Abstract
Enhanced proliferation and survival are common features of cancer cells. Cancer cells are metabolically reprogrammed which aids in their survival in nutrient-poor environments. Indeed, changes in metabolism of glucose and glutamine are essential for tumor progression. Thus, metabolic reprogramming is now well accepted as a hallmark of cancer. Recent findings suggest that reprogramming of lipid metabolism also occurs in cancer cells, since lipids are used for biosynthesis of membranes, post-translational modifications, second messengers for signal transduction, and as a source of energy during nutrient deprivation. The tumor suppressor p53 is a transcription factor that controls the expression of proteins involved in cell cycle arrest, DNA repair, apoptosis, and senescence. p53 also regulates cellular metabolism, which appears to play a key role in its tumor suppressive activities. In this review article, we summarize non-canonical functions of wild-type and mutant p53 on lipid metabolism and discuss their association with cancer progression.
Keywords: p53, lipid metabolism, cancer, mevalonate pathway, fatty acid oxidation
1. Introduction
Cancer cells have an extraordinary ability to adapt themselves to adverse environments. In other words, cancers can change their characteristics by modifying pathways or altering protein expression so that they can survive and expand in various environments. Cancer cells usually reside in poor oxygen and nutrition environments, and hence attempt to reprogram cellular metabolism [1].
There are common modifications in metabolic pathways that are well correlated with cancer progression [2]. Warburg et al. [3] first observed that cancer cells exhibit an increase in glucose uptake and glycolysis as an adaptive event in nutrient deprived conditions. This leads to enhanced glucose dependence and increased lactate production in cancer cells, also referred to as the Warburg effect. However, glucose is not the only biomolecule on which cancer cells rely. Glutamine consumption is also high in cancer cells, likely due to an increase in demand of carbon structures and amino-nitrogen which are required for nucleotide, protein, and lipid synthesis [4]. Besides these changes in glucose and amino acid metabolism, fast-proliferating cancer cells also have high avidity for lipids (fatty acids and cholesterol) which are mainly used for biosynthesis of structural components of the cells (membranes), as well as for production of energy during nutrient deprivation [5]. In physiological conditions, lipid synthesis is restricted to specialized tissues, such as liver and adipose tissues, while normal cells from other tissues obtain lipids directly from the bloodstream [5]. On the other hand, cancer cells often gain the ability to synthesize lipids and show enhanced lipid uptake [6]. Several studies have shown that upregulation of enzymes involved in the synthesis of fatty acids and cholesterol (mevalonate pathway) is required for tumor progression [5,6,7,8]. Specifically, a wide variety of tumors have increased expression of acetyl-CoA-carboxylase (ACC) [6] that catalyzes the conversion of acetyl-CoA to malonyl-CoA, the rate-limiting step in the lipid biosynthesis [5]. Another enzyme involved in lipid metabolism and highly overexpressed in cancers is fatty acid synthase (FAS), an enzyme that converts acetyl-CoA to palmitic acid [9]. High expression of this enzyme is also correlated with poor prognosis in cancer patients [9]. Additionally, tumors manifest an increase in the expression of ATP citrate lyase (ACLY) which catalyzes the conversion of citrate to oxaloacetic acid and acetyl-CoA, as well as an increase in transcriptional activities of the sterol regulatory element binding proteins (SREBPs); both ACLY and SREBPs promote cholesterol production [10].
Tumor suppressor p53 is the most frequently mutated human gene in cancer [11]. p53 suppresses tumorigenesis by regulating transcription of numerous downstream target genes that govern cell cycle progression and cell death [12]. p53 also regulates the expression of metabolism-associated proteins, and some of them play key roles in tumor suppression [13,14,15]. Previous reports have shown that both wild-type and mutant p53 are involved in the glucose metabolism and nucleotide synthesis by regulating levels or activities of glucose-6-phosphate dehydrogenase (G6PD) [16]; TP53-induced glycolysis and the apoptosis regulator (TIGAR) [17]; glucose transporter 1 (GLUT1) [18]; deoxycytidine kinase (dCK); and many other nucleotide metabolism-related proteins [19], which is described in details elsewhere. In this review article, we summarize studies that focus on the contributions of both wild-type and mutant p53 to lipid metabolism.
2. Regulation of Lipid Metabolism by Wild-Type p53
There is an increasing number of studies showing that p53 regulates lipid metabolism by transcriptional control or protein–protein interaction. The following are effectors associated with lipid metabolism whose activities or levels are regulated by p53 (Table 1).
Table 1.
Targets of wild-type p53 in lipid metabolism.
