Abstract
The NF-κB/IκB signaling pathway is a critical regulator of cell survival in cancer. Here, we report that combined down-regulation of growth arrest- and DNA-damage-inducible proteins (GADD)45α and γ expression by NF-κB is an essential step for various cancer types to escape programmed cell death. We demonstrate that inhibition of NF-κB in cancer cells results in GADD45α- and γ-dependent induction of apoptosis and inhibition of tumor growth. Inhibition of GADD45α and γ in cancer cells by small interfering RNA abrogates apoptosis induction by the inhibitor of NF-κB and blocks c-Jun N-terminal kinase activation, whereas overexpression of GADD45α and γ activates c-Jun N-terminal kinase and induces apoptosis. These results establish an unambiguous role for the GADD45 family as an essential mediator of cell survival in cancer cells with implications for cancer chemotherapy and novel drug discovery.
In addition to its role in the immune system and in mediating inflammatory responses, the NF-κB/inhibitor of NF-κB (IκB) pathway has been directly implicated in tumorigenesis, cancer cell survival, apoptosis, invasion, and metastasis (1, 2). Constitutive activation of NF-κB is frequently observed in various cancer types, including prostate cancer and is a critical step for cancer cells to escape programmed cell death and to survive proapoptotic stimuli by numerous triggers (3–5). Furthermore, resistance of cancer cells to chemotherapeutic agents can be at least partially explained by deregulated NF-κB activation (6). Although several facets of the prosurvival pathway of NF-κB action have been elucidated, many aspects of the exact molecular mechanism remain unknown.
c-Jun N-terminal kinase (JNK) activation plays a role in UV-induced apoptosis, which also activates NF-κB (7). Originally, JNK activation and inhibition experiments indicated a proapoptotic role for JNK in tumor necrosis factor α (TNF-α) signaling (8). Nevertheless, detailed analysis of the TNF-α pathway did not support the proapoptotic function of JNK. NF-κB has been postulated to induce the expression of a JNK inhibitor that contributes to the antiapoptotic function of NF-κB (9). The growth arrest- and DNA-damage-inducible proteins (GADD)45 gene family encodes three related GADD proteins, GADD45α, β, and γ (10). GADD45 proteins are primarily nuclear proteins that interact with various cell-cycle-related proteins (10, 11). The role of GADD45 in apoptosis remains unclear. The only member of the GADD45 family that has been, until now, shown to be involved in the antiapoptotic effect of NF-κB is GADD45β (12). NF-κB-induced cell survival has been proposed to be mediated by induction of GADD45β expression and down-regulation of JNK activity (12). However, this hypothesis has been challenged, because mice lacking GADD45β do not express a defect in apoptosis induction or JNK activation (13). Interestingly, all three GADD45 family members interact in vitro with the upstream kinase mitogen-activated protein kinase kinase kinase 4 (MEKK4; also known as MTK1), which activates both p38 and JNK. GADD45 proteins bind a site in MEKK4 near the inhibitory domain, relieve autoinhibition, and activate MEKK4 kinase, leading to JNK activation and apoptosis (14).
Because NF-κB impinges on multiple aspects of tumor progression and apoptosis, it is critical to decipher the detailed molecular signal transduction pathways involved in NF-κB action as a prerequisite to design therapeutic strategies to interfere with this pathway. We have explored the involvement and relevance of the different members of the GADD45 family in this pathway and demonstrate here that NF-κB-mediated repression of GADD45α and γ is necessary and sufficient for cancer cell survival. We show that apoptosis induction upon inhibition of NF-κB is due to induction of GADD45α and γ expression and that GADD45α- and γ-, but not β-, dependent JNK activity contributes to apoptosis in cancer cells.
Materials and Methods
Plasmids and Adenovirus (Ad) Constructs. The NF-κB p50 and p65 JNK1, JNK2, and mitogen-activated proteinase kinase kinase 4 (MKK4) expression vectors and the Ad under control of the cytomegalovirus (CMV) promoter encoding β-galactosidase (β-gal) (Ad5CMVβ-gal) have been described (3, 15). The pcDNA3-GADD45α, pcDNA3-GADD45β, pcDNA3-GADD45γ, pGL3-GADD45α-luciferase, and pcDNA3-c-Myc constructs were kindly provided by H. Saito (Dana–Farber Cancer Institute, Boston), D. G. Tenen (Beth Israel Deaconess Medical Center), and M. B. Greenberg (Harvard Medical School). The Ad under control of the CMV promoter encoding the IκBα gene (Ad5CMVIκB) was provided by F. Brennan (Kennedy Institute of Rheumatology, London) (16). The pCDNA3-GADD45α-Flag, -GADD45β-Flag, and -GADD45γ-Flag plasmids containing the GADD45α, GADD45β, and GADD45γ coding sequences in-frame with an N-terminal Flag tag were generated by PCR using pcDNA3-GADD45α, pcDNA3-GADD45β, and pcDNA3-GADD45γ as a template, respectively, and were inserted into appropriate restriction sites inside the polylinker of the pCDNA3-Flag vector.
Real-Time PCR. Total RNA was harvested by using the program qiashredder (Qiagen, Valencia, CA) and the RNeasy minikit (Qiagen). Real-time PCR was performed as described (3). The primers are described in Supporting Text, which is published as supporting information on the PNAS web site.
