Abstract
Cell death in skeletal component cells, including chondrocytes, osteoblasts, and osteocytes, plays roles in skeletal development, maintenance, and repair as well as in the pathogenesis of osteoarthritis and osteoporosis. Chondrocyte proliferation, differentiation, and apoptosis are important steps for endochondral ossification. Although the inactivation of P53 and RB is involved in the pathogenesis of osteosarcomas, the deletion of p53 and inactivation of Rb are insufficient to enhance chondrocyte proliferation, indicating the presence of multiple inhibitory mechanisms against sarcomagenesis in chondrocytes. The inflammatory processes induced by mechanical injury and chondrocyte death through the release of danger-associated molecular patterns (DAMPs) are involved in the pathogenesis of posttraumatic osteoarthritis. The overexpression of BCLXL increases bone volume with a normal structure and maintains bone during aging by inhibiting osteoblast apoptosis. p53 inhibits osteoblast proliferation and enhances osteoblast apoptosis, thereby reducing bone formation, but also exerts positive effects on osteoblast differentiation through the Akt–FoxOs pathway. Apoptotic osteocytes release ATP, which induces the receptor activator of nuclear factor κ-B ligand (Rankl) expression and osteoclastogenesis, from pannexin 1 channels. Osteocyte death ultimately results in necrosis; DAMPs are released to the bone surface and promote the production of proinflammatory cytokines, which induce Rankl expression, and osteoclastogenesis is further enhanced.
Keywords: osteoarthritis, p53, Rb, ATP, DAMPs, Rankl, BCLXL, FoxO, osteoarthritis, apoptosis, necrosis
1. Chondrocyte Death
1.1. Chondrocyte Proliferation and Apoptosis
1.1.1. Runx2, Indian Hedgehog (Ihh), and Parathyroid Hormone-Related Peptide (Pthrp) Regulate Chondrocyte Proliferation and Differentiation
The growth plate is composed of reservoir, proliferating, prehypertrophic, hypertrophic, and terminal hypertrophic chondrocytes. The expression of Runx2 is up-regulated in prehypertrophic chondrocytes, and chondrocyte hypertrophy is mainly regulated by Runx2 and, to a lesser extent, by Runx3. Runx2 induces the expression of Ihh, which is also strongly expressed in prehypertrophic chondrocytes [1,2]. Ihh enhances chondrocyte proliferation [3]. It also induces the expression of Pthrp, which inhibits Runx2 expression and chondrocyte hypertrophy through the protein kinase A (PKA) signaling pathway in a negative feedback loop [4,5,6]. Vascular invasion occurs at the layer of terminal hypertrophic chondrocytes. The preosteoblasts surrounding cartilage invade the layer of terminal hypertrophic chondrocytes with blood vessels. The terminal hypertrophic chondrocyte layer is invaded by osteoclasts, and preosteoblasts attach to the invaded surface of the terminal hypertrophic chondrocyte layer and become osteoblasts, which produce bone matrix proteins. Runx2 enhances vascular invasion and cartilage matrix degradation through the induction of Vegf and Mmp13, respectively, in terminal hypertrophic chondrocytes [7,8,9]. Terminal hypertrophic chondrocytes gradually die by apoptosis or become osteoblasts, and the terminal hypertrophic chondrocyte layer is replaced with bone [10,11]. Therefore, chondrocyte apoptosis is observed in the terminal hypertrophic chondrocyte layer, but is mostly absent in the other layers under physiological conditions.
1.1.2. Cyclin D1 Plays an Important Role in Chondrocyte Proliferation
The progression of the cell cycle is controlled by the sequential synthesis of individual cyclins. Cyclin D activates Cdk4 and Cdk6 in the G1 phase, cyclin E activates Cdk2 in the G1/S phase, cyclin A activates Cdk2 and Cdk1 in the S phase, and cyclin B activates Cdk1 in the M phase [12]. Cyclin D1 is known to be regulated by numerous extracellular growth factors and hormones, including Pthrp, transforming growth factor β (TGFβ), Ihh, and Wnt5b, during chondrocyte proliferation [13,14,15]. Cyclin D1 is also a well-established human oncogene because the amplification and overexpression of Cyclin D1 are involved in many kinds of cancers [16]. In the growth plate, Cyclin D1 is strongly expressed in the proliferating layer [15], and Cyclin D1-deficient mice show dwarfism with a diminished proliferating layer in the growth plate [13,15,17,18]. Cdk4 or Cdk6 activated by Cyclin Ds phosphorylates Rb family proteins (pRb, p130, and p110), resulting in the release of E2f transcription factors. This leads to the transcriptional activation of E2f-responsive genes that are essential for DNA synthesis, including cyclins E and A [16]. Rb family proteins play an important role in chondrocyte proliferation and differentiation [19,20].
1.1.3. Can the Overexpression of Both Cdk6 and Cyclin D1 Induce Chondrocyte Proliferation?
We examined the functions of Cdk6 and Cyclin D1 in chondrocyte proliferation and differentiation by generating transgenic mice overexpressing Cdk6, Cyclin D1, or both Cdk6 and Cyclin D1 under the control of the Col2a1 promoter, which directs transgene expression to chondrocytes [21]. Cdk6 or Cyclin D1 single transgenic embryos show normal phenotypes, whereas Cdk6/Cyclin D1 double transgenic mice show dwarfism, retarded chondrocyte maturation, the increased incorporation of BrdU, and markedly enhanced chondrocyte apoptosis. Although more chondrocytes in Cdk6/Cyclin D1 double transgenic mice enter the S phase, many fail to complete the cell cycle and die by apoptosis, indicating that the overexpression of both Cdk6 and Cyclin D1 is insufficient to enhance chondrocyte proliferation, but successfully induces apoptosis. However, many in vitro and in vivo studies have shown that the overexpression of Cyclin D1 enhances cell proliferation, and Cyclin D1 plays an important oncogenic role in many cancers [16,21,22,23]. Rb is highly phosphorylated, while unphosphorylated p107 levels are elevated in Cdk6/Cyclin D1 double transgenic mice [21]. p107 is a target gene of E2f, and p107 is also up-regulated in Rb−/− and p130−/− mouse embryonic fibroblasts [24,25,26]. Furthermore, the expression of the E2f target gene Cdc6 is up-regulated, whereas that of E2f target genes, including Cyclin E, Dihydrofolate reductase (Dhfr), Cdc25a, and B-Myb, is down-regulated in Cdk6/Cyclin D1 double transgenic mice [21]. The introduction of siRNA for p107 reverses the expression of these down-regulated E2f target genes. The deletion of p53 almost completely rescues chondrocyte apoptosis, but fails to enhance chondrocyte proliferation in Cdk6/Cyclin D1 double transgenic mice. These findings indicate that the overexpression of Cdk6/Cyclin D1 enhances G1/S cell cycle transition by phosphorylating Rb, and also that chondrocytes fail to complete their cell cycle and undergo p53-dependent apoptosis through the dysregulation of E2f target genes due to the up-regulation of p107 [21]. Therefore, the deletion of p53 in addition to the inactivation of Rb is insufficient to enhance chondrocyte proliferation, indicating the presence of multiple inhibitory mechanisms against sarcomagenesis in chondrocytes [21].
1.1.4. p107 Plays a Key Role in Chondrocyte Proliferation and Apoptosis
p107−/− mice show the most severe inhibition of chondrocyte differentiation among the knockout mice of Rb family genes. The inhibition of chondrocyte differentiation is further enhanced in p107−/−p130−/− or p107−/−Rb−/− mice and chondrocyte proliferation also increases without elevations in chondrocyte apoptosis [19,20,27]. Therefore, the phenotypic differences that exist between p107−/−p130−/− and p107−/−Rb−/− mice and Cdk6/Ccnd1 double transgenic mice are enhanced chondrocyte proliferation and the lack of chondrocyte apoptosis in p107−/−p130−/− and p107−/−Rb−/− mice. When Cdk6 and Cyclin D1 are overexpressed, Rb, p107, and p130 are expected to be phosphorylated, leading to the release of repressing E2fs (E2f4, 5) and activation of activating E2fs (E2f1–3), both of which enhance the transcription of E2f target genes including p107. Therefore, the phosphorylation of Rb, p107, and p130 enhances the transcription of p107 by E2f, and unphosphorylated p107 is continuously supplied. Under physiological conditions, unphosphorylated p107 associates with repressing E2fs, whereas unphosphorylated p107 also associates with activating E2fs when p107 is up-regulated, resulting in the dysregulation of E2f target gene expression, as reported in Cdk6/Cyclin D1 double transgenic mice [28,29]. Therefore, p107 is a key regulatory molecule in chondrocyte proliferation and apoptosis.
