Abstract
The observation of radiation-induced bystander responses, in which cells respond to their neighbors being irradiated, has important implications for understanding mechanisms of radiation action particularly after low-dose exposure. Much of this questions the current dogma of direct DNA damage driving response in irradiated systems. In this study, we have used a charged-particle microbeam to target individual helium ions (3He2+) to individual cells within a population of radioresistant glioma cells cultured alone or in coculture with primary human fibroblasts. We found that even when a single cell within the glioma population was precisely traversed through its cytoplasm with one 3He2+ ion, bystander responses were induced in the neighboring nonirradiated glioma or fibroblasts so that the yield of micronuclei was increased by 36% for the glioma population and 78% for the bystander fibroblast population. Importantly, the yield of bystander-induced micronuclei was independent of whether the cytoplasm or nucleus of a cell was targeted. The bystander responses were fully eliminated when the populations were treated with 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide or filipin, which scavenge nitric oxide (NO) and disrupt membrane rafts, respectively. By using the probe 4-amino-5-methylamino-2′,7′-difluorofluorescein, it was found that the NO level in the glioma population was increased by 15% after 1 or 10 cytoplasmic traversals, and this NO production was inhibited by filipin. This finding shows that direct DNA damage is not required for switching on of important cell-signaling mechanisms after low-dose irradiation and that, under these conditions, the whole cell should be considered a sensor of radiation exposure.
Recently, increasing experimental evidence has shown that the traversal of a cell nucleus by tracks of ionizing radiation is not a prerequisite for triggering a cellular response (1). Increases of chromosomal damage (2, 3), genomic instability (4–6), mutations (3, 7, 8), and malignant transformation (9, 10) have been observed in nonexposed cells that are in the vicinity of traversed cells or are recipients of conditioned medium from irradiated cultures. Although the mechanisms by which these bystander effects are induced are not well understood, several reports indicate that they can be mediated by gap junctional intercellular communication (11) and/or by soluble factors such as reactive oxygen species (12), cytokines (13), and nitric oxide (NO) (14, 15) released from irradiated cells. In addition, it has been recently shown that after exposure to conventional α-particles, mutations are induced in bystander cells by way of a cell-membrane-dependent signaling pathway (16).
These observations have profound consequences for current models of radiation risk, in that they suggest that physical dose and its targets within irradiated tissues may not be the sole driver of carcinogenic pathways and that cell–cell signaling processes between exposed and nonexposed cells may be important. This finding may be of particular importance for exposures at low doses, equivalent to environmental exposures where cells at risk only see single tracks of radiation at any one time (17). Bystander responses may also be relevant to the therapeutic environment because a mechanistic understanding of them may lead to approaches to enhance bystander responses in tumors and also possibly to protect surrounding normal tissue. Bystander effects are already a key requirement in gene-therapy approaches in which low transfection levels of therapeutic agents lead to the requirement for bystander-mediated toxicity (18, 19).
So far, most of the reported bystander responses are generated from nuclear irradiation. When conventional α-particle irradiations are used as a tool to study the bystander effect, only the nucleus, but not the targeted cytoplasm, has been considered in calculations of the fraction of targeted cells (11, 20). However, it has been found that cytoplasmic extracts from irradiated cells can induce DNA fragmentation in normal nuclei (21), and direct irradiation of the cell cytoplasm does cause cell killing and genetic mutations (22). Thus, the cytoplasm is also an important target for the genotoxic effects of ionizing radiation. It is largely unknown whether irradiation of the cytoplasm only can subsequently elicit any bystander response in nonirradiated cells. In this work, we used a sophisticated charged-particle microbeam to deliver exact numbers of helium ions through the cytoplasm of restricted numbers of cells within a population. Using a radioresistant glioma line, known to produce a significant bystander response, we found that, even when a single cell was irradiated through its cytoplasm only, a bystander response measured as the formation of micronuclei (MN) was induced in neighboring nonirradiated cells with the same or different genotypes to the targeted cells. Moreover, we found that NO, produced probably by the sphingomyelin pathway, was involved in the bystander response. These findings suggested that direct targeting of nuclear DNA is not always required for expression of a radiation-induced bystander effect.