| Targets | Effects of Wtp53 | Biological Consequence | Reference |
|---|---|---|---|
| G6PD | Inhibit the activity by physical binding. | Loss of p53 activates G6PD and the pentose pathway, leading to lipid accumulation in the liver. | [16] |
| SREBP-1 | Transcriptionally repress the expression. | Disruption of p53 in ob/ob mice restores the expression of lipogenic enzymes regulated by SREBP-1. | [20] |
| SIRT1 | A complex of p53 and Foxo3a transactivates SIRT1. | In p53−/− mice, nutrient starvation fails to increase SIRT1. It remains unclear whether increased lipid accumulation in p53−/− mice is due to attenuated SIRT1 levels. |
[21] |
| Aromatase | Transcriptionally increase the expression. | p53−/− mice have lower levels of aromatase, resulting in higher levels of testosterone and lipid accumulation, which is nullified by transgenic expression of aromatase. | [22] |
| Acad11 | Transcriptionally increase the expression. | Although Acad11 plays a key role in p53-mediated OXPHOS and cell survival upon glucose starvation, it is unclear whether increased Acad11 levels by p53 enhance fatty acid β-oxidation and how enhanced fatty acid β-oxidation contributes to cell survival. | [23] |
| Lipin1 | Transcriptionally increase the expression. | Glucose restriction in C2C12 cells phosphorylates p53, leading to upregulation of Lipin1 and fatty acid oxidation. | [24] |
| MCD | Transcriptionally increase the expression. | Mdm2C305F mice show attenuated MCD induction and increased fatty acid accumulation in the liver under ribosomal stress, due to lack of inhibitory effects of RPs on Mdm2 and reduction in the p53 activity. | [25] |
| DHRS3 | Transcriptionally increase the expression. | Activation of p53 upregulates DHRS3 which is associated with lipid droplets accumulation. | [26,27] |
| Caveolin 1 | Transcriptionally increase the expression. | Overexpression of p53 upregulates Caveolin 1, leading to redution in intracellular free choleserol and viable cell growth. | [28] |
2.1. Glucose-6-Phosphate Dehydrogenase (G6PD)
G6PD is a rate-limiting enzyme that catalyzes the first step in the pentose phosphate pathway (PPP). G6PD activation also increases NADPH production, which is required for lipid biosynthesis (Table 1) [29,30]. Interestingly, the carboxy (C)-terminal region of wild-type p53 directly binds with G6PD and inhibits its function (Figure 1A) [16]. Also, G6PD activity is increased in mouse embryonic fibroblasts (MEFs) and several tissues from p53−/− mice compared with those from wild-type mice [16]. Moreover, p53−/− MEFs and p53−/− colorectal carcinoma cell line HCT116 have increase in glucose uptake, PPP influx, and lipid accumulation, as compared with their counterparts having wild-type p53. A lack of p53 also results in a G6PD-dependent increase in NADPH in HCT116 cells [16]. These observations suggest that wild-type p53 reduces production of NADPH and inhibits accumulation of lipids by its direct binding to G6PD (Figure 1B). Importantly, an elevated expression of G6PD is correlated with poor clinical prognosis in esophageal squamous cell carcinoma [31]. Given that enhanced lipid biosynthesis is a common feature of cancer cells, inhibition of G6PD activity by p53 could contribute to p53-mediated tumor suppression.
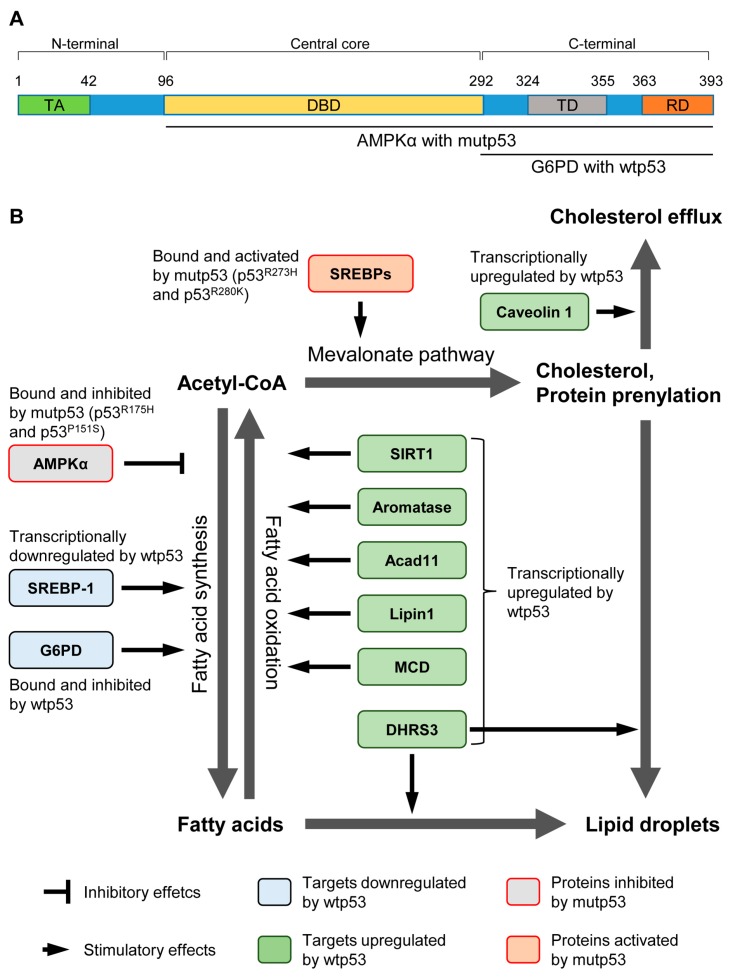
Figure 1.
Regulation of lipid metabolism by wild-type and mutant p53. (A) Schematic representation of functional domains in p53 and regions which interact with G6PD and AMPKα. TA: transactivation domain, DBD: DNA-binding domain, TD: tetramerization domain, RD: regulatory domain; (B) Wild-type p53 (wtp53) can regulate lipid metabolism by direct protein–protein interaction or transcriptional control of proteins involved in fatty acid synthesis, fatty acid oxidation, the mevalonate pathway, cholesterol efflux, and lipid droplet formation. Generally, wtp53 inhibits the fatty acid synthesis and lipid accumulation. In contrast, mutant p53 (mutp53) enhances fatty acid synthesis by inhibitory interaction with AMPKα. Also, mutp53 cooperates with SREBPs to upregulate enzymes involved in the mevalonate pathway.
