD11b+ microglia via intraventricular ganciclovir infusion enabled the examination of relative contribution of resident and infiltrating myeloid cells in GFP+ bone marrow chimeras [187,188]. Brain-resident cells comprised the majority of tumour-associated macrophages, and their selective reduction slowed tumour growth and decreased blood vessel density. This suggests microglia may be particularly responsible for the progression of glioma, and raises the possibility that infiltrating myeloid cells are in fact protective. To confirm this hypothesis, further phenotypic characterization will be required to identify cells as microglia. Once the phenotypic and functional diversity of glioma-associated myeloid cells has been clarified, it may be possible to target specific subsets directly to enhance protective functions or mitigate dysfunctional interactions with the tumour microenvironment.
One strategy for overcoming robust immunosuppression in the glioma microenvironment is enhancing the presentation of tumour-associated antigen to T cells prior to encountering the tumour. In humans, this strategy has mostly revolved around dendritic cell-based vaccines, which were initiated for other cancers in the 1990s and have undergone various modifications to improve immunogenicity [189]. Currently there are dozens of phase I–II clinical trials employing vaccination with autologous dendritic cells loaded with whole tumour lysate, and purified or synthetic antigen peptides [190,191,192,193], with some indications of success associated with decreases in number of Treg cells following treatment [194]. However, as of yet none have been particularly successful at addressing the poor antigen-specific T cell response rates observed so far. This is possibly due to systemic immunosuppression caused by the glioblastoma itself [195]. In the future it may become possible to enhance dendritic cell function by altering genes involved in antigen presentation or response to systemic immunosuppression, or it may be necessary to target a different subset of cells for optimal response, such as tumour-associated macrophages/microglia [196]. Interestingly, transplantation of gene-modified hematopoietic stem cells (the precursors to infiltrating macrophages) has also been tested in humans, but only for the purpose of mediating the myeloablative side-effects of high dose chemotherapy [197,198]. Nonetheless, these studies demonstrate that HSC transplantation is feasible, which indicates that gene therapy involving HSCs may be a promising avenue for further investigation.
5. From Bench to Bedside: Targets for Future Research
Since the role of immune surveillance in the brain was first recognised, much has been learned about the role of myeloid cells in the initiation and resolution of CNS disorders. In particular, the past several years have seen the development of new, reliable methods of genetic modification that have greatly accelerated our understanding of the CNS during health and disease. Despite this, current strategies for harnessing the potential of the myeloid cell are yet to mature into reliable, efficacious treatments.
We suspect this is because we are only just beginning to understand the true heterogeneity of the hematopoietic system, particularly relating to cells found in the neurovascular unit. Despite recent insights regarding the ontogeny and phenotype of these cells, many researchers continue to refer to all phagocytic cells in the CNS as “microglia”. Therefore readers are strongly encouraged to evaluate the literature regarding neuro-inflammation and microglial activation with care, and to refrain from equating microglia and CNS macrophages in their own research. This can now be achieved relatively easily, as many reporter systems are available [10], and the transcriptional profiles of the two major subsets are well characterized [22,23,24,34]. Additionally, novel methods of depleting specific subsets of cells are under development [199], which may assist with the functional characterization of the heterogeneous subsets of microglia and other myeloid cells without confounding effects of irradiation on brain tissue, for example [200]. Additionally, a closer examination of the heterogeneous states between the two poles of M1 and M2 activation in macrophages is needed to understand which factors are most desirable for neuroprotection. M2 activation is generally considered the desirable, neurotrophic phenotype, and M1 macrophages are thought to encourage neurodegeneration. However, growing evidence suggests that both phenotypes, or perhaps an intermediary state, are required for different phases of the neuro-immune response [201]. In addition, this schema may be particularly inappropriate for characterizing responses of resident microglia to pathological insults [157]. Undoubtedly, progress in fate mapping and identification of the various subsets of myeloid cells will enable us to explore this issue further.
As cell-based therapies enter the clinic, new strategies for monitoring transplanted cells in vivo will be required. The capacity to monitor the spatial distribution of myeloid cell homing will enable clinicians to monitor the co-localization of transplanted cells with existing biomarkers of pathology, which may enable them to examine outcomes earlier than is currently possible. The development of new imaging modalities and reporter systems has progressed far in animal trials; however, challenges associated with retention of labels, sensitivity, long-term safety, and viability of reporters have limited its use in the clinic to date [202]. Until this has been optimized, clinicians may benefit from the use of existing biomarkers of neuro-immune activity such as translocator protein (18 kDa, TSPO) expression for the localization of activated microglia and infiltrating myeloid cells during disease and therapy [203]. Positron emission tomography using TSPO ligands as markers of microglial activation have been investigated for use in neurodegenerative diseases and multiple sclerosis [204,205], and are under investigation for prediction and monitoring of disease progression [203,206]. Molecular imaging with TSPO ligands has also been applied for non-invasive monitoring of glioma in a null-background mouse model [207], and is under consideration for monitoring of key events in glioma progression in humans [208]. Future work should focus on novel applications for existing non-invasive imaging techniques, or identify additional strategies for non-invasive monitoring of cell trafficking and associated neuro-immune activity.
Moving forward, myeloid-based cellular therapy appears to be heading in three general directions: the enhancement of de novo immunomodulatory functions via in vitro stimulation with drugs, peptides or antigens; the insertion or removal of genetic information to enhance neuroprotection or eliminate neurodegenerative responses; or use as a “Trojan horse” for microvesicles containing therapeutic molecules, enzymes or gene vectors intended for neurons [209,210] (Figure 3). In the gene modification arena, optimized methods including lentiviral/adenoviral vectors and, most recently, customizable nucleases such as zinc finger nucleases [211] and CRIPSR-cas9 [212] have recently been developed, although it remains to be seen whether the efficiency, stability, and long-term safety of these systems are sufficient for widespread use [213]. Likewise, microvesicle and nanoparticle-loaded cell delivery is beginning to receive attention as a viable method of CNS therapeutic delivery, with initial trials indicating it is possible to target the CNS with loaded macrophages [214]. Further study will likely be needed to establish the optimal components and safe clearance of these delivery vehicles.
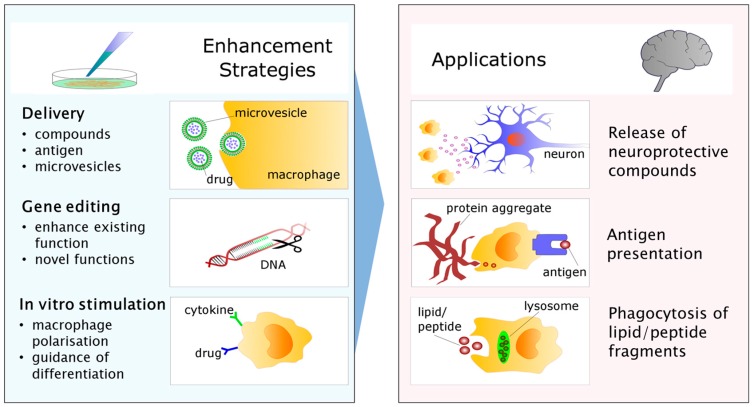
Figure 3.
