Abstract
The physiologic function of the progressive hyperleptinemia of diet-induced obesity is unknown. However, that lipotoxicity in nonadipose tissues of congenitally unleptinized obese rodents is far greater than in hyperleptinemic diet-induced obesity rodents has suggested an antilipotoxic role. To test this hypothesis, mice with severe lipotoxic cardiomyopathy, induced transgenically by cardiomyocyte-specific overexpression of the acyl CoA synthase (ACS) gene, were made hyperleptinemic by treatment with recombinant adenovirus containing the leptin cDNA. Normoleptinemic control ACS-transgenic mice developed severe dilated cardiomyopathy with thickened left ventricular walls and profound impairment of systolic function on echocardiogram; histologically, there was severe myofiber disorganization and interstitial fibrosis, with intracytoplasmic lipid vacuoles identifiable by electron microscope. By contrast, the hearts of hyperleptinemic ACS-transgenic mice appeared normal, with normal echocardiograms and cardiac triglyceride (TG) contents. Their lower myocardial TG content was ascribed primarily to profound lowering of plasma TG and free fatty acids; free fatty acids were 17% of normal at 8 weeks. Additionally, enhanced myocardial AMP-activated protein kinase phosphorylation may have increased fatty acid oxidation, thereby contributing to the lowering of lipid stores. We conclude that obesity-level hyperleptinemia protects the heart from lipotoxicity.
Keywords: leptin, SIRT1, apoptosis, AMP-activated protein kinase, triglycerides
The physiologic role of the hyperleptinemia that accompanies diet-induced obesity is unknown. However, syndromes of congenital leptin deficiency (1–4) or resistance (5–7) are associated with early onset of widespread ectopic lipid deposition and lipotoxicity that can be ameliorated by restoring leptin action (2–4, 8, 9). That lipotoxicity is usually absent early in the course of diet-induced obesity has suggested that an important physiologic role of its hyperleptinemia is to protect the nonadipose tissues from lipid overaccumulation (7), at least in the initial period of fat storage.
To test this hypothesis, we have used a model of myocardial steatosis and lipotoxicity induced by cardiomyocyte-specific transgenic overexpression of the acyl CoA synthase (ACS) gene (10). These mice are normal except for severe lipotoxic cardiomyopathy caused, not by defective leptin action, but by increased import of fatty acids. They develop echocardiographic evidence of left ventricular dysfunction, biochemical and electron microscopic evidence of ectopic lipid deposition, and histologic evidence of myofiber disorganization and interstitial fibrosis. The mice die prematurely with a dilated cardiomyopathy (10).
If the antilipotoxic hypothesis is correct, the induction of obesity-level hyperleptinemia in these lean normoleptinemic transgenic mice should prevent their lipotoxic cardiomyopathy. The present study was designed to test this premise.
Materials and Methods
Animals. Breeding pairs of MHCα-ACS-transgenic mice were provided by J.E.S. Mice were bred, genotyped, and housed in individual cages with a constant temperature and 12 h of light alternating with 12 h of darkness. All mice were fed standard chow (Teklad 4% mouse/rat diet, Teklad, Madison, WI) ad libitum.
Adenovirus Transfer of Leptin cDNA. Recombinant adenovirus containing either the leptin cDNA (AdCMV-leptin) or, as a control, the β-galactosidase (β-gal) cDNA (AdCMV-β-gal) was prepared as described (11). A total of 3 × 109 plaque-forming units of adenovirus was administered intravenously to 6-week-old ACS-transgenic mice weighing ≈20 g anesthetized with xylazine and ketamine-HCl.
Plasma Measurements. Plasma triglyceride (TG) levels were measured by the glycerol phosphate oxidase-Trinder TG kit (Sigma). Plasma free fatty acids (FFA) were measured with the Wako NEFA kit (Wako Chemical USA, Richmond, VA). Plasma leptin was assayed with the Linco leptin assay kit (Linco Research, St. Charles, MO)
TG Content of Heart. Mice were anesthetized with a mixture of xylazine and ketamine-HCl. After death, hearts were perfused with PBS (pH 7.4), dissected, and placed in liquid nitrogen immediately. Total lipids from hearts were extracted and dried under N2 gas. Cardiac TG content was assayed as described (7).
