Abstract
The P3k oncoprotein [homolog of the catalytic subunit p110α of class 1A phosphoinositide 3-kinase (PI3K)] and its downstream effector Akt induce oncogenic transformation in cultures of chicken embryo fibroblasts (CEF). The winged helix transcription factor FoxO1 is a growth-attenuating and proapoptotic protein and serves as a substrate of Akt. Here we show that FoxO1 expression is constitutively suppressed in CEF transformed by P3k or Akt. The FoxO1 protein level is high in serum-starved normal CEF, but platelet-derived growth factor treatment induces rapid phosphorylation and disappearance of FoxO1. PI3K inhibitors or the proteasome inhibitor lactacystin interfere with this process. These data suggest that phosphorylation-dependent degradation of FoxO1 by means of proteasomes plays a role in oncogenic transformation by P3k and Akt. A dominant negative mutant of FoxO1 containing the repressor domain of the Drosophila Engrailed protein induces partial oncogenic transformation of CEF and interferes with FoxO1-dependent transcriptional activation. The FoxG1 oncoprotein also inhibits transcriptional activation by FoxO1. Inhibition of FoxO1, albeit by different mechanisms, appears to be a common denominator of the PI3K and FoxG1 oncogenic pathways.
Keywords: phosphoinositide 3-kinase, oncogenic transformation, transcriptional repression
Constitutive activation of the phosphoinositide 3-kinase (PI3K)/Akt signal pathway is found in various human cancers (1, 2). The oncogenic potential of PI3K is also evident in experimental systems. Two avian retroviruses, avian sarcoma virus (ASV)16 and ASV8905, induce oncogenic transformation in cultures of chicken embryo fibroblasts (CEF) and cause hemangiosarcomas in chickens to carry the v-p3k oncogene. This cell-derived insert in the viral genome codes for a homologue of the catalytic subunit p110α of class 1A PI3K (3, 4). Oncogenic transformation by the v-P3k oncoprotein requires activation of the Ser-Thr kinase Akt. Constitutively active forms of Akt also induce transformation of CEF and cause hemangiosarcomas in young chickens (5). Akt phosphorylates many protein substrates to carry out diverse cellular functions that affect cell growth, survival, and metabolism (6–11). Important among these Akt targets are the transcriptional regulators belonging to the family of FoxO proteins. Members of this family share the winged helix motif (or forkhead box) as a DNA-binding domain (12, 13). In higher eukaryotes, FoxO proteins have diverse functions, extending from the control of metabolism to the regulation of cell replication (14). These proteins operate at the crossroads of two important signaling pathways, one originating with PI3K and Akt, the other with transforming growth factor type β (TGF-β). FoxO1, FoxO3a, and FoxO4 are phosphorylated by Akt, and this phosphorylation induces exclusion from the nucleus, binding to the 14-3-3 protein and prevents transcriptional regulation by FoxO (15–19). Recent findings suggest degradation of FoxO1 and FoxO3a upon phosphorylation by Akt in certain settings (20, 21). FoxO proteins interact with Smad3 and Smad4 to form a TGF-β-mediated transcriptional activation complex that targets p21Cip1, resulting in an attenuation of cell growth (22). The functions of the FoxO proteins are further modulated by another Fox family transcription factor, FoxG1. FoxG1 acts as a transcriptional repressor, has oncogenic potential (23–27), and can address some of the same targets as the FoxO proteins but can repress instead of activate these targets (22).
Here we report that in CEF transformed by P3k or Akt, the FoxO1 protein is down-regulated by means of degradation in proteasomes. We also demonstrate partial transformation of CEF by FoxO1 constructs that have been modified to function as transcriptional repressors and hence as antagonists of FoxO1. These observations suggest that oncogenic transformation by P3k and Akt requires drastic loss of the transcriptional regulator function of FoxO1. Such loss of function is also induced by the FoxO1 repressor constructs or by the Fox family protein FoxG1 and has similar oncogenic consequences.