Strategies for enhancing myeloid cell therapy for the CNS. The phagocytic capacity of myeloid cells may be adapted for use as delivery vehicles for compounds, antigens, or microvesicles loaded with therapeutic substances destined for the brain. Pictured is a macrophage in the process of taking up drug-loaded nanoparticles intended for delivery into the CNS. Gene editing enables the up/downregulation of existing functions to enhance protective, or eliminate damaging responses to disease. It is also possible to introduce expression of enzymes not usually present in these cells through this process. Another strategy for guiding myeloid cell-based therapies in the CNS involves in vitro manipulation of polarisation or differentiation, for example with cytokines or drugs known to guide the cells into a more neuroprotective phenotype. As a result of in vitro manipulation, myeloid cells returned to the circulation may then be better capable of ameliorating brain pathology by one of several mechanisms. For example, CNS-infiltrating myeloid cells may be better equipped to release compounds with growth or survival-promoting effects on nearby diseased neurons. Alternatively, it may be possible to enhance the capacity of infiltrating cells to present brain-derived antigens to the adaptive immune system, thus enhancing clearance of unwanted protein products. Myeloid cells may also be used to enhance the clearance of excess waste or diseased tissue independently via phagocytosis.
In summary, much progress has been made in the understanding of myeloid cell function. However, much more remains to be learned regarding the functional and associated molecular heterogeneity of myeloid subsets and their transport in and out of the brain. Current advances in neuro-immunology, genetics, and biotechnology hold great potential for the treatment of some of the most persistent and devastating illnesses affecting the CNS. We anticipate many new insights will be gained as a result of these efforts.
Acknowledgments
Richard Banati gratefully acknowledges support as a Distinguished Researcher at the Australian Nuclear Science and Technology Organisation.
Abbreviations
| CNS | Central nervous system |
| BBB | Blood–brain barrier |
| CSF | Cerebrospinal fluid |
| ISF | Interstitial fluid |
| EAE | Experimental autoimmune encephalitis |
| CAA | Cerebral amyloid angiopathy |
| AD | Alzheimer’s disease |
| MS | Multiple sclerosis |
| LSD | Lysosomal storage disease |
| TSPO | Translocator protein (18kDa) |
| HSC | Hematopoietic stem cell |
Author Contributions
Meredith Harrison-Brown drafted the manuscript; Richard Banati and Guo-Jun Liu critically revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Collins P.Y., Patel V., Joestl S.S., March D., Insel T.R., Daar A.S. Grand challenges in global mental health: A consortium of researchers, advocates and clinicians announces here research priorities for improving the lives of people with mental illness around the world, and calls for urgent action and investment. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fineberg N.A., Haddad P.M., Carpenter L., Gannon B., Sharpe R., Young A.H., Joyce E., Rowe J., Wellsted D., Nutt D.J., et al. The size, burden and cost of disorders of the brain in the UK. J. Psychopharmacol. 2013;27:761–770. doi: 10.1177/0269881113495118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittchen H.U., Jacobi F., Rehm J., Gustavsson A., Svensson M., Jönsson B., Olesen J., Allgulander C., Alonso J., Faravelli C., et al. The size and burden of mental disorders and other disorders of the brain in europe 2010. Eur. Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickey W.F. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 6.Prinz M., Priller J., Sisodia S.S., Ransohoff R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt B., Carare R.O., Bechmann I., Flügel A., Laman J.D., Weller R.O. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016;132:317–338. doi: 10.1007/s00401-016-1606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziv Y., Ron N., Butovsky O., Landa G., Sudai E., Greenberg N., Cohen H., Kipnis J., Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 9.Menasria R., Canivet C., Piret J., Boivin G. Infiltration pattern of blood monocytes into the central nervous system during experimental herpes simplex virus encephalitis. PLoS ONE. 2015;10:e0145773. doi: 10.1371/journal.pone.0145773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greter M., Lelios I., Croxford A.L. Microglia versus myeloid cell nomenclature during brain inflammation. Front. Immunol. 2015;6:249. doi: 10.3389/fimmu.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampron A., Lessard M., Rivest S. Effects of myeloablation, peripheral chimerism, and whole-body irradiation on the entry of bone marrow-derived cells into the brain. Cell Transplant. 2012;21:1149–1159. doi: 10.3727/096368911X593154. [DOI] [PubMed] [Google Scholar]
- 12.Yona S., Jung S. Monocytes: Subsets, origins, fates and functions. Curr. Opin. Hematol. 2010;17:53–59. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- 13.Chow A., Brown B.D., Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat. Rev. Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 14.Ginhoux F., Merad M. Microglia arise from extra-embryonic yolk sac primitive progenitors. Med. Sci. 2011;27:719. doi: 10.1051/medsci/2011278013. [DOI] [PubMed] [Google Scholar]
- 15.Kierdorf K., Erny D., Goldmann T., Sander V., Schulz C., Perdiguero E.G., Wieghofer P., Heinrich A., Riemke P., Holscher C., et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 16.Schulz C., Perdiguero E.G., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S.E.W., Pollard J.W., et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 17.Mildner A., Schmidt H., Nitsche M., Merkler D., Hanisch U.-K., Mack M., Heikenwalder M., Bruck W., Priller J., Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 18.Kierdorf K., Katzmarski N., Haas C.A., Prinz M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS ONE. 2013;8:e58544. doi: 10.1371/journal.pone.0058544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 20.Butovsky O., Jedrychowski M.P., Moore C.S., Cialic R., Lanser A.J., Gabriely G., Koeglsperger T., Dake B., Wu P.M., Doykan C.E., et al. Identification of a unique TGF-β dependent molecular and functional signature in microglia. Nat. Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beutner C., Linnartz-Gerlach B., Schmidt S.V., Beyer M., Mallmann M.R., Staratschek-Jox A., Schultze J.L., Neumann H. Unique transcriptome signature of mouse microglia. Glia. 2013;61:1429–1442. doi: 10.1002/glia.22524. [DOI] [PubMed] [Google Scholar]