Echocardiography. Longitudinal noninvasive transthoracic echo-cardiograms were performed in unsedated mice 8 weeks after administration of either AdCMV-leptin or AdCMV-β-gal as a control. Transthoracic echocardiographic examination was performed by using a General Electric Vivid7 Pro machine equipped with a 12-mHz transducer. Motion mode (M-mode) and 2D echo images were obtained in the parasternal long- and short-axis views. Fractional shortening was calculated from M-mode images as the left ventricular end-diastolic dimension (LVEDD) minus the left ventricular end-systolic dimension divided by LVEDD.
Electron Microscopy of Myocardium. Cardiac muscle fragments were fixed with 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). After rinsing in phosphate buffer, postfixation in 1% osmic acid for1hat room temperature, and en bloc staining with uranyl acetate, specimens were processed for epoxy embedding (Polybed R812; Fluka). Thin sections were stained with uranyl acetate and lead citrate and photographed in a JEOL 1200 EX electron microscope.
Histology. Hearts were perfused with paraformaldehyde, fixed overnight, and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin/eosin or Masson's trichrome for collagen.
Quantitative Real-Time RT-PCR. Total RNA was extracted from hearts and livers by the TRIzol isolation method according to the manufacturer's protocol (Life Technologies, Rockville, MD). All reactions were done in triplicate. The real-time amount of all mRNA was calculated by using the standard curve method. 18S mRNA was used as the invariant control for all studies. Primer sequences of genes used for quantification of mRNA by real-time PCR are shown in Table 1.
Table 1. Primers used for real-time PCR.
| Name | GenBank no. | Forward primer | Reverse primer |
|---|---|---|---|
| ACC1 | AF374169 | CCCAGCAGAATAAAGCTACTTTGG | TCCTTTTGTGCAACTAGGAACGT |
| ACC2 | AF290178 | AACTCCCTGCCAAGCTCATG | GGAGGGCCAGGTGTCATTG |
| ACO | NM_017340 | GGCCAACTATGGTGGACATCA | ACCAATCTGGCTGCACGAA |
| CPT-1 | NM_013495 | CCTGGGCATGATTGCAAAG | ACGCCACTCACGATGTTCTTC |
| FAS | XM_126624 | CCTGGATAGCATTCCGAACCT | AGCACATCTCGAAGGCTACACA |
| GPAT | M77003 | ATCTTCAGAACAGCAAAATCGAAA | CAGCGGAAAACTCCAAATCC |
| PPARα | X57638 | CTGCAGAGCAACCATCCAGAT | GCCGAAGGTCCACCATTTT |
| PPARγ | AF156666 | TCAGAGGGACAAGGATTCATGA | CACCAAAGGGCTTCCGCAGGCT |
| SPT | NM_009269 | CCTCCAAGCATCAGGGTTGT | GGATGCAGCCCTCTGTAGCT |
| PGC-Iα | AB025784 | GCGCCAGCCAACACTCA | TGGGTGTGGTTTGCATGGT |
| SREBP-Ic | L16995 | GCAACACTGGCAGAGATCTACGT | TGGCGGGCACTACTTAGGAA |
| CD36 | AF111268 | GGACCATTGGCGATGAGAAA | CCAGGCCCAGGAGCTTTATT |
| MCD | NM_053477 | CAGAGGACCGGCTACGCTAT | CAGCTTACTGATGTGGTGGAAGA |
| SCD-1 | NM_009127 | CCAGAATGACGTGTACGAATGG | GCGTGTGTTTCTGAGAACTTGTG |
| SCD-4 | NM_183216 | GGCTTTCCAGAATGACGTGTATG | GCGTGTGTTTCTGAGAACTTGTG |
| Bax | NM_007528 | CCAAGAAGCTGAGCGAGTGTC | CCTCTGCAGCTCCATATTGCT |
| Bcl2 | NM_009741 | TGGGATGCCTTTGTGGAACT | GAGACAGCCAGGAGAAATCAAAC |
| SIRT1 | NM_019812 | GCCAAACTTTGTTGTAACCCTGTA | TGGTGGCAACTCTGATAAATGAA |
| UCP-1 | U63419 | ACTGGAGGTGTGGCAGTGTTC | TGGGCTTGCATTCTGACCTT |
| UCP-2 | NM_011672 | TGTTGATGTGGTCAAGACGAGAT | CATGGTAAGGGCACAGTGA |
| UCP-3 | NM_009464 | CATCACAAGAAATGCCATTGTCA | TCCAGCAACTTCTCCTTGATGA |
ACO, acyl CoA oxidase; CPT, carnitine palmitoyl transferase; GPAT, glycerophosphate acyl transferase; PPAR, peroxisome proliferator-activated receptor; SPT, serine palmitoyl transferase; PGC, PPARγ coactivator, SREBP, sterol regulatory element-binding protein; UCP, uncoupling protein; FAS, fatty acid synthase; SCD, stearoyl-CoA desaturase.