Materials and Methods
Culture of CEF for Transfection and Transformation Assays. CEF were prepared from White Leghorn embryos obtained from Charles River Laboratories (28). DNA was transfected into CEF by using the dimethyl sulfoxide/polybrene method (5). Assays for focus formation by using replication-competent avian sarcoma (RCAS) vectors that express myristoylated P3K or myristoylated Akt were performed as described in refs. 4, 5, and 29.
Serum Starvation and Platelet-Derived Growth Factor (PDGF) Stimulation. For serum starvation, cells were cultured in Ham's F-10 medium with 0.5% FCS and 0.1% chicken serum. After 40 h, the medium was replaced with plain F-10 medium, and the culture was further incubated for 2 h. The cells were then stimulated with 50 ng/ml PDGF (Life Technologies) for 15 min. For drug treatment, rapamycin (10 ng/ml), wortmannin (100 nM), LY294002 (20 μM), PD98059 (20 μM), or lactacystin (10 μM) was added to the culture 30 min before the addition of PDGF.
Western Blots. Cells were lysed in Nonidet P-40 lysis buffer (20 mM Tris·HCl, pH 7.5/150 mM NaCl/10% glycerol/1% Nonidet P-40/10 mM NaF/1 mM sodium pyrophosphate/1 mM sodium orthovanadate) containing a protease inhibitor mixture (Complete, Boehringer Mannheim, which is now Roche Molecular Biochemicals). Lysates containing 60 μg of protein were separated by SDS/PAGE and transferred to Immobilon P membranes (Millipore). The membranes were blocked with 5% nonfat dry milk, Tris-buffered saline, and 0.05% Tween 20 for 1 h at room temperature and then probed with anti-FoxO1 (Cell Signaling Technology, Beverly, MA) to detect FoxO1, anti-phospho-FoxO1 (Ser-256, Cell Signaling Technology) to detect phosphorylated FoxO1, and anti-α-tubulin (ICN) as an internal control.
Plasmid Construction. Human FoxO1 cDNA (a kind gift from F. Barr, University of Pennsylvania, Philadelphia) was subcloned into the pBSFI adaptor plasmid as described in ref. 30. The AU1 epitope tag was attached to the amino terminus of FoxO1 by PCR using the 5′-AU1FoxO1 primer (5′-TGCTCTAGACCATGGACACCTACAGATACATC-3′) and the 3′-FoxO1 primer (5′-CGCTCTTGACCATCCACTCG-3′), followed by ligation of the XbaI/BglII fragment of the PCR products with XbaI/BglII-digested pBSFI-FoxO1. The FoxO1-Eng fusion construct was generated as follows: The MluI fragment spanning the transcriptional repressor domain of Engrailed (Eng) (a kind gift from M. Nishizawa, The Scripps Research Institute) was subcloned into pBSFI to generate pBSFI-Eng. The NcoI fragment of AU1-FoxO1 was then ligated into NcoI-digested pBSFI-Eng. FoxO1(AA)-Eng was constructed by introducing alanine substitutions in the Akt phosphorylation sites of FoxO1-Eng with Kunkel's method (31). FoxO1(AA)/H215R-Eng was prepared by introducing a point mutation at codon 215 by PCR with the 5′-H215R primer (5′-GCTCGAATTCAATTCGTCGTAATCTGTCC-3′) and the 3′-H215R primer (5′-GCTCCCACCCTCTGGATTGAGCATC-3′) and with pBSFI-FoxO1 as a template. The EcoRI/PflMI fragment of the PCR products was ligated into EcoRI/PflMI-digested pBSFI-FoxO1(AA)-Eng. The SfiI fragments carrying these constructs were subcloned into the replication-competent avian retroviral vector RCAS.Sfi as described (5). For reporter assays, the SfiI fragments were subcloned into pcDNA3.Sfi. The pGL3-cytomegalovirus (CMV)-3xIRS firefly luciferase reporter construct was built by subcloning annealed oligonucleotides 3xIRS-1 (5′-TCGATTCAAAATAAGTTTGTTTTGCTTCAAAATAAGTTTGTTTTGCTTCAAAATAAGTTTGTTTTGCGTAC-3′) and 3xIRS-2 (5′-GCAAAACAAACTTATTTTGAAGCAAAACAAACTTATTTTGAAGCAAAACAAACTTATTTTGAAT-3′) into XhoI/KpnI-digested pGL3-CMV with a silent mutation in the luciferase gene to remove a putative Fox-binding site (32). Akt and FoxG1 plasmids have been described in refs. 5 and 24.
