Abstract
In addition to the traditional renin–angiotensin system, a great deal of evidence favors the existence of numerous independent tissue-specific renin–angiotensin systems. We report that mast cells are an additional source of renin and constitute a unique extrarenal renin–angiotensin system. We use renin-specific antibodies to demonstrate that cardiac mast cells contain renin. Extending this observation to the human mast cell line HMC-1, we show that these mast cells also express renin. The HMC-1 renin RT-PCR product is 100% homologous to Homo sapiens renin. HMC-1 cells also contain renin protein, as demonstrated both by immunoblot and immunocytochemical analyses. Renin released from HMC-1 cells is active; furthermore, HMC-1 cells are able to synthesize renin. It is known that, in the heart, mast cells are found in the interstitium in close proximity to nerves and myocytes, which both express angiotensin II receptors. Inasmuch as myocardial interstitium contains angiotensinogen and angiotensin-converting enzyme, and because we were able to detect renin only in mast cells, we postulate that the release of renin from cardiac mast cells is the pivotal event triggering local formation of angiotensin II. Because of the ubiquity of mast cells, our results represent a unique paradigm for understanding local renin–angiotensin systems, not just in the heart, but in all tissues. Our findings provide a rationale for targeting mast cells in conjunction with renin–angiotensin system inhibitors in the management of angiotensin II-related dysfunctions.
Traditionally the renin–angiotensin system (RAS) has been viewed as a circulating axis, whereby renin is released into the circulation from the kidneys in response to decreased renal perfusion pressure, decreased delivery of NaCl at the macula densa, and/or increased renal sympathetic nerve activity (1). The rate-limiting step in the formation of angiotensin II (ANG II) is the proteolytic action of renin, which cleaves angiotensinogen (Aogen) to the intermediate angiotensin I (ANG I). ANG I is then converted to ANG II by angiotensin-converting enzyme (ACE) at the endothelial surface (2, 3).
In addition to this conventional pathway, many tissues, including heart and brain, are thought to be capable of local ANG II production via tissue-specific RAS (4, 5). Although Aogen, ANG I, and ACE, have been demonstrated in various organs, the presence of renin of extrarenal origin has been more difficult to prove and remains controversial. In this investigation, experiments were designed to determine whether renin could be detected in native tissue other than kidney. Because ANG II plays such a crucial role in cardiovascular disease, we focused our efforts on heart tissue, which we screened for renin by using an established polyclonal anti-renin Ab made to recombinant human renin (6). We found that cardiac mast cells were immunopositive for renin. In addition, we used a cultured mast cell line to extrapolate our observations from fixed heart slices to living cells.
Our findings indicate that mast cells are a previously undescribed source of renin, which once released, activates local RAS. These results represent a unique paradigm for understanding local formation of ANG II in all organs.
Materials and Methods
Tissue Preparation. Pathogen-free Sprague–Dawley rats of both sexes (Charles River Breeding Laboratories), weighing between 150 and 300 g, were used for these experiments. Rats were killed according to approved International Animal Care and Use Committee guidelines. Briefly, rats were anesthetized with CO2 vapor and exsanguinated, and the hearts were rapidly excised and mounted by aortic cannulation in a Langendorff apparatus. The hearts were washed for 20 min with Krebs–Henseleit buffer to remove blood and fixed by perfusion with 4% paraformaldehyde (PF) at pH 7.4. After 10 min of perfusion, the hearts were detached from the apparatus and immersed in 4% PF for one additional hour before rinsing and storing in 30% sucrose for 3 h to cryoprotect. Kidneys were removed, washed free of blood, fixed, and cryoprotected as above. The hearts or kidneys were then embedded in tissue freezing medium (Electron Microscopy Sciences) and snap-frozen in liquid nitrogen. A Bright cryostat (model OTF) was used to prepare frozen sections of 10 μm, which were collected onto clean Fisher Superfrost Plus slides and stored at –80°C until they were ready for immunohistochemistry.
Immuno- and Histochemical Staining. Kidney and heart. Slides containing frozen tissue sections were washed for 5 min in PBS. The sections were then permeabilized for 10 min at 37°C with a solution containing 4% FBS and 0.3% Triton X-100 dissolved in PBS. After washing the sections with PBS, 10% FBS was applied to the sections for 1 h at 37°C to block nonspecific binding before adding antibodies. After this, primary Ab was applied to the sections for 2 h at 37°C, followed by three washes in PBS. Next, sections were exposed to secondary Ab (all purchased from Molecular Probes) for 1 h at 37°C. Sections were then washed as described above, followed by fixation for 3 min with 4% PF. After washing with PBS, sections were mounted in Vectashield anti-fading solution (Vector Laboratories, Burlingame, CA). In experiments where tissue was stained with toluidine blue (Sigma) (0.25% in acetic acid, pH 2.0), the procedure of Kiernan et al. (7) was followed. In instances where tissues were costained, staining with toluidine blue was performed after the tissue had been immunostained.