1.2. Chondrocyte Death in Osteoarthritis (OA)
1.2.1. Is Chondrocyte Death Involved in the Pathogenesis of OA?
OA is a common degenerative joint disease that is characterized by the progressive breakdown of articular cartilage in addition to changes in other joint components including the subchondral bone, menisci, synovium, ligaments, capsule, and muscles. A number of processes, including chondrocyte hypertrophy, inflammatory processes, and chondrocyte death, are involved in the pathogenesis of OA [9,30,31]. Apoptosis, autophagic cell death, and necrosis have been detected in OA cartilage [32]. If cells die through apoptosis or autophagy, death is completed by the removal of cells through engulfment by scavengers. In these cases, the integrity of cytoplasmic membranes is maintained when phagocytosis occurs. Although chondrocytes are isolated by an extracellular matrix, similar to osteocytes, as described later, the terminal phase of apoptosis or autophagic cell death is not engulfed by phagocytes, a transition to necrosis ensues, and cells are eliminated by cell disruption; this process is called secondary necrosis [33]. Therefore, the mixed morphology of apoptosis, autophagic cell death, and necrosis has been reported in OA cartilage [34]. Similar to osteocytes, autophagy also protects chondrocytes from apoptosis, and its failure leads to apoptosis [35,36].
Previous studies reported positive correlations between chondrocyte death by apoptosis and the severity of OA [37,38,39,40,41,42,43]. Furthermore, a significant decrease has been reported in chondrocyte numbers in articular cartilage with aging [39,44,45]. The intra-articular administration of caspase inhibitors has been shown to inhibit chondrocyte death and cartilage degradation in OA rabbit models [46,47]. In contrast, a recent study using a 3D confocal cartilage imaging technique showed that chondrocyte death by diphtheria toxin did not result in cartilage damage [48]. Therefore, it currently remains controversial whether chondrocyte death itself causes cartilage degradation.
1.2.2. Chondrocyte Death during Mechanical Injury May Contribute to the Development of OA
Chondrocyte death may be induced by mechanical injury, the loss of the extracellular matrix, loss of growth factors, or excessive levels of reactive oxygen species (ROS) [49] (Figure 1). Abnormal mechanical loading is a major risk factor for the development of OA. Injurious mechanical loading on articular cartilage causes collagen degradation, the loss of glycosaminoglycan (GAG), and chondrocyte apoptosis [50,51,52]. The importance of the extracellular matrix for chondrocyte survival has been demonstrated in integrin α1 knockout mice, which showed an increase in the number of apoptotic chondrocytes because integrins function as receptors that connect extracellular matrix proteins to various intracellular cytoskeletal proteins [53]. Col2a1-deficient mice also show an increase in the number of apoptotic chondrocytes in articular cartilage [54].
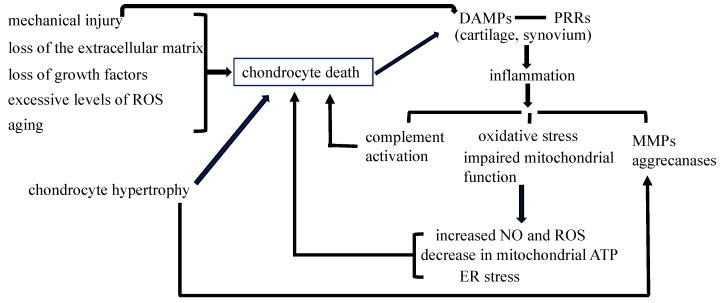
Figure 1.
Chondrocyte death in osteoarthritis. Mechanical injury, the loss of the extracellular matrix, loss of growth factors, and excessive levels of reactive oxygen species (ROS) induce chondrocyte death in articular cartilage. Chondrocyte hypertrophy in articular cartilage induces the destruction of the cartilage matrix through the induction of matrix metalloproteinases (MMPs) and aggrecanases, and leads to chondrocyte apoptosis. The number of dead chondrocytes also increases in articular cartilage during aging. DAMPs (danger-associated molecular patterns) released by mechanical injury and chondrocyte secondary necrosis initiate non-infectious inflammatory responses through PRRs (pattern recognition receptors) expressed in osteoarthritis (OA) cartilage and the synovium, causing complement activation, oxidative stress, impaired mitochondrial function, and the induction of MMPs and aggrecanases. Complement activation, an increase in nitric oxide (NO) and ROS, a decrease in mitochondrial ATP, and endoplasmic reticulum (ER) stress further enhance chondrocyte death, resulting in the progression of osteoarthritis. The contribution of chondrocyte death to inflammatory processes needs to be evaluated. The inflammatory processes induced by mechanical injury contribute to the development of posttraumatic OA; however, their significance in non-posttraumatic OA remains unclear.
Mechanical injury increases danger-associated molecular patterns (DAMPs), including released and degraded cartilage matrix constituents, S100 family molecules, the high-mobility group box 1 (HMGB1) protein, purine metabolites, heat-shock proteins, and uric acid, which initiate non-infectious inflammatory responses through various pattern recognition receptors (PRRs) expressed in OA cartilage and the synovium, including toll-like receptor 2 (TLR2), TLR4, and the receptor for advanced glycation end-products (RAGE) [30]. Chondrocyte apoptosis or autophagic death also increases DAMPs because both result in secondary necrosis [33]. However, the significance of the DAMPs released from necrotic chondrocytes still needs to be investigated because the ablation of chondrocytes by diphtheria toxin prior to the operation for surgical OA reduces cartilage damage [48]. In this case, DAMPs must be considered to have already been released from necrotic chondrocytes and cartilage may contain no sufficient DAMPs when mechanical injury occurs.
1.2.3. Non-Infectious Inflammatory Processes through PRRs Are Involved in the Pathogenesis of Posttraumatic OA
The signaling pathway through PRRs induces synovitis, transduces inflammation, increases the expression of matrix metalloproteinases (MMPs) and aggrecanases, and induces oxidative stress and cell death [55,56]. Inflammatory signals increase oxidative stress and impair mitochondrial function, an increase in nitric oxide (NO) and ROS induces chondrocyte apoptosis, and a decrease in mitochondrial ATP generation contributes to chondrocyte death [57,58,59,60,61]. Complement may be activated by DAMPs, and activated complement components may induce chondrocyte death [62,63]. Furthermore, oxidative stress and mitochondrial dysfunction cause endoplasmic reticulum (ER) stress, and the unsuccessful resolution of ER stress by the unfolded protein response (UPR) promotes chondrocyte apoptosis via Chop [64] (Figure 1). Adenosine monophosphate-activated protein kinase (AMPK) activity regulates energy metabolism via downstream mediators including the NAD+-dependent protein deacetylase SIRT1, and SIRT1 inhibits chondrocyte apoptosis [65,66,67]. Chondrocyte apoptosis increases during aging, and may be a base for the onset of OA. Inflammatory processes promote chondrocyte apoptosis during the progression of OA (Figure 1). However, the contribution of chondrocyte death to inflammatory processes needs to be further clarified. Although inflammatory processes contribute to the development of posttraumatic OA, their significance in the pathogenesis of non-posttraumatic OA remains to be evaluated.