Orthotopic Implantation of DU145 Tumor Cells. DU145 cells infected with AdCMVβ-gal or AdCMVIκB or uninfected cells (2 × 106 cells in 50 μl of MEM) were used for implantation and were carefully injected under the prostatic capsule of 8-week-old male severe combined immunodeficient (SCID) beige mice as described (17).
Western Blot Analysis. Western blots were performed as described in Supporting Text by using anti-pan-JNK, anti-phospho-JNK, anti-phospho-c-jun, and anti-phospho-MKK4 antibodies from Cell Signaling Technology (Beverly, MA), and anti-GADD45α, anti-GADD45β, anti-GADD45γ, and anti-c-Myc antibodies from Santa Cruz Biotechnology.
Kinase Assays. JNK kinase activity was measured by using the SAPK/JNK assay kit (Cell Signaling Technology) according to the manufacturer's protocol.
Proliferation and Apoptosis Assays. Proliferation assays were performed by using the Rapid Cell Viability assay (Oncogene Research Products, San Diego) according to the manufacturer's protocol. Apoptosis was assayed in tissue culture supernatants by the cell-death detection (nuclear matrix protein) ELISA (Oncogene Research Products) according to the manufacturer's protocol.
IL-6 ELISA. IL-6 in the serum of SCID mice was assayed by an ELISA (BioSource International, Camarillo, CA) as described (3).
Infection Assays and DNA Transfection Assays. DU145, PC-3, SKBR3, MDA-231, Caki, and UOK cells at 106 cells per ml were infected with Ad5CMVIκB or Ad5CMVβ-gal at a multiplicity of infection of 1,000 (3). Transfections were carried out by using Lipofectamine Plus (Invitrogen) as described (3) in two experiments and repeated three times using different plasmid preparations with similar results.
Small Interfering RNA (siRNA) Oligonucleotides and Transfections. The sense-strand sequence for each siRNA (Qiagen) is described (a complementary oligonucleotide was synthesized for each): GADD45α siRNA, 5′-AACGTCGACCCCGATAACGTG; GADD45β siRNA, 5′-AAGTTGATGAATGTGGACCCA; and GADD45γ siRNA, 5′-AACGAGGACGCCTGGAAGGAT. A total of 50 μM of RNA duplexes was transfected into cells by using TKO transfection reagent (Mirus, Madison, WI) and was tested for specificity and efficiency (see Supporting Text).
siRNA Lentiviral Vectors. Single 83-mer oligonucleotides were designed, containing an XbaI site at the 5′ end and sense and antisense siRNA strands intermediated by a short spacer, plus a partial sequence of the H1-RNA promoter at the 3′ end. Standard PCR procedures (Advantage 2 PCR kit, Clontech) were performed by using specific siRNA oligonucleotides and T3 primer plus pSuper-like plasmids (18) as a template to provide H1-mediated siRNA cassettes with an additional XbaI site at the 3′ end. PCR products were purified (Qiagen), digested with XbaI, and cloned into the 3′ LTR NheI site of a CMV-GFP lentiviral vector as described (18). The LV-siGFP construct (control) was kindly donated by O. Singer (Salk Institute for Biological Studies). The following siRNA oligonucleotides were used: 5′-TGTCTAGACAAAAACGTCGACCCCGATAACGTGtctcttgaaCACGTTATCGGGGTCGACGGGGGATCTGTGGTCTCATACA-3′ for GADD45α; 5′-CTGTCTAGACAAAAAGTTGATGAATGTGGACCCAtctcttgaaTGGGTCCACATTCATCAACGGGGATCTGTGGTCTCATACA-3′ for GADD45β; and 5′-CTGTCTAGACAAAAACGAGGACGCCTGGAAGGATtctcttgaaATCCTTCCAGGCGTCCTCGGGGGATCTGTGGTCTCATACA-3′ for GADD45γ.
Vesicular stomatitis virus G envelope protein-pseudotyped lentiviruses were prepared and purified as described (18–20). Vector concentrations were analyzed by immunocapture p24-gag ELISA (Alliance, DuPont/NEN, Boston) (19).
Results
NF-κB Plays an Important Role in Tumor Growth, Cell Survival, and Apoptosis in Cancer Cells. Several potential explanations for the cell-survival activity of NF-κB have been proposed. Nevertheless, many controversies and open questions regarding the precise molecular mechanisms remain. To gain further insight into the NF-κB-dependent survival pathway in cancer cells, we infected DU145 prostate cancer cells that contain constitutively active NF-κB (3) with Ad5CMVIκB or Ad5CMVβ-gal as a control. Blockage of NF-κB resulted in inhibition of cell proliferation (Fig. 6A, which is published as supporting information on the PNAS web site) and apoptosis induction within 72 h (Fig. 1A). An Ad encoding soluble FasL as a positive control for apoptosis induced apoptosis significantly faster than interference with NF-κB. NF-κB inhibition induced apoptosis in breast and renal cancer cells as well, demonstrating that escape from programmed cell death due to deregulated NF-κB activation is a common feature of various cancer types (Fig. 6B). We corroborated our Ad data by using a lentivirus system encoding a dominant-negative IκBα mutant (DNIκBαM) (see Supporting Text). This IκBα mutant protein lacks the IκB kinase phosphorylation sites (Ser-32 and Ser-36) in the proline, glutamate, serine, and threonine domain. IκBαM cannot be degraded by the proteasome and the NF-κB/IκB complex remains in the cytoplasm. Infection with LV-IκBαM led to inhibition of proliferation and induction of apoptosis in cancer cells when compared with a lentivirus encoding the GFP gene (LV-GFP control) (Fig. 6 C and D). LV-GFP infection did not affect proliferation or apoptosis by itself when compared with noninfected cells.