2.2. Sterol Regulatory Element-Binding Protein-1 (SREBP-1)
SREBPs are a family of basic helix–loop–helix leucine zipper transcription factors that control the expression of a range of lipogenic enzymes required for the synthesis of cholesterol, fatty acid, triacylglycerol, and phospholipid (Table 1) [32]. Specifically, SREBP-1, but not SREBP-2, is shown to be well correlated with fatty acids synthesis induced by refeeding following fasting in mice [33]. SREBP-1 is frequently upregulated in multiple types of cancer, including glioblastoma and prostate cancer, and contributes towards tumor progression [34,35]. Also, levels of SREBP-1 are found to be negatively correlated with p53 levels in mice when fasting followed by refeeding [20]. Interestingly, ob/ob mice show reduced levels of SREBP-1 and its target enzymes with increase in p53 levels [20]. Moreover, p53 deletion in ob/ob mice partially restores the levels of SREBP-1 and its downstream targets, including fatty acid synthase (FAS) [20]. Mechanistically, the exogenous expression of p53 in p53-null Saos2 osteosarcoma cells reduces the promoter activity of the SREBP-1c gene (Figure 1B) [20]. However, it still remains unclear how significantly a decrease in SREBP-1 levels contributes to p53-mediated tumor suppression.
2.3. Sirtuin 1 (SIRT1)
SIRT1 is an evolutionarily conserved NAD+-dependent protein deacetylase that targets proteins involved in fat cell maturation and accumulation, nutrient sensing, and regulation of cellular metabolism [36]. In hepatocyte-specific SIRT1 knockout mice fed a high fat diet (HFD), there is a decrease in PPARα signaling and fatty acid β-oxidation, leading to an accumulation of fatty acids in the liver and hepatic steatosis (Figure 1B) [37]. Also, knockdown of SIRT1 leads to an increase in acetylation and activities of the liver X receptor (LXR) and SREBP-1 transcription factors, both of which contribute to the accumulation of lipids [38,39,40].
Interestingly, SIRT1 mRNA expression is induced by a complex of p53 and the forkhead transcription factor Foxo3a [21]. Upon nutrient starvation, p53 binds with Foxo3a, and this complex transactivates SIRT1 through two p53-responsive elements in its promoter (Table 1) [21]. In p53−/− mice, nutrient starvation fails to increase the SIRT1 expression [21]. Although p53−/− mice show higher levels of lipid accumulation when fed a HFD as compared with wild-type mice [22], it remains unclear whether this is caused by attenuation in SIRT1 levels in p53−/− mice.
2.4. Aromatase
Aromatase is the enzyme that catalyzes the final step in the biosynthesis of estrogens and is encoded by the Cyp19 gene. Disruption of Cyp19 in mice leads to increased adipocyte volume, hyperleptinemia, hyperinsulinemia, and hypercholesterolemia, when compared to wild-type mice, indicating that aromatase plays a key role in lipid metabolism [41]. A recent study from Wang et al. [22] shows that conversion of testosterone by aromatase is impaired in p53−/− mice, since p53−/− mice have higher levels of testosterone and lower levels of β-estradiol in the blood than wild-type mice when fed a HFD. They also show that p53 can transcriptionally upregulate aromatase [22]. Moreover, increased lipid accumulation in the livers of p53−/− mice is substantially nullified by transgenic expression of aromatase [22]. Given that Cyp19 (aromatase) knockout mice show accumulation of lipid droplets in the liver [22,41], these observations strongly indicate that reduced aromatase activity following loss of p53 is at least partially responsible for the lipid accumulation in the p53−/− livers (Table 1). Thus, p53 regulates lipid metabolism in an aromatase-dependent manner (Figure 1B).
2.5. Acyl-CoA Dehydrogenase Family Member 11 (Acad11)
Acad11 belongs to a family of enzymes that catalyze the first step in every cycle of fatty acid β-oxidation in mitochondria [42]. Acad11 is shown to be a p53 target and contributes to pro-survival function of p53 during glucose starvation (Figure 1B) [23]. Expression of Acad11 is significantly higher following doxorubicin treatment in wild-type MEFs than that in p53−/− MEFs, due to the binding of p53 to its responsive elements present in introns of the Acad11 gene [23]. Intriguingly, upon glucose starvation in MEFs expressing HRasV12, p53 promotes oxidative phosphorylation (OXPHOS) and cell survival in a manner dependent on Acad11 [23]. However, it is unclear whether increased Acad11 levels by p53 enhance fatty acid β-oxidation and how enhanced fatty acid β-oxidation contributes to cell survival under low glucose conditions (Table 1).
2.6. Lipin 1
Lipin 1 is a nuclear protein that is required for normal adipose tissue development [43]. Lipin 1 upregulates PPARα and activates the mitochondrial fatty acid oxidative metabolism (Table 1) [44]. Assaily et al. [24] show that p53 can upregulate Lipin 1 mRNA levels through three p53 binding sites in intron 1. Also, γ-irradiation in wild-type mice, but not in p53−/− mice, upregulates Lipin 1 mRNA levels in the spleen, thymus, and bone morrow [24]. Additionally, glucose restriction in mouse myoblast C2C12 cells phosphorylates p53 at Ser18 (Ser15 in humans), leading to the upregulation of Lipin 1 and fatty acid oxidation (Table 1) [24]. These observations indicate that increased Lipin 1 by p53 upon glucose deprivation enhances fatty acid oxidation (Figure 1B) [24]. However, it still remains unknown whether Lipin 1 plays a role in p53-mediated tumor suppression.