Primary polyclonal rabbit anti-renin Ab against human recombinant renin (6) was applied to rat kidney or heart sections at a dilution of 1:500. The corresponding secondary Ab used was Alexa Fluor 488 donkey anti-rabbit IgG (green) diluted 1:300 or 1:500 for kidney or heart, respectively. For competition experiments, an excess of human renin (Calbiochem) was combined with the polyclonal rabbit anti-renin Ab overnight, before proceeding with immunostaining.
Both a monoclonal mouse anti-renin Ab against rat renin (Swant, Bellinzona, Switzerland) and a polyclonal rabbit anti-histamine Ab (Accurate Chemicals) were applied to rat heart sections at a dilution of 1:100 and 1:500, respectively. For these sections, the secondary Abs used were a 1:500 dilution of both Alexa Fluor 594 goat anti-mouse IgG (red) and Alexa Fluor 488 donkey anti-rabbit IgG (green).
Heart sections that were colabeled with the rabbit anti-renin Ab (1:500) and mouse anti-cathepsin-D Ab (1:500) (Oncogene) were subsequently stained by using the following secondary Abs: Alexa Fluor 594 donkey anti-rabbit IgG (red) (1:300) and Alexa Fluor 488 goat anti-mouse IgG (green) (1:300).
The goat anti-synapsin Ia/b Ab (Santa Cruz Biotechnology), a specific label of neurons (8) was applied to heart sections at a dilution of 1:300, and the polyclonal rabbit anti-renin Ab was applied at a dilution of 1:500. The following secondary Abs were used: Alexa Fluor 488 goat anti-mouse IgG (green) (1:300) and Alexa Fluor 594 donkey anti-rabbit IgG (red) (1:500).
HMC-1 cells. The human mastocytoma cell line, HMC-1, was kindly provided to us by I. Biaggioni (Vanderbilt University, Nashville, TN) and J. H. Butterfield (Mayo Clinic, Rochester, MN). Cells were maintained in suspension culture at high density in Iscove's modified Dulbecco's medium supplemented with 10% FBS and kept at 37°C, 5% CO2. For immunocytochemistry, HMC-1 cells were grown on standard 22-mm glass coverslips for 48 h and rinsed free of media with PBS. After this, cells were fixed and permeabilized in PBS containing 3.7% PF and 0.3% Triton X-100. Cells were washed with PBS for 3 min and then incubated for 30 min at 37°C with 1% BSA to block nonspecific binding. After this, the cells were incubated with polyclonal rabbit anti-renin Ab (1:400) for 1 h at room temperature. The cells were washed three times for 5 min each with PBS, and then exposed to Alexa Fluor 488 donkey anti-rabbit IgG (green) (1:400) for 1 h at room temperature. After washing with PBS as above, the coverslip was mounted onto a microscope slide with Vectashield.
Tissue sections or cells were examined either with an inverted epifluorescent microscope (Nikon Diaphot) interfaced to a frame-transfer type cooled charge-coupled device (Roper Scientific) and processed with metaf luor/metamorph software (Universal Imaging) or with a Leica TCS SP2 confocal microscope. Digital images were imported into photoshop 5.0 (Adobe Systems, Mountain View, CA) for minimal processing.
Renin Activity. Pooled confluent flasks or individual wells of HMC-1 cells were pelleted and resuspended in Hepes buffer (pH 5.7, 1 mM EDTA) containing the mast cell degranulating agent compound 48/80 (100 or 20 μg/ml; Sigma). After 30 min, cells were spun down, and the renin-containing supernatant incubated with increasing concentrations of human Aogen (Sigma). Renin activity (ANG I formed) was then determined by using a GammaCoat Plasma Renin Activity 125I RIA kit (DiaSorin, Stillwater, MN). The selective renin inhibitor BILA2157 was generously provided to us by Boehringer Ingelheim.
RT-PCR. Total RNA was extracted from human kidney tissue and HMC-1 cells by using RNA STAT-60 reagent (Tel-Test, Friendswood, TX). One microgram of total RNA from each sample was reverse-transcribed and assayed by RT-PCR using a Onestep RT-PCR kit (Qiagen, Valencia, CA). Sense and antisense primers specific for human renin at exons 4 and 7 of the renin gene were 5′-TCTCAGCCAGGACATCATCA-3′ and 5′-AGTGGAAATTCCCTTCGTAA-3′, respectively (9), thus avoiding coamplification of genomic DNA coding for renin. Sense and antisense primers for β-actin were 5′-GCTCGTCGTCGACAACGGCTC-3′ and 5′-GCTCTTCTACTGGGTCTAGTACAAAC-3′. The amplification profile used was: 50°C for 30 min, 95°C for 15 min, then 94°C for 30 sec, 55°C for 30 sec, 72°C for 1 min (40 cycles), and finally 72°C for 10 min. PCR products generated were ≈288 and ≈350 bp for renin and β-actin, respectively. PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining. The renin RT-PCR product from HMC-1 RNA was extracted from the agarose gel by using GENECLEAN II (QBIOgene, Carlsbad, CA); after ethanol precipitation to further purify and concentrate the DNA, samples were sent to the DNA Sequencing Resource Center (The Rockefeller University, New York) and run on a SpectruMedix 9610 DNA sequencer.