- 22.Moore C.S., Ase A.R., Kinsara A., Rao V.T.S., Michell-Robinson M., Leong S.Y., Butovsky O., Ludwin S.K., Séguéla P., Bar-Or A., et al. P2Y12 expression and function in alternatively activated human microglia. Neurol. Neuroimmunol. Neuroinflamm. 2015;2:e80. doi: 10.1212/NXI.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Fernhoff N.B., Mulinyawe S.B., Bohlen C.J., Adil A., Tucker A., et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro Xavier A.L., Kress B.T., Goldman S.A., Lacerda de Menezes J.R., Nedergaard M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J. Neurosci. 2015;35:11848–11861. doi: 10.1523/JNEUROSCI.1217-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesler C.T., Liao S., Munn L.L., Padera T.P. Lymphatic vessels in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013;5:111–124. doi: 10.1002/wsbm.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212:991. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhardt B., Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: Function and dysfunction. Semin. Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 29.Carare R.O., Bernardes-Silva M., Newman T.A., Page A.M., Nicoll J.A.R., Perry V.H., Weller R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 30.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., et al. A paravascular pathway facilitates csf flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4:1–11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iliff J.J., Wang M., Zeppenfeld D.M., Venkataraman A., Plog B.A., Liao Y., Deane R., Nedergaard M. Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain. J. Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shechter R., London A., Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: Absolute barriers versus educational gates. Nat. Rev. Immunol. 2013;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- 33.Chinnery H.R., Ruitenberg M.J., McMenamin P.G. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J. Neuropathol. Exp. Neurol. 2010;69:896–909. doi: 10.1097/NEN.0b013e3181edbc1a. [DOI] [PubMed] [Google Scholar]
- 34.Goldmann T., Wieghofer P., Jordao M.J.C., Prutek F., Hagemeyer N., Frenzel K., Amann L., Staszewski O., Kierdorf K., Krueger M., et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prodinger C., Bunse J., Krüger M., Schiefenhövel F., Brandt C., Laman J.D., Greter M., Immig K., Heppner F., Becher B., et al. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445–458. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 36.Barkauskas D.S., Evans T.A., Myers J., Petrosiute A., Silver J., Huang A.Y. Extravascular CX3CR1+ cells extend intravascular dendritic processes into intact central nervous system vessel lumen. Microsc. Microanal. 2013;19:778–790. doi: 10.1017/S1431927613000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassmann H., Zimprich F., Vass K., Hickey W.F. Microglial cells are a component of the perivascular glia limitans. J. Neurosci. Res. 1991;28:236–243. doi: 10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- 38.Kida S., Pantazis A., Weller R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 39.Hohsfield L.A., Humpel C. Migration of blood cells to β-amyloid plaques in alzheimer’s disease. Exp. Gerontol. 2015;65:8–15. doi: 10.1016/j.exger.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood–brain barrier. J. Neural Transm. 2006;113:477–485. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- 41.Williams J.L., Holman D.W., Klein R.S. Chemokines in the balance: Maintenance of homeostasis and protection at cns barriers. Front. Cell. Neurosci. 2014;8:336. doi: 10.3389/fncel.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 43.Paul D., Ge S., Lemire Y., Jellison E.R., Serwanski D.R., Ruddle N.H., Pachter J.S. Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J. Neuroinflamm. 2014;11:10. doi: 10.1186/1742-2094-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal S., Anderson P., Durbeej M., van Rooijen N., Ivars F., Opdenakker G., Sorokin L.M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai A.Y., McLaurin J. Clearance of amyloid-β peptides by microglia and macrophages: The issue of what, when and where. Fut. Neurol. 2012;7:165–176. doi: 10.2217/fnl.12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tietz S., Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015;209:493. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larochelle C., Alvarez J.I., Prat A. How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 48.Winkler E.A., Bell R.D., Zlokovic B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler E.A., Bell R.D., Zlokovic B.V. Pericyte-specific expression of PDGF β receptor in mouse models with normal and deficient PDGF β receptor signaling. Mol. Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nisancioglu M.H., Mahoney W.M., Kimmel D.D., Schwartz S.M., Betsholtz C., Genové G. Generation and characterization of rgs5 mutant mice. Mol. Cell. Biol. 2008;28:2324–2331. doi: 10.1128/MCB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunz J., Krause D., Kremer M., Dermietzel R. The 140-kDa protein of blood-brain barrier-associated pericytes is identical to aminopeptidase N. J. Neurochem. 1994;62:2375–2386. doi: 10.1046/j.1471-4159.1994.62062375.x. [DOI] [PubMed] [Google Scholar]
- 52.Trost A., Lange S., Schroedl F., Bruckner D., Motloch K.A., Bogner B., Kaser-Eichberger A., Strohmaier C., Runge C., Aigner L., et al. Brain and retinal pericytes: Origin, function and role. Front. Cell. Neurosci. 2016 doi: 10.3389/fncel.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krueger M., Bechmann I. Cns pericytes: Concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 54.Sakuma R., Kawahara M., Nakano-Doi A., Takahashi A., Tanaka Y., Narita A., Kuwahara-Otani S., Hayakawa T., Yagi H., Matsuyama T., et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J. Neuroinflamm. 2016;13:57. doi: 10.1186/s12974-016-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kovac A., Erickson M.A., Banks W.A. Brain microvascular pericytes are immunoactive in culture: Cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J. Neuroinflamm. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakagomi T., Nakano-Doi A., Kawamura M., Matsuyama T. Do vascular pericytes contribute to neurovasculogenesis in the central nervous system as multipotent vascular stem cells? Stem Cells Dev. 2015;24:1730–1739. doi: 10.1089/scd.2015.0039. [DOI] [PubMed] [Google Scholar]
- 57.Özen I., Deierborg T., Miharada K., Padel T., Englund E., Genové G., Paul G. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128:381–396. doi: 10.1007/s00401-014-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armulik A., Genové G., Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Prasad S., Cucullo L. Pericytes and astrocytes crosstalk: Understanding perivascular synergism at the bbb. FASEB J. 2013;27:lb720. [Google Scholar]