Reporter Assays. Reporter assays were performed by using CEF. Cells were seeded into MP-24 tissue culture plates at 4 × 104 cells per well. On the next day, the cultures were transfected with 50 ng of pGL3-CMV-3xIRS firefly luciferase reporter driven by three copies of the insulin response sequence: the consensus binding site for FoxO1, 200 ng of pcDNA3 expression vector carrying FoxO1, Akt, or FoxG1 constructs, and 5 ng of pRL-CMV, a Renilla luciferase construct, as internal control. After 40 h of incubation, the cultures were washed once with PBS and then lysed in 250 μl of Passive lysis buffer (Promega) according to the manufacturer's protocol. Firefly luciferase activities and Renilla luciferase activities were measured by using the Dual-Luciferase reporter assay system (Promega) with a Berthold Biolumat model LB 9501. Firefly luciferase activities were normalized against Renilla luciferase activities.
Results and Discussion
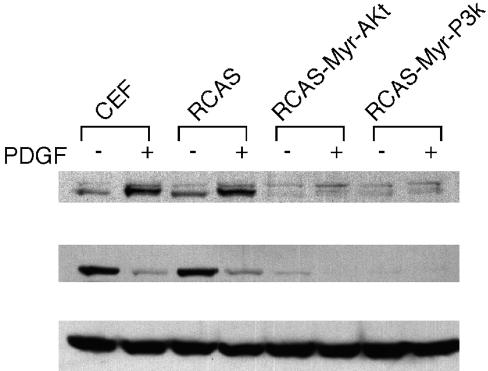
The direct genetic link between Akt and the FoxO family protein DAF-16 in Caenorhabditis elegans and similar links in mammalian systems prompted us to examine the phosphorylation status of endogenous FoxO1 in Akt-transformed CEF with a phospho-specific anti-FoxO1 Ab. This Ab recognizes FoxO1 that is phosphorylated at Ser-256, the major Akt-dependent phosphorylation site residing in the winged helix DNA-binding domain. Uninfected CEF, CEF transformed by Akt or P3k, and CEF infected with the RCAS vector alone were serum-starved and then stimulated with PDGF for 15 min. Background phosphorylation of FoxO1 was observed in normal or RCAS vector-infected CEF, and PDGF greatly enhanced the phosphorylation as expected (Fig. 1 Top). However, contrary to our expectation, phosphorylated FoxO1 was below detectable levels in Akt- or P3k-transformed CEF, even after PDGF stimulation. The same blot was probed with a phosphorylation-independent FoxO1 Ab. In normal CEF, the presence of FoxO1 was observed, but FoxO1 disappeared 15 min after PDGF stimulation (Fig. 1 Middle). However, in Akt- or P3k-transformed CEF, FoxO1 was not detectable, irrespective of PDGF stimulation. This result may explain the failure to detect phosphorylated FoxO1 in P3k- and in Akt-infected CEF. Equal loading of the lysates was confirmed with an anti-α-tubulin Ab (Fig. 1 Bottom). These results suggest that P3k and Akt regulate FoxO1 protein levels possibly by affecting production or stability. The data cannot be explained by phosphorylation-dependent changes in cellular distribution.
Fig. 1.
FoxO1 protein levels and phosphorylation in Akt/P3k-transformed CEF. Uninfected CEF, RCAS vector-infected CEF, and CEF transformed by myristoylated Akt (Myr-Akt) or myristoylated P3k (Myr-P3k) were serum-starved for 40 h and then stimulated or not with 50 ng/ml PDGF-BB for 15 min. Lysates were separated by 10% SDS/PAGE and transferred to a poly(vinylidene difluoride) membrane. The membrane was probed with anti-phospho-FoxO1 (Ser-256, Cell Signaling Technology) (Top). The same blot was stripped and probed with anti-FoxO1 (phosphorylation-independent, Cell Signaling Technology) (Middle) and with anti-α-tubulin Ab (ICN) (Bottom).