2. Osteoblast Death
2.1. Roles of Apoptosis in the Regulation of Bone Mass and Osteoblast Differentiation
Osteoblast Apoptosis Regulates Bone Mass
Osteoblast apoptosis plays an important role in bone development and maintenance. Between 60% and 80% of the osteoblasts that originally assemble at the resorption pit have been estimated to die by apoptosis. Furthermore, bone loss caused by a sex steroid deficiency, glucocorticoid (GC) excess, or aging has been partly attributed to apoptosis in osteoblasts and osteocytes, and parathyroid hormone (PTH), bisphosphonate, and calcitonin exert anabolic effects on bone by inhibiting osteoblast and osteocyte apoptosis [68,69,70,71,72,73,74,75]. In GC excess, autophagy appears to protect osteocytes from the negative effects of GCs; however, when these effects reach a certain threshold, the process of autophagy induces apoptosis in cells. Excess GCs increase the levels of the pro-apoptotic factors, Bim and Bak; decrease that of the pro-survival factor, BclXL; up-regulate the expression of the p53 protein; increase ROS levels; inhibit Akt; and enhance ER stress [35].
The overexpression of BCLXL inhibits osteoblast apoptosis and increases the mineral density and trabecular and cortical bone volumes of femurs as well as the cancellous bone volume of vertebrae due to enhanced bone formation. The bone structure is similar to, but stronger than that in wild-type mice and the increased bone mass is maintained during aging in both sexes [76]. The deletion of Bak and Bax in osteoblasts also increases the mineral density and trabecular bone volume of femurs, whereas the cortical bone volume of femurs and cancellous bone volume of vertebrae are not increased and cortical bone shows severe porosity in aged mice [77]. Since the expression of Rankl and Vegf is up-regulated in aged mice, osteoblast maturation may be inhibited or osteocyte death may occur. Furthermore, the deletion of Bak and Bax in osteoblasts appears to be insufficient for the efficient inhibition of osteoblast apoptosis.
2.2. Osteoblast Apoptosis and Osteoblast Differentiation
2.2.1. Osteoblast Apoptosis Suppresses Osteoblast Differentiation In Vitro by Reducing Cell Density during Cultivation
Our group and other groups described skeletal development in Bcl2−/− mice and the differentiation and functions of Bcl2−/− osteoblasts and osteoclasts [78,79,80]. Bcl2−/− mice show an increased bone mass due to enhanced apoptosis in and the impaired differentiation of osteoclasts. However, the viability and differentiation of Bcl2−/− osteoblasts are controversial in these studies. In vitro osteoblast cultures revealed that osteoblast apoptosis occurred similarly in Bcl2−/− and wild-type osteoblasts or was enhanced in Bcl2−/− osteoblasts [78,79]. In vitro osteoblast cultures also showed that the differentiation of Bcl2−/− osteoblasts was similar to or impaired more than that of wild-type osteoblasts [78,79]. In our analyses, osteoblast apoptosis was found to be increased in Bcl2−/− mice in vivo and Bcl2−/− osteoblasts in vitro. Furthermore, in situ hybridization and real-time RT-PCR analyses have shown that osteoblast marker gene expression is increased in Bcl2−/− mice, and in vitro osteoblastogenesis is also accelerated in Bcl2−/− osteoblasts [80]. This discrepancy in osteoblast differentiation in Bcl2−/− mice has been attributed to the different findings obtained on osteoblastogenesis in vitro. When primary osteoblasts were seeded at a concentration of 2.5 × 104/cm2, osteoblast differentiation occurred similarly in wild-type and Bcl2−/− osteoblasts. However, when primary osteoblasts were seeded at a higher concentration of 1 × 105/cm2, osteoblast differentiation was greater in Bcl2−/− osteoblasts than in wild-type osteoblasts [80]. Osteoblastogenesis in BCL2-overexpressing primary osteoblasts in vitro is also controversial, and was previously reported to be enhanced and inhibited [81]. The inhibition of osteoblastogenesis in BCL2-overexpressing primary osteoblasts is also dependent on the cell density seeded because the overexpression of BCL2 enhances osteoblast differentiation by increasing cell density through the inhibition of apoptosis in vitro [81]. In accordance with in vitro findings, osteoblast differentiation is inhibited in osteoblast-specific BCL2 transgenic mice [81]. This is in contrast to Bcl2−/− osteoblasts for which osteoblast apoptosis was found to be enhanced during cultivation [80]. Therefore, the discrepancy in the differentiation of Bcl2−/− osteoblasts is explained by a reduced cell density during cultivation, which decelerates osteoblast differentiation, due to increases in apoptosis in Bcl2−/− osteoblasts.
2.2.2. Apoptotic Responses Enhance Osteoblast Differentiation through the p53–Akt–FoxO Pathway
Enhanced osteoblast differentiation in Bcl2−/− mice is partly explained by the induction and activation of FoxOs [80]. FoxO1 and FoxO3a induce osteoblast differentiation [80,82,83,84]. FoxO proteins are inactivated through the phosphorylation of Thr24, S256, and S319 in FoxO1 and that of Thr32, S253, and S315 in FoxO3 by Akt, and are activated through the phosphorylation of S212 in FoxO1 and S207 in FoxO3a by Mst1, and Akt and Mst1 are activated by phosphorylation [85] (Figure 2). In Bcl2−/− calvariae, FoxO1 and FoxO3a mRNA expression is up-regulated, the phosphorylation of THr24 in FoxO1 and Thr32 in FoxO3a is reduced, the phosphorylation of S207 in FoxO3a is mildly reduced, and the expression of FoxO target genes, including FasL, Gadd45a, and Bim, is up-regulated [80]. In accordance with these findings, the phosphorylation of Akt is severely reduced and that of Mst1 is mildly reduced. Therefore, FoxOs are activated through the inactivation of Akt in Bcl2−/− calvariae [80] (Figure 2). p53 mRNA and protein expression is up-regulated, and the expression of its target genes—Pten and Igfbp3—is also increased in Bcl2−/− calvariae [80]. Thus, the activation of Akt is inhibited, at least in part, by the p53-dependent inactivation of Akt, leading to the activation of FoxOs (Figure 2). These findings indicate that apoptotic responses enhance osteoblast differentiation through the p53–Akt–FoxO pathway.
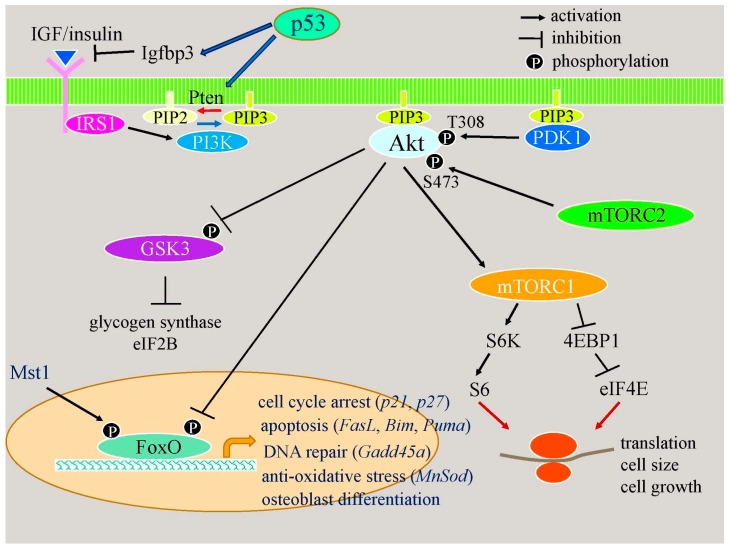
Figure 2.
Acceleration of osteoblast differentiation through the p53–Akt–FoxO pathway. p53 induces the expression of Igfbp3 and Pten. Igfbp3 inhibits IGF/insulin binding to the receptor, and Pten dephosphorylates PIP3 to PIP2. PIP3 recruits Akt to the cell membrane and Akt is activated by PDK1 and mTORC2. The decrease in PIP3 inactivates Akt. Akt regulates metabolism, cell growth, cell survival, cell proliferation, and cell differentiation through GSK3, mTORC1, and FoxOs. Akt phosphorylates FoxOs to inactivate them. FoxOs are involved in cell cycle arrest, apoptosis, DNA repair, anti-oxidative stress, and osteoblast differentiation.