- 60.Armulik A., Genove G., Mae M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 61.Vallières L., Sawchenko P.E. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J. Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bechmann I., Priller J., Kovac A., Bontert M., Wehner T., Klett F.F., Bohsung J., Stuschke M., Dirnagl U., Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur. J. Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 63.Bechmann I., Kwidzinski E., Kovac A.D., Simbürger E., Horvath T., Gimsa U., Dirnagl U., Priller J., Nitsch R. Turnover of rat brain perivascular cells. Exp. Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y., Jorstad N.L., Shiao C., Cherne M.K., Khademi S.B., Montine K.S., Montine T.J., Keene C.D. Perivascular, but not parenchymal, cerebral engraftment of donor cells after non-myeloablative bone marrow transplantation. Exp. Mol. Pathol. 2013;95:7–17. doi: 10.1016/j.yexmp.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hickey W.F., Kimura H. Perivascular microglial cells of the cns are bone marrow-derived and present antigen in vivo. Science. 1988;239:290. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 66.Sofroniew M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Man S., Ubogu E.E., Ransohoff R.M. Inflammatory cell migration into the central nervous system: A few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winger R.C., Koblinski J.E., Kanda T., Ransohoff R.M., Muller W.A. Rapid remodeling of tight junctions during paracellular diapedesis in a human model of the blood–brain barrier. J. Immunol. 2014;193:2427–2437. doi: 10.4049/jimmunol.1400700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burns A.R., Walker D.C., Brown E.S., Thurmon L.T., Bowden R.A., Keese C.R., Simon S.I., Entman M.L., Smith C.W. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J. Immunol. 1997;159:2893–2903. [PubMed] [Google Scholar]
- 70.Von Wedel-Parlow M., Schrot S., Lemmen J., Treeratanapiboon L., Wegener J., Galla H.J. Neutrophils cross the BBB primarily on transcellular pathways: An in vitro study. Brain Res. 2011;1367:62–76. doi: 10.1016/j.brainres.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 71.Wewer C., Seibt A., Wolburg H., Greune L., Schmidt M.A., Berger J., Galla H.J., Quitsch U., Schwerk C., Schroten H., et al. Transcellular migration of neutrophil granulocytes through the blood-cerebrospinal fluid barrier after infection with streptococcus suis. J. Neuroinflamm. 2011;8:51. doi: 10.1186/1742-2094-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buhler L.A., Samara R., Guzman E., Wilson C.L., Krizanac-Bengez L., Janigro D., Ethell D.W. Matrix metalloproteinase-7 facilitates immune access to the CNS in experimental autoimmune encephalomyelitis. BMC Neurosci. 2009;10:1–12. doi: 10.1186/1471-2202-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babcock A.A., Kuziel W.A., Rivest S., Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J. Neurosci. 2003;23:7922. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsieh C.L., Niemi E.C., Wang S.H., Lee C.C., Bingham D., Zhang J., Cozen M.L., Charo I., Huang E.J., Liu J., et al. CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J. Neurotrauma. 2014;31:1677–1688. doi: 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang D., Wang J., Kivisakk P., Rollins B.J., Ransohoff R.M. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific t helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dogan R.-N.E., Elhofy A., Karpus W.J. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of tnf- and inos-expressing macrophages and myeloid dendritic cells. J. Immunol. 2008;180:7376–7384. doi: 10.4049/jimmunol.180.11.7376. [DOI] [PubMed] [Google Scholar]
- 77.Stowe A.M., Wacker B.K., Cravens P.D., Perfater J.L., Li M.K., Hu R., Freie A.B., Stüve O., Gidday J.M. CCL2 upregulation triggers hypoxic preconditioning-induced protection from stroke. J. Neuroinflamm. 2012;9:33. doi: 10.1186/1742-2094-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toft-Hansen H., Buist R., Sun X.-J., Schellenberg A., Peeling J., Owens T. Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J. Immunol. 2006;177:7242–7249. doi: 10.4049/jimmunol.177.10.7242. [DOI] [PubMed] [Google Scholar]
- 79.Schreiber R.C., Krivacic K., Kirby B., Vaccariello S.A., Wei T., Ransohoff R.M., Zigmond R.E. Monocyte chemoattractant protein (MCP)-1 is rapidly expressed by sympathetic ganglion neurons following axonal injury. NeuroReport. 2001;12:601–606. doi: 10.1097/00001756-200103050-00034. [DOI] [PubMed] [Google Scholar]
- 80.Flügel A., Hager G., Horvat A., Spitzer C., Singer G.M.A., Graeber M.B., Kreutzberg G.W., Schwaiger F.W. Neuronal MCP-1 expression in response to remote nerve injury. J. Cereb. Blood Flow Metab. 2001;21:69–76. doi: 10.1097/00004647-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 81.D’Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factorα signaling during peripheral organ inflammation. J. Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parenti G., Andria G., Ballabio A. Lysosomal storage diseases: From pathophysiology to therapy. Annu. Rev. Med. 2015;66:471–486. doi: 10.1146/annurev-med-122313-085916. [DOI] [PubMed] [Google Scholar]
- 83.Krivit W., Sung J.H., Shapiro E.G., Lockman L.A. Microglia: The effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transpl. 1995;4:385–392. doi: 10.1016/0963-6897(95)00021-O. [DOI] [PubMed] [Google Scholar]
- 84.Platt F.M., Boland B., van der Spoel A.C. Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012;199:723. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Archer L.D., Langford-Smith K.J., Bigger B.W., Fildes J.E. Mucopolysaccharide diseases: A complex interplay between neuroinflammation, microglial activation and adaptive immunity. J. Inherit. Metab. Dis. 2014;37:1–12. doi: 10.1007/s10545-013-9613-3. [DOI] [PubMed] [Google Scholar]
- 86.Cartier N., Aubourg P. Hematopoietic stem cell gene therapy in hurler syndrome, globoid cell leukodystrophy, metachromatic leukodystrophy and X-adrenoleukodystrophy. Curr. Opin. Mol. Ther. 2008;10:471–478. [PubMed] [Google Scholar]
- 87.Krall W.J., Challita P.M., Perlmutter L.S., Skelton D.C., Kohn D.B. Cells expressing human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation. Blood. 1994;83:2737–2748. [PubMed] [Google Scholar]
- 88.Biffi A., de Palma M., Quattrini A., del Carro U., Amadio S., Visigalli I., Sessa M., Fasano S., Brambilla R., Marchesini S., et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J. Clin. Investig. 2004;113:1118–1129. doi: 10.1172/JCI200419205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyake N., Miyake K., Karlsson S., Shimada T. Successful treatment of metachromatic leukodystrophy using bone marrow transplantation of HoxB4 overexpressing cells. Mol. Ther. 2010;18:1373–1378. doi: 10.1038/mt.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng Y., Rozengurt N., Ryazantsev S., Kohn D.B., Satake N., Neufeld E.F. Treatment of the mouse model of mucopolysaccharidosis I with retrovirally transduced bone marrow. Mol. Genet. Metab. 2003;79:233–244. doi: 10.1016/S1096-7192(03)00116-1. [DOI] [PubMed] [Google Scholar]