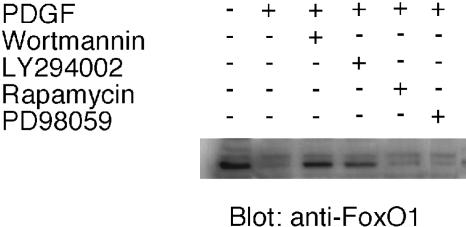
To identify the pathway extending from the PDGF receptor to the down-regulation of FoxO1, a similar experiment was performed by using pharmacological inhibitors of PI3K (wortmannin and LY294002), extracellular signal-regulated kinase (PD98059), and target of rapamycin (TOR). PDGF-induced down-regulation of FoxO1 was specifically inhibited by wortmannin and LY294002 but not by PD98059 or rapamycin (Fig. 2). Equal loading of the lysates was confirmed with an anti-α-tubulin Ab (data not shown). These results suggest that the PDGF-induced down-regulation of FoxO1 depends on PI3K activity but does not involve the tuberous sclerosis-TOR branch of Akt signaling.
Fig. 2.
Effect of PI3K inhibitors on FoxO1 down-regulation. Uninfected CEF were serum-starved for 40 h, incubated with rapamycin (10 ng/ml), wortmannin (100 nM), LY294002 (20 μM), or the extracellular signal-regulated kinase inhibitor PD98059 (20 μM) for 30 min, and then stimulated with PDGF for 15 min. The membrane was probed with anti-FoxO1 Ab.
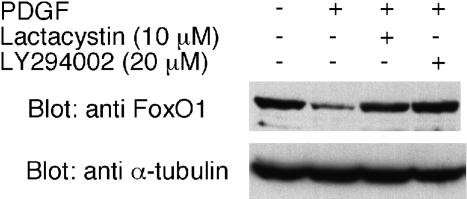
To test the possibility that down-regulation of FoxO1 upon PDGF stimulation is regulated by protein degradation, we incubated the cells before PDGF stimulation with 10 μM lactacystin, a proteasome inhibitor. A Western blot probed with anti-FoxO1 Ab demonstrated that lactacystin prevents the PDGF-induced down-regulation of FoxO1 as does Ly294002. This result strongly suggests the involvement of protein degradation in the regulation of FoxO1 (Fig. 3). Our finding is consistent with the recent report on insulin-induced degradation of FoxO1 in HepG2 cells (20). Matsuzaki et al. (20) found that FoxO1 phosphorylation by Akt leads to exclusion of FoxO1 from the nucleus and that the phosphophorylated FoxO1 is ubiquitinated in the cytoplasm followed by degradation in the 26 S proteasome. However, in HEPG2 cells the degradation of FoxO1 after insulin stimulation proceeds significantly slower (with a half-life of 4–6 h) than in CEF, where FoxO1 is undetectable 15 min after stimulation by PDGF. The extremely rapid turnover of FoxO1 in PDGF-stimulated CEF suggests that CEF are highly sensitive to growth inhibition by FoxO1. Another recent study of FL5.12 murine pro-B lymphocytic cells also showed that phosphorylation of FoxO1 (and of FoxO3a) and of tuberin by Akt results in degradation by means of proteasomes (21).
Fig. 3.
Effect of lactacystin on FoxO1 down-regulation. CEF were serum-starved for 40 h, incubated with lactacystin (10 μM) or LY294002 (20 μM) for 30 min, and then stimulated with PDGF for 15 min. The membrane was probed with anti-FoxO1 Ab or with anti-α-tubulin Ab for a loading control.