Materials and Methods
Cell Culture and Treatments. Human primary fibroblast AG01522 (AG0) with WT p53 (wtp53) (11) and human glioblastoma T98G cells with mutated p53 (mp53) (23) were used for this study. AG0 cells were grown in α-MEM containing ribonucleosides supplemented with 18% FCS and 1% nonessential amino acids. T98G cells were maintained in RPMI medium 1640 supplemented with 10% FCS and 0.01% sodium pyruvate. Both culture media contained 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. All cultures were maintained at 37°C in an atmosphere of 95% air and 5% CO2. One day before microbeam irradiation, ≈1,000 plateau phase T98G cells were seeded in the central area (5 mm in diameter) of a specially designed microbeam dish consisting of a 3-μm-thick Mylar film base and cultured in RPMI medium 1640. In other experiments, T98G cells and AG0 cells were seeded in two separate regions ≈5 mm apart within the same microbeam dish and subsequently cocultured in α-MEM. T98G cells also had optimal growth in the α-MEM medium. The regions prepared for cell seeding had been pretreated with 1.7 μg/cm2 Cell-Tak adhesive (Collaborative Biomedical Products, Bedford, MA).
The cell cytoplasm was stained with 0.1 μg/ml Nile red 10 min before irradiation, and the cell nucleus was stained with 0.2 μg/ml Hoechst 33342 1 h before irradiation, enabling individual cytoplasm or nucleus to be identified by the microbeam system (24). During irradiation, the cells were maintained in the serum-free medium containing 10 mM Hepes to avoid any possibility of the production of short-lived SCE-inducing factor that might result from serum-containing medium exposed to α-particles (25). Immediately after irradiation, the medium was replaced with 2 ml of complete medium to allow cell culture for 24 h until further treatment for MN measurement. In some cases, cells were treated with 20 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (c-PTIO; Molecular Probes), a NO scavenger, during and after irradiation or with 0.5 μg/ml filipin (Sigma) 1 h before irradiation. Filipin abrogates signaling through glycosphingolipid-enriched membrane microdomains (GEMs) or rafts in variant cells (16, 26).
Microbeam Irradiation. The Gray Cancer Institute microbeam system was used for this study. It allows individual charged particles to be delivered to cells with high reproducibility (single ion delivered with >99% efficiency) and high accuracy (>99% within 2 μm) (24, 27). The position of each Nile red-stained cell cytoplasm or Hoechst-stained nucleus was found by using a computerized imaging system, and its coordinates were stored to be revisited and irradiated automatically. For the cytoplasmic irradiation, 1 or 10 randomly selected or 100% of T98G cells within the population were traversed individually at a single location through their cytoplasm with a precise number of 3.5 MeV (1 eV = 1.602 × 10–19 J), 100 keV/μm helium-3 ions (3He2+) at least 5 μm from nucleus. An automated scanning system involving recognition of both nuclear and cytoplasmic signals was used. Alternatively, for nuclear irradiation, the randomly selected cells were individually traversed through the center of the nucleus. Identical dishes of cells exposed with 1 or 10 3He2+ ions targeted through the medium 100 μm from cells were used as nonirradiated controls.
MN Scoring. The cytokinesis block technique was used to assay for MN in situ. After irradiation (24 h), the culture medium was replaced with one containing 1 μg/ml cytochalasin B. The cells were incubated for a further 26 h for T98G or for 48 h for AG0 and then fixed with methanol/acetic acid [9:1 (vol/vol)] for 20 min. Air-dried cells were stained with 10 μg/ml acridine orange. MN were scored in the binucleated cells and classified according to standard criteria (28). The MN yield, YMN, was calculated as the ratio of the number of MN to the scored number of binucleated cells.
NO Measurement. The cellular NO level was assayed in situ by using 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (Molecular Probes), which is cell-permeant and essentially nonfluorescent until it reacts with NO derivates such as N2O3, produced by oxidization of NO, to yield highly fluorescent benzotriazole. In brief, 24 h after cytoplasmic traversal, cells were treated with 3.5 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate for 45 min at 37°C. After the excess probe was removed, the cells were incubated for an additional 20 min to allow complete deesterification of the intracellular diacetates. The fluorescence images of 100–200 randomly selected cells per dish were captured by using a 3CCD color camera (Photonic Science, East Sussex, U.K.) on a fluorescent microscope (Zeiss Axioskop) with manual UV-light shutter and filters. The exposure conditions were normalized to allow quantitative comparisons of the relative fluorescence intensity of the cells between groups.