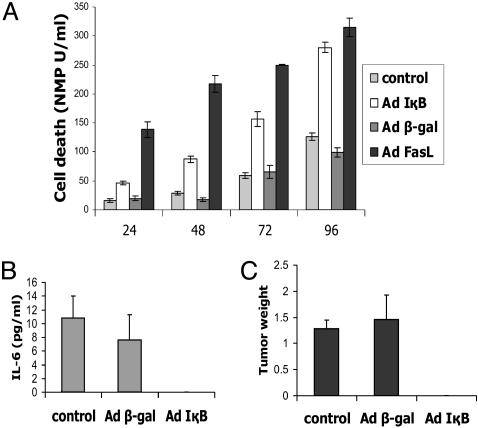
Fig. 1.
Inhibition of NF-κB induces apoptosis in prostate cancer cells and inhibits tumor formation in SCID mice. (A) Apoptosis assay. DU145 cells were infected with AdCMVIκBα or AdCMVβ-gal and apoptosis was measured 24, 48, and 72 h after infection. Data are means ± SD of three independent infections for each virus at each time point. (B and C) Inhibition of tumor formation by IκBα expression. A quantity of 2 × 106 DU145 cells infected with AdCMVIκBα or AdCMVβ-gal were implanted orthotopically into the prostate of SCID mice. The IL-6 expression (B) and the size of the tumors and tumor weight (C) were measured 2 months after implantation.
To determine whether blockage of NF-κB has an effect on tumor formation in vivo, DU145 cells infected with AdCMVIκB or AdCMVβ-gal, as well as uninfected cells, were orthotopically implanted into the prostate of SCID mice. Two months later, the mice were examined for tumor formation, tumor weight, and IL-6 expression in the serum. In contrast to the control cells, blockage of NF-κB completely abrogated tumor growth and IL-6 expression of implanted DU145 cells (Fig. 1 B and C). These results demonstrate that NF-κB plays an important role in tumor growth and cell survival.
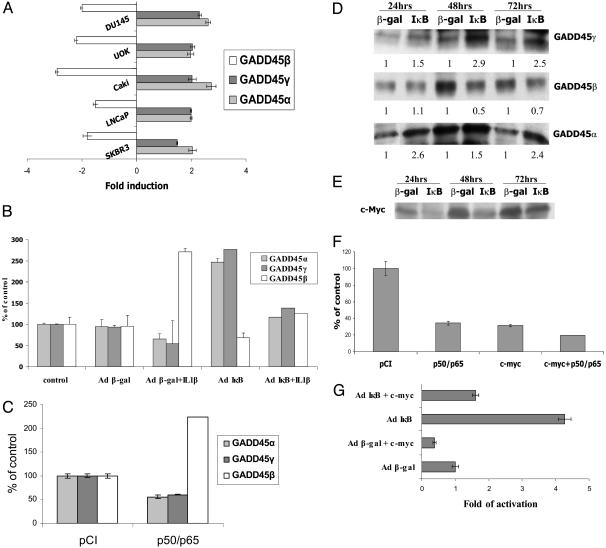
Expression of the GADD45 Family Members Is Tightly Regulated by the NF-κB/IκB Signaling Pathway Through c-Myc Expression. The GADD45 family encodes three related proteins, GADD45α, β, and γ (10), that play a role in the G2/M checkpoint (21, 22). Because of the controversy of GADD45β involvement in cell-survival effects of NF-κB (12), we examined whether GADD45β or the other two members of the GADD45 family play a critical role in NF-κB-mediated cell survival. We determined the expression level of the GADD45 family members in DU145 cells and other prostate, breast, and renal cancer cell lines after infection with AdCMVIκB or AdCMVβ-gal. Real-time PCR demonstrated that inhibition of NF-κB induces GADD45α and γ expression 1.5- to 3-fold and reduces GADD45β expression 1.5- to 3-fold in the various cancer cell types, indicating that all three members of the GADD45 family are regulated by NF-κB, although in opposite directions, and that this NF-κB-dependent differential effect on the GADD45 family is a common pathway in cancer cells (Fig. 2A). Because inhibition of NF-κB increases GADD45α and γ, but decreases β, expression, we expected that activation of NF-κB will result in opposite effects. To explore this notion, we performed real-time PCR analysis on total RNA isolated from U138MG glioma cells stimulated with IL-1β,an NF-κB activator, and then infected them with either Ad5CMVIκB or Ad5CMVβ-gal. IL-1β stimulation indeed led to a decrease in GADD45α and γ gene expression and a concomitant increase in GADD45β expression (Fig. 2B). This IL-1β effect depended on NF-κB because overexpression of IκB abrogated the effect on the GADD45 family (Fig. 2B). This result was further corroborated by real-time PCR in LNCaP prostate cancer cells transfected with expression vectors for NF-κB p50 and p65. Concomitant expression of NF-κB p50 and p65 repressed GADD45α and γ gene expression and induced GADD45β expression (Fig. 2C). These data demonstrate that constitutively active NF-κB in cancer cells changes the ratio of expression of the three GADD45 family members, which can be reversed by inhibition of NF-κB and indicate that the balance of the three GADD45 members may play a critical role in escape from programmed cell death. To confirm that differences in mRNA expression correlate with protein expression, protein extracts obtained from DU145 cells 24, 48, and 72 h after infection with Ad5CMVIκB or Ad5CMVβ-gal were analyzed by Western blotting. The Western blots corroborated our real-time PCR data showing that overexpressed IκB enhances GADD45α and γ protein expression and reduces GADD45β protein levels in DU145 cells (Fig. 2D).