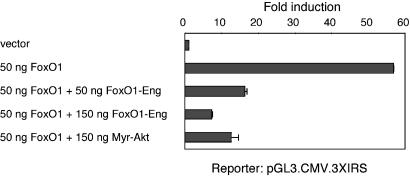
Because FoxO proteins are growth-inhibitory, a decrease in FoxO1 may be required for oncogenic transformation induced by P3k or Akt. This possibility was explored with a FoxO1 chimeric construct that could function as an antagonist of WT FoxO1. It was shown previously that a deletion mutant of FoxO1 that contains the DNA-binding domain but lacks the transactivation domain can function as a dominant negative (33, 34). Following a similar strategy, we made an artificial repressor construct, FoxO1-Eng, in which the carboxyl-terminal activator domain of FoxO1 was replaced by the repressor domain of the Drosophila Eng protein. This construct was tested for the ability to inhibit FoxO1-induced transcriptional activation in CEF, by using a reporter with three copies of an insulin-responsive sequence upstream of the CMV minimal promoter of pGL3-CMV. As shown in Fig. 4, FoxO1-Eng inhibited FoxO1-induced transcription in a dose-dependent manner, and this inhibition was as strong as that caused by myristoylated Akt. These results suggest that FoxO1-Eng fusion constructs can be used as dominant negative forms of FoxO1 in CEF.
Fig. 4.
Effects of FoxO1-Eng fusion constructs on FoxO1-induced transcriptional activation in CEF. CEF were transfected with pGL3-CMV-3xIRS, the firefly luciferase reporter construct carrying three copies of the FoxO1-binding motif upstream of the CMV minimal promoter, together with FoxO1 constructs in the pcDNA3 vector as indicated. Data were calibrated for transfection efficiency by cotransfection of the Renilla luciferase reporter construct pRL-CMV.
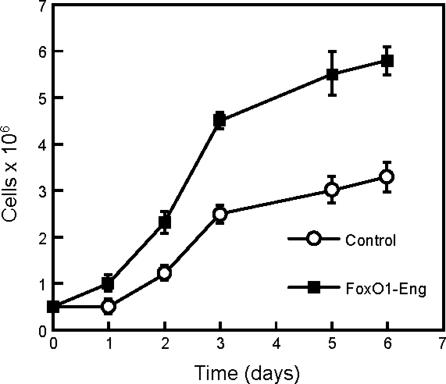
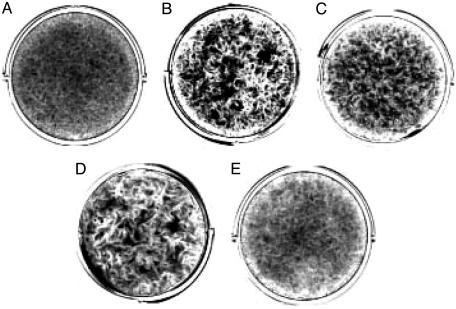
To test the effects of FoxO1-Eng on cell proliferation, FoxO1-Eng was expressed in CEF with the RCAS vector. These FoxO1-Eng-CEF grew faster and to a higher saturation density than CEF transfected with the empty RCAS vector (Fig. 5). Transfection with RCAS-FoxO1-Eng also induced foci of transformed cells when the cells were cultured under nutrient agar (Fig. 6). A second construct, FoxO1(AA)-Eng, which carried alanine mutations in the Akt phosphorylation sites of FoxO1, also induced focus formation, indicating that Akt-mediated phosphorylation of the chimeric FoxO1 protein plays no role in this transformation process. However, FoxO1(AA)/H215R-Eng, a third construct that failed to bind DNA because of an additional mutation in the winged helix DNA-binding domain, failed to induce transformation. The enhanced growth properties of FoxO1-Eng- and FoxO1(AA)-Eng-transfected CEF did not include anchorage independence, nor did the FoxO1 repressor constructs induce tumors in young chickens. The severe down-regulation of FoxO1 in P3k- or Akt-transformed CEF and the partial transforming activity of the FoxO1-Eng repressor construct both point to an involvement of FoxO1 in PI3K-dependent oncogenic transformation. Overexpression of FoxO1 in CEF would be expected to inhibit transformation induced by P3k or Akt. This possibility could not be tested because FoxO1 could not be stably expressed in CEF for reasons that may have to do with its growth-inhibitory activities.
Fig. 5.