Western Blotting. Samples of rat kidney homogenate (20 μg per lane), HMC-1 lysate (50 μg per lane), and cathepsin D (CD) (500 μg per lane; Sigma) were prepared with 2× Novex Tris-glycine SDS sample buffer (Invitrogen) and boiled for 5 min, before separation on 12% Tris-glycine SDS-polyacrylamide minigels (Invitrogen). Electrophoresis was carried out at 200 V, 40 mA per gel for 1 h. Gels were soaked in transfer buffer (25 mM Tris-base/0.2 M glycine/10% methanol, pH 8.5) and electrotransferred to poly(vinylidene difluoride) (PVDF) membranes (Immobilon-P, Millipore) for 90 min at 25 V, 100 mA at room temperature. Membranes were blocked for at least 2 h in blocking buffer [Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% (wt/vol) nonfat dry milk]. Primary antibodies were incubated with the PVDF overnight at 4°C, diluted appropriately in primary Ab dilution buffer (TBS containing 0.1% Tween 20, 5% BSA). The PVDF was washed three times with TBS, and then horseradish peroxidase-coupled secondary Ab was added at a 1:2,000 dilution in blocking buffer for 1 h. After three further TBS washes, the protein of interest was detected by using enhanced chemiluminescence (LumiGLO; Cell Signaling Technology) and by exposure to x-ray film (Biomax MR, Kodak).
Results
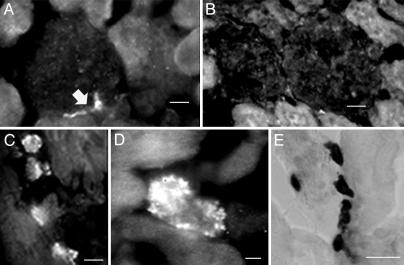
To establish specificity of the polyclonal anti-renin Ab for renin, we first screened sections of rat kidney. Cryostat sections (10 μm) of PF-fixed rat kidney were incubated with the anti-renin Ab followed by Alexa Fluor 488 donkey anti-rabbit IgG. Fig. 1A is a representative section of kidney stained with the Ab; it shows that the vascular pole of the glomerulus, the site of renin synthesis in the kidney, immunoreacted with the anti-renin Ab (see arrow). As a negative control, rat kidney sections were treated with anti-renin Ab preadsorbed with an excess of human renin. As shown in Fig. 1B, there was no immunostaining under this condition, thus demonstrating the specificity of the anti-renin Ab.
Fig. 1.
Immunostaining of kidney and heart with anti-renin antibody. (A) The polyclonal anti-renin Ab (1:500) exhibits specific binding to rat kidney at the vascular pole of the glomerulus (arrow). (Scale bar = 10 μm.) (B) No staining was seen in sections exposed to the polyclonal anti-renin Ab (1:500) preadsorbed with an excess of human renin. (Scale bar, 10 μm.) (C and D) Staining of sections of rat ventricle prepared with the polyclonal anti-renin Ab (1:500) and viewed with a ×40 (C) or ×100 (D) objective. (Scale bars, 10 μmin C and 4 μmin D.) (E) Staining of mast cells in rat ventricle with toluidine blue. (Scale bar, 20 μm.)
Sections cut from intact rat heart were screened for renin protein by indirect immunofluorescence microscopy with the polyclonal anti-renin Ab. Cryostat sections (10 μm) of fixed rat ventricle were incubated with the anti-renin Ab followed by Alexa-Fluor conjugated anti-rabbit IgG. When sections were stained with the anti-renin Ab, this exposed a subpopulation of immunoreactive cells. Representative sections from ventricle are shown in Fig. 1 C (×40) and D (×100), and demonstrate immunoreactivity to the anti-renin Ab in these sections. These granulated cells were the only cells in the section to be stained with the anti-renin Ab. No other cell type in the ventricle, such as myocytes and nerves, immunoreacted with the Ab. These immunopositive cells were frequently observed in the interstitial space. Based on their granular appearance, size, and location, these cells were visually identified as cardiac mast cells.