2.2.3. p53 Reduces Bone Formation by Inhibiting Osteoblast Proliferation and Enhancing Osteoblast Apoptosis
Mice with activated mutations in p53 exhibit osteopenia [86]. Furthermore, conditional knockout mice of Mdm2, which negatively regulates p53 activity, using the 3.6-kb Col1a1 promoter in Cre transgenic mice resulted in reduced bone formation, and the differentiation of primary osteoblasts was inhibited in vitro [87]. The phenotypes of p53-deficient mice are controversial, with bone mass and bone formation being normal or increased [88,89,90]. The introduction of p53 into wild-type and p53−/− osteoblasts inhibits osteoblast differentiation [90]. However, the expression levels of p53 and osteocalcin have also been reported to be positively related [91]. Since the deletion of p53 enhances proliferation and inhibits apoptosis, the deletion of p53 may increase cell density in cultures, leading to the acceleration of osteoblast differentiation in vitro because osteoblast differentiation is dependent on cell density in vitro [76,80,81]. We found that skeletal development and chondrocyte differentiation in p53−/− embryos were similar to those in wild-type embryos, whereas primary chondrocytes from p53−/− embryos differentiated more rapidly than those from wild-type embryos in micromass cultures due to increased cell density (unpublished observation). The discrepancy in chondrocyte differentiation in vivo and in vitro is explained by chondrocyte proliferation being increased in p53−/− chondrocytes in vitro, but not in the growth plate of p53−/− mice, which demonstrates that the deletion of p53 is insufficient to increase chondrocyte proliferation in vivo [21]. An increase in osteoblast numbers due to enhanced proliferation and reduced apoptosis may also lead to an increase in bone formation in p53−/− mice, as reported previously [90]. Therefore, a major function of p53 in bone formation is to inhibit the proliferation of and enhance apoptosis in osteoblasts, leading to reduced bone formation. p53 also has a positive effect on osteoblast differentiation. p53 and FoxOs have similar functions, including inhibition of the cell cycle, DNA repair, and the induction of cell death, and have many overlapping target genes, such as p21, Wip1, Gadd45a, Pa26, and Puma [92]. The inhibition of osteoblast proliferation in Bcl2−/− mice is, at least in part, due to the up-regulation of p53 and FoxOs [80].
3. Osteocyte Death
3.1. Osteocyte Death and Bone Remodeling
3.1.1. ATP Released from Apoptotic Cells through Pannexin Channels Enhances Bone Resorption
Osteocytes, which are embedded in the bone matrix, form a network that is composed of two communication systems in bone. One is an intracellular communication system via gap junction-coupled cell processes, and the other is an extracellular communication system through canaliculi, through which osteocyte processes pass (Figure 3 and Figure 4). Both communication systems are extended to osteoblasts on the bone surface [93]. The extracellular communication system and hemichannels, which are transportation pathways between the processes and canaliculi, are required for the survival of osteocytes, which acquire nutrients, oxygen, and survival signals through them [33,81]. Two junctional proteins—connexins and pannexins—form membrane channels permeable to large molecules in vertebrates [94]. In bone cells, gap junctions are mainly formed by connexin 43 (Gja1), and hemichannels are formed by Gja1 and pannexins. There are three members in the pannexin family: pannexin 1 (Panx1) and pannexin 3 (Panx3) are expressed in bone cells, with Panx1 already being extensively examined [95]. In apoptotic cells, Panx1 undergoes caspase-mediated cleavage at the C terminus for activation, and activated Panx1 channels release adenosine triphosphate (ATP) as a “find me” signal necessary for macrophage recruitment [96]. Extracellular ATP binds to P2Y G-protein-coupled receptors and P2X ligand-gated ion channels—both of which are expressed in osteoblasts, osteocytes, and osteoclasts—and activate them [97]. The activation of P2Y1 receptors (P2Y1R) enhances the receptor activator of nuclear factor κ-B ligand (Rankl) expression in osteoblasts, the activation of P2Y6R in osteoclasts increases their survival, the activation of P2X7R in osteoclast precursor cells allows membrane fusion to form multinucleated osteoclasts, and P2X7R activates Panx1 for ATP release [95,98,99,100,101] (Figure 3). Since Rankl induces osteoclastogenesis and activates osteoclast functions, the activation of P2YR and P2XR by ATP leads to enhanced bone resorption.
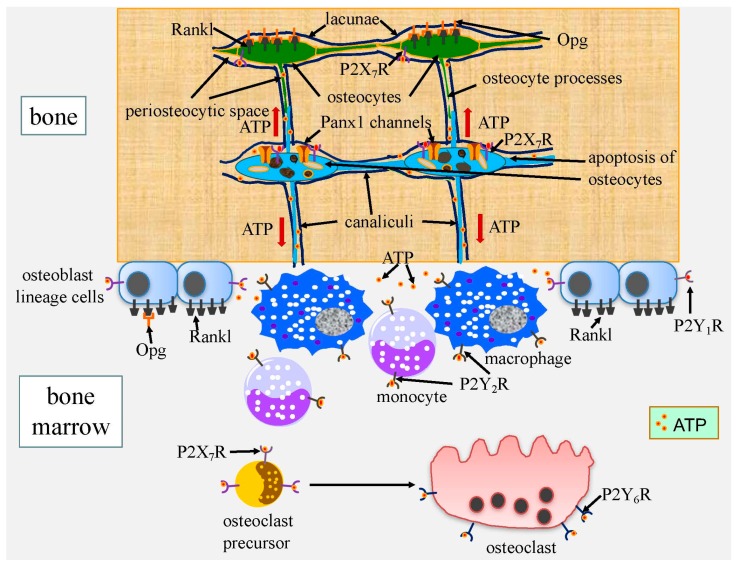
Figure 3.
Induction of bone resorption by ATP released from apoptotic osteocytes. In apoptotic osteocytes, active Panx1 channels, which release ATP, are formed by the caspase-mediated cleavage of the C terminus. P2X7 receptor (P2X7R), which is activated by extracellular ATP, forms a complex with Panx1 and enhances ATP release. Extracellular ATP recruits macrophages and monocytes through P2Y2R, increases nuclear factor κ-B ligand (Rankl) expression in osteocytes through P2X7R and in osteoblasts through P2Y1R, increases osteoclast survival through P2Y6R, and enhances the membrane fusion of osteoclast precursor cells to form multinucleated osteoclasts through P2X7R. The up-regulation of Rankl in osteocytes will reduce the release of osteoprotegerin (Opg), which is secreted by osteocytes, to the bone surface. It has been shown that Panx1 and P2X7R are required for Rankl up-regulation in osteocytes in fatigued bone. However, the functions of ATP released from apoptotic osteocytes shown here have not yet been directly proven in damaged bone.
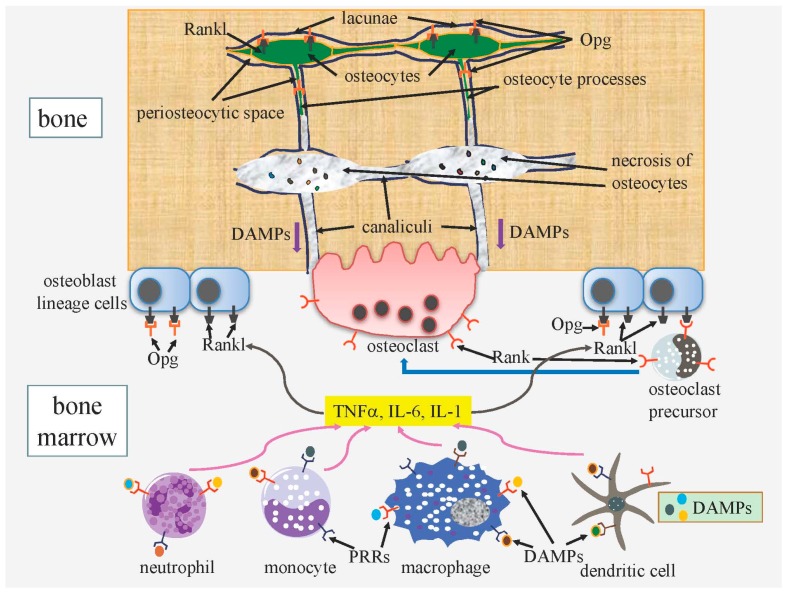
Figure 4.
