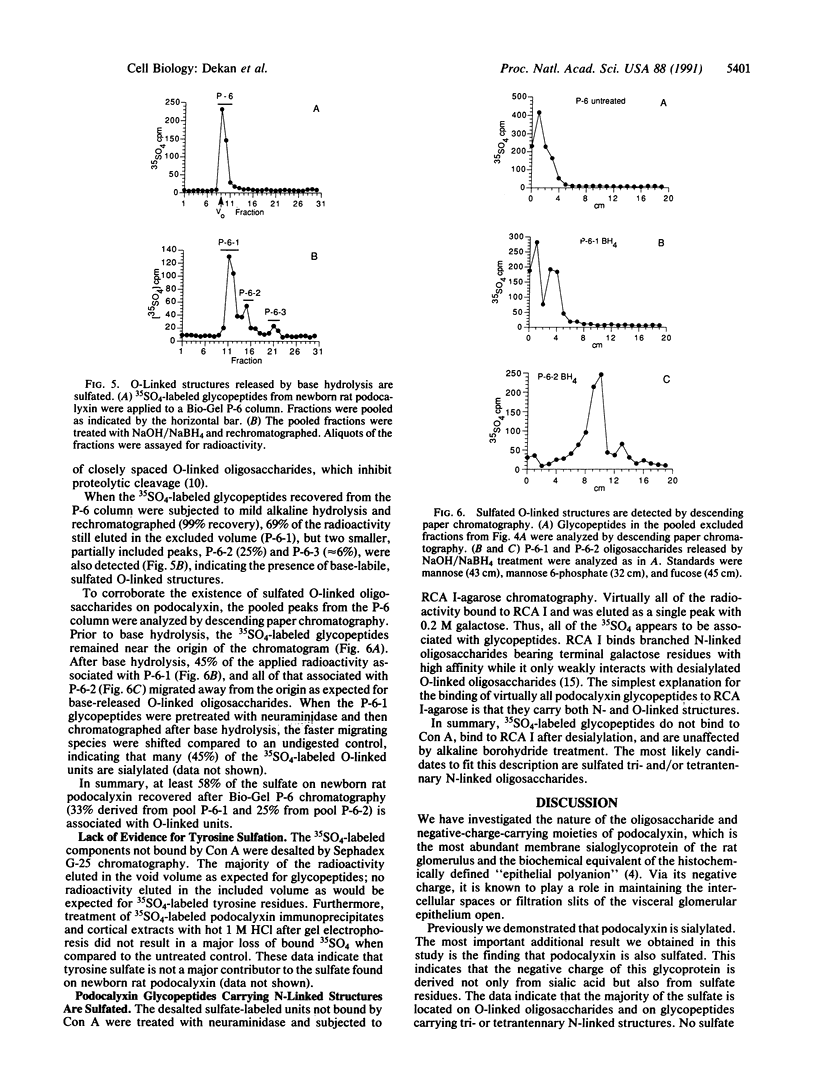
Abstract
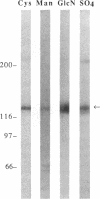
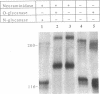
Podocalyxin is the major sialoprotein of the rat glomerulus. Its function is to maintain the filtration slits of the glomerular epithelium open by virtue of its high net negative charge. We have used biosynthetic labeling and oligosaccharide analysis to characterize the anionic-charge-carrying moieties on this protein. Kidney slices from 2-day-old rats were biosynthetically labeled with [35S]Cys, [3H]Man, [3H]GlcN, and 35SO4, after which podocalyxin was immunoprecipitated and purified by SDS/PAGE. All these labels were incorporated into podocalyxin. Immunoprecipitates were subjected to digestion with specific glycosidases or digested with Pronase followed by chromatographic analysis of the released glycopeptides. Podocalyxin was susceptible to digestion with N-Glycanase and O-Glycanase, indicating the presence of both N- and O-linked oligosaccharides. Approximately 30% of the [3H]GlcN-labeled glycopeptides bound to Con A, confirming the presence of high mannose, hybrid, or biantennary N-linked structures; alkaline borohydride treatment confirmed the presence of O-linked oligosaccharides. Analysis of the 35SO4-labeled glycopeptides indicated that both the N- and O-linked structures were sulfated. We conclude that in newborn rat kidney (i) podocalyxin contains both O- and N-linked oligosaccharides [high mannose or hybrid type, biantennary, and complex (sialylated) type], (ii) podocalyxin is sulfated, and (iii) sulfate is located on both O-linked oligosaccharides and on glycopeptides carrying tri- or tetrantennary N-linked structures. These results indicate that the net negative charge of podocalyxin is most likely derived from sulfate as well as from sialic acid residues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979 Oct 10;254(19):9795–9799. [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Choi H. U., Meyer K. The structure of a sulfated glycoprotein of chick allantoic fluid. J Biol Chem. 1974 Feb 10;249(3):932–939. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- Davis C. G., Elhammer A., Russell D. W., Schneider W. J., Kornfeld S., Brown M. S., Goldstein J. L. Deletion of clustered O-linked carbohydrates does not impair function of low density lipoprotein receptor in transfected fibroblasts. J Biol Chem. 1986 Feb 25;261(6):2828–2838. [PubMed] [Google Scholar]
- Gabel C. A., Bergmann J. E. Processing of the asparagine-linked oligosaccharides of secreted and intracellular forms of the vesicular stomatitis virus G protein: in vivo evidence of Golgi apparatus compartmentalization. J Cell Biol. 1985 Aug;101(2):460–469. doi: 10.1083/jcb.101.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz A., Watson C., Johnson A. R., Roberts M. K. Sulfated glycoproteins secreted by human vascular endothelial cells. J Biol Chem. 1982 Nov 25;257(22):13581–13586. [PubMed] [Google Scholar]