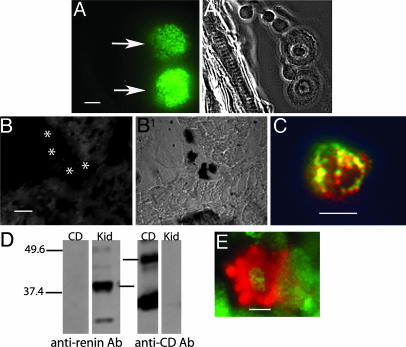
To ascertain that sections of rat heart indeed possess an identifiable mast cell population, cryostat sections of PF-fixed rat ventricle were examined after staining with toluidine blue, a classical histochemical stain for mast cells (7). Fig. 1E is a typical transmitted light image showing toluidine-blue staining in a fixed section. Every section analyzed contained toluidine-blue positive cells. To determine whether the cells stained with the anti-renin Ab were also toluidine-blue positive, sections were costained with both reagents. Fig. 2 A and A1 is a section of rat ventricle viewed with epifluorescence and transmitted light. The arrows point to two cells that immunoreacted with the anti-renin Ab (Fig. 2 A) (green). These same cells were also stained with toluidine blue (Fig. 2 A1), thereby supporting our contention that the anti-renin Ab reacts with renin in cardiac mast cells.
Fig. 2.
Presence of immunoreactive renin in cardiac mast cells. (A and A1) Costained section of rat ventricle with the polyclonal anti-renin Ab (A) and toluidine blue (A1). (Scale bar, 5 μm.) (B and B1) A section of rat ventricle prepared with the polyclonal anti-renin Ab (1:500) preadsorbed with an excess of human renin (B) and stained with toluidine blue (B1). The toluidine-blue-stained mast cells (B1) did not immunoreact with the preadsorbed anti-renin Ab (see asterisks in B). (C) A cardiac mast cell colabeled with the monoclonal anti-renin Ab (red) (1:100) and an antihistamine Ab (green) (1:500). (Scale bar, 10 μm.) Areas stained with both Abs appear yellow. (D) Western blot of rat kidney homogenate (Kid) (20 μg per lane) and pure CD (500 μg per lane) probed with the polyclonal anti-renin Ab (1:12,500) and an anti-CD Ab (1:100). (E) A cardiac mast cell colabeled with anti-CD Ab (green) (1:500) and the polyclonal anti-renin Ab (red) (1:500). (Scale bar, 5 μm.)
The specificity of the anti-renin Ab for mast cell renin was also determined in sections of heart (Fig. 2 B and B1). A section of ventricle was treated with the anti-renin Ab preadsorbed with excess human renin (Fig. 2B) and then stained with toluidine blue, as a means of identifying mast cells (Fig. 2B1). This section contained toluidine-blue-positive mast cells, as shown in the transmitted light image. Their corresponding position is indicated by the asterisks in the fluorescence image; clearly, these cells did not immunoreact with the preadsorbed anti-renin Ab. These results are further proof that the anti-renin Ab is recognizing renin in cardiac mast cells.
Inasmuch as mast cells contain histamine, heart sections were stained with an antihistamine Ab as a definitive measure for classifying the subpopulation of cells that stained with the anti-renin Ab. Sections of rat heart were costained with the polyclonal rabbit anti-histamine Ab (green) and the monoclonal mouse anti-renin Ab (red). Overlapping areas of staining appear yellow. The histamine-containing mast cells stained with both antibodies, as shown in Fig. 2C, corroborating our initial identification of these renin-containing cells as mast cells. No other staining was observed.
Mast cells also contain CD, a protease capable of cleaving Aogen to ANG I, via a renin-independent pathway (10). blast analysis reveals that renin and CD are 60% homologous at the amino acid level. Experiments were next performed to ensure that the polyclonal anti-renin Ab did not cross react with CD, which is present in endosomal and lysosomal compartments of mast cells (11, 12). We first performed a Western blot analysis using kidney homogenate and pure CD, which were probed with the polyclonal anti-renin Ab (1:12,500) (Fig. 2D). Gels were loaded with purified CD in one lane and rat kidney homogenate was loaded in the other, and gels were probed with the anti-renin Ab. Kidney homogenate displayed bands consistent with the presence of renin at ≈42 kDa, whereas there was no reactivity to the anti-renin Ab in the lane loaded with pure CD. The other half of the gel, also loaded with rat kidney homogenate and CD, was probed with the anti-CD Ab (1:100). The lane loaded with CD showed distinct bands typical of CD at 48 and 34 kDa, the lower band corresponding to a subunit of CD. The kidney homogenate displayed a faint band at 48 kDa, consistent with the known paucity of CD in the kidney (13). Importantly, there was no band associated with renin in the homogenate. These results demonstrate that the anti-renin and anti-CD antibodies do not cross-react with CD and renin, respectively
Because mast cells contain CD, sections of rat heart were costained with both anti-renin and anti-CD antibodies to determine whether renin and CD could be colocalized to the same cells. Sections of frozen rat ventricle were exposed to the polyclonal anti-renin Ab (red) and CD Ab (green). Mast cells in the heart stained with both antibodies, and according to two-dimensional analysis, did so in separate compartments (Fig. 2E). No other cells stained with both antibodies.