Fig. 2.
Expression of GADD45 family members is regulated by the NF-κB/IκB signaling pathway through c-Myc expression. (A) Real-time PCR analysis of GADD45α, β, and γ after inhibition of NF-κB in different cancer cell lines. Total RNA was collected from DU145, SKBR3, LNCaP, Caki, and UOK cells infected with Ad5CMVβ-gal or Ad5CMVIκB. Each RNA was normalized to GAPDH. (B) Real-time PCR analysis of GADD45α, β, and γ upon activation of NF-κB. Total RNA was collected from U138 MG cells, treated with IL-1β for 6 h, and infected with Ad5CMVβ-gal or Ad5CMVIκB or without Ad infection (control). Each RNA was normalized to GAPDH. (C) Real-time PCR analysis of GADD45α and γ in prostate cancer cells transfected with NF-κB. Total RNA was collected from LNCaP cells transfected with p50 and p65 NF-κB expression vectors or pCI vector as a control. Each RNA was normalized to GAPDH. (D) Western blot analysis of GADD45α, β, and γ after inhibition of NF-κB. Protein extracts were obtained 24, 48, and 72 h after infection with Ad5CMVβ-gal or Ad5CMVIκB. (E) Western blot analysis of c-Myc after inhibition of NF-κB. Protein extracts were obtained 24, 48, and 72 h after infection with Ad5CMVβ-gal or Ad5CMVIκB. (F) Transcriptional activity of the GADD45α promoter. LNCaP cells were transfected with the GADD45α promoter-luciferase construct with the NF-κB p50 and p65 expression vectors or c-Myc expression vector. Luciferase activity was determined 16 h later. Data are means ± SD of two results of one representative transfection normalized to the amount of β-gal expression. Luciferase activity of the GADD45α promoter is shown as percentage of control of the pCI parental vector as indicated on the left. (G) Transcriptional activity of the GADD45α promoter in DU145 cells after infection with Ad5CMVβ-gal or Ad5CMVIκB and transfection of c-Myc expression vector or parental vector. Luciferase activity of the GADD45α promoter is shown as fold induction over the β-gal control.
To determine the mechanism of NF-κB-mediated repression of GADD45α and γ expression, we focused on the transcriptional regulation of the GADD45α promoter. The c-myc transcription factor had been previously shown to repress the GADD45α promoter by a polymerase II recruitment mechanism (23), and the c-Myc gene is a known target for NF-κB. To evaluate the relevance of c-Myc for NF-κB-mediated regulation of GADD45α gene expression, we analyzed expression of c-Myc protein in DU145 cells after infection with Ad5CMVIκB or Ad5CMVβ-gal. Blockage of NF-κB led to strong inhibition of c-Myc expression (Fig. 2E). To test whether c-Myc overexpression or activated NF-κB repress the GADD45α promoter, a GADD45α promoter-luciferase construct was cotransfected into LNCaP cells with either a c-Myc expression vector, NF-κB p50 and p65 expression vectors, or a combination thereof, as well as the parental vector as a control. NF-κB p50/p65 and c-Myc drastically reduced the promoter activity down to 34% and 31% of the control, respectively, and the combination further reduced GADD45α promoter activity (Fig. 2F). In contrast, inhibition of NF-κB by infection with Ad5CMVIκB resulted in a 4-fold transcriptional stimulation of the GADD45α promoter as compared with Ad5CMVβ-gal (Fig. 2G). Concomitant transfection with the c-Myc expression vector significantly reverted the effect of IκB on the GADD45α promoter down to 1.6-fold (Fig. 2G). These results provide strong evidence that induction of the GADD45α gene upon inhibition of NF-κB is at least partially due to decreased expression of c-Myc.