Growth curve of CEF expressing FoxO1-Eng. CEF transfected with RCAS vector alone or with RCAS-FoxO1-Eng were seeded at 5 × 105 cells per 60-mm plate on day 0 and cultured in cloning medium (5). Cell numbers were counted at indicated times by using a Beckman Coulter counter.
Fig. 6.
Tests for focus formation by the FoxO1-Eng constructs. CEF were transfected with 500 ng of DNA constructs by using the dimethyl sulfoxide/polybrene method. The cells were overlaid with nutrient agar for 21 days and then fixed and stained with crystal violet. (A) Empty RCAS vector. (B) RCAS FoxO1-Eng. (C) ASV31(FoxG1). (D) RCAS FoxO1(AA)-Eng. (E) RCAS FoxO1(AA)/H215R-Eng.
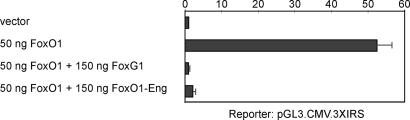
A connection between FoxO proteins and oncogenic transformation is also supported by the actions of another Fox family protein, FoxG1. FoxG1 was originally identified as brain factor 1; it was also recovered as the retroviral oncoprotein Qin in ASV31 (35, 36). Expression of FoxG1 is restricted to the telencephalon (36, 37). During embryonal development, this telencephalon-specific expression of FoxG1 prevents premature differentiation of neuroepithelial cells and is essential for the normal formation of the brain (38–40). FoxG1 knock-out mice are born but are not viable. Overexpression of FoxG1 in the developing avian brain leads to excessive growth of the neuroepithelium in the telencephalon, probably by interfering with apoptosis (41). Oncogenic transformation induced by retroviral expression of FoxG1 is correlated with transcriptional repression (23–25). FoxG1 binds to the consensus sequence TGTAAACAAA (25), which is closely similar to GTAAACAA, identified as the binding motif for FoxO proteins (42). In cotransfections of FoxO1 and FoxG1 with the pGL3-CMV-3xIRS reporter, FoxG1 strongly inhibited FoxO1-mediated transcriptional activation (Fig. 7). FoxG1 and FoxO proteins may therefore share target genes that are negatively regulated by the former and positively regulated by the latter.
Fig. 7.
Effects of FoxG1 and FoxO1-Eng fusion constructs on FoxO1-induced transcriptional activation in CEF. CEF were transfected with pGL3-CMV-3xIRS, the firefly luciferase reporter construct carrying three copies of the FoxO1-binding motif upstream of the CMV minimal promoter, together with effector constructs in the pcDNA3 vector as indicated. Data were calibrated for transfection efficiency by cotransfection of the Renilla luciferase reporter construct pRL-CMV.
A critical function of the FoxO proteins is the transcriptional activation of p21Cip1 in response to TGF-β (22). The promoter region of p21Cip1 contains contiguous FoxO- and Smad-binding sites that are targeted by Smad–FoxO complexes. FoxG1 binds to the FoxO–Smad complex but does not prevent this complex from interacting with its composite binding site. However, it appears that the ternary complex may now recruit corepressors and so may turn the FoxO1–Smad-mediated transcriptional activation into repression. Oncogenic transformation induced by ectopic expression of FoxG1 may therefore involve the down-regulation of p21Cip1, converting the TGF-β-mediated positive signal into a negative one. Inactivation of the p21Cip1-dependent cytostatic activity of TGF-β may also be part of the oncogenic mechanism induced by a gain of function in PI3K and Akt. This effect is based on a direct action on FoxO, making it unavailable for transcriptional regulation. Our data show that control of FoxO protein stability is an important aspect of PI3K-mediated oncogenicity. Interference with FoxO-mediated transcription is also important for Myr-dependent stimulation of cell growth (43). Another branch of PI3K signaling is essential for oncogenic transformation: the activation of the TOR kinase, and, presumably, TOR-dependent enhanced synthesis of specific proteins (5, 11, 44). The interactions of transcriptional controls, operating through FoxO1 and translational controls and mediated by TOR in PI3K oncogenesis, require additional study.