Immunoblotting. Hearts were lysed in RIPA buffer (0.15 mM NaCl/0.05 mM Tris·HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) with 10 μg/ml leupeptin and 10 μg/ml aprotinin and processed for immunoblotting with antiphospho-AMP-activated protein kinase (AMPK) (Thr-172) (Cell Signaling Technology, Beverly, MA).
Results
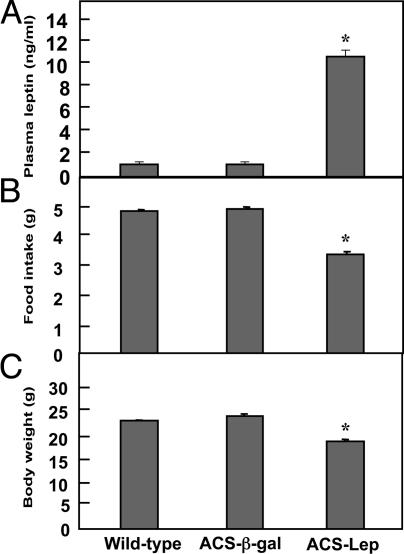
Effects of Hyperleptinemia on Clinical Parameters. Six-week-old transgenic mice with high-level cardiac expression of ACS received an i.v. injection of recombinant adenovirus containing either AdCMV-leptin or AdCMV-β-gal. During the first week after AdCMV-leptin treatment, plasma leptin levels ranged between 40 and 50 ng/ml, well above the 4 ng/ml hyperleptinemia previously reported in rats at the start of a high-fat diet (7). However, at 8 weeks after AdCMV-leptin treatment, leptin levels had declined to 11.1 ± 0.45 ng/ml (Fig. 1A), well below the ≈25 ng/ml mean leptin level after 10 weeks of high-fat feeding (7). Leptin levels averaged 1.2 ± 0.06 ng/ml in both AdCMV-β-gal-treated ACS-transgenic control mice and untreated wild-type controls.
Fig. 1.
Comparison of various parameters in ACS-transgenic mice treated with AdCMV-leptin (ACS-Lep) or AdCMV-β-gal (ACS-β-gal) and untreated wild-type controls. (A) Mean (±SEM) plasma leptin level at 14 weeks of age (8 weeks after AdCMV treatments). (B) Mean (±SEM) food intake for the three groups. (C) Mean (±SEM) body weight for the three groups of mice. *, P < 0.01.
Food intake (Fig. 1B) and body weight (Fig. 1C) were significantly reduced in the hyperleptinemic ACS-transgenic mice compared to wild-type and AdCMV-β-gal-treated transgenic mice. On postmortem dissection of hyperleptinemic mice, no fat tissue could be identified in fat depots, confirming earlier observations (11, 12).
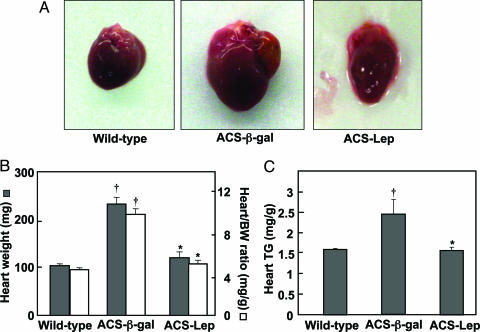
Effects of Hyperleptinemia on the Cardiac Phenotype of ACS-Transgenic Mice. Severe dilated cardiomyopathy was grossly apparent in normoleptinemic ACS-transgenic control mice. There was marked hypertrophy and dilatation of all chambers (Fig. 2A), with a doubling of heart weight and heart weight/body weight ratio compared to the wild-type group (Fig. 2B). In striking contrast, the hearts of hyperleptinemic ACS-transgenic mice were normal in size, appearance, weight, and heart/body weight ratio. The cardiac TG content of the AdCMV-β-gal-treated ACS transgenic mice was significantly higher than that of wild-type mice (P < 0.01) but was normal in AdCMV-leptin-treated ACS-transgenic mice (Fig. 2C).
Fig. 2.