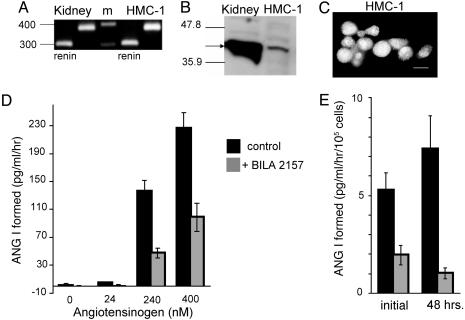
The immunohistochemical results demonstrated that cardiac mast cells express immunoreactive renin. We next explored whether mast cell-derived renin is active when released from mast cells. To address this question, we used the chymase-deficient human mast cell line, HMC-1 (14). Because the presence of renin in mast cells had never been reported, before attempting to measure renin activity, we determined whether HMC-1 express renin mRNA and renin protein. Total RNA (1 μg) was extracted from HMC-1, reversed transcribed, and assayed by PCR using sense and antisense primers specific for human renin at exons 4 and 7 of the renin gene (9), to avoid coamplification of genomic DNA coding for renin. Fig. 3A is an ethidium bromide-stained gel showing that the HMC-1 renin PCR product (lane 4) is similar to renin product from human kidney (lane 1). To further characterize the RT-PCR product from HMC-1, the DNA band was extracted from the gel and sequenced at the Rockefeller University Sequencing Facility. The reported nucleotide sequence was then compared to the known sequence of Homo sapiens renin mRNA by blast analysis (GenBank accession no. NM-000537.2). There was 100% homology between the sequence from HMC-1 and human renin, establishing a precedent for the presence of renin in mast cells.
Fig. 3.
HMC-1 cells express active renin. (A) Total RNA extracted from human kidney and the human mast cell line HMC-1 was reverse transcribed. cDNA was amplified by PCR using specific primers for human renin (lanes 1 and 4) and β-actin controls (lanes 2 and 5). A 100-bp marker ladder (m) was run between kidney and HMC-1 RT-PCR products. (B) Western blot probing rat kidney homogenate (20 μg per lane) and HMC-1 cells (50 μg per lane) for renin with the polyclonal anti-renin Ab (1:12,500). The arrow shows the ≈42-kDa band for renin. (C) Immunocytochemical staining of HMC-1 cells with the polyclonal anti-renin Ab (1:400). (Scale bar, 10 μm.) (D) ANG I formed (renin activity, pg/ml/hr), from HMC-1 cell releasate incubated with increasing concentrations of human Aogen, with or without BILA2157 (100 nM) (mean ± SEM; n = 3). (E) Measurement of ANG I formed from HMC-1 cell releasate initially and 48 h later (renin activity, pg/ml/hr per 105 cells). Releasate was incubated with 240 nM Aogen in the absence or presence of BILA2157 (100 nM) (mean ± SEM; n = 12).
HMC-1 cells also express renin protein. Western analysis of HMC-1 homogenate, probed with the anti-renin Ab, showed a ≈42-kDa band for renin (Fig. 3B). The abundance of renin protein was much greater in kidney than in HMC-1 homogenate. One possible explanation for this difference in the quantity of protein expressed in kidney and HMC-1 may be the differential expression of glycosylated and nonglycosylated renin isoforms found in kidney (15). In addition, the cells immunoreacted with the anti-renin Ab (Fig. 3C). To determine that renin protein in HMC-1 is active, i.e., it cleaves Aogen to form ANG I, cells were grown to confluence and degranulated with the mast cell-degranulating compound 48/80 (16). The cell releasate was incubated with increasing concentrations of Aogen and then assayed for ANG I formed (i.e., renin activity) by RIA. A concentration-response curve for ANG I formation as a function of Aogen concentration was generated on the HMC-1 releasate (Fig. 3D). The amount of ANG I formed increased with Aogen concentration. To account for a possible contribution to ANG I formation by CD, which is also capable of cleaving Aogen, although at a rate 105 times slower than renin (17), ANG I was generated either in the absence or presence of the specific renin inhibitor, BILA2157 (100 nM; IC50 = 1 nM) (18). As shown in Fig. 3D, ≈70% of the total ANG I formed originates from renin. These results demonstrate that mast cell-derived renin is active.
Further experiments with HMC-1 cells were also designed to determine whether these cells are capable of resynthesizing renin once degranulated. Releasates were analyzed from the same population of HMC-1 exposed to compound 48/80 (20 μg/ml) 48 h apart, and analyzed for BILA2157-sensitive renin activity. As shown in Fig. 3E, ≈70% of the ANG I activity measured in the presence of 240 nM Aogen is caused by BILA2157-sensitive renin, both initially and 48 h after degranulation with 48/80. These results indicate that HMC-1 can resynthesize renin.