Statistical Analysis. Statistical analysis was done on the means of the data obtained from at least three independent experiments. Two replicates were counted for each experimental point in each experiment to determine the MN yield. All results are presented as means ± SEM. Significance was assessed by using Student's t test at P < 0.01.
Results
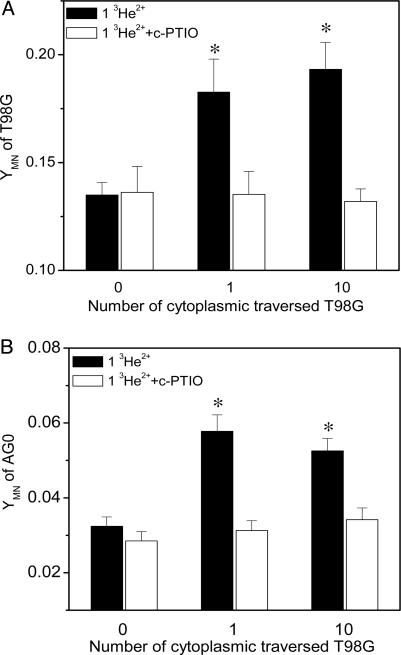
Direct Evidence of Bystander Responses Induced by Cytoplasmic Irradiation. When only a single human glioblastoma T98G cell within the population was traversed by a single 3He2+ ion, through its cytoplasm, chromosomal damage, measured as additional MN, was detected in the population 24 h after irradiation. The yield of MN increased by 36% (P < 0.01) from 0.135 in the nonirradiated controls to 0.183 (Fig. 1A), indicating that MN were produced in the neighboring nontraversed T98G cells. Moreover, it was found that the MN yield in the T98G population when 10 cells were targeted through their cytoplasm was not significantly different from that observed when a single cell was targeted, suggesting that the bystander response is independent of the number of cells targeted. This deduction was further confirmed by the finding that targeting all the cells within the T98G population through their cytoplasm only did not increase MN yields above bystander levels (see Fig. 3A).
Fig. 1.
Cytoplasmic traversal-induced MN production in T98G population (A) and bystander AG0 population (B) with or without c-PTIO treatment. One or 10 cells in the T98G population were individually irradiated through the cytoplasm with one helium ion (*, P < 0.01 compared with nonirradiated control). YMN, yield of MN.
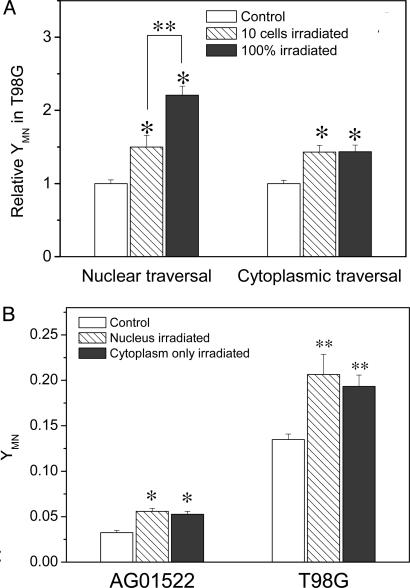
Fig. 3.
(A) Relative yield of MN (YMN) produced in T98G cells where either 10 or 100% of cells were irradiated with one helium ion through the nucleus or cytoplasm only. The MN yield of the nonirradiated T98G control was normalized as 1 (*, P < 0.01 compared with nonirradiated T98G control; **, P < 0.01 between 10-cell irradiation and 100% of cell irradiation). (B) Cytoplasmic traversal and nuclear traversal-induced MN produced in either the T98G or bystander AG0 populations. Ten cells in the T98G population were individually irradiated through cytoplasm or nucleus with one helium ion, respectively, (* and **, P < 0.01 compared with nonirradiated AG0 and T98G control, respectively).
To investigate whether the targeted T98G cells are harmful to neighboring normal cells, human primary fibroblast AG0 cells, previously also shown to exhibit bystander responses (29, 30), were cocultured with the irradiated T98G population. It was found that when only a single T98G cell was irradiated through its cytoplasm with one 3He2+ ion, 24 h after irradiation, the yield of MN in the nonirradiated AG0 population was increased by 78% (P < 0.01), compared with the control cocultured with a nonirradiated T98G population (Fig. 1B). Again, increasing the number of targeted T98G cells did not enhance the MN production in the bystander AG0 population. The results in Fig. 1 give direct evidence that the cytoplasmic traversal of even a single cell can trigger bystander responses not only in the same population but also in a neighboring population with a different genotype and radiosensitivity.