- 91.Sergijenko A., Langford-Smith A., Liao A.Y., Pickford C.E., McDermott J., Nowinski G., Langford-Smith K.J., Merry C.L.R., Jones S.A., Wraith J.E., et al. Myeloid/microglial driven autologous hematopoietic stem cell gene therapy corrects a neuronopathic lysosomal disease. Mol. Ther. 2013;21:1938–1949. doi: 10.1038/mt.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S., et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 93.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I., Morena F., Calabria A., et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: An ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 94.Caughey B., Lansbury P.T. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 95.Winklhofer K.F., Tatzelt J., Haass C. The two faces of protein misfolding: Gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Treusch S., Cyr D.M., Lindquist S. Amyloid deposits: Protection against toxic protein species? Cell Cycle. 2009;8:1668–1674. doi: 10.4161/cc.8.11.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agadjanyan M.G., Petrovsky N., Ghochikyan A. A fresh perspective from immunologists and vaccine researchers: Active vaccination strategies to prevent and reverse alzheimer’s disease. Alzheimer’s Dement. 2015;11:1246–1259. doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P., Ferris S., et al. Two phase 3 trials of bapineuzumab in mild-to-moderate alzheimer’s disease. N. Engl. J. Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayeux R. Handbook of Clinical Neurology. Volume 89. Elsevier; Amsterdam, The Netherlands: 2008. Alzheimer’s disease: Epidemiology; pp. 195–205. [DOI] [PubMed] [Google Scholar]
- 100.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 101.Bisht K., Sharma K.P., Lecours C., Sanchez M.G., El Hajj H., Milior G., Olmos-Alonso A., Gomez-Nicola D., Luheshi G., Vallieres L., et al. Dark microglia: A new phenotype predominantly associated with pathological states. Glia. 2016;64:826–839. doi: 10.1002/glia.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Streit W.J., Braak H., Xue Q.-S., Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Streit W.J. Microglial senescence: Does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Streit W.J., Xue Q.-S., Tischer J., Bechmann I. Microglial pathology. Acta Neuropathol. Commun. 2014;2:142. doi: 10.1186/s40478-014-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., et al. Complement and microglia mediate early synapse loss in alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Audoy-Rémus J., Bozoyan L., Dumas A., Filali M., Lecours C., Lacroix S., Rivest S., Tremblay M.-E., Vallières L. Gpr84 deficiency reduces microgliosis, but accelerates dendritic degeneration and cognitive decline in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2015;46:112–120. doi: 10.1016/j.bbi.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 107.Simard A.R., Soulet D., Gowing G., Julien J.-P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 108.Town T., Laouar Y., Pittenger C., Mori T., Szekely C.A., Tan J., Duman R.S., Flavell R.A. Blocking tgf-β–smad2/3 innate immune signaling mitigates alzheimer-like pathology. Nat. Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naert G., Rivest S. Cc chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Majumdar A., Chung H., Dolios G., Wang R., Asamoah N., Lobel P., Maxfield F.R. Degradation of fibrillar forms of Alzheimer’s amyloid β-peptide by macrophages. Neurobiol. Aging. 2008;29:707–715. doi: 10.1016/j.neurobiolaging.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S.K., Younkin S., et al. Trem2 variants in Alzheimer’s disease. N. Engl. J. Med. 2012;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez Murcia J.D., Schmutz C., Munger C., Perkes A., Gustin A., Peterson M., Ebbert M.T.W., Norton M.C., Tschanz J.T., Munger R.G., et al. Assessment of TREM2 rs75932628 association with Alzheimer’s disease in a population-based sample: The cache county study. Neurobiol. Aging. 2013;34:2889.e2811–2889.e2813. doi: 10.1016/j.neurobiolaging.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang T., Tan L., Zhu X.-C., Zhang Q.-Q., Cao L., Tan M.-S., Gu L.-Z., Wang H.-F., Ding Z.-Z., Zhang Y.-D., et al. Upregulation of trem2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39:2949–2962. doi: 10.1038/npp.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jay T.R., Miller C.M., Cheng P.J., Graham L.C., Bemiller S., Broihier M.L., Xu G., Margevicius D., Karlo J.C., Sousa G.L., et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015;212:287. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colonna M., Wang Y. Trem2 variants: New keys to decipher alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016;17:201–207. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- 116.Kleinberger G., Yamanishi Y., Suárez-Calvet M., Czirr E., Lohmann E., Cuyvers E., Struyfs H., Pettkus N., Wenninger-Weinzierl A., Mazaheri F., et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 2014;6:243–286. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 117.Keage H.A.D., Matthews F.E., Yip A., Gao L., McCracken C., McKeith I.G., Rubinsztein D.C., Brayne C., Function M.R.C.C., Ageing S. APOE and ACE polymorphisms and dementia risk in the older population over prolonged follow-up: 10 years of incidence in the MRC CFA study. Age Ageing. 2010;39:104–111. doi: 10.1093/ageing/afp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao L., Lin S., Bales K.R., Gelfanova V., Koger D., DeLong C., Hale J., Liu F., Hunter J.M., Paul S.M. Macrophage-mediated degradation of β-amyloid via an apolipoprotein E isoform-dependent mechanism. J. Neurosci. 2009;29:3603–3612. doi: 10.1523/JNEUROSCI.5302-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang Y., Cudaback E., Jorstad N.L., Hemingway J.F., Hagan C.E., Melief E.J., Li X., Yoo T., Khademi S.B., Montine K.S., et al. APOE3, but not APOE4, bone marrow transplantation mitigates behavioral and pathological changes in a mouse model of Alzheimer disease. Am. J. Pathol. 2013;183:905–917. doi: 10.1016/j.ajpath.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ibach B., Haen E. Acetylcholinesterase inhibition in Alzheimer’s disease. Curr. Pharm. Des. 2004;10:231–251. doi: 10.2174/1381612043386509. [DOI] [PubMed] [Google Scholar]
- 121.Takata K., Kitamura Y., Saeki M., Terada M., Kagitani S., Kitamura R., Fujikawa Y., Maelicke A., Tomimoto H., Taniguchi T., et al. Galantamine-induced amyloid-β clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J. Biol. Chem. 2010;285:40180–40191. doi: 10.1074/jbc.M110.142356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moon J.H., Kim S.Y., Lee H.G., Kim S.U., Lee Y.B. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar β amyloid peptide (1-42)-stimulated microglia. Exp. Mol. Med. 2008;40:11–18. doi: 10.3858/emm.2008.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orgogozo J.-M., Gilman S., Dartigues J.-F., Laurent B., Puel M., Kirby L.C., Jouanny P., Dubois B., Eisner L., Flitman S., et al. Subacute meningoencephalitis in a subset of patients with ad after aβ42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.WNL.0000073623.84147.A8. [DOI] [PubMed] [Google Scholar]