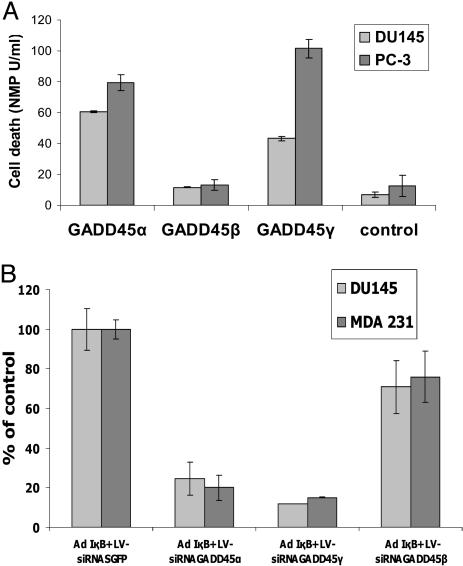
IκBα-Induced GADD45 Expression Is Essential for Apoptosis Induction. The role of the GADD45 family in growth arrest and apoptosis is not entirely clear (24–26). In particular, enhanced GADD45β expression has been suggested to either increase cell survival or induce growth arrest (9, 11). To elucidate the relevance of GADD45 genes in IκB-mediated apoptosis, we directly tested the effect of overexpressed GADD45 family genes on apoptosis in DU145 and PC-3 cells. Transfection of GADD45α and γ, but not GADD45β, induced apoptosis in both cell lines (Fig. 3A). Interestingly, GADD45β did not reduce the basal level of apoptosis, as would have been expected based on its up-regulation by activated NF-κB. To unequivocally determine the relevance of the GADD45 family for IκB-mediated apoptosis, we used siRNAs to inhibit endogenous GADD45α, β, and γ expression. siRNA oligonucleotides specific for the different members of the GADD45 family, as well as a siRNA oligonucleotide against GFP as control, were either transiently transfected into DU145 cells or stably expressed by lentivirus vectors. The specificity of the GADD45α, β, and γ siRNA oligonucleotides was validated in control experiments as described in detail in Supporting Text, and Fig. 7, which is published as supporting information on the PNAS web site. Inhibition of IκB-mediated GADD45α and γ up-regulation dramatically reduced apoptosis (Fig. 3B) by 80–90%, whereas blocking of GADD45β had only a marginal effect on apoptosis induction. Again, blocking of GADD45β did not enhance apoptosis, as would have been expected based on the data published (12). Our data provide the strongest evidence that the GADD45α and γ genes are critical and essential mediators of apoptosis induction upon inhibition of NF-κB. These results further imply that NF-κB induced escape from programmed cell death in cancer cells is, for a large part, dependent on down-regulation of GADD45α and γ expression.
Fig. 3.
IκBα-induced GADD45 expression is essential for apoptosis induction. (A) Apoptosis of prostate cancer cells after transfection with GADD45α, β, and γ. DU145 and PC-3 cells were transfected with GADD45α, β, and γ expression vectors (1μg) and apoptosis was measured after 48 h. Data are means ± SD of three independent transfections for each vector. (B) Apoptosis assay of DU145 prostate cancer cells and MDA-231 breast cancer cells after infection with Ad5CMVIκB or AdCMVβ-gal and infections with lentiviruses encoding GADD45α, β, and γ siRNA duplexes. Data are means ± SD of three independent transfections.
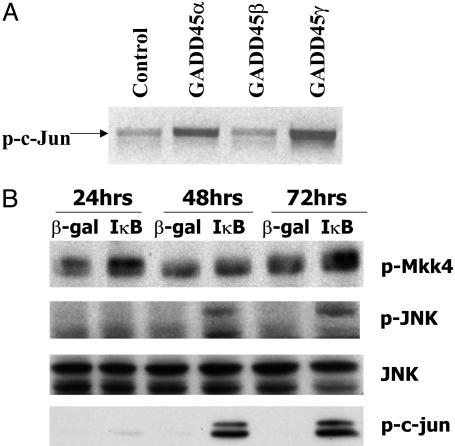
GADD45α and γ Induction in Response to Blockage of NF-κB Is Essential for JNK Activation. The GADD45 family has been linked to activation of JNK kinase (21) in several different settings, although, in the context of NF-κB signaling, GADD45β has been suggested to inhibit JNK activation (12, 27). Whereas JNK activation has been implicated in apoptosis, the importance of JNK in apoptosis remains controversial (28, 29). NF-κB has been postulated to prevent sustained JNK activation through up-regulation of GADD45β (7–9, 15). Nevertheless, GADD45β knockout mice do not express a defect in apoptosis or a change in JNK activity (12). This controversy about JNK and apoptosis may be related to the use of different systems and inducers in different studies and possibly striking differences between cancer cell-associated effects versus inflammatory processes.