A proposed scheme for the enhanced osteoclastogenesis by osteocyte death. Any type of osteocyte death ultimately results in necrosis because dead osteocytes are not phagocytosed. DAMPs (danger-associated molecular patterns) released from necrotic osteocytes pass through canaliculi and reach the bone surface. Macrophages, dendritic cells, neutrophils, and monocytes are stimulated by DAMPs through PRRs (pattern recognition receptors), and produce TNFα, IL-6, and IL-1, which stimulate the expression of Rankl in osteoblast lineage cells. Osteoclast precursors differentiate into osteoclasts through Rankl-Rank signaling. Opg released from osteocytes to the bone surface negatively regulates osteoclastogenesis and osteoclast activity. This scheme is predicted from previous studies, but has not been directly proven in damaged bone.
Osteocyte death occurs during aging, after menopause, under unloading, and pathological conditions such as microcracks, and the death of osteocytes is closely coupled to bone resorption [102,103,104]. The microdamage of bone has been associated with osteocyte apoptosis and targeted bone resorption [105,106]. Furthermore, bone resorption is markedly enhanced by the induction of osteocyte death [107]. Cells mainly die through one of three pathways, i.e., apoptosis, autophagic cell death, and necrosis, under physiological and pathological conditions [108]. In osteocyte apoptosis, ATP released from Panx1 channels enhances Rankl expression in the neighboring osteocytes and Rankl up-regulation and bone resorption are attenuated in Panx1−/− mice and P2X7R−/− mice [109,110]. Therefore, ATP released from Panx1 channels, which are activated by P2X7R, in apoptotic osteocytes enhances Rankl expression in the neighboring osteocytes and osteoblasts, recruits macrophages, and increases osteoclasts in the neighboring bone surface and vascular canals (Figure 3).
3.1.2. DAMPs Released from Necrotic Osteocytes Further Enhance Bone Resorption
Osteocyte death is distinct because osteocytes are isolated in the bone matrix and apoptosis and autophagic cell death end in secondary necrosis due to the absence of phagocytosis by scavengers [33]. Necrosis leads to the rupture of the cytoplasmic membrane, with most of the intracellular content being released into the extracellular environment [111]. Immunostimulatory molecules including DAMPs, such as S100 family molecules, the HMGB1 protein, purine metabolites, heat-shock proteins, and uric acid, are released through canaliculi to the bone surface and vascular canals in bone (Figure 4). Released DAMPs bind to PRRs, such as TLR2, TLR4, and RAGE, on macrophages, dendritic cells, monocytes, and neutrophils, and promote the production of proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1, which induces the expression of Rankl in osteoblast lineage cells [33,112,113,114,115,116]. Therefore, osteocyte necrosis further enhances Rankl expression and osteoclastogenesis, which have been triggered by ATP released from apoptotic osteocytes, and induced and activated osteoclasts dissolve the neighboring bone surface, leading to the remodeling of damaged bone parts (Figure 4). Since bone resorption after osteocyte apoptosis is attenuated in Panx1−/− mice [110], the release of ATP from Panx1 channels appears to be a prerequisite for bone resorption. Although the scheme in Figure 4 for bone resorption by osteocyte necrosis is predicted from previous studies, direct evidence for this scheme has not yet been obtained in damaged bone.
3.2. Functions of Osteocytes
3.2.1. Do Live Osteocytes Inhibit Bone Resorption?
A number of mouse models generated in order to elucidate the functions of osteocytes have encountered difficulties due to the death of osteocytes because this process induces bone remodeling as a repair process (Figure 3 and Figure 4). Osteocyte ablation by diphtheria toxin, the deletion of Gja1 (connexin 43), which constitutes gap junction channels and hemichannels, and osteocyte-specific transgenic mice expressing mutant Gja1, which blocks gap junction channels and hemichannels, cause osteocyte apoptosis, leading to secondary necrosis, enhanced bone resorption, and bone remodeling [107,117,118]. The extracellular communication system formed by canaliculi is essential for the bone remodeling process because ATP and the immunostimulatory molecules released from apoptotic cells and necrotic cells, respectively, pass through canaliculi to reach the bone surface or vascular canals (Figure 3 and Figure 4). Most osteocytes die in osteoblast-specific BCl2 transgenic mice using the 2.3-kb Col1a1 promoter because the osteocyte processes and canaliculi are severely reduced, possibly due to the formation of a complex of BCL2, actin, and gelsolin, which reduces gelsolin-severing activity in order to increase actin polymerization [81,119]. In BCl2 transgenic mice, osteocyte death does not induce bone resorption because the extracellular communication system is also disrupted [120]. Osteoclastogenesis and bone resorption are reduced and bone formation is enhanced, leading to an increased bone mass in BCl2 transgenic mice. These findings indicate that the osteocyte network stimulates osteoclastogenesis and bone resorption and inhibits osteoblastogenesis and bone formation under physiological conditions. Furthermore, these osteocyte functions are augmented under unloaded conditions [33,120].
3.2.2. Regulation of the Release of Osteocyte-Derived Opg to the Bone Surface May Be a Major Role for Rankl on Osteocytes
Osteocytes strongly express Rankl and Opg, which is a secreted decoy receptor of Rankl [120,121,122]. The osteocyte-specific deletion of Rankl results in the inhibition of bone resorption, indicating that osteocytes are a major source of Rankl [121,122]. However, the direct interaction of osteocyte processes and osteoclast precursors appears to be limited and the physiological significance of soluble Rankl from osteocytes is unclear. We previously proposed that a major function of Rankl on osteocytes is the regulation of Opg release to the bone surface and vascular canals by trapping Opg [33]. Opg secreted from osteocytes is trapped by Rankl on the surface of osteocytes; however, some Opg is released to the bone surface and vascular canals through canaliculi and binds to Rankl on the surface of osteoblasts, thereby inhibiting osteoclastogenesis and the activation of osteoclasts [33]. This needs to be proved through the generation of osteocyte-specific Opg/Rankl double knockout mice. In the region with dead osteocytes, the release of Opg to the bone surface through canaliculi will be reduced by the up-regulated expression of Rankl in the neighboring osteocytes. The reduced release of Opg will further enhance osteoclastogenesis and osteoclast activity (Figure 3 and Figure 4).
4. Conclusions
Chondrocyte proliferation is strictly regulated in vivo and acceleration of the cell cycle results in p53-dependent apoptosis. Although the inhibition of osteoblast apoptosis increases bone mass, the effects of apoptosis on bone formation are largely dependent on p53, which exerts opposite effects on bone formation by inhibiting the proliferation of and enhancing apoptosis in osteoblasts and also by accelerating osteoblast differentiation through the Akt–FoxOs pathway. Chondrocyte death is involved in the onset and progression of posttraumatic OA. Osteocyte apoptosis is predicted to enhance bone resorption through the release of ATP, and subsequent osteocyte necrosis is predicted to enhance bone resorption through the release of DAMPs, leading to the replacement of damaged bone.
Acknowledgments
This work was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Japan (#26221310).