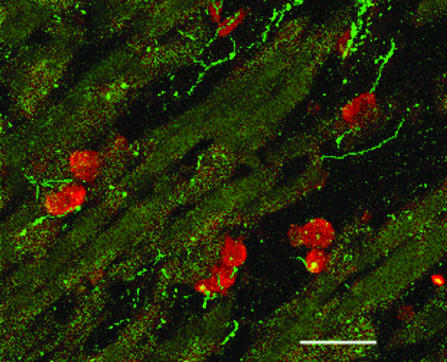
Inasmuch as mast cells are known to degranulate in response to myocardial ischemia/reperfusion (19), and ANG II is known to enhance norepinephrine release from cardiac nerve endings (20, 21), we analyzed the spatial relationship between cardiac mast cells and nerves in heart. Fig. 4 is a section of rat ventricle stained both with the anti-renin Ab (red) and anti-synapsin I1/b Ab (green) viewed with a confocal microscope. This representative section demonstrates that mast cells are closely apposed to the nerves in heart. These results suggest that mast cell degranulation, as occurs with myocardial ischemia/reperfusion, may be the pivotal event in local RAS activation, initiation of ANG II formation, and stimulation of ANG II receptors expressed on nerve endings, causing excessive release of norepinephrine.
Fig. 4.
A confocal image of rat ventricle (0.5 μm) colabeled with the polyclonal anti-renin Ab (red) (1:500) and an anti-synapsin Ab (green) (1:300). The mast cells stained red and cardiac nerves stained green. (Scale bar, 40 μm.)
Discussion
The traditional RAS is defined by the formation of ANG II in the blood from kidney-derived renin and activation of high-affinity receptors in target organs (5). The identification of tissue-specific RAS, where locally generated ANG II acts on resident receptors, has gained considerable attention, especially in heart (4), brain (22), eye (23, 24), and testis (25). Here, we report that mast cells constitute an additional source of renin that contributes to tissue-specific RAS.
Our experiments focus on the heart because of the numerous cardiovascular effects of ANG II. We used an immunohistochemical approach to identify a previously undescribed source of renin residing in cardiac mast cells. Renin mRNA and protein has been demonstrated in cultured canine cardiac myocytes (26), and renin mRNA has been demonstrated in fibroblasts, as well as endothelial and coronary vascular smooth muscle cells (4, 27–29). Other studies conclude that there is no evidence for renin synthesis in normal adult rat myocytes (30). When we used anti-renin Abs, we observed immunostaining in mast cells in intact fixed heart sections. We confirmed this finding at the level of confocal and epifluorescence microscopy. As shown in Figs. 1 and 4, no other cell type in the heart immunoreacted with the anti-renin Ab at detectable levels. In addition, in preliminary experiments on surgical specimens of human right atrium, we were able to demonstrate selective immunoreactivity to renin in mast cells (unpublished data).
We next assessed whether or not mast cells express the gene for human renin. For this test, we used the human mast cell line HMC-1. RT-PCR of total RNA from the HMC-1 cells, using primers specific for human renin at exons 4 and 7, generated a PCR product, which when sequenced was 100% homologous to H. sapiens renin. This finding confirmed that the HMC-1 cells are capable of expressing renin. It is known that alternative splicing of the human renin gene generates kidney-, lung-, and brain-specific mRNA isoforms (31). These three human renin mRNA variants result from alternative tissue-specific transcriptional start sites within intron A. We do not yet know whether mast cell renin is a splice variant of human renin, and this will require future testing.
Western blotting and immunocytochemical analysis of HMC-1 show that these cells express renin protein in addition to the mRNA (Fig. 3). Furthermore, analysis of the HMC-1 releasate shows that this renin protein is active and can cleave Aogen to form ANG I. These results show that the renin released from mast cells is functional.
HMC-1 cells appear to be capable of synthesizing renin, in that renin activity was measured in the releasate from cells that had been degranulated 48 h earlier (Fig. 3E). This result is consistent with other reports showing recovery of mast cell granule-associated cytokines after repeated degranulation events (32). Inasmuch as HMC-1 are a model of native mast cells, our data suggest that mast cells are capable of renin synthesis in situ and do not acquire renin by internalization of circulating renin. Mast cells are not known to express either the mannose-6-phosphate receptor (33) or renin receptor (34), two possible means by which renin has been postulated to bind to the surface of cells, which could lead to its internalization. Our studies in the HMC-1 cells also rule out this possibility.
Based on our evidence that mast cells are a source of renin, we speculate that, with mast cell degranulation, ANG II can be generated in the interstitial space of the myocardium in close association with vessels, myocytes, and nerves. It is known that, in the heart, mast cells are found in the interstitial space (35). Both Aogen and ACE (36, 37) are present in cardiac interstitial fluid. With mast cell degranulation, as occurs in myocardial ischemia/reperfusion, renin released from mast cells acts directly on interstitial Aogen, which may then be converted to ANG II by available ACE. Thus, release of mast cell-derived renin is the pivotal event initiating local RAS activation and ANG II formation in heart. Because mast cells are closely apposed to cardiac nerves and myocytes (Fig. 4), locally formed ANG II can then act on the resident population of Angiotensin AT1 receptors and exert its pathophysiological effects.