Soluble Bystander Signaling Factor. Because the cells were sparsely grown in one or two separated regions of the microbeam dish and no direct gap junctional intercellular communication existed between cells, radiation-induced soluble signaling factor(s) must be responsible for the bystander responses triggered by the cytoplasmic irradiation. Previously, we had demonstrated that when the nucleus of a T98G is targeted with microbeam-delivered 3He2+ ions, NO is subsequently involved in the bystander-induced cellular damage. To investigate whether NO contributes to these cytoplasmic traversal-induced bystander responses, we treated the cell populations with c-PTIO, a NO scavenger, during and after microbeam irradiation. It was found that this treatment completely eliminated the bystander MN induction in both populations of irradiated T98G and neighboring nonirradiated AG0 cells (see Fig. 1). Therefore, NO is also involved in the cytoplasmic irradiation-induced bystander response.
Bystander Response Mediated by Cell Membrane Signaling. The cytoplasmic irradiation-induced cellular damage suggests that some subcellular organelles outside of the cell nuclei may play a role as a radiation target. As a carrier of enzymes and receptors and the pathway for intracellular signaling molecule release, the cell membrane could be an important candidate involved in the radiation response. Thus, we treated cells with filipin to disrupt membrane GEMs. Fig. 2A illustrates that this treatment inhibited the induction of MN in the T98G population when 1 or 10 cells had been individually traversed through their cytoplasm with five 3He2+ ions. Moreover, the bystander MN induction in the nonirradiated AG0 population, which had been cocultured with the cytoplasmic-traversed T98G population, was also inhibited by this filipin treatment (Fig. 2B). Therefore, the cytoplasmic traversal-induced bystander responses were probably mediated through membrane GEMs.
Fig. 2.
Cytoplasmic traversal-induced MN production in the T98G population (A) and bystander AG0 population (B) with or without filipin treatment. One or 10 cells in the T98G population were individually irradiated through the cytoplasm with five helium ions (*, P < 0.01 compared with nonirradiated control).
Influence of Particle Number on the Bystander MN Induction. The data above showed that when the cytoplasm of a few T98G cells was traversed by one or five 3He2+ ions, the yield of MN in the irradiated T98G population (Figs. 1 A and 2 A) and that of the bystander nonirradiated AG0 population (Figs. 1B and 2B) were not significantly influenced by the number of particles. Thus, the physical dose is not a key factor in the cytoplasmic traversal-induced bystander response, and the energy deposited in the cytoplasm from one 3He2+ ion may be enough to fully trigger the response. These data are consistent with the finding that bystander responses are independent of the radiation dose and the linear energy transfer when a few cells within a confluent human primary fibroblast culture are targeted (30). They are also in agreement with the report that the medium harvested from cells irradiated with either 0.5 Gy or 5 Gy of γ-rays induces the same bystander responses (31).
Comparing the Bystander Responses Induced by Cytoplasmic or Nuclear Traversal. Fig. 3A illustrates that, when the fraction of cells with cytoplasmic traversals increases from ≈1% (10 cells) to 100%, the yield of MN in the T98G population does not increase. This result was in contrast to the situation where energy is deposited in the nucleus (and the overlying and underlying cytoplasm). For an increasing proportion of cells targeted through the nucleus, a greater response of MN induction was observed because of increasing direct effect (32).
The accepted paradigm for radiation effects at the cellular level is that direct nuclear irradiation is more serious than direct cytoplasmic traversal (33). To investigate whether the bystander response induced by nuclear irradiation is greater than that induced by cytoplasmic irradiation, we also measured the bystander MN formation induced by microbeam-targeted nuclear traversal. Fig. 3B illustrates representative bystander MN yields in both the nonirradiated T98G population and the cocultured AG0 population where 10 cells have been individually targeted through the cytoplasm or nucleus with one 3He2+, respectively. It was found that the yields of MN were not significantly different between nuclear-traversed and cytoplasmic-traversed T98G populations with both of them significantly higher than the nonirradiated control (P < 0.01). In addition, the T98G populations traversed through either nucleus or cytoplasm induced similar MN production in the bystander nonirradiated AG0 population.