- 124.Bayer A.J., Bullock R., Jones R.W., Wilkinson D., Paterson K.R., Jenkins L., Millais S.B., Donoghue S. Evaluation of the safety and immunogenicity of synthetic aβ42 (an1792) in patients with ad. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 125.Nicoll J.A.R., Barton E., Boche D., Neal J.W., Ferrer I., Thompson P., Vlachouli C., Wilkinson D., Bayer A., Games D., et al. Aβ species removal after aβ42 immunization. J. Neuropathol. Exp. Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 126.Patton R.L., Kalback W.M., Esh C.L., Kokjohn T.A., Van Vickle G.D., Luehrs D.C., Kuo Y.-M., Lopez J., Brune D., Ferrer I., et al. Amyloid-β peptide remnants in an-1792-immunized alzheimer’s disease patients: A biochemical analysis. Am. J. Pathol. 2006;169:1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farlow M.R., Andreasen N., Riviere M.-E., Vostiar I., Vitaliti A., Sovago J., Caputo A., Winblad B., Graf A. Long-term treatment with active aβ immunotherapy with cad106 in mild Alzheimer’s disease. Alzheimer’s Res. Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pfeifer M., Boncristiano S., Bondolfi L., Stalder A., Deller T., Staufenbiel M., Mathews P.M., Jucker M. Cerebral hemorrhage after passive anti-aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 129.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., et al. The antibody aducanumab reduces aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 130.Boyd T.D., Bennett S.P., Mori T., Governatori N., Runfeldt M., Norden M., Padmanabhan J., Neame P., Wefes I., Sanchez-Ramos J., et al. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimer’s Dis.: JAD. 2010;21:507–518. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vogel D.Y.S., Kooij G., Heijnen P.D.A.M., Breur M., Peferoen L.A.N., van der Valk P., de Vries H.E., Amor S., Dijkstra C.D. GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur. J. Immunol. 2015;45:1808–1819. doi: 10.1002/eji.201444960. [DOI] [PubMed] [Google Scholar]
- 132.Hooten K.G., Beers D.R., Zhao W., Appel S.H. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:364–375. doi: 10.1007/s13311-014-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O., Burrell J.R., Zoing M.C. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 134.McGoldrick P., Joyce P.I., Fisher E.M.C., Greensmith L. Rodent models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2013;1832:1421–1436. doi: 10.1016/j.bbadis.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 135.Clement A.M., Nguyen M.D., Roberts E.A., Garcia M.L., Boillée S., Rule M., McMahon A.P., Doucette W., Siwek D., Ferrante R.J., et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 136.Beers D.R., Henkel J.S., Xiao Q., Zhao W., Wang J., Yen A.A., Siklos L., McKercher S.R., Appel S.H. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao W., Beers D.R., Appel S.H. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2013;8:888–899. doi: 10.1007/s11481-013-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zondler L., Müller K., Khalaji S., Bliederhäuser C., Ruf W.P., Grozdanov V., Thiemann M., Fundel-Clemes K., Freischmidt A., Holzmann K., et al. Peripheral monocytes are functionally altered and invade the cns in als patients. Acta Neuropathol. 2016;132:391–411. doi: 10.1007/s00401-016-1548-y. [DOI] [PubMed] [Google Scholar]
- 139.Miller R.G., Mitchell J.D., Moore D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD001447. [DOI] [PubMed] [Google Scholar]
- 140.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/WNL.47.6_Suppl_4.233S. [DOI] [PubMed] [Google Scholar]
- 141.Liu B.S., Ferreira R., Lively S., Schlichter L.C. Microglial SK3 and SK4 currents and activation state are modulated by the neuroprotective drug, riluzole. J. Neuroimmune Pharmacol. 2013;8:227–237. doi: 10.1007/s11481-012-9365-0. [DOI] [PubMed] [Google Scholar]
- 142.Ohta Y., Nagai M., Miyazaki K., Tanaka N., Kawai H., Mimoto T., Morimoto N., Kurata T., Ikeda Y., Matsuura T., et al. Neuroprotective and angiogenic effects of bone marrow transplantation combined with granulocyte colony-stimulating factor in a mouse model of amyotrophic lateral sclerosis. Cell Med. 2011;2:69–83. doi: 10.3727/215517910X582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miller R.G., Zhang R., Block G., Katz J., Barohn R., Kasarskis E., Forshew D., Gopalakrishnan V., McGrath M.S. NP001 regulation of macrophage activation markers in ALS: A phase I clinical and biomarker study. Amyotroph. Lateral Scler. Frontotemporal Degener. 2014;15:601–609. doi: 10.3109/21678421.2014.951940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Van der Valk P., de Groot C.J.A. Staging of multiple sclerosis (MS) lesions: Pathology of the time frame of MS. Neuropathol. Appl. Neurobiol. 2000;26:2–10. doi: 10.1046/j.1365-2990.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 145.Brendecke S.M., Prinz M. Do not judge a cell by its cover—Diversity of CNS resident, adjoining and infiltrating myeloid cells in inflammation. Semin. Immunopathol. 2015;37:591–605. doi: 10.1007/s00281-015-0520-6. [DOI] [PubMed] [Google Scholar]
- 146.Ajami B., Bennett J.L., Krieger C., McNagny K.M., Rossi F.M.V. Infiltrating monocytes trigger eae progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 147.Banati R.B., Gehrmann J., Schubert P., Kreutzberg G.W. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 148.Mildner A., Mack M., Schmidt H., Bruck W., Djukic M., Zabel M.D., Hille A., Priller J., Prinz M. Ccr2+ly-6chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 149.Miron V.E., Boyd A., Zhao J.-W., Yuen T.J., Ruckh J.M., Shadrach J.L., van Wijngaarden P., Wagers A.J., Williams A., Franklin R.J.M., et al. M2 microglia/macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ruckh J.M., Zhao J.-W., Shadrach J.L., van Wijngaarden P., Rao T.N., Wagers A.J., Franklin R.J.M. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Greter M., Heppner F.L., Lemos M.P., Odermatt B.M., Goebels N., Laufer T., Noelle R.J., Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 152.Kivisäkk P., Imitola J., Rasmussen S., Elyaman W., Zhu B., Ransohoff R.M., Khoury S.J. Localizing CNS immune surveillance: Meningeal APCs activate T cells during eae. Ann. Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shemer A., Jung S. Differential roles of resident microglia and infiltrating monocytes in murine CNS autoimmunity. Semin. Immunopathol. 2015;37:613–623. doi: 10.1007/s00281-015-0519-z. [DOI] [PubMed] [Google Scholar]