Adenosine and neuropeptide Y (NPY) are two of many potential mediators of mast cell degranulation in ischemia. Adenosine, which is known to activate mast cells (38), is elevated in ischemia (39). NPY, coreleased with norepinephrine from sympathetic nerve endings, (40), also activates mast cells (41). NPY release may act as a potential positive feedback mechanism by which sympathetic activation in myocardial ischemia leads to mast cell degranulation, renin release, and ANG II formation further stimulating sympathetic nerves. In conclusion, we present evidence that mast cells represent a source of extrarenal renin, which, when released, can initiate the local formation of ANG II. Because of the omnipresence of mast cells, our results represent a unique paradigm for understanding local RAS, not just in the heart, but in all organs. Our findings provide a rationale for the use of selective renin inhibitors, mast cell stabilizers, and ANG II receptor blockers to alleviate the dysfunctions associated with mast cell degranulation. In as much as hyperactive RAS and excessive release of catecholamines are hallmarks of congestive heart failure, our findings may form the basis for new approaches in the management of heart disease.
Acknowledgments
We thank Ms. R. Mora and Drs. G. Kreitzer and D. Gardner for their technical assistance. This work was supported by National Institutes of Health Grants DK60726 (to R.B.S.), HL34215 (to R.L.), HL46403 (to R.L.), DK56365 (to D.H.), and DK45218 (to D.H.) and Minority Access to Research Careers Grant F31GM 64875 (to A.C.R.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RAS, renin–angiotensin system; ANG I, angiotensin I; ANG II, angiotensin II; ACE, angiotensin-converting enzyme; PF, paraformaldehyde; CD, cathepsin D; Aogen, angiotensinogen.
References
- 1.Valtin, H. S. & Schaefer, J. A. (1995) in Renal Function (Little, Brown, Boston), pp. 135–138.
- 2.Peach, M. J. (1977) Physiol. Rev. 57, 313–370. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, D. J. (1987) J. Clin. Invest. 79, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dostal, D. E. & Baker, K. M. (1999) Circ. Res. 85, 643–650. [DOI] [PubMed] [Google Scholar]
- 5.Davisson, R. L. (2003) Am. J. Physiol. 285, R498–R511. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, W. G., Gahnem, F., Catanzaro, D. F., James, G. D., Camargo, M. J., Laragh, J. H. & Sealey, J. E. (1996) Hypertension 27, 1121–1133. [DOI] [PubMed] [Google Scholar]
- 7.Kiernan, J. (1981) Histological and Histochemical Methods: Theory and Practice (Pergamon, Oxford).
- 8.Sudhof, T. C., Czernik, A. J., Kao, H. T., Takei, K., Johnston, P. A., Horiuchi, A., Kanazir, S. D., Wagner, M. A., Perin, M. S. & De Camilli, P. (1989) Science 245, 1474–1480. [DOI] [PubMed] [Google Scholar]
- 9.Becker, B. N., Jacobson, L. M., Becker, Y. T., Radke, N. A., Heisey, D. M., Oberley, T. D., Pirsch, J. D., Sollinger, H. W., Brazy, P. C. & Kirk, A. D. (2000) Transplantation 69, 1485–1491. [DOI] [PubMed] [Google Scholar]
- 10.Jensen, B., Kramer, B. K. & Kurtz, A. (1997) Hypertension 29, 1148–1155. [DOI] [PubMed] [Google Scholar]
- 11.Baram, D., Adachi, R., Medalia, O., Tuvim, M., Dickey, B. F., Mekori, Y. A. & Sagi-Eisenberg, R. (1999) J. Exp. Med. 189, 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demoz, M., Castino, R., Dragonetti, A., Raiteri, E., Baccino, F. M. & Isidoro, C. (1999) J. Cell Biochem. 73, 370–378. [PubMed] [Google Scholar]
- 13.Goto, M., Mizunashi, K., Kimura, N. & Furukawa, Y. (2001) J. Am. Soc. Nephrol. 12, 1965–1970. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson, G., Blom, T., Kusche-Gullberg, M., Kjellen, L., Butterfiled, J. H., Sundstrom, C., Nillson, K. & Hellman, L. (1994) Scand. J. Immunol. 39, 489–498. [DOI] [PubMed] [Google Scholar]
- 15.Katz, S. & Opsahl, J. A. (1995) in Hypertension: Pathology, Diagnosis, and Management, ed. Laragh, J. H. & Brenner, M. (Raven, New York), pp. 1489–1502.
- 16.Levi, R. & Allan, G. (1980) in Drug-Induced Heart Disease, ed. Bristow, M. (Elsevier, New York), pp. 377–395.