Evidence for NO Production. To confirm the result of c-PTIO treatment (see Fig. 1) and obtain more direct evidence of NO being involved in the cytoplasmic irradiation-induced bystander responses, NO derivates were assayed in situ by using 4-amino-5-methylamino-2′,7′-difluorofluorescein 24 h after irradiation. A representative experiment showed that, when only one T98G cell was traversed through its cytoplasm with one 3He2+, the percentage of NO-positive cells was increased from 25% in the nonirradiated control to 55% (Fig. 4A) and that the relative fluorescence intensity was increased by 15% in the whole population (Fig. 4B). Moreover, the NO level did not vary when the number of cytoplasmic-targeted cells increased from 1 to 10, which supports the finding that increasing the numbers of cytoplasmically traversed cells does not enhance the bystander MN induction, as shown above.
Fig. 4.
Percentage of NO-positive cells (A) and relative NO-induced fluorescence intensity (B) in the cytoplasmic-traversed T98G population with or without treatment with c-PTIO or filipin. The fluorescence intensity of the nonirradiated T98G was considered as 1. A few cells within the T98G population were individually traversed through cytoplasm with one helium ion (*, P < 0.01 compared with nonirradiated control).
An interesting finding was that, when the T98G cells was pretreated with filipin before irradiation, the NO production in the population exposed to cytoplasmic irradiation was fully inhibited (Fig. 4), which demonstrates that NO production induced by cytoplasmic traversal is possibly mediated by cell membrane GEMs. In addition, as expected, the treatment of cells with c-PTIO also effectively diminished the NO level in the cytoplasm-irradiated population.
Further measurement showed that no significant change occurred in the NO level in the nonirradiated AG0 population before and after coculturing with the cytoplasmic-traversed T98G population (data not shown). These data also suggest that NO is not released from the bystander AG0 cells when they are perturbed by the cytoplasmic-irradiated T98G cells. This finding is consistent with the reports that inducible NO synthase cannot be expressed in cells with WT p53 after irradiation (34).
Discussion
Earlier studies with polonium-tipped microneedles to deliver α-particles of restricted range to either the nucleus or cytoplasm of Chinese hamster ovary cells strongly suggested that the nucleus was the critical site for cellular survival and mitotic delay, and the irradiation of cytoplasm was largely innocuous (35, 36). By using a charged-particle microbeam, however, to localize radiation exposure, recent evidence demonstrates that irradiation of cellular cytoplasm with exact number of α-particles results in genetic mutation although this cytoplasmic irradiation is largely nonlethal (22). The present study shows that, even when only a single cell is traversed through its cytoplasm by a precise number of helium-3 ions (as surrogates for α-particles), chromosomal damage measured as MN formation can be induced in other vicinal nonirradiated cells possessing the same or different genotypes to the targeted cell. This result provides direct evidence that extranuclear irradiation can induce extracellular bystander responses. These studies were performed in a radioresistant glioma cell line known to induce a NO-mediated bystander response after nuclear irradiation (32). A bystander response was also observed in cocultured primary human fibroblasts, which are significantly more radiosensitive than the glioma cells and also have been reported to show significant bystander responses to targeted particle irradiation (29, 30). The background level of spontaneous chromosomal damage is significantly different between these two cell lines (see Figs. 1 and 2). Despite this difference, the AG0 cells showed the larger percentage increase in responses under bystander conditions, suggesting that the underlying radiosensitivity of a cell population may be important in determining its sensitivity to bystander signals.
It has been suggested that reactive oxygen species play an important role in cytoplasmic irradiation-induced cellular effects (22). Our observation that bystander MN induction is eliminated when the cell populations are treated with c-PTIO, a NO scavenger, indicates that NO is involved in the cytoplasmic traversal-induced bystander response (see Fig. 1). The in situ measurement of NO shows that the NO level can be increased by 15% in the T98G population where only one cell had been targeted through its cytoplasm (Fig. 4). In general, NO molecules are believed to be induced by the activation of inducible NO synthase through the conversion of l-arginine to l-citrulline in the presence of NADPH and oxygen (37).