Acknowledgments
We thank Hershey S. Filoteo and Jeffrey Ludwig for excellent technical support and Gail Yoder for expert help with the manuscript. This is the Scripps Research Institute manuscript no. 16263-MEM. This work was supported by grants from the National Cancer Institute, National Institutes of Health.
Abbreviations: PI3K, phosphoinositide 3-kinase; CEF, chicken embryo fibroblasts; TOR, target of rapamycin; RCAS, replication-competent avian sarcoma; Eng, Engrailed; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor type β; CMV, cytomegalovirus; ASV, avian sarcoma virus.
References
- 1.Vivanco, I. & Sawyers, C. L. (2002) Nat. Rev. Cancer 2, 489–501. [DOI] [PubMed] [Google Scholar]
- 2.Samuels, Y., Wang, Z., Bardelli, A., Silliman, N., Ptak, J., Szabo, S., Yan, H., Gazdar, A., Powell, S. M., Riggins, G. J., et al. (2004) Science 304, 554. [DOI] [PubMed] [Google Scholar]
- 3.Chang, H. W., Aoki, M., Fruman, D., Auger, K. R., Bellacosa, A., Tsichlis, P. N., Cantley, L. C., Roberts, T. M. & Vogt, P. K. (1997) Science 276, 1848–1850. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, M., Schetter, C., Himly, M., Batista, O., Chang, H. W. & Vogt, P. K. (2000) J. Biol. Chem. 275, 6267–6275. [DOI] [PubMed] [Google Scholar]
- 5.Aoki, M., Batista, O., Bellacosa, A., Tsichlis, P. & Vogt, P. K. (1998) Proc. Natl. Acad. Sci. USA 95, 14950–14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alessi, D. R. & Cohen, P. (1998) Curr. Opin. Genet. Dev. 8, 55–62. [DOI] [PubMed] [Google Scholar]
- 7.Chan, T. O., Rittenhouse, S. E. & Tsichlis, P. N. (1999) Annu. Rev. Biochem. 68, 965–1014. [DOI] [PubMed] [Google Scholar]
- 8.Datta, S. R., Brunet, A. & Greenberg, M. E. (1999) Genes Dev. 13, 2905–2927. [DOI] [PubMed] [Google Scholar]
- 9.Downward, J. (1998) Curr. Opin. Cell Biol. 10, 262–267. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor, M. A. & Alessi, D. R. (2001) J. Cell Sci. 114, 2903–2910. [DOI] [PubMed] [Google Scholar]
- 11.Aoki, M. & Vogt, P. K. (2004) Curr. Top. Microbiol. Immunol. 279, 321–338. [DOI] [PubMed] [Google Scholar]
- 12.Weigel, D. & Jackle, H. (1990) Cell 63, 455–456. [DOI] [PubMed] [Google Scholar]
- 13.Kaestner, K. H., Knochel, W. & Martinez, D. E. (2000) Genes Dev. 14, 142–146. [PubMed] [Google Scholar]
- 14.Accili, D. & Arden, K. C. (2004) Cell 117, 421–426. [DOI] [PubMed] [Google Scholar]
- 15.Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. C., Blenis, J. & Greenberg, M. E. (1999) Cell 96, 857–868. [DOI] [PubMed] [Google Scholar]
- 16.Brunet, A., Kanai, F., Stehn, J., Xu, J., Sarbassova, D., Frangioni, J. V., Dalal, S. N., DeCaprio, J. A., Greenberg, M. E. & Yaffe, M. B. (2002) J. Cell Biol. 156, 817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahill, C. M., Tzivion, G., Nasrin, N., Ogg, S., Dore, J., Ruvkun, G. & Alexander-Bridges, M. (2001) J. Biol. Chem. 276, 13402–13410. [DOI] [PubMed] [Google Scholar]
- 18.Biggs, W. H., III, Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. (1999) Proc. Natl. Acad. Sci. USA 96, 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kops, G. J., de Ruiter, N. D., De Vries-Smits, A. M., Powell, D. R., Bos, J. L. & Burgering, B. M. (1999) Nature 398, 630–634. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki, H., Daitoku, H., Hatta, M., Tanaka, K. & Fukamizu, A. (2003) Proc. Natl. Acad. Sci. USA 100, 11285–11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plas, D. R. & Thompson, C. B. (2003) J. Biol. Chem. 278, 12361–12366. [DOI] [PubMed] [Google Scholar]