We applied a mass spectrometric assay to identify protein–protein interaction partners of GADD45 proteins in vivo, resulting in identification of MEKK4, the upstream kinase of JNK, as an in vivo interactor of GADD45γ (Supporting Text and Fig. 8, which is published as supporting information on the PNAS web site). Western blot analysis by using an anti-MEKK4 antibody confirmed that MEKK4 interacts with GADD45γ and α (Fig. 8). GADD45 family members have been shown to interact in vitro with MEKK4 and colocalize in the cytoplasm (12–14). This interaction activates MEKK4 kinase, leading to MKK4 and subsequently JNK activation (12–14). To determine whether GADD45 family members could activate MKK4, we transfected GADD45 expression vectors with a MKK4-Flag expression vector into DU145 cells. MKK4 protein was immunoprecipitated with the anti-Flag antibody, subjected to an in vitro kinase assay, and analyzed by Western blot by using antibodies against phospho-JNK, a specific MKK4 substrate. Only GADD45α and γ, but not β, enhanced MKK4 activity, resulting in enhanced phosphorylation of JNK (Fig. 8). To further evaluate the contribution of JNK in GADD45α- and γ-dependent IκB-mediated apoptosis, we determined whether GADD45 family members activate JNK in prostate cancer cells. We transfected expression vectors for the GADD45 family as well as the parental vector into DU145 cells and analyzed cell extracts for JNK activation by using antibodies against phospho-c-jun, a specific JNK substrate. Only GADD45α and γ, but not β, activated JNK (Fig. 4A). To test whether blocking of NF-κB, which enhances GADD45α and γ expression leads to JNK activation, protein extracts were obtained from DU145 cells after infection with Ad5CMVIκB or Ad5CMVβ-gal for 24, 48, and 72 h and were analyzed by Western blotting. IκB overexpression up-regulated phosphorylation of MKK4 (Fig. 4B). As a consequence, phospho-JNK expression was detected at 48 and 72 h after IκB infection, but not in the β-gal control, whereas equal amounts of total JNK were detected in all lanes (Fig. 4B). Furthermore, active JNK directly correlated with enhanced phosphorylation of c-jun (Fig. 4B).
Fig. 4.
JNK activation in response to blockage of NF-κB due to induction of GADD45α and γ. (A) JNK kinase assays after transfection with GADD45α, β, and γ. DU145 cells were transfected with GADD45α, β, and γ expression vectors (1 μg) and analyzed by using the SAPK/JNK assay kit (Cell Signaling Technology). (B) DU145 cells were infected with AdCMVIκBα or AdCMVβ-gal and protein extracts were collected 48 h after infection. The proteins were analyzed by Western blots (Top and Middle) by using anti-MKK4 antibody (Cell Signaling Technology), anti-phospho-JNK (Cell Signaling Technology), and anti-total JNK (Cell Signaling Technology), and c-jun phosphorylation (Bottom) showed MKK4 and JNK activity in prostate cancer cells at 24, 48, and 72 h after infection with each virus.
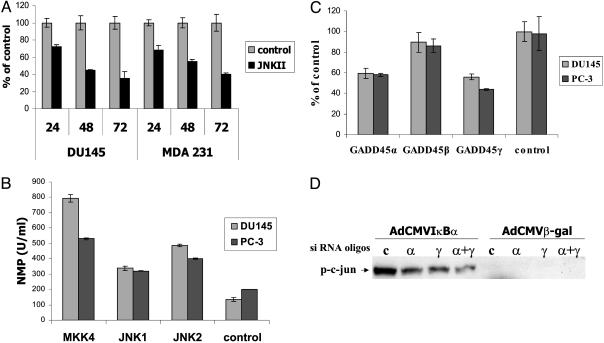
Induction of GADD45 Expression Is Essential for JNK Activation and Apoptosis Induction. To establish the relevance of JNK activation in mediating apoptosis in DU145 cells, we incubated DU145 cells infected with Ad5CMVIκB or Ad5CMVβ-gal in the absence or presence of a specific JNK inhibitor, JNKII. Compared with the control, apoptosis of DU145 cells expressing IκB was significantly reduced, but not abolished in JNKII-treated cells (Fig. 5A), showing that JNK contributes to but is not absolutely essential for IκB-mediated apoptosis. To investigate whether JNK can induce apoptosis in cancer cells, DU145 cells were analyzed for apoptosis after transfection with expression vectors encoding JNK1, JNK2, or the upstream kinase MKK4 (Fig. 5B). MKK4, JNK1, and JNK2 enhanced apoptosis compared with the control. We determined the relevance of JNK activation for apoptosis induction by GADD45α and γ in DU145 cells overexpressing GADD45 genes in the absence or presence of the JNK inhibitor. JNK inhibition resulted in a decrease rather than abrogation of cell death in GADD45α- and γ-expressing cells, but not in GADD45β-expressing cells, supporting the notion that GADD45α- and γ-induced apoptosis is partially mediated by means of JNK activation (Fig. 5C). We further demonstrated that siRNA-mediated inhibition of up-regulation of GADD45α and γ expression in response to IκB drastically reduced JNK activation, which correlates with the reduction in apoptosis (Fig. 5, and Fig. 9, which is published as supporting information on the PNAS web site). Our results clearly indicate that the JNK pathway contributes to GADD45-dependent apoptosis in cancer cells, but other pathways are likely to play important roles as well.
Fig. 5.
Induction of GADD45 expression is essential for JNK activation and apoptosis induction. (A) Apoptosis of prostate and breast cancer cells 48 h after infection with Ad5CMVIκB or AdCMVβ-gal and treatment with the JNK inhibitor JNKII SP600125 (100 nM, Calbiochem). Data are means ± SD of three independent infections. (B) Apoptosis of two prostate cancer cell lines after transfection with expression vectors (1 μg) for the JNK family members, JNK1 and 2, and its upstream regulator MKK4. Data are means ± SD of three independent transfections. (C) Apoptosis of prostate cancer cells after transfection with the GADD45 family members (1 μg) and treatment with the JNK inhibitor, SP600125 (100 nM). Data are means ± SD of three independent transfections. Apoptosis is shown as the percentage of apoptosis for each GADD45 family gene in the absence of the JNK inhibitor. (D) Kinase assay showing inhibition of JNK kinase activity by GADD45 siRNA duplexes (50 nM) in prostate cancer cells infected with Ad5CMVIκB or AdCMVβ-gal.