It is therefore of interest to know the pathway for NO production induced by the cytoplasmic irradiation. Our investigation shows that, when the membrane GEMs containing sphingomyelin (38) are disrupted by filipin, the production of intercellular NO (Fig. 4) and the subsequent bystander responses (Fig. 2) are fully inhibited. It has been found that the sphingomyelinases can be activated by irradiation (39, 40) even in isolated membranes devoid of nuclei (41). The activated sphingomyelinases will stimulate the expression of inducible NO synthase so that NO is produced (42, 43). Thus, most likely, the cytoplasmic traversal-induced NO production is mediated upstream by the membrane sphingomyelinase by the sphingomyelin signal-transduction pathway.
NO itself does not induce DNA strand breaks (44). Thus, the bystander MN induction should result from NO-downstream products and/or NO derivatives. It has been reported that some cytokines such as tumor growth factor type β can be produced by NO stress in a time- and dose-dependent fashion (45, 46) and that cells treated with tumor growth factor type β1 indeed undergo a time-dependent increase in DNA cleavage (47) where reactive oxygen species may be involved (13). Therefore, cytokines including tumor growth factor type β1 are likely to be candidates for NO-downstream-signaling factors involved in the cytoplasmic traversal-induced bystander responses.
Compared with nuclear traversal, cytoplasmic traversal of every cell within a population has less effect on the irradiated cells (22). However, the present study indicates that cytoplasmic traversal can induce a bystander response similar to that induced by nuclear traversal. Also, targeting every cell through the cytoplasm induces the same level of effect as that observed when only one cell is targeted and a bystander response is produced. This result is in contrast to the situation where every cell is targeted through the nucleus and a large effect is observed because of direct damage in cellular DNA (see Fig. 3A). Taken together with the observation that cytoplasmic traversal, either in 1 or 10 cells with one or five particles, elicits similar bystander MN induction, it can be suggested that a signal-amplification process might play an important role in the bystander response. When a few cells in the population are individually targeted through their cytoplasm, signaling factors, perhaps including cytokines, produced downstream of NO are released from targeted cells. These factors may attack several vicinal nonirradiated cells simultaneously, such that the newly damaged cells will release further reactive species and cause other cells to be damaged so that a measurable bystander response is produced.
In summary, our results show that, after cytoplasmic traversal of a T98G cell, NO is produced through a cell membrane (GEMs)-related pathway and this contributes to the cytoplasmic traversal-induced bystander responses in both glioma cells and cocultured primary fibroblasts. It has been known that radiation-induced NO regulates tumor microenvironments leading to angiogenesis and vasorelaxation (48, 49). Therefore, understanding the mechanisms will make it possible to develop techniques for decreasing radiation damage and potential carcinogenesis in normal tissues by reducing bystander signaling or maximizing cell sterilization during radiotherapy by amplifying bystander responses in tumors. For radiation risk, it is clear that the biological penumbra of localized irradiation of one or a few cells within the population is much greater than the physical dose penumbra and if this translates into in vivo responses, this will need to be included in models of radiation risk.
Acknowledgments
We thank Stuart Gilchrist and Bob Sunderland for assistance with the microbeam irradiations. This work was supported by Cancer Research UK and the Gray Cancer Institute.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NO, nitric oxide; MN, micronucleus; c-PTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide; GEMs, glycosphingolipid-enriched membrane microdomains.