2.7. Malonyl-CoA Decarboxylase (MCD)
MCD that catalyzes the conversion of malonyl-CoA to acetyl-CoA is a key enzyme in lipid metabolism and enhances fatty acid oxidation, hence preventing fatty acids synthesis and lipid accumulation [45]. Importantly, a recent study using an inducible p53ERTAM MEF cell system identifies MCD as a p53 target; activation of p53 via Mdm2 inhibition with nutlin-3 significantly increases MCD mRNA levels in wild-type, but not p53−/−, MEFs through p53’s binding to the promoter and intron regions of the MCD gene [25]. Liu et al. [25] also generate mice having a cancer-associated mutation in the Mdm2 gene at codon 305 (Mdm2C305F) which disrupts the interaction of Mdm2 with ribosomal proteins (RPs), RPL11 and RPL5; during ribosomal stress, these RPs bind to Mdm2 and inhibit Mdm2’s function towards p53, hence activating p53 [46]. Interestingly, under ribosomal stress by fasting, Mdm2C305F mice show attenuated MCD induction, increase in malonyl-CoA, and increased fatty acid accumulation in the liver, due to the failure of RPs to bind with Mdm2 and the subsequent reduction in the p53 activity, as compared with mice having wild-type Mdm2 (Table 1) [25]. Thus, p53 inhibits lipid accumulation in the liver by transactivating MCD (Figure 1B). However, the contribution of MCD to p53-mediated tumor suppression needs to be investigated.
2.8. Dehydrogenase/Reductase 3 (DHRS3)
DHRS3, also known as retinal short-chain dehydrogenase/reductase (retSDR1), is a highly conserved member of the short chain alcohol dehydrogenase/reductase superfamily and is involved in maintaining the cellular supply of retinol metabolites [47]. DHSRS3 is localized in the endoplasmic reticulum (ER) and lipid droplets, both of which are storage vesicles containing lipid, such as cholesterol and retinol esters [48]. Additionally, overexpression of DHRS3 in U2OS cells increases lipid droplet accumulation (Figure 1B) [26]. Doxorubicin treatment is shown to increase the DHRS3 mRNA expression in HCT116 cells having wild-type p53, but not HCT116 p53-null cells (Table 1) [27]. Indeed, there are two p53 responsible elements in the DHRS3 promoter (Figure 1B) [27]. Deisenroth et al. [26] also show that overexpression of p53 in 3T3-L1 preadipocytes increases lipid droplet formation and the DHRS3 mRNA expression is increased upon p53 induction in MEFs expressing p53ERTAM in the absence of functional MDM2 (Mdm2-null or Mdm2C462A). Thus, p53 is involved in the accumulation of lipid droplets. However, it remains unclear whether p53-induced lipid droplet accumulation contributes to tumor suppression.
2.9. Caveolin 1
Caveolin 1 is the main component of the caveolae plasma membranes present in many different cell types [49]. Caveolin 1 is a scaffolding protein which binds to free cholesterol and is implicated in free cholesterol efflux [50]. Bist et al. [28] report that p53 binds to the promoter of caveolin 1 in human skin fibroblasts. Overexpression of wild-type p53 results in an increase in the caveolin 1 promoter activity and a decrease in intracellular free cholesterol in human skin fibroblasts, whereas mutant p53 fails to do so, suggesting that p53 increases cholesterol efflux, possibly through the upregulation of caveolin 1 (Figure 1B) [28]. Reduced intracellular free cholesterol through p53-induced caveolin 1 is correlated with decreased viable cell growth of human skin fibroblasts (Table 1) [28]. In line with this study, overexpression of caveolin 1 is shown to increase cholesterol efflux and reduce the proliferation of human skin fibroblasts [51]. However, dysregulation of caveolin 1 expression is found in multiple types of cancer [52,53,54]. It should be clarified in which cellular context caveolin 1 functions as a tumor suppressor or an oncogene and whether altered intracellular cholesterol levels by caveolin 1 could suppress or promote cancer progression.
3. Roles of Mutant p53 in the Lipid Metabolism
Mutations in p53 are common events in the majority of cancers. Increasing evidence has demonstrated that many p53 mutants contribute to cancer progression by altering activities of other transcription factors, such as p63, p73, Ets2, and Nrf2, as their gain-of-function (GOF) activities [55,56,57]. Intriguingly, p53R273H and p53R280K GOF mutants are shown to bind with SREBPs, leading to their activation that causes the upregulation of enzymes involved in the mevalonate pathway (Figure 1B) [58]. This leads to an increase in cholesterol synthesis and protein prenylation (farnesylation and geranylgeranylation), which accelerates growth in the 3-dimensional culture of breast cancer cells (Table 2) [58]. Also, the presence of mutations in p53 correlates with high levels of enzymes involved in the mevalonate pathway in human breast cancer tissues [58]. These observations suggest the possibility that mutant p53 contributes to breast cancer progression through the upregulation of cholesterol production and protein prenylation.
Table 2.