- 22.Seoane, J., Le, H. V., Shen, L., Anderson, S. A. & Massague, J. (2004) Cell 117, 211–223. [DOI] [PubMed] [Google Scholar]
- 23.Sonderegger, C. K. & Vogt, P. K. (2003) Oncogene 22, 1749–1757. [DOI] [PubMed] [Google Scholar]
- 24.Li, J., Thurm, H., Chang, H. W., Iacovoni, J. S. & Vogt, P. K. (1997) Proc. Natl. Acad. Sci. USA 94, 10885–10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, J., Chang, H. W., Lai, E., Parker, E. J. & Vogt, P. K. (1995) Cancer Res. 55, 5540–5544. [PubMed] [Google Scholar]
- 26.Tan, K., Shaw, A. L., Madsen, B., Jensen, K., Taylor-Papadimitriou, J. & Freemont, P. S. (2003) J. Biol. Chem. 278, 20507–20513. [DOI] [PubMed] [Google Scholar]
- 27.Yao, J., Lai, E. & Stifani, S. (2001) Mol. Cell. Biol. 21, 1962–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogt, P. K. (1969) in Fundamental Techniques in Virology, eds. Habel, K. & Salzman, N. P. (Academic, New York), pp. 316–326.
- 29.Hughes, S. H., Greenhouse, J. J., Petropoulos, C. J. & Sutrave, P. (1987) J. Virol. 61, 3004–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempf, B. E. & Vogt, P. K. (1999) Cell Growth Differ. 10, 813–818. [PubMed] [Google Scholar]
- 31.Kunkel, T. A., Bebenek, K. & McClary, J. (1991) Methods Enzymol. 204, 125–139. [DOI] [PubMed] [Google Scholar]
- 32.Sonderegger, C. K., Narisawa-Saito, M. & Vogt, P. K. (2003) Oncogene 22, 1908–1915. [DOI] [PubMed] [Google Scholar]
- 33.Leenders, H., Whiffield, S., Benoist, C. & Mathis, D. (2000) Eur. J. Immunol. 30, 2980–2990. [DOI] [PubMed] [Google Scholar]
- 34.Nakae, J., Kitamura, T., Silver, D. L. & Accili, D. (2001) J. Clin. Invest. 108, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, J. & Vogt, P. K. (1993) Proc. Natl. Acad. Sci. USA 90, 4490–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao, W. & Lai, E. (1992) Neuron 8, 957–966. [DOI] [PubMed] [Google Scholar]
- 37.Chang, H. W., Li, J., Kretzschmar, D. & Vogt, P. K. (1995) Proc. Natl. Acad. Sci. USA 92, 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xuan, S., Baptista, C. A., Balas, G., Tao, W., Soares, V. C. & Lai, E. (1995) Neuron 14, 1141–1152. [DOI] [PubMed] [Google Scholar]
- 39.Hanashima, C., Shen, L., Li, S. C. & Lai, E. (2002) J. Neurosci. 22, 6526–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanashima, C., Li, S. C., Shen, L., Lai, E. & Fishell, G. (2004) Science 303, 56–59. [DOI] [PubMed] [Google Scholar]
- 41.Ahlgren, S., Vogt, P. K. & Bronner-Fraser, M. (2003) J. Neurobiol. 57, 337–349. [DOI] [PubMed] [Google Scholar]
- 42.Furuyama, T., Nakazawa, T., Nakano, I. & Mori, N. (2000) Biochem. J. 349, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouchard, C., Marquardt, J., Bras, A., Medema, R. H., Eilers, M. (2004) EMBO J. 23, 2830–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoki, M., Blazek, E. & Vogt, P. K. (2001) Proc. Natl. Acad. Sci. USA 98, 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]