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Yoshida C.A., Yamamoto H., Fujita T., Furuichi T., Ito K., Inoue K., Yamana K., Zanma A., Takada K., Ito Y., et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inada M., Yasui T., Nomura S., Miyake S., Deguchi K., Himeno M., Sato M., Yamagiwa H., Kimura T., Yasui N., et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.St-Jacques B., Hammerschmidt M., McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwamoto M., Kitagaki J., Tamamura Y., Gentili C., Koyama E., Enomoto H., Komori T., Pacifici M., Enomoto-Iwamoto M. Runx2 expression and action in chondrocytes are regulated by retinoid signaling and parathyroid hormone-related peptide (PTHrP) Osteoarthr. Cartil. 2003;11:6–15. doi: 10.1053/joca.2002.0860. [DOI] [PubMed] [Google Scholar]
- 5.Li T.F., Dong Y., Ionescu A.M., Rosier R.N., Zuscik M.J., Schwarz E.M., O’Keefe R.J., Drissi H. Parathyroid hormone-related peptide (PTHrP) inhibits Runx2 expression through the PKA signaling pathway. Exp. Cell Res. 2004;299:128–136. doi: 10.1016/j.yexcr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Vortkamp A., Lee K., Lanske B., Segre G.V., Kronenberg H.M., Tabin C.J. Regulation of rate of cartilage differentiation by indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 7.Himeno M., Enomoto H., Liu W., Ishizeki K., Nomura S., Kitamura Y., Komori T. Impaired vascular invasion of Cbfa1-deficient cartilage engrafted in the spleen. J. Bone Miner. Res. 2002;17:1297–1305. doi: 10.1359/jbmr.2002.17.7.1297. [DOI] [PubMed] [Google Scholar]
- 8.Zelzer E., Glotzer D.J., Hartmann C., Thomas D., Fukai N., Soker S., Olsen B.R. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev. 2001;106:97–106. doi: 10.1016/S0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 9.Komori T. Regulation of bone development and extracellular matrix protein genes by Runx2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 10.Yang L., Tsang K.Y., Tang H.C., Chan D., Cheah K.S. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA. 2014;111:12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X., von der Mark K., Henry S., Norton W., Adams H., de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10:e1004820. doi: 10.1371/journal.pgen.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malumbres M. Revisiting the “Cdk-centric” view of the mammalian cell cycle. Cell Cycle. 2005;4:206–210. doi: 10.4161/cc.4.2.1410. [DOI] [PubMed] [Google Scholar]
- 13.Beier F., Ali Z., Mok D., Taylor A.C., Leask T., Albanese C., Pestell R.G., LuValle P. TGFβ and PTHrP control chondrocyte proliferation by activating cyclin D1 expression. Mol. Biol. Cell. 2001;12:3852–3863. doi: 10.1091/mbc.12.12.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long F., Zhang X.M., Karp S., Yang Y., McMahon A.P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y., Topol L., Lee H., Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 16.Musgrove E.A., Caldon C.E., Barraclough J., Stone A., Sutherland R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 17.Fantl V., Stamp G., Andrews A., Rosewell I., Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 18.Sicinski P., Donaher J.L., Parker S.B., Li T., Fazeli A., Gardner H., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 19.Cobrinik D., Lee M.H., Hannon G., Mulligan G., Bronson R.T., Dyson N., Harlow E., Beach D., Weinberg R.A., Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- 20.Rossi F., MacLean H.E., Yuan W., Francis R.O., Semenova E., Lin C.S., Kronenberg H.M., Cobrinik D. p107 and p130 Coordinately regulate proliferation, Cbfa1 expression, and hypertrophic differentiation during endochondral bone development. Dev. Biol. 2002;247:271–285. doi: 10.1006/dbio.2002.0691. [DOI] [PubMed] [Google Scholar]
- 21.Ito K., Maruyama Z., Sakai A., Izumi S., Moriishi T., Yoshida C.A., Miyazaki T., Komori H., Takada K., Kawaguchi H., et al. Overexpression of Cdk6 and Ccnd1 in chondrocytes inhibited chondrocyte maturation and caused p53-dependent apoptosis without enhancing proliferation. Oncogene. 2013;33:1862–1871. doi: 10.1038/onc.2013.130. [DOI] [PubMed] [Google Scholar]
- 22.Wang T.C., Cardiff R.D., Zukerberg L., Lees E., Arnold A., Schmidt E.V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 23.Mueller A., Odze R., Jenkins T.D., Shahsesfaei A., Nakagawa H., Inomoto T., Rustgi A.K. A transgenic mouse model with cyclin D1 overexpression results in cell cycle, epidermal growth factor receptor, and p53 abnormalities. Cancer Res. 1997;57:5542–5549. [PubMed] [Google Scholar]
- 24.Zhu L., Xie E., Chang L.S. Differential roles of two tandem E2F sites in repression of the human p107 promoter by retinoblastoma and p107 proteins. Mol. Cell. Biol. 1995;15:3552–3562. doi: 10.1128/MCB.15.7.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkhart D.L., Wirt S.E., Zmoos A.F., Kareta M.S., Sage J. Tandem E2F binding sites in the promoter of the p107 cell cycle regulator control p107 expression and its cellular functions. PLoS Genet. 2010;6:e1001003. doi: 10.1371/journal.pgen.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurford R.K., Jr., Cobrinik D., Lee M.H., Dyson N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 27.Landman A.S., Danielian P.S., Lees J.A. Loss of pRB and p107 disrupts cartilage development and promotes enchondroma formation. Oncogene. 2012;32:4798–4805. doi: 10.1038/onc.2012.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komori T. Regulation of Rb family proteins by Cdk6/Ccnd1 in growth plates. Cell Cycle. 2013;12:2161–2162. doi: 10.4161/cc.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calbo J., Parreno M., Sotillo E., Yong T., Mazo A., Garriga J., Grana X. G1 cyclin/cyclin-dependent kinase-coordinated phosphorylation of endogenous pocket proteins differentially regulates their interactions with E2F4 and E2F1 and gene expression. J. Biol. Chem. 2002;277:50263–50274. doi: 10.1074/jbc.M209181200. [DOI] [PubMed] [Google Scholar]
- 30.Liu-Bryan R., Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamli Z., Sharif M. Chondrocyte apoptosis: A cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 2011;14:159–166. doi: 10.1111/j.1756-185X.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn K., D’Lima D.D., Hashimoto S., Lotz M. Cell death in cartilage. Osteoarthr. Cartil. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Komori T. Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res. 2013;352:191–198. doi: 10.1007/s00441-012-1546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roach H.I., Aigner T., Kouri J.B. Chondroptosis: A variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9:265–277. doi: 10.1023/B:APPT.0000025803.17498.26. [DOI] [PubMed] [Google Scholar]
- 35.Komori T. Glucocorticoid signaling and bone biology. Horm. Metab. Res. 2016;48:755–763. doi: 10.1055/s-0042-110571. [DOI] [PubMed] [Google Scholar]
- 36.Bohensky J., Shapiro I.M., Leshinsky S., Watanabe H., Srinivas V. PIM-2 is an independent regulator of chondrocyte survival and autophagy in the epiphyseal growth plate. J. Cell. Physiol. 2007;213:246–251. doi: 10.1002/jcp.21117. [DOI] [PubMed] [Google Scholar]
- 37.Mistry D., Oue Y., Chambers M.G., Kayser M.V., Mason R.M. Chondrocyte death during murine osteoarthritis. Osteoarthr. Cartil. 2004;12:131–141. doi: 10.1016/j.joca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Thomas C.M., Fuller C.J., Whittles C.E., Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthr. Cartil. 2007;15:27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto S., Ochs R.L., Komiya S., Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 40.Blanco F.J., Guitian R., Vazquez-Martul E., de Toro F.J., Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 41.Sharif M., Whitehouse A., Sharman P., Perry M., Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004;50:507–515. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- 42.Kim H.A., Lee Y.J., Seong S.C., Choe K.W., Song Y.W. Apoptotic chondrocyte death in human osteoarthritis. J. Rheumatol. 2000;27:455–462. [PubMed] [Google Scholar]