Targets of mutant p53 in lipid metabolism.
| Targets | Effects of Mutp53 | Biological Consequence | References |
|---|---|---|---|
| SREBPs | Bind and activate the transcription activity. | In breast cancer cells expressing mutp53, increased activities of SREBPs enhance the mevalonate pathway and accelerate growth in the 3D culture. | [58] |
| AMPK | Bind and inhibit the kinase activity. | GOF p53 mutants bind to and inhibit AMPK activity. It remains unclear how significantly the mutp53’s inhibitory effect on AMPK contributes to fatty acid synthesis and tumor progression. | [59] |
A recent study has identified AMP-activated protein kinase (AMPK) as a novel binding partner of mutant p53 [59]. AMPK is a heterotrimeric protein kinase and is activated by low nutrient or energy levels [60]. Activated/phosphorylated AMPK inhibits fatty acid synthesis by phosphorylation and inhibition of both acetyl-CoA-carboxylase (ACC) and SREBP-1 (Table 2), thus linking availability of nutrients with lipid metabolism [61,62]. Zhou et al. [59] show that the ectopic expression of p53 GOF mutants (p53R175H and p53P151S) inhibits AMPK activity and subsequently reduces phosphorylation of ACC under glucose and serum starvation in a p53-null head and neck squamous cell carcinoma (HNSCC) cell line, UMSCC1. AMPK activity is also reduced in MEFs expressing p53R172H (equivalent with human p53R175H), but not in p53−/− MEFs [59]. In HNSCC mouse models, mutant p53 levels in tumors are negatively correlated with phosphorylation of AMPK [59]. Moreover, the downregulation of mutant p53 in Tu138 (p53P151S) and SK-Br-3 (p53R175H) cell lines increases AMPK activity upon energy stress induced by inhibition of GLUT1, one of the glucose transporters [59]. Thus, mutant p53 inhibits the activity of AMPK. The observed inhibition of AMPK activity is caused by the interaction of AMPK subunit α (AMPKα) with mutant p53 (p53R175H and p53P151S) at the central core DNA-binding domain and C-terminal region (Figure 1A) [59]. Thus, p53 GOF mutants enhance not only tumor progression, but could also increase fatty acid synthesis by inhibiting AMPK (Figure 1B). It remains unclear how significantly the inhibitory effect of mutant p53 on AMPK activity contributes to the fatty acid synthesis and tumor progression.
4. Summary and Future Perspectives
In this review article, we focus on lipid metabolism pathways that are directly associated with p53, including fatty acid synthesis, fatty acid oxidation, cholesterol synthesis, cholesterol efflux, and lipid droplet formation. Other pathways in lipid metabolism include the synthesis of phospholipids, triglycerides, and ceramides [6]. However, to the best of our knowledge, there is no direct evidence showing the involvement of p53 in these processes.
Interestingly, recent data from our laboratory have demonstrated that mevalonte-5-phosphate (MVP), an intermediate metabolite in the mevalonate pathway, is involved in the stabilization of conformational p53 mutants, since the inhibition of MVP production leads to the degradation of conformational mutant p53 [63]. Given that stabilization of mutant p53 is required for its GOF activities [11,58,64,65,66,67], our study reveals a novel mechanism by which the mevalonate pathway contributes to cancer progression. Thus, the mevalonate pathway promotes tumorigenesis by two mechanisms: (1) by enhancing protein prenylation, which is required for the activation of proteins involved in proliferation and migration, such as Ras and Rho; and (2) by stabilizing conformational mutant p53. It should be noted that the efficacy of statins (cholesterol lowering drugs) that inhibit the mevalonate pathway on tumor suppression is promising, yet they still remain controversial [68,69,70,71,72]. Our study may suggest the importance of knowledge of the p53 status in tumors treated with statins to determine their efficacy.
Inhibition of fatty acid synthesis is shown to induce apoptosis in a pancreatic cancer cell line [73]. On the other hand, in a xenograft mouse model of myc-overexpressing triple negative breast cancer cells, inhibition of fatty acid oxidation reduces tumor growth [74]. Altering the balance of fatty acid synthesis and oxidation in tumors might be important to suppress cancer progression in a context-dependent manner. Additionally, the efficacy of different types of inhibitors for the lipid metabolism pathways on tumor suppression should be investigated in the future.
In vivo roles of p53-regulated, lipid metabolism-related proteins—SIRT1 and aromatase—in tumor progression have been shown using mouse models. Loss of one allele of SIRT1 (SIRT1+/−) in mice accelerates tumor onset and incidence in the p53+/− background, indicating that SIRT1 haplo-insufficiency facilities tumorigenesis [75], while overexpression of SIRT1, specifically in the gut, reduces the number and size of intestinal tumors in APCmin/+ mice [76]. McPherson et al. [77] show that aromatase knockout mice develop prostate hyperplasia. On the other hand, overexpression of aromatase in testis and the mammary gland increases the incidence of testicular cancer and mammary ductal adenocarcinomas, respectively [78,79]. Thus, in vivo roles of aromatase in tumor development could be cell type- and tissue contextdependent. Nonetheless, future studies testing in vivo roles of other p53 targets associated with lipid metabolism in tumorigenesis are needed to better understand contributions of these proteins to p53-mediated tumor suppression.
In summary, loss of p53 activity and mutations in p53 helps accelerate lipid accumulation, which could further contribute to cancer progression. Thus, lipid metabolism pathways can be targeted for cancer therapy, particularly for cancers lacking p53 or having p53 mutations.