Discussion
In this study, we have, for the first time, to our knowledge, demonstrated that NF-κB-mediated cell-survival mechanisms in cancer cells are absolutely dependent on two GADD45 family members, GADD45α and γ. We demonstrate that down-regulation of GADD45α and γ protein expression, presumably through induction of c-Myc, is an essential step in NF-κB-dependent escape from programmed cell death in cancer cells. We show that JNK activity contributes to apoptosis in cancer cells and depends on GADD45α and γ, but not β. De Smaele et al. (12) previously implicated up-regulation of GADD45β and down-regulation of JNK activity to NF-κB-mediated cell survival in fibroblasts in response to TNF-α. However, these data were recently challenged by experiments performed in GADD45β-deficient murine embryonic fibroblasts (13) that did not exhibit any defect with regard to cell survival or apoptosis induction by TNF-α and no difference in JNK activity. This controversy surrounding GADD45β and NF-κB has left the question wide open as to how NF-κB inhibits programmed cell death. Our data confirm that NF-κB down-regulates GADD45β expression in cancer cells as well; nevertheless, we do not observe a major contribution of GADD45β to cell survival or apoptosis induction, but instead observe an indispensable involvement of GADD45α and γ in cell survival and apoptosis. These results are consistent with the GADD45β-deficient murine embryonic fibroblast data, suggesting that GADD45β may play some role in cell survival, but that there is redundancy of the two other GADD45 family members that counteract the activity of GADD45β. Part of the answer may be the change in the ratio of GADD45β protein to GADD45α and γ proteins that dictates cell survival versus programmed cell death. However, it is not entirely clear whether GADD45β is an antiapoptotic or proapoptotic protein, because other reports demonstrated that GADD45β acts in similar fashion as GADD45α and γ, including being able to activate rather than inhibit JNK. Our data indicate that in contrast to GADD45α and γ that activate JNK GADD45β does not have any significant effect on JNK in our cancer cell system. De Smaele et al. (12) suggested that GADD45α and γ expression is NF-κB-independent in murine embryonic fibroblasts and T cells. This result is not consistent with our findings, but may suggest that GADD45 regulation by NF-κB varies in different cell types or in response to different stimuli or in cancer versus normal cells.
Our data clearly implicate up-regulation of GADD45α and γ in apoptosis induction of cancer cells in response to blockade of NF-κB. This up-regulation appears to be at least partially mediated by repression of the survival gene and oncogene c-Myc. Screening of published transcriptional profiling data sets and other published reports (www.ncbi.nih.gov/geo) reveals that the majority of proapoptotic stimuli enhance expression of GADD45α or γ. We have evidence that up-regulation of GADD45α and γ is an essential step for apoptosis induction in cancer cells by a variety of proapoptotic agents, including agents that do not inhibit NF-κB (L.F.Z., A.C., Y.W., R.G.C., and T.A.L., unpublished data) indicating that GADD45α and γ play an unambiguous and universal role in cancer cell survival. These data, furthermore, provide the rationale to determine whether reduced expression or loss of activity of GADD45α or γ plays a general role in escape from programmed cell death of cancer cells, even in settings not affected by NF-κB. Our results establish the GADD45 family as an essential and critical mediator of cell survival in cancer cells and support the need for the exploitation of approaches to induce programmed cell death in cancer by targeting GADD45.
Supplementary Material
Acknowledgments
We thank Lyuba Varticovski, Steve Goldring, and Rosana Kapeller for comments on the manuscript. This work was supported by National Institutes of Health Grants 1RO1 CA85467 and P50 CA090381 and the Hershey Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ad, adenovirus; CMV, cytomegalovirus; IκB, inhibitor of NFκB; Ad5CMVIκB, Ad encoding the IκBα gene; β-gal, β-galactosidase; Ad5CMVβ-gal, Ad carrying the β-gal gene; GADD, growth arrest- and DNA-damage-inducible proteins; JNK, c-Jun N-terminal kinase; TNF-α, tumor necrosis factor α; MEKK4, mitogen-activated proteinase kinase kinase kinase 4; MKK4, mitogen-activated protein kinase kinase 4; SCID, severe combined immunodeficient; siRNA, small interfering RNA.