- Horvat R., Hovorka A., Dekan G., Poczewski H., Kerjaschki D. Endothelial cell membranes contain podocalyxin--the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986 Feb;102(2):484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Sharkey D. J., Farquhar M. G. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984 Apr;98(4):1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Vernillo A. T., Farquhar M. G. Reduced sialylation of podocalyxin--the major sialoprotein of the rat kidney glomerulus--in aminonucleoside nephrosis. Am J Pathol. 1985 Mar;118(3):343–349. [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney T. P., Adelstein E., Morris D. A., Mawhinney A. M., Barbero G. J. Structure determination of five sulfated oligosaccharides derived from tracheobronchial mucus glycoproteins. J Biol Chem. 1987 Mar 5;262(7):2994–3001. [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Miettinen A., Dekan G., Farquhar M. G. Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol. 1990 Oct;137(4):929–944. [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Pinter A., Compans R. W. Sulfated components of enveloped viruses. J Virol. 1975 Oct;16(4):859–866. doi: 10.1128/jvi.16.4.859-866.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. O-linked glycosylation of retroviral envelope gene products. J Virol. 1988 Mar;62(3):1016–1021. doi: 10.1128/jvi.62.3.1016-1021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W., Caulfield J. P., Farquhar M. G. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978 Aug;39(2):90–100. [PubMed] [Google Scholar]
- Roux L., Holojda S., Sundblad G., Freeze H. H., Varki A. Sulfated N-linked oligosaccharides in mammalian cells. I. Complex-type chains with sialic acids and O-sulfate esters. J Biol Chem. 1988 Jun 25;263(18):8879–8889. [PubMed] [Google Scholar]
- Sadler J. E., Paulson J. C., Hill R. L. The role of sialic acid in the expression of human MN blood group antigens. J Biol Chem. 1979 Mar 25;254(6):2112–2119. [PubMed] [Google Scholar]
- Schnabel E., Anderson J. M., Farquhar M. G. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990 Sep;111(3):1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E., Dekan G., Miettinen A., Farquhar M. G. Biogenesis of podocalyxin--the major glomerular sialoglycoprotein--in the newborn rat kidney. Eur J Cell Biol. 1989 Apr;48(2):313–326. [PubMed] [Google Scholar]
- Seiler M. W., Rennke H. G., Venkatachalam M. A., Cotran R. S. Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest. 1977 Jan;36(1):48–61. [PubMed] [Google Scholar]
- Smith P. L., Baenziger J. U. A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science. 1988 Nov 11;242(4880):930–933. doi: 10.1126/science.2460923. [DOI] [PubMed] [Google Scholar]
- Sundblad G., Holojda S., Roux L., Varki A., Freeze H. H. Sulfated N-linked oligosaccharides in mammalian cells. II. Identification of glycosaminoglycan-like chains attached to complex-type glycans. J Biol Chem. 1988 Jun 25;263(18):8890–8896. [PubMed] [Google Scholar]