Acknowledgments
We thank Swathi V. Iyer and Atul Ranjan for helpful discussion and editing the manuscript. This paper is supported by NIH 5-R01-CA174735-03 (Tomoo Iwakuma) grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Keijer J., van Dartel D.A. Reprogrammed metabolism of cancer cells as a potential therapeutic target. Curr. Pharm. Des. 2014;20:2580–2594. doi: 10.2174/13816128113199990483. [DOI] [PubMed] [Google Scholar]
- 2.Hirschey M.D., DeBerardinis R.J., Diehl A.M., Drew J.E., Frezza C., Green M.F., Jones L.W., Ko Y.H., Le A., Lea M.A., et al. Dysregulated metabolism contributes to oncogenesis. Semin. Cancer Biol. 2015;35(Suppl.):S129–S150. doi: 10.1016/j.semcancer.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos C.R., Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 6.Beloribi-Djefaflia S., Vasseur S., Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zahra Bathaie S., Ashrafi M., Azizian M., Tamanoi F. Mevalonate pathway and human cancers. Curr. Mol. Pharm. 2016;9 doi: 10.2174/1874467209666160112123205. [DOI] [PubMed] [Google Scholar]
- 8.Ribas V., Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016;5:22. doi: 10.1186/s40169-016-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie E., Schulze A., Zechner R., Walther T.C., Farese R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo D., Bell E.H., Mischel P., Chakravarti A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr. Pharm. Des. 2014;20:2619–2626. doi: 10.2174/13816128113199990486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed-Pastor W.A., Prives C. Mutant p53: One name, many proteins. Genes Dev. 2012;26:1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivlin N., Brosh R., Oren M., Rotter V. Mutations in the p53 tumor suppressor gene: Important milestones at the various steps of tumorigenesis. Genes Cancer. 2011;2:466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkers C.R., Maddocks O.D., Cheung E.C., Mor I., Vousden K.H. Metabolic regulation by p53 family members. Cell Metab. 2013;18:617–633. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein I., Ezra O., Rivlin N., Molchadsky A., Madar S., Goldfinger N., Rotter V. p53, a novel regulator of lipid metabolism pathways. J. Hepatol. 2012;56:656–662. doi: 10.1016/j.jhep.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Liu J., Zhang C., Hu W., Feng Z. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett. 2015;356:197–203. doi: 10.1016/j.canlet.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang P., Du W., Wang X., Mancuso A., Gao X., Wu M., Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011;13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bensaad K., Tsuruta A., Selak M.A., Vidal M.N., Nakano K., Bartrons R., Gottlieb E., Vousden K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C., Liu J., Liang Y., Wu R., Zhao Y., Hong X., Lin M., Yu H., Liu L., Levine A.J., et al. Tumour-associated mutant p53 drives the Warburg effect. Nat. Commun. 2013;4:2935. doi: 10.1038/ncomms3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kollareddy M., Dimitrova E., Vallabhaneni K.C., Chan A., Le T., Chauhan K.M., Carrero Z.I., Ramakrishnan G., Watabe K., Haupt Y., et al. Regulation of nucleotide metabolism by mutant p53 contributes to its gain-of-function activities. Nat. Commun. 2015;6:7389. doi: 10.1038/ncomms8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yahagi N., Shimano H., Matsuzaka T., Najima Y., Sekiya M., Nakagawa Y., Ide T., Tomita S., Okazaki H., Tamura Y., et al. p53 activation in adipocytes of obese mice. J. Biol. Chem. 2003;278:25395–25400. doi: 10.1074/jbc.M302364200. [DOI] [PubMed] [Google Scholar]
- 21.Nemoto S., Fergusson M.M., Finkel T. Nutrient availability regulates sirt1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Zhao X., Gao X., Mei Y., Wu M. A new role of p53 in regulating lipid metabolism. J. Mol. Cell Biol. 2013;5:147–150. doi: 10.1093/jmcb/mjs064. [DOI] [PubMed] [Google Scholar]
- 23.Jiang D., LaGory E.L., Kenzelmann Broz D., Bieging K.T., Brady C.A., Link N., Abrams J.M., Giaccia A.J., Attardi L.D. Analysis of p53 transactivation domain mutants reveals ACAD11 as a metabolic target important for p53 pro-survival function. Cell Rep. 2015;10:1096–1109. doi: 10.1016/j.celrep.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assaily W., Rubinger D.A., Wheaton K., Lin Y., Ma W., Xuan W., Brown-Endres L., Tsuchihara K., Mak T.W., Benchimol S. ROS-mediated p53 induction of lpin1 regulates fatty acid oxidation in response to nutritional stress. Mol. Cell. 2011;44:491–501. doi: 10.1016/j.molcel.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., He Y., Jin A., Tikunov A.P., Zhou L., Tollini L.A., Leslie P., Kim T.H., Li L.O., Coleman R.A., et al. Ribosomal protein-Mdm2-p53 pathway coordinates nutrient stress with lipid metabolism by regulating mcd and promoting fatty acid oxidation. Proc. Natl. Acad. Sci. USA. 2014;111:E2414–E2422. doi: 10.1073/pnas.1315605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deisenroth C., Itahana Y., Tollini L., Jin A., Zhang Y. p53-inducible DHRS3 is an endoplasmic reticulum protein associated with lipid droplet accumulation. J. Biol. Chem. 2011;286:28343–28356. doi: 10.1074/jbc.M111.254227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirschner R.D., Rother K., Muller G.A., Engeland K. The retinal dehydrogenase/reductase retSDR1/DHRS3 gene is activated by p53 and p63 but not by mutants derived from tumors or EEC/ADULT malformation syndromes. Cell Cycle. 2010;9:2177–2188. doi: 10.4161/cc.9.11.11844. [DOI] [PubMed] [Google Scholar]