- 17.Hackenthal, E., Hackenthal, R. & Hilgenfeldt, U. (1978) Biochim. Biophys. Acta 522, 574–588. [DOI] [PubMed] [Google Scholar]
- 18.Simoneau, B., Lavallee, P., Anderson, P. C., Bailey, M., Bantle, G., Berthiaume, S., Chabot, C., Fazal, G., Halmos, T., Ogilvie, W. W., et al. (1999) Bioorg. Med. Chem. 7, 489–508. [DOI] [PubMed] [Google Scholar]
- 19.Kruger, P. G., Ellingsen, T. & Saetersdal, T. S. (1990) J. Exp. Pathol. 5, 29–38. [PubMed] [Google Scholar]
- 20.Seyedi, N., Win, T., Lander, H. M. & Levi, R. (1997) Circ. Res. 81, 774–784. [DOI] [PubMed] [Google Scholar]
- 21.Reid, A. C., Mackins, C. J., Seyedi, N., Levi, R. & Silver, R. B. (2004) Am. J. Physiol. 286, H1448–H1454. [DOI] [PubMed] [Google Scholar]
- 22.Davisson, R. L., Oliverio, M. I., Coffman, T. M. & Sigmund, C. D. (2000) J. Clin. Invest. 106, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deinum, J., Derkx, F. H., Danser, A. H. & Schalekamp, M. A. (1990) Endocrinology 126, 1673–1682. [DOI] [PubMed] [Google Scholar]
- 24.Wagner, J., Jan Danser, A. H., Derkx, F. H., de Jong, T. V., Paul, M., Mullins, J. J., Schalekamp, M. A. & Ganten, D. (1996) Br. J. Ophthalmol. 80, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung, P. S., Wong, T. P., Lam, S. Y., Chan, H. C. & Wong, P. Y. (2000) Life Sci. 66, 1317–1324. [DOI] [PubMed] [Google Scholar]
- 26.Barlucchi, L., Leri, A., Dostal, D. E., Fiordaliso, F., Tada, H., Hintze, T. H., Kajstura, J., Nadal-Ginard, B. & Anversa, P. (2001) Circ. Res. 88, 298–304. [DOI] [PubMed] [Google Scholar]
- 27.Endo-Mochizuki, Y., Mochizuki, N., Sawa, H., Takada, A., Okamoto, H., Kawaguchi, H., Nagashima, K. & Kitabatake, A. (1995) Heart Vessels 10, 285–293. [DOI] [PubMed] [Google Scholar]
- 28.Dostal, D. E. (2000) Regul. Pept. 91, 1–11. [DOI] [PubMed] [Google Scholar]
- 29.Bader, M., Peters, J., Baltatu, O., Müller, D. N., Luft, F. C. & Ganten, D. (2001) J. Mol. Med. 79, 76–102. [DOI] [PubMed] [Google Scholar]
- 30.Katz, S., Opsahl, J. A & Forbis, L. M. (2001) Basic Res. Cardiol. 96, 659–668. [DOI] [PubMed] [Google Scholar]
- 31.Sinn, P. L. & Sigmund, C. D. (2000) Physiol. Genomics 3, 25–31. [DOI] [PubMed] [Google Scholar]
- 32.Xiang, Z., Block, M., Lofman, C. & Nilsson, G. (2001) J. Allergy Clin. Immunol. 108, 116–121. [DOI] [PubMed] [Google Scholar]
- 33.Peters, J., Farrenkopf, R., Clausmeyer, S., Zimmer, J., Kantachuvesiri, S., Sharp, M. G. F. & Mullins, J. J. (2002) Circ. Res. 90, 1135–1141. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen, G., Delarue, F., Burcklè, C., Bouzhir, L., Giller, T. & Sraer, J. D. (2002) J. Clin. Invest. 109, 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marone, G., De Crescenzo, G., Adt, M., Patella, V., Arbustini, E. & Genovese, A. (1995) Immunopharmacology 31, 1–18. [DOI] [PubMed] [Google Scholar]
- 36.Dell'italia, L. J., Meng, Q. C., Balcells, E., Wei, C. C., Palmer, R., Hageman, G. R., Durand, J., Hankes, G. H. & Oparil, S. (1997) J. Clin. Invest. 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Lannoy, L., Schuijt, M. O., Saxena, P. R., Schalekamp, M. & Danser, A. (2001) J. Hypertension 19, 959–965. [DOI] [PubMed] [Google Scholar]
- 38.Feoktistov, I. & Biaggioni, I. (1995) J. Clin. Invest. 96, 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belardinelli, L., Linden, J. & Berne, R. (1989) Prog. Cardiovasc. Dis. 32, 73–97. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg, J., Franco-Cereceda, A., Lacroix, J. S. & Pernow, J. (1990) Ann. N.Y. Acad. Sci. 611, 166–174. [DOI] [PubMed] [Google Scholar]
- 41.Arzubiaga, C., Morrow, J., Roberts, L. J., Jr., & Biaggioni, I. (1991) J. Allergy Clin. Immunol. 87, 88–93. [DOI] [PubMed] [Google Scholar]