- 154.Yamasaki R., Lu H., Butovsky O., Ohno N., Rietsch A.M., Cialic R., Wu P.M., Doykan C.E., Lin J., Cotleur A.C., et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miron V.E., Franklin R.J.M. Macrophages and cns remyelination. J. Neurochem. 2014;130:165–171. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- 156.Vogel D.Y.S., Vereyken E.J.F., Glim J.E., Heijnen P.D.A.M., Moeton M., van der Valk P., Amor S., Teunissen C.E., van Horssen J., Dijkstra C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 2013;10:1–12. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ransohoff R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 158.Yednock T.A., Cannon C., Fritz L.C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4βl integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 159.Bauer M., Brakebusch C., Coisne C., Sixt M., Wekerle H., Engelhardt B., Fässler R. Β1 integrins differentially control extravasation of inflammatory cell subsets into the cns during autoimmunity. Proc. Natl. Acad. Sci. USA. 2009;106:1920–1925. doi: 10.1073/pnas.0808909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weber C., Alon R., Moser B., Springer T. Sequential regulation of α4β1 and α5β1 integrin avidity by cc chemokines in monocytes: Implications for transendothelial chemotaxis. J. Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berger J.R., Koralnik I.J. Progressive multifocal leukoencephalopathy and natalizumab—Unforeseen consequences. N. Engl. J. Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 162.Nikfar S., Rahimi R., Abdollahi M. A meta-analysis of the efficacy and tolerability of interferon-β in multiple sclerosis, overall and by drug and disease type. Clin. Ther. 2010;32:1871–1888. doi: 10.1016/j.clinthera.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 163.Kasper L.H., Reder A.T. Immunomodulatory activity of interferon-beta. Ann. Clin. Transl. Neurol. 2014;1:622–631. doi: 10.1002/acn3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Floris S., Ruuls S.R., Wierinckx A., van der Pol S.M., Dopp E., van der Meide P.H., Dijkstra C.D., de Vries H.E. Interferon-β directly influences monocyte infiltration into the central nervous system. J. Neuroimmunol. 2002;127:69–79. doi: 10.1016/S0165-5728(02)00098-X. [DOI] [PubMed] [Google Scholar]
- 165.Jiang H., Milo R., Swoveland P., Johnson K.P., Panitch H., Dhib-Jalbut S. Interferon β-1b reduces interferon γ-induced antigen-presenting capacity of human glial and b cells. J. Neuroimmunol. 1995;61:17–25. doi: 10.1016/0165-5728(95)00072-A. [DOI] [PubMed] [Google Scholar]
- 166.Ransohoff R.M., Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 167.Kocur M., Schneider R., Pulm A.-K., Bauer J., Kropp S., Gliem M., Ingwersen J., Goebels N., Alferink J., Prozorovski T., et al. IFNβ secreted by microglia mediates clearance of myelin debris in CNS autoimmunity. Acta Neuropathol. Commun. 2015;3:20. doi: 10.1186/s40478-015-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hosmane S., Tegenge M.A., Rajbhandari L., Uapinyoying P., Kumar N.G., Thakor N., Venkatesan A. Toll/interleukin-1 receptor domain-containing adapter inducing interferon-β mediates microglial phagocytosis of degenerating axons. J. Neurosci. 2012;32:7745–7757. doi: 10.1523/JNEUROSCI.0203-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ekta Franscina P., Chittaranjan A. Interferon-related depression: A primer on mechanisms, treatment, and prevention of a common clinical problem. Curr. Neuropharmacol. 2016;14:743–748. doi: 10.2174/1570159X14666160106155129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Burt R.K., Balabanov R., Han X., Sharrack B., Morgan A., Quigley K., Yaung K., Helenowski I.B., Jovanovic B., Spahovic D. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313:275–284. doi: 10.1001/jama.2014.17986. [DOI] [PubMed] [Google Scholar]
- 171.Gross C.C., Jonuleit H., Wiendl H. Fulfilling the dream: Tolerogenic dendritic cells to treat multiple sclerosis. Eur. J. Immunol. 2012;42:569–572. doi: 10.1002/eji.201242402. [DOI] [PubMed] [Google Scholar]
- 172.Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 173.Raϊch-Regué D., Grau-López L., Naranjo-Gómez M., Ramo-Tello C., Pujol-Borrell R., Martínez-Cáceres E., Borràs F.E. Stable antigen-specific T-cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur. J. Immunol. 2012;42:771–782. doi: 10.1002/eji.201141835. [DOI] [PubMed] [Google Scholar]
- 174.Mansilla M.J., Sellès-Moreno C., Fàbregas-Puig S., Amoedo J., Navarro-Barriuso J., Teniente-Serra A., Grau-López L., Ramo-Tello C., Martínez-Cáceres E.M. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci. Ther. 2015;21:222–230. doi: 10.1111/cns.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mansilla M.J., Contreras-Cardone R., Navarro-Barriuso J., Cools N., Berneman Z., Ramo-Tello C., Martínez-Cáceres E.M. Cryopreserved vitamin D3-tolerogenic dendritic cells pulsed with autoantigens as a potential therapy for multiple sclerosis patients. J. Neuroinflamm. 2016;13:113. doi: 10.1186/s12974-016-0584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ohgaki H., Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 177.Ohgaki H., Dessen P., Jourde B., Horstmann S., Nishikawa T., di Patre P.-L., Burkhard C., Schüler D., Probst-Hensch N.M., Maiorka P.C., et al. Genetic pathways to glioblastoma. Cancer Res. 2004;64:6892. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 178.Yang P., Wang Y., Peng X., You G., Zhang W., Yan W., Bao Z., Wang Y., Qiu X., Jiang T. Management and survival rates in patients with glioma in China (2004–2010): A retrospective study from a single-institution. J. Neuro-Oncol. 2013;113:259–266. doi: 10.1007/s11060-013-1103-9. [DOI] [PubMed] [Google Scholar]
- 179.Li W., Graeber M.B. The molecular profile of microglia under the influence of glioma. Neuro-Oncology. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fecci P.E., Mitchell D.A., Whitesides J.F., Xie W., Friedman A.H., Archer G.E., Herndon J.E., Bigner D.D., Dranoff G., Sampson J.H. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 181.Gustafson M.P., Lin Y., New K.C., Bulur P.A., O’Neill B.P., Gastineau D.A., Dietz A.B. Systemic immune suppression in glioblastoma: The interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-Oncology. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chang A.L., Miska J., Wainwright D.A., Dey M., Rivetta C.V., Yu D., Kanojia D., Pituch K.C., Qiao J., Pytel P., et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76:5671–5682. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hambardzumyan D., Gutmann D.H., Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016;19:20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lombardi G., Rumiato E., Bertorelle R., Saggioro D., Farina P., Della Puppa A., Zustovich F., Berti F., Sacchetto V., Marcato R., et al. Clinical and genetic factors associated with severe hematological toxicity in glioblastoma patients during radiation plus temozolomide treatment: A prospective study. Am. J. Clin. Oncol. 2015;38:514–519. doi: 10.1097/COC.0b013e3182a790ea. [DOI] [PubMed] [Google Scholar]