- 28.Bist A., Fielding C.J., Fielding P.E. p53 regulates caveolin gene transcription, cell cholesterol, and growth by a novel mechanism. Biochemistry. 2000;39:1966–1972. doi: 10.1021/bi991721h. [DOI] [PubMed] [Google Scholar]
- 29.Pandolfi P.P., Sonati F., Rivi R., Mason P., Grosveld F., Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanton R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64:362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Li X., Zhang X., Fan R., Gu H., Shi Y., Liu H. Glucose-6-phosphate dehydrogenase expression is correlated with poor clinical prognosis in esophageal squamous cell carcinoma. Eur. J. Surg. Oncol. 2015;41:1293–1299. doi: 10.1016/j.ejso.2015.08.155. [DOI] [PubMed] [Google Scholar]
- 32.Eberle D., Hegarty B., Bossard P., Ferre P., Foufelle F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie. 2004;86:839–848. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Horton J.D., Bashmakov Y., Shimomura I., Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. USA. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo D., Prins R.M., Dang J., Kuga D., Iwanami A., Soto H., Lin K.Y., Huang T.T., Akhavan D., Hock M.B., et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci. Signal. 2009;2 doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ettinger S.L., Sobel R., Whitmore T.G., Akbari M., Bradley D.R., Gleave M.E., Nelson C.C. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64:2212–2221. doi: 10.1158/0008-5472.CAN-2148-2. [DOI] [PubMed] [Google Scholar]
- 36.Schug T.T., Li X. Sirtuin 1 in lipid metabolism and obesity. Ann. Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purushotham A., Schug T.T., Xu Q., Surapureddi S., Guo X., Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X., Zhang S., Blander G., Tse J.G., Krieger M., Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 39.Ponugoti B., Kim D.H., Xiao Z., Smith Z., Miao J., Zang M., Wu S.Y., Chiang C.M., Veenstra T.D., Kemper J.K. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker A.K., Yang F., Jiang K., Ji J.Y., Watts J.L., Purushotham A., Boss O., Hirsch M.L., Ribich S., Smith J.J., et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones M.E., Thorburn A.W., Britt K.L., Hewitt K.N., Wreford N.G., Proietto J., Oz O.K., Leury B.J., Robertson K.M., Yao S., et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He M., Pei Z., Mohsen A.W., Watkins P., Murdoch G., van Veldhoven P.P., Ensenauer R., Vockley J. Identification and characterization of new long chain acyl-CoA dehydrogenases. Mol. Genet. Metab. 2011;102:418–429. doi: 10.1016/j.ymgme.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterfy M., Phan J., Xu P., Reue K. Lipodystrophy in the FLD mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 44.Finck B.N., Gropler M.C., Chen Z., Leone T.C., Croce M.A., Harris T.E., Lawrence J.C., Jr., Kelly D.P. Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Laurent G., German N.J., Saha A.K., de Boer V.C., Davies M., Koves T.R., Dephoure N., Fischer F., Boanca G., Vaitheesvaran B., et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell. 2013;50:686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindstrom M.S., Jin A., Deisenroth C., White Wolf G., Zhang Y. Cancer-associated mutations in the Mdm2 zinc finger domain disrupt ribosomal protein interaction and attenuate Mdm2-induced p53 degradation. Mol. Cell. Biol. 2007;27:1056–1068. doi: 10.1128/MCB.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zolfaghari R., Chen Q., Ross A.C. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G578–G588. doi: 10.1152/ajpgi.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beilstein F., Carriere V., Leturque A., Demignot S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp. Cell Res. 2016;340:172–179. doi: 10.1016/j.yexcr.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Liu P., Rudick M., Anderson R.G. Multiple functions of caveolin-1. J. Biol. Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 50.Murata M., Peranen J., Schreiner R., Wieland F., Kurzchalia T.V., Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fielding C.J., Bist A., Fielding P.E. Intracellular cholesterol transport in synchronized human skin fibroblasts. Biochemistry. 1999;38:2506–2513. doi: 10.1021/bi981012o. [DOI] [PubMed] [Google Scholar]
- 52.Freeman M.R., Yang W., di Vizio D. Caveolin-1 and prostate cancer progression. Adv. Exp. Med. Biol. 2012;729:95–110. doi: 10.1007/978-1-4614-1222-9_7. [DOI] [PubMed] [Google Scholar]
- 53.Anwar S.L., Wahyono A., Aryandono T., Haryono S.J. Caveolin-1 in breast cancer: Single molecule regulation of multiple key signaling pathways. Asian Pac. J. Cancer Prev. 2015;16:6803–6812. doi: 10.7314/APJCP.2015.16.16.6803. [DOI] [PubMed] [Google Scholar]
- 54.Nwosu Z.C., Ebert M.P., Dooley S., Meyer C. Caveolin-1 in the regulation of cell metabolism: A cancer perspective. Mol. Cancer. 2016;15:71. doi: 10.1186/s12943-016-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J., Reumers J., Couceiro J.R., de Smet F., Gallardo R., Rudyak S., Cornelis A., Rozenski J., Zwolinska A., Marine J.C., et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011;7:285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- 56.Do P.M., Varanasi L., Fan S., Li C., Kubacka I., Newman V., Chauhan K., Daniels S.R., Boccetta M., Garrett M.R., et al. Mutant p53 cooperates with ETS2 to promote etoposide resistance. Genes Dev. 2012;26:830–845. doi: 10.1101/gad.181685.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walerych D., Lisek K., Sommaggio R., Piazza S., Ciani Y., Dalla E., Rajkowska K., Gaweda-Walerych K., Ingallina E., Tonelli C., et al. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat. Chem. Biol. 2016;18:897–909. doi: 10.1038/ncb3380. [DOI] [PubMed] [Google Scholar]