- 43.Hashimoto S., Takahashi K., Amiel D., Coutts R.D., Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998;41:1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 44.Todd Allen R., Robertson C.M., Harwood F.L., Sasho T., Williams S.K., Pomerleau A.C., Amiel D. Characterization of mature vs. aged rabbit articular cartilage: Analysis of cell density, apoptosis-related gene expression and mechanisms controlling chondrocyte apoptosis. Osteoarthr. Cartil. 2004;12:917–923. doi: 10.1016/j.joca.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Adams C.S., Horton W.E., Jr. Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat. Rec. 1998;250:418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 46.D’Lima D., Hermida J., Hashimoto S., Colwell C., Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 47.Dang A.C., Warren A.P., Kim H.T. Beneficial effects of intra-articular caspase inhibition therapy following osteochondral injury. Osteoarthr. Cartil. 2006;14:526–532. doi: 10.1016/j.joca.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Zhang M., Mani S.B., He Y., Hall A.M., Xu L., Li Y., Zurakowski D., Jay G.D., Warman M.L. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J. Clin. Investig. 2016;126:2893–2902. doi: 10.1172/JCI83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Del Carlo M., Jr., Loeser R.F. Cell death in osteoarthritis. Curr. Rheumatol. Rep. 2008;10:37–42. doi: 10.1007/s11926-008-0007-8. [DOI] [PubMed] [Google Scholar]
- 50.Clements K.M., Bee Z.C., Crossingham G.V., Adams M.A., Sharif M. How severe must repetitive loading be to kill chondrocytes in articular cartilage? Osteoarthr. Cartil. 2001;9:499–507. doi: 10.1053/joca.2000.0417. [DOI] [PubMed] [Google Scholar]
- 51.D’Lima D.D., Hashimoto S., Chen P.C., Colwell C.W., Jr., Lotz M.K. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthr. Cartil. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 52.Clements K.M., Hollander A.P., Sharif M., Adams M.A. Cyclic loading can denature type II collagen in articular cartilage. Connect. Tissue Res. 2004;45:174–180. doi: 10.1080/03008200490514121. [DOI] [PubMed] [Google Scholar]
- 53.Zemmyo M., Meharra E.J., Kuhn K., Creighton-Achermann L., Lotz M. Accelerated, aging-dependent development of osteoarthritis in α1 integrin-deficient mice. Arthritis Rheum. 2003;48:2873–2880. doi: 10.1002/art.11246. [DOI] [PubMed] [Google Scholar]
- 54.Yang C., Li S.W., Helminen H.J., Khillan J.S., Bao Y., Prockop D.J. Apoptosis of chondrocytes in transgenic mice lacking collagen II. Exp. Cell Res. 1997;235:370–373. doi: 10.1006/excr.1997.3692. [DOI] [PubMed] [Google Scholar]
- 55.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schelbergen R.F., Blom A.B., van den Bosch M.H., Sloetjes A., Abdollahi-Roodsaz S., Schreurs B.W., Mort J.S., Vogl T., Roth J., van den Berg W.B., et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64:1477–1487. doi: 10.1002/art.33495. [DOI] [PubMed] [Google Scholar]
- 57.Johnson K., Jung A., Murphy A., Andreyev A., Dykens J., Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000;43:1560–1570. doi: 10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 58.Blanco F.J., Rego I., Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011;7:161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 59.Kurz B., Lemke A., Kehn M., Domm C., Patwari P., Frank E.H., Grodzinsky A.J., Schunke M. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50:123–130. doi: 10.1002/art.11438. [DOI] [PubMed] [Google Scholar]
- 60.Maneiro E., Lopez-Armada M.J., de Andres M.C., Carames B., Martin M.A., Bonilla A., del Hoyo P., Galdo F., Arenas J., Blanco F.J. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 2005;64:388–395. doi: 10.1136/ard.2004.022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DelCarlo M., Loeser R.F. Chondrocyte cell death mediated by reactive oxygen species-dependent activation of pkc-betai. Am. J. Physiol. Cell physiol. 2006;290:802–811. doi: 10.1152/ajpcell.00214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q., Rozelle A.L., Lepus C.M., Scanzello C.R., Song J.J., Larsen D.M., Crish J.F., Bebek G., Ritter S.Y., Lindstrom T.M., et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bohana-Kashtan O., Ziporen L., Donin N., Kraus S., Fishelson Z. Cell signals transduced by complement. Mol. Immunol. 2004;41:583–597. doi: 10.1016/j.molimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Uehara Y., Hirose J., Yamabe S., Okamoto N., Okada T., Oyadomari S., Mizuta H. Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthr. Cartil. 2014;22:1007–1017. doi: 10.1016/j.joca.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Takayama K., Ishida K., Matsushita T., Fujita N., Hayashi S., Sasaki K., Tei K., Kubo S., Matsumoto T., Fujioka H., et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 66.Gagarina V., Gabay O., Dvir-Ginzberg M., Lee E.J., Brady J.K., Quon M.J., Hall D.J. SIRT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62:1383–1392. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabay O., Oppenhiemer H., Meir H., Zaal K., Sanchez C., Dvir-Ginzberg M. Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SIRT1 mice. Ann. Rheum. Dis. 2012;71:613–616. doi: 10.1136/ard.2011.200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Brien C.A., Jia D., Plotkin L.I., Bellido T., Powers C.C., Stewart S.A., Manolagas S.C., Weinstein R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 69.Kousteni S., Bellido T., Plotkin L.I., O’Brien C.A., Bodenner D.L., Han L., Han K., DiGregorio G.B., Katzenellenbogen J.A., Katzenellenbogen B.S., et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell. 2001;104:719–730. doi: 10.1016/S0092-8674(02)08100-X. [DOI] [PubMed] [Google Scholar]
- 70.Stanislaus D., Yang X., Liang J.D., Wolfe J., Cain R.L., Onyia J.E., Falla N., Marder P., Bidwell J.P., Queener S.W., et al. In vivo regulation of apoptosis in metaphyseal trabecular bone of young rats by synthetic human parathyroid hormone (1–34) fragment. Bone. 2000;27:209–218. doi: 10.1016/S8756-3282(00)00309-4. [DOI] [PubMed] [Google Scholar]
- 71.Gohel A., McCarthy M.B., Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–5347. doi: 10.1210/en.140.11.5339. [DOI] [PubMed] [Google Scholar]
- 72.Weinstein R.S., Jilka R.L., Parfitt A.M., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plotkin L.I., Weinstein R.S., Parfitt A.M., Roberson P.K., Manolagas S.C., Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Investig. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jilka R.L., Weinstein R.S., Bellido T., Roberson P., Parfitt A.M., Manolagas S.C. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Investig. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomkinson A., Reeve J., Shaw R.W., Noble B.S. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab. 1997;82:3128–3135. doi: 10.1210/jc.82.9.3128. [DOI] [PubMed] [Google Scholar]
- 76.Moriishi T., Fukuyama R., Miyazaki T., Furuichi T., Ito M., Komori T. Overexpression of BCLXL in osteoblasts inhibits osteoblast apoptosis and increases bone volume and strength. J. Bone Miner. Res. 2016;31:1366–1380. doi: 10.1002/jbmr.2808. [DOI] [PubMed] [Google Scholar]
- 77.Jilka R.L., O’Brien C.A., Roberson P.K., Bonewald L.F., Weinstein R.S., Manolagas S.C. Dysapoptosis of osteoblasts and osteocytes increases cancellous bone formation but exaggerates cortical porosity with age. J. Bone Miner. Res. 2014;29:103–117. doi: 10.1002/jbmr.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamashita J., Datta N.S., Chun Y.-H.P., Yang D.-Y., Carey A.A., Kreider J.M., Goldstein S.A., McCauley L.K. Role of Bcl2 in osteoclastogenesis and PTH anabolic actions in bone. J. Bone Miner. Res. 2007;23:621–632. doi: 10.1359/jbmr.071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagase Y., Iwasawa M., Akiyama T., Kadono Y., Nakamura M., Oshima Y., Yasui T., Matsumoto T., Hirose J., Nakamura H., et al. Anti-apoptotic molecule Bcl-2 regulates the differentiation, activation, and survival of both osteoblasts and osteoclasts. J. Biol. Chem. 2009;284:36659–36669. doi: 10.1074/jbc.M109.016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moriishi T., Kawai Y., Komori H., Rokutanda S., Eguchi Y., Tsujimoto Y., Asahina I., Komori T. Bcl2 deficiency activates foxo through akt inactivation and accelerates osteoblast differentiation. PLoS ONE. 2014;9:e86629. doi: 10.1371/journal.pone.0086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moriishi T., Maruyama Z., Fukuyama R., Ito M., Miyazaki T., Kitaura H., Ohnishi H., Furuichi T., Kawai Y., Masuyama R., et al. Overexpression of Bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosis. PLoS ONE. 2011;6:e27487. doi: 10.1371/journal.pone.0027487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teixeira C.C., Liu Y., Thant L.M., Pang J., Palmer G., Alikhani M. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J. Biol. Chem. 2010;285:31055–31065. doi: 10.1074/jbc.M109.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siqueira M.F., Flowers S., Bhattacharya R., Faibish D., Behl Y., Kotton D.N., Gerstenfeld L., Moran E., Graves D.T. FOXO1 modulates osteoblast differentiation. Bone. 2011;48:1043–1051. doi: 10.1016/j.bone.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ambrogini E., Almeida M., Martin-Millan M., Paik J.-H., DePinho R.A., Han L., Goellner J., Weinstein R.S., Jilka R.L., O’Brien C.A. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11:136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tzivion G., Dobson M., Ramakrishnan G. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta. 2011;1813:1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 86.Tyner S.D., Venkatachalam S., Choi J., Jones S., Ghebranious N., Igelmann H., Lu X., Soron G., Cooper B., Brayton C., et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 87.Lengner C.J., Steinman H.A., Gagnon J., Smith T.W., Henderson J.E., Kream B.E., Stein G.S., Lian J.B., Jones S.N. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J. Cell Biol. 2006;172:909–921. doi: 10.1083/jcb.200508130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sakai A., Sakata T., Tanaka S., Okazaki R., Kunugita N., Norimura T., Nakamura T. Disruption of the p53 gene results in preserved trabecular bone mass and bone formation after mechanical unloading. J. Bone Miner. Res. 2002;17:119–127. doi: 10.1359/jbmr.2002.17.1.119. [DOI] [PubMed] [Google Scholar]
- 89.Okazaki R., Sakai A., Ootsuyama A., Sakata T., Nakamura T., Norimura T. Trabecular bone mass and bone formation are preserved after limb immobilisation in p53 null mice. Ann. Rheum. Dis. 2004;63:453–456. doi: 10.1136/ard.2003.011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 2006;172:115–125. doi: 10.1083/jcb.200507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwartz K.A., Lanciloti N.J., Moore M.K., Campione A.L., Chandar N. p53 transactivity during in vitro osteoblast differentiation in a rat osteosarcoma cell line. Mol. Carcinog. 1999;25:132–138. doi: 10.1002/(SICI)1098-2744(199906)25:2<132::AID-MC8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 92.Zhang X., Tang N., Hadden T.J., Rishi A.K. Akt, foxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 93.Marks S.C., Odgren P.R. Structure and Development of the Skeleton. Volume 1. Academic Press; London, UK: 2002. pp. 3–15. [Google Scholar]
- 94.Scemes E., Spray D.C., Meda P. Connexins, pannexins, innexins: Novel roles of “hemi-channels”. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Penuela S., Gehi R., Laird D.W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta. 2013;1828:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 96.Chekeni F.B., Elliott M.R., Sandilos J.K., Walk S.F., Kinchen J.M., Lazarowski E.R., Armstrong A.J., Penuela S., Laird D.W., Salvesen G.S., et al. Pannexin 1 channels mediate ′find-me′ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gallagher J.A. ATP P2 receptors and regulation of bone effector cells. J. Musculoskelet. Neuronal Interact. 2004;4:125–127. [PubMed] [Google Scholar]
- 98.Buckley K.A., Hipskind R.A., Gartland A., Bowler W.B., Gallagher J.A. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone. 2002;31:582–590. doi: 10.1016/S8756-3282(02)00877-3. [DOI] [PubMed] [Google Scholar]
- 99.Korcok J., Raimundo L.N., Du X., Sims S.M., Dixon S.J. P2Y6 nucleotide receptors activate NF-κB and increase survival of osteoclasts. J. Biol. Chem. 2005;280:16909–16915. doi: 10.1074/jbc.M410764200. [DOI] [PubMed] [Google Scholar]
- 100.Lemaire I., Falzoni S., Zhang B., Pellegatti P., di Virgilio F. The P2X7 receptor and Pannexin-1 are both required for the promotion of multinucleated macrophages by the inflammatory cytokine GM-CSF. J. Immunol. 2011;187:3878–3887. doi: 10.4049/jimmunol.1002780. [DOI] [PubMed] [Google Scholar]
- 101.Iglesias R., Locovei S., Roque A., Alberto A.P., Dahl G., Spray D.C., Scemes E. P2X7 receptor-pannexin1 complex: Pharmacology and signaling. Am. J. Physiol. Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Noble B.S., Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol. Cell. Endocrinol. 2000;159:7–13. doi: 10.1016/S0303-7207(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 103.Cardoso L., Herman B.C., Verborgt O., Laudier D., Majeska R.J., Schaffler M.B. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J. Bone Miner. Res. 2009;24:597–605. doi: 10.1359/jbmr.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emerton K.B., Hu B., Woo A.A., Sinofsky A., Hernandez C., Majeska R.J., Jepsen K.J., Schaffler M.B. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46:577–583. doi: 10.1016/j.bone.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verborgt O., Gibson G.J., Schaffler M.B. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J. Bone Miner. Res. 2000;15:60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 106.Noble B. Bone microdamage and cell apoptosis. Eur. Cells Mater. 2003;6:46–55; discusssion 55. doi: 10.22203/ecm.v006a05. [DOI] [PubMed] [Google Scholar]
- 107.Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 109.Kennedy O.D., Laudier D.M., Majeska R.J., Sun H.B., Schaffler M.B. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014;64:132–137. doi: 10.1016/j.bone.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheung W.Y., Fritton J.C., Morgan S.A., Seref-Ferlengez Z., Basta-Pljakic J., Thi M.M., Suadicani S.O., Spray D.C., Majeska R.J., Schaffler M.B. Pannexin-1 and P2X7-receptor are required for apoptotic osteocytes in fatigued bone to trigger rankl production in neighboring bystander osteocytes. J. Bone Miner. Res. 2016;31:890–899. doi: 10.1002/jbmr.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zong W.X. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 112.Messmer D., Yang H., Telusma G., Knoll F., Li J., Messmer B., Tracey K.J., Chiorazzi N. High mobility group box protein 1: An endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 113.Park J.S., Svetkauskaite D., He Q., Kim J.Y., Strassheim D., Ishizaka A., Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 114.Lotze M.T., Tracey K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 115.Ibrahim Z.A., Armour C.L., Phipps S., Sukkar M.B. RAGE and TLRs: Relatives, friends or neighbours? Mol. Immunol. 2013;56:739–744. doi: 10.1016/j.molimm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 116.O’Brien C.A. Control of RANKL gene expression. Bone. 2010;46:911–919. doi: 10.1016/j.bone.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bivi N., Condon K.W., Allen M.R., Farlow N., Passeri G., Brun L.R., Rhee Y., Bellido T., Plotkin L.I. Cell autonomous requirement of connexin 43 for osteocyte survival: Consequences for endocortical resorption and periosteal bone formation. J. Bone Miner. Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu H., Gu S., Riquelme M.A., Burra S., Callaway D., Cheng H., Guda T., Schmitz J., Fajardo R.J., Werner S.L., et al. Connexin 43 channels are essential for normal bone structure and osteocyte viability. J. Bone Miner. Res. 2015;30:436–448. doi: 10.1002/jbmr.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ke H., Parron V.I., Reece J., Zhang J.Y., Akiyama S.K., French J.E. BCL2 inhibits cell adhesion, spreading, and motility by enhancing actin polymerization. Cell Res. 2010;20:458–469. doi: 10.1038/cr.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moriishi T., Fukuyama R., Ito M., Miyazaki T., Maeno T., Kawai Y., Komori H., Komori T. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of rankl in osteoblasts and sost in osteocytes at unloading. PLoS ONE. 2012;7:e40143. doi: 10.1371/journal.pone.0040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiong J., Onal M., Jilka R.L., Weinstein R.S., Manolagas S.C., O’Brien C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q., Bonewald L.F., Kodama T., Wutz A., Wagner E.F., et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]