References
- 1.Morgan, W. F. (2003) Oncogene 22, 7094–7099. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande, A., Goodwin, E. H., Bailey, S. M., Marrone, B. L. & Lehnert, B. E. (1996) Radiat. Res. 145, 260–267. [PubMed] [Google Scholar]
- 3.Nagasawa, H. & Little, J. B. (1999) Radiat. Res. 152, 552–557. [PubMed] [Google Scholar]
- 4.Seymour, C. B. & Mothersill, C. (1997) Radiat. Oncol. Investig. 5, 106–110. [DOI] [PubMed] [Google Scholar]
- 5.Limoli, C. L., Ponnaiya, B., Corcoran, J. J., Giedzinski, E., Kaplan, M. I., Hartmann, A. & Morgan, W. F. (2000) Adv. Space Res. 25, 2107–2117. [DOI] [PubMed] [Google Scholar]
- 6.Morgan, W. F. (2003) Radiat. Res. 159, 567–580. [DOI] [PubMed] [Google Scholar]
- 7.Zhou, H., Randers-Pehrson, G., Waldren, C. A., Vannais, D., Hall, E. J. & Hei, T. K. (2000) Proc. Natl. Acad. Sci. USA 97, 2099–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little, J. B. (2000) Carcinogenesis 21, 397–404. [DOI] [PubMed] [Google Scholar]
- 9.Sawant, S. G., Randers-Pehrson, G., Geard, C. R., Brenner, D. J. & Hall, E. J. (2001) Radiat. Res. 155, 397–401. [DOI] [PubMed] [Google Scholar]
- 10.Lewis, D. A., Mayhugh, B. M., Qin, Y., Trott, K. & Mendonca, M. S. (2001) Radiat. Res. 156, 251–258. [DOI] [PubMed] [Google Scholar]
- 11.Azzam, E. I., de Toledo, S. M. & Little, J. B. (2001) Proc. Natl. Acad. Sci. USA 98, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayanan, P. K., Goodwin, E. H. & Lehnert, B. E. (1997) Cancer Res. 57, 3963–3971. [PubMed] [Google Scholar]
- 13.Iyer, R. & Lehnert, B. E. (2000) Cancer Res. 60, 1290–1298. [PubMed] [Google Scholar]
- 14.Shao, C., Furusawa, Y., Aoki, M., Matsumoto, H. & Ando, K. (2002) Int. J. Radiat. Biol. 78, 837–844. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto, H., Hayashi, S., Hatashita, M., Ohnishi, K., Shioura, H., Ohtsubo, T., Kitai, R., Ohnishi, T. & Kano, E. (2001) Radiat. Res. 155, 387–396. [DOI] [PubMed] [Google Scholar]
- 16.Nagasawa, H., Cremesti, A., Kolesnick, R., Fuks, Z. & Little, J. B. (2002) Cancer Res. 62, 2531–2534. [PubMed] [Google Scholar]
- 17.Brenner, D. J., Doll, R., Goodhead, D. T., Hall, E. J., Land, C. E., Little, J. B., Lubin, J. H., Preston, D. L., Preston, R. J., Puskin, J. S., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 13761–13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesnil, M., Piccoli, C., Tiraby, G., Willecke, K. & Yamasaki, H. (1996) Proc. Natl. Acad. Sci. USA 93, 1831–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesnil, M. & Yamasaki, H. (2000) Cancer Res. 60, 3989–3999. [PubMed] [Google Scholar]
- 20.Nagasawa, H. & Little, J. B. (1992) Cancer Res. 52, 6394–6396. [PubMed] [Google Scholar]
- 21.Kurihara, H., Torigoe, S., Omura, M., Saito, K., Kurihara, M. & Matsubara, S. (1998) Radiat. Res. 150, 269–274. [PubMed] [Google Scholar]
- 22.Wu, L. J., Randers-Pehrson, G., Xu, A., Waldren, C. A., Geard, C. R., Yu, Z. & Hei, T. K. (1999) Proc. Natl. Acad. Sci. USA 96, 4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto, H., Shimura, M., Omatsu, T., Okaichi, K., Majima, H. & Ohnishi, T. (1994) Cancer Lett. 87, 39–46. [DOI] [PubMed] [Google Scholar]
- 24.Folkard, M., Vojnovic, B., Prise, K. M., Bowey, A. G., Locke, R. J., Schettino, G. & Michael, B. D. (1997) Int. J. Radiat. Biol. 72, 375–385. [DOI] [PubMed] [Google Scholar]
- 25.Lehnert, B. E. & Goodwin, E. H. (1997) Cancer Res. 57, 2164–2171. [PubMed] [Google Scholar]
- 26.Orlandi, P. A. & Fishman, P. H. (1998) J. Cell Biol. 141, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkard, M., Vojnovic, B., Hollis, K. J., Bowey, A. G., Watts, S. J., Schettino, G., Prise, K. M. & Michael, B. D. (1997) Int. J. Radiat. Biol. 72, 387–395. [DOI] [PubMed] [Google Scholar]
- 28.Albertini, R. J., Anderson, D., Douglas, G. R., Hagmar, L., Hemminki, K., Merlo, F., Natarajan, A. T., Norppa, H., Shuker, D. E., Tice, R., et al. (2000) Mutat. Res. 463, 111–172. [DOI] [PubMed] [Google Scholar]
- 29.Belyakov, O. V., Malcolmson, A. M., Folkard, M., Prise, K. M. & Michael, B. D. (2001) Br. J. Cancer 84, 674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao, C., Furusawa, Y., Kobayashi, Y., Funayama, T. & Wada, S. (2003) FASEB J. 17, 1422–1427. [DOI] [PubMed] [Google Scholar]
- 31.Lyng, F. M., Seymour, C. B. & Mothersill, C. (2000) Br. J. Cancer 83, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao, C., Stewart, V., Folkard, M., Michael, B. D. & Prise, K. M. (2003) Cancer Res. 63, 8437–8442. [PubMed] [Google Scholar]
- 33.Hall, E. J. (2000) Radiobiology for the Radiologist (Lippincott, Philadelphia).