- 185.Zhang L., Alizadeh D., Van Handel M., Kortylewski M., Yu H., Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57:1458–1467. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 186.Galarneau H., Villeneuve J., Gowing G., Julien J.-P., Vallières L. Increased glioma growth in mice depleted of macrophages. Cancer Res. 2007;67:8874. doi: 10.1158/0008-5472.CAN-07-0177. [DOI] [PubMed] [Google Scholar]
- 187.Müller A., Brandenburg S., Turkowski K., Müller S., Vajkoczy P. Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int. J. Cancer. 2015;137:278–288. doi: 10.1002/ijc.29379. [DOI] [PubMed] [Google Scholar]
- 188.Brandenburg S., Müller A., Turkowski K., Radev Y.T., Rot S., Schmidt C., Bungert A.D., Acker G., Schorr A., Hippe A., et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016;131:365–378. doi: 10.1007/s00401-015-1529-6. [DOI] [PubMed] [Google Scholar]
- 189.Anguille S., Smits E.L., Lion E., van Tendeloo V.F., Berneman Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–e267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 190.Phuphanich S., Wheeler C.J., Rudnick J.D., Mazer M., Wang H., Nuño M.A., Richardson J.E., Fan X., Ji J., Chu R.M., et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2013;62:125–135. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu J.S., Liu G., Ying H., Yong W.H., Black K.L., Wheeler C.J. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 192.Yamanaka R., Homma J., Yajima N., Tsuchiya N., Sano M., Kobayashi T., Yoshida S., Abe T., Narita M., Takahashi M., et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: Results of a clinical phase I/II trial. Clin. Cancer Res. 2005;11:4160. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 193.Mitchell D.A., Batich K.A., Gunn M.D., Huang M.-N., Sanchez-Perez L., Nair S.K., Congdon K.L., Reap E.A., Archer G.E., Desjardins A., et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Prins R.M., Wang X., Soto H., Young E., Lisiero D.N., Fong B., Everson R., Yong W.H., Lai A., Li G., et al. Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients. J. Immunother. 2013;36:152–157. doi: 10.1097/CJI.0b013e3182811ae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.De Vleeschouwer S., Rapp M., Sorg R.V., Steiger H.-J., Stummer W., van Gool S., Sabel M. Dendritic cell vaccination in patients with malignant gliomas: Current status and future directions. Neurosurgery. 2006;59:988–1000. doi: 10.1227/01.NEU.0000245595.38957.3E. [DOI] [PubMed] [Google Scholar]
- 196.Li W., Holsinger R.M.D., Kruse C.A., Flügel A., Graeber M.B. The potential for genetically altered microglia to influence glioma treatment. CNS Neurol. Disord. Drug Targets. 2013;12:750–762. doi: 10.2174/18715273113126660171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Adair J.E., Beard B.C., Trobridge G.D., Neff T., Rockhill J.K., Silbergeld D.L., Mrugala M., Kiem H.-P. Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci. Transl. Med. 2012;4:133–157. doi: 10.1126/scitranslmed.3003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adair J.E., Johnston S.K., Mrugala M.M., Beard B.C., Guyman L.A., Baldock A.L., Bridge C.A., Hawkins-Daarud A., Gori J.L., Born D.E., et al. Gene therapy enhances chemotherapy tolerance and efficacy in glioblastoma patients. J. Clin. Investig. 2014;124:4082–4092. doi: 10.1172/JCI76739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Waisman A., Ginhoux F., Greter M., Bruttger J. Homeostasis of microglia in the adult brain: Review of novel microglia depletion systems. Trends Immunol. 2015;36:625–636. doi: 10.1016/j.it.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 200.Betlazar C., Middleton R.J., Banati R.B., Liu G.-J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016;9:144–156. doi: 10.1016/j.redox.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shechter R., Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: No longer ‘if’ but ‘how’. J. Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- 202.Srinivas M., Aarntzen E.H.J.G., Bulte J.W.M., Oyen W.J., Heerschap A., de Vries I.J.M., Figdor C.G. Imaging of cellular therapies. Adv. Drug Deliv. Rev. 2010;62:1080–1093. doi: 10.1016/j.addr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 203.Liu G.-J., Middleton R.J., Hatty C.R., Kam W.W.-Y., Chan R., Pham T., Harrison-Brown M., Dodson E., Veale K., Banati R.B. The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 2014;24:631–653. doi: 10.1111/bpa.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cagnin A., Brooks D.J., Kennedy A.M., Gunn R.N., Myers R., Turkheimer F.E., Jones T., Banati R.B. In Vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 205.Banati R.B., Newcombe J., Gunn R.N., Cagnin A., Turkheimer F., Heppner F., Price G., Wegner F., Giovannoni G., Miller D.H., et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis. Brain. 2000;123:2321. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- 206.Giannetti P., Politis M., Su P., Turkheimer F., Malik O., Keihaninejad S., Wu K., Reynolds R., Nicholas R., Piccini P. Microglia activation in multiple sclerosis black holes predicts outcome in progressive patients: An in vivo [(11)C](R)-PK11195-PET pilot study. Neurobiol. Dis. 2014;65:203–210. doi: 10.1016/j.nbd.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 207.Banati R.B., Middleton R.J., Chan R., Hatty C.R., Kam W.W., Quin C., Graeber M.B., Parmar A., Zahra D., Callaghan P., et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014;5:5452. doi: 10.1038/ncomms6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Roncaroli F., Su Z., Herholz K., Gerhard A., Turkheimer F.E. TSPO expression in brain tumours: Is TSPO a target for brain tumour imaging? Clin. Transl. Imaging. 2016;4:145–156. doi: 10.1007/s40336-016-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fais S., Logozzi M., Lugini L., Federici C., Azzarito T., Zarovni N., Chiesi A. Exosomes: The ideal nanovectors for biodelivery. Biol. Chem. 2013;394:1–15. doi: 10.1515/hsz-2012-0236. [DOI] [PubMed] [Google Scholar]
- 210.Yim N., Ryu S.-W., Choi K., Lee K.R., Lee S., Choi H., Kim J., Shaker M.R., Sun W., Park J.-H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016 doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Miller J.C., Holmes M.C., Wang J., Guschin D.Y., Lee Y.-L., Rupniewski I., Beausejour C.M., Waite A.J., Wang N.S., Kim K.A., et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 2007;25:778–785. doi: 10.1038/nbt1319. [DOI] [PubMed] [Google Scholar]
- 212.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Klyachko N.L., Haney M.J., Zhao Y., Manickam D.S., Mahajan V., Suresh P., Hingtgen S.D., Mosley R.L., Gendelman H.E., Kabanov A.V., et al. Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine. 2014;9:1403–1422. doi: 10.2217/nnm.13.115. [DOI] [PMC free article] [PubMed] [Google Scholar]