- 58.Freed-Pastor W.A., Mizuno H., Zhao X., Langerod A., Moon S.H., Rodriguez-Barrueco R., Barsotti A., Chicas A., Li W., Polotskaia A., et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou G., Wang J., Zhao M., Xie T.X., Tanaka N., Sano D., Patel A.A., Ward A.M., Sandulache V.C., Jasser S.A., et al. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol. Cell. 2014;54:960–974. doi: 10.1016/j.molcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardie D.G., Ross F.A., Hawley S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park S.H., Gammon S.R., Knippers J.D., Paulsen S.R., Rubink D.S., Winder W.W. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J. Appl. Physiol. 2002;92:2475–2482. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- 62.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y., et al. AMPK phosphorylates and inhibits srebp activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parrales A., Ranjan A., Iyer S.V., Padhye S., Weir S.J., Roy A., Iwakuma T. DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway. Nat. Cell Biol. 2016;18:1233–1243. doi: 10.1038/ncb3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyer S.V., Parrales A., Begani P., Narkar A., Adhikari A.S., Martinez L.A., Iwakuma T. Allele-specific silencing of mutant p53 attenuates dominant-negative and gain-of-function activities. Oncotarget. 2016;7:5401–5415. doi: 10.18632/oncotarget.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu H.B., Yang K., Xie Y.Q., Lin Y.W., Mao Q.Q., Xie L.P. Silencing of mutant p53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells. World J. Surg. Oncol. 2013;11:22. doi: 10.1186/1477-7819-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bossi G., Lapi E., Strano S., Rinaldo C., Blandino G., Sacchi A. Mutant p53 gain of function: Reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene. 2006;25:304–309. doi: 10.1038/sj.onc.1209026. [DOI] [PubMed] [Google Scholar]
- 67.Lim L.Y., Vidnovic N., Ellisen L.W., Leong C.O. Mutant p53 mediates survival of breast cancer cells. Br. J. Cancer. 2009;101:1606–1612. doi: 10.1038/sj.bjc.6605335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanfilippo K.M., Keller J., Gage B.F., Luo S., Wang T.F., Moskowitz G., Gumbel J., Blue B., O’Brian K., Carson K.R. Statins are associated with reduced mortality in multiple myeloma. J. Clin. Oncol. 2016;19:JCO683482. doi: 10.1200/JCO.2016.68.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moon H., Hill M.M., Roberts M.J., Gardiner R.A., Brown A.J. Statins: Protectors or pretenders in prostate cancer? Trends Endocrinol. Metab. 2014;25:188–196. doi: 10.1016/j.tem.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Baandrup L., Dehlendorff C., Friis S., Olsen J.H., Kjaer S.K. Statin use and risk for ovarian cancer: A Danish nationwide case-control study. Br. J. Cancer. 2015;112:157–161. doi: 10.1038/bjc.2014.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu L.L., Hsieh M.C., Chow J.M., Liu S.H., Chang C.L., Wu S.Y. Statins improve outcomes of nonsurgical curative treatments in hepatocellular carcinoma patients. Medicine. 2016;95:e4639. doi: 10.1097/MD.0000000000004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldvaser H., Rizel S., Hendler D., Neiman V., Shepshelovich D., Shochat T., Sulkes A., Brenner B., Yerushalmi R. The association between treatment for metabolic disorders and breast cancer characteristics. Int. J. Endocrinol. 2016;2016:4658469. doi: 10.1155/2016/4658469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishi K., Suzuki K., Sawamoto J., Tokizawa Y., Iwase Y., Yumita N., Ikeda T. Inhibition of fatty acid synthesis induces apoptosis of human pancreatic cancer cells. Anticancer Res. 2016;36:4655–4660. doi: 10.21873/anticanres.11016. [DOI] [PubMed] [Google Scholar]
- 74.Camarda R., Zhou A.Y., Kohnz R.A., Balakrishnan S., Mahieu C., Anderton B., Eyob H., Kajimura S., Tward A., Krings G., et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016;22:427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang R.H., Sengupta K., Li C., Kim H.S., Cao L., Xiao C., Kim S., Xu X., Zheng Y., Chilton B., et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Firestein R., Blander G., Michan S., Oberdoerffer P., Ogino S., Campbell J., Bhimavarapu A., Luikenhuis S., de Cabo R., Fuchs C., et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McPherson S.J., Wang H., Jones M.E., Pedersen J., Iismaa T.P., Wreford N., Simpson E.R., Risbridger G.P. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/en.142.6.2458. [DOI] [PubMed] [Google Scholar]
- 78.Fowler K.A., Gill K., Kirma N., Dillehay D.L., Tekmal R.R. Overexpression of aromatase leads to development of testicular Leydig cell tumors: An in vivo model for hormone-mediated testicularcancer. Am. J. Pathol. 2000;156:347–353. doi: 10.1016/S0002-9440(10)64736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diaz-Cruz E.S., Sugimoto Y., Gallicano G.I., Brueggemeier R.W., Furth P.A. Comparison of increased aromatase versus ERα in the generation of mammary hyperplasia and cancer. Cancer Res. 2011;71:5477–5487. doi: 10.1158/0008-5472.CAN-10-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]