- 34.Matsumoto, H., Hayashi, S., Hatashita, M., Shioura, H., Ohtsubo, T., Kitai, R., Ohnishi, T., Yukawa, O., Furusawa, Y. & Kano, E. (2000) Int. J. Radiat. Biol. 76, 1649–1657. [DOI] [PubMed] [Google Scholar]
- 35.Munro, T. R. (1970) Radiat. Res. 44, 747–757. [PubMed] [Google Scholar]
- 36.Munro, T. R. (1970) Radiat. Res. 42, 451–470. [PubMed] [Google Scholar]
- 37.Hobbs, A. J., Fukuto, J. M. & Ignarro, L. J. (1994) Proc. Natl. Acad. Sci. USA 91, 10992–10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons, K. & Ikonen, E. (1997) Nature 387, 569–572. [DOI] [PubMed] [Google Scholar]
- 39.Komatsu, M., Takahashi, T., Abe, T., Takahashi, I., Ida, H. & Takada, G. (2001) Biochim. Biophys. Acta 1533, 47–54. [DOI] [PubMed] [Google Scholar]
- 40.Magnoni, C., Euclidi, E., Benassi, L., Bertazzoni, G., Cossarizza, A., Seidenari, S. & Giannetti, A. (2002) Toxicol. In Vitro 16, 349–355. [DOI] [PubMed] [Google Scholar]
- 41.Haimovitz-Friedman, A., Kan, C. C., Ehleiter, D., Persaud, R. S., McLoughlin, M., Fuks, Z. & Kolesnick, R. N. (1994) J. Exp. Med. 180, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsuyama, K., Shichiri, M., Marumo, F. & Hirata, Y. (1998) Endocrinology 139, 4506–4512. [DOI] [PubMed] [Google Scholar]
- 43.Pahan, K., Sheikh, F. G., Khan, M., Namboodiri, A. M. & Singh, I. (1998) J. Biol. Chem. 273, 2591–2600. [DOI] [PubMed] [Google Scholar]
- 44.Wink, D. A., Hanbauer, I., Grisham, M. B., Laval, F., Nims, R. W., Laval, J., Cook, J., Pacelli, R., Liebmann, J., Krishna, M., et al. (1996) Curr. Top. Cell. Regul. 34, 159–187. [DOI] [PubMed] [Google Scholar]
- 45.Vodovotz, Y., Chesler, L., Chong, H., Kim, S. J., Simpson, J. T., DeGraff, W., Cox, G. W., Roberts, A. B., Wink, D. A. & Barcellos-Hoff, M. H. (1999) Cancer Res. 59, 2142–2149. [PubMed] [Google Scholar]
- 46.Ayache, N., Boumediene, K., Mathy-Hartert, M., Reginster, J. Y., Henrotin, Y. & Pujol, J. P. (2002) Osteoarthritis Cartilage 10, 344–352. [DOI] [PubMed] [Google Scholar]
- 47.Cain, K., Inayat-Hussain, S. H., Couet, C., Qin, H. M. & Oberhammer, F. A. (1996) Cytometry 23, 312–321. [DOI] [PubMed] [Google Scholar]
- 48.Sonveaux, P., Brouet, A., Havaux, X., Gregoire, V., Dessy, C., Balligand, J. L. & Feron, O. (2003) Cancer Res. 63, 1012–1019. [PubMed] [Google Scholar]
- 49.Sonveaux, P., Dessy, C., Brouet, A., Jordan, B. F., Gregoire, V., Gallez, B., Balligand, J. L. & Feron, O. (2002) FASEB J. 16, 1979–1981. [DOI] [PubMed] [Google Scholar]