References
- 1.Li, Q. & Verma, I. M. (2002) Nat. Rev. Immunol. 2, 725–734. [DOI] [PubMed] [Google Scholar]
- 2.Karin, M., Cao, Y., Greten, F. R. & Li, Z. W. (2002) Nat. Rev. Cancer 2, 301–310. [DOI] [PubMed] [Google Scholar]
- 3.Zerbini, L. F., Wang, Y., Cho, J. Y. & Libermann, T. A. (2003) Cancer Res. 63, 2206–2215. [PubMed] [Google Scholar]
- 4.Chauhan, D., Uchiyama, H., Akbarali, Y., Urashima, M., Yamamoto, K., Libermann, T. A. & Anderson, K. C. (1996) Blood 87, 1104–1112. [PubMed] [Google Scholar]
- 5.Oya, M., Takayanagi, A., Horiguchi, A., Mizuno, R., Ohtsubo, M., Marumo, K., Shimizu, N. & Murai, M. (2003) Carcinogenesis 24, 377–384. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin, A. S. (2001) J. Clin. Invest. 107, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tournier, C., Hess, P., Yang, D. D., Xu, J., Turner, T. K., Nimnual, A., Bar-Sagi, D., Jones, S. N., Flavell, R. A. & Davis, R. J. (2000) Science 288, 870–874. [DOI] [PubMed] [Google Scholar]
- 8.Verheij, M., Bose, R., Lin, X. H., Yao, B., Jarvis, W. D., Grant, S., Birrer, M. J., Szabo, E., Zon, L. I., Kyriakis, J. M., et al. (1996) Nature 380, 75–79. [DOI] [PubMed] [Google Scholar]
- 9.Tang, G., Minemoto, Y., Dibling, B., Purcell, N. H., Li, Z., Karin, M. & Lin, A. (2001) Nature 414, 313–317. [DOI] [PubMed] [Google Scholar]
- 10.Hall, P. A., Kearsey, J. M., Coates, P. J., Norman, D. G., Warbrick, E. & Cox, L. S. (1995) Oncogene 10, 2427–2433. [PubMed] [Google Scholar]
- 11.Chen, I. T., Akamatsu, M., Smith, M. L., Lung, F. D., Duba, D., Roller, P. P., Fornace, A. J., Jr., & O'Connor, P. M. (1996) Oncogene 12, 595–607. [PubMed] [Google Scholar]
- 12.De Smaele, E., Zazzeroni, F., Papa, S., Nguyen, D. U., Jin, R., Jones, J., Cong, R. & Franzoso, G. (2001) Nature 15, 308–313. [DOI] [PubMed] [Google Scholar]
- 13.Amanullah, A., Azam, N., Balliet, A., Hollander, C., Hoffman, B., Fornace, A. J., Jr., & Liebermann, D. (2003) Nature 424, 741. [DOI] [PubMed] [Google Scholar]
- 14.Mita, H., Tsutsui, J., Takekawa, M., Witten, E. A. & Saito, H. (2002) Mol. Cell. Biol. 22, 4544–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sluss, H. K., Barrett, T., Derijard, B. & Davis, R. J. (1994) Mol. Cell. Biol. 14, 8376–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bondeson, J., Brennan, F., Foxwell, B. & Feldmann, M. (2000) J. Rheumatol. 27, 2078–2089. [PubMed] [Google Scholar]
- 17.Zhou, J. R., Yu, L., Zhong, Y., Nassr, R. L., Franke, A. A., Gaston, S. M. & Blackburn, G. L. (2002) Prostate 53, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiscornia, G., Singer, O., Ikawa, M. & Verma, I. M. (2003) Proc. Natl. Acad. Sci. USA 100, 1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naldini, L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M. & Trono, D. (1996) Science 272, 263–267. [DOI] [PubMed] [Google Scholar]
- 20.Pfeifer, A. & Verma, I. M. (2001) Annu. Rev. Genomics Hum. Genet. 2, 177–211. [DOI] [PubMed] [Google Scholar]
- 21.Takekaw, M. & Saito, H. (1998) Cell 95, 521–530. [DOI] [PubMed] [Google Scholar]
- 22.Jin, S., Tong, T., Fan, W., Fan, F., Antinore, M. J., Zhu, X., Mazzacurati, L., Li, X., Petrik, K. L., Rajasekaran, B., et al. (2002) Oncogene 21, 8696–8704. [DOI] [PubMed] [Google Scholar]
- 23.Barsyte-Lovejoy, D., Mao, D. Y. & Penn, L. Z. (2004) Oncogene 23, 3481–3486. [DOI] [PubMed] [Google Scholar]
- 24.Harki, D. P., Bean, J. M., Miklos, D., Song, Y. H., Truong, V. B., Englert, C., Christians, F. C., Ellisen, L. W., Maheswaran, S., Oliner, J. D. & Haber, D. A. (1998) Cell 97, 575–586. [DOI] [PubMed] [Google Scholar]
- 25.Lu, B., Yu, H., Chow, C., Li, B., Zheng, W., Davis, R. J. & Flavell, R. A. (2001) Immunity 14, 583–590. [DOI] [PubMed] [Google Scholar]
- 26.Tran, H., Brunet, A., Grenier, J. M., Datta, S. R., Fornace, A. J., Jr., DiStefano, P. S., Chiang, L. W. & Greenberg, M. E. (2002) Science 29, 6530–6534. [DOI] [PubMed] [Google Scholar]
- 27.Vairapandi, M., Balliet, A. G., Hoffman, B. & Liebermann, D. A. (2002) J. Cell. Physiol. 192, 327–338. [DOI] [PubMed] [Google Scholar]
- 28.Fornace, A. J., Jr., Jackman, J., Hollander, M. C., Hoffman-Liebermann, B. & Liebermann, D. A. (1992) Ann. N.Y. Acad. Sci. 663, 139–153. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis, J. M. (2001) Nature 414, 265–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.