Comparison of cardiac parameters in ACS-transgenic mice treated with AdCMV-leptin or AdCMV-β-gal and untreated wild-type controls. (A) Gross appearance of a representative heart from each group. The heart of an AdCMV-β-gal-treated mouse (ACS-β-gal) exhibits striking enlargement with a dilated left atrium, whereas that of an AdCMV-leptin-treated mouse (ACS-Lep) appears normal. (B) Heart weight (filled bars) and heart weight/body weight ratio (open bars) in the three groups of mice. (C) Mean (±SEM) cardiac TG content in the three groups of mice. *, P < 0.01 vs. AdCMV-β-gal-treated ACS-transgenic mice; †, P < 0.01 vs. wild-type controls.
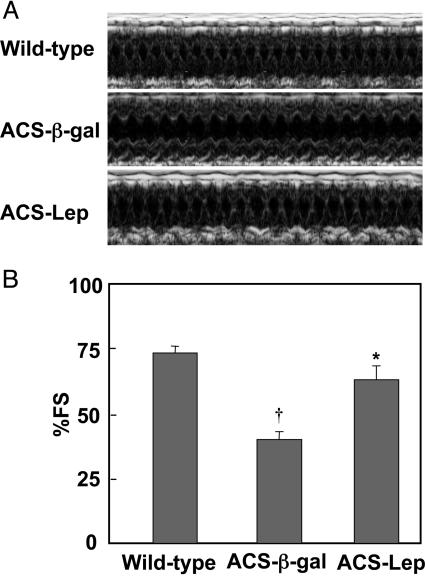
Transthoracic echocardiograms in AdCMV-β-gal-treated ACS-transgenic mice revealed markedly impaired systolic cardiac function with depressed fractional shortening on motion mode (M-mode) images and thickening of the anterior and posterior walls of the left ventricle (Fig. 3). The hyperleptinemic ACS-transgenic group, by contrast, exhibited normal fractional shortening (P < 0.01).
Fig. 3.
A representative transthoracic echocardiogram from each of the three groups of mice (A) and a comparison of percent fractional shortening (% FS) in the three groups of mice (B). *, P < 0.01 vs. AdCMV-β-gal-treated ACS-transgenic mice; †, P < 0.01 vs. wild-type controls. M-mode, motion mode.
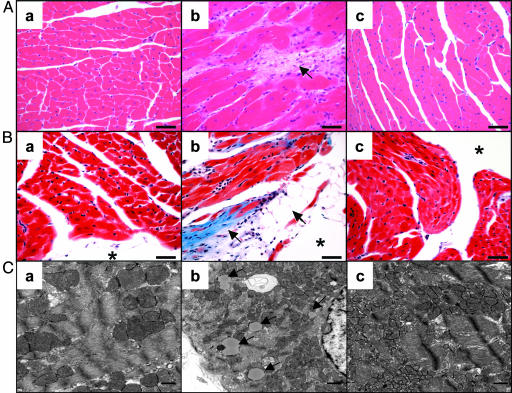
Effects of Hyperleptinemia on Cardiac Histology. Hematoxylin/eosin staining of hearts of control ACS-transgenic mice revealed myofiber disorganization, enlarged cardiomyocytes, and interstitial fibrosis (Fig. 4A). Trichrome stains highlighted the collagen deposits in the subendocardium and interstitium. Myocytes had large unilocular vacuoles consistent with lipid droplets, resembling adipocytes (Fig. 4B). On electron microscopy, intracytoplasmic lipid vacuoles could be identified in AdCMV-β-gal-treated ACS transgenic mice (Fig. 4C) but not in those of AdCMV-leptin-treated or wild-type mice. AdCMV-leptin-treated ACS-transgenic hearts were morphologically indistinguishable from the wild-type hearts.
Fig. 4.
Comparison of the myocardial histology of wild-type (a), ACS-transgenic treated with AdCMV-β-gal (b), and ACS-transgenic treated with AdCMV-leptin (c) mice. (A) Hematoxylin/eosin stain showing myofiber disorganization, cardiomyocyte enlargement, and interstitial fibrosis in the AdCMV-β-gal-treated ACS-transgenic group but a normal appearance in the hyperleptinemic group. (Bar, 40 μm.) (B) Trichrome stain of the hearts showing collagen deposition in the subendocardium and interstitium; cells resembling adipocytes can be seen near the lumen of the heart. The hyperleptinemic ACS-transgenic group is entirely normal. (Bar, 40 μm.) (C) Electron microscopic appearance of myocardial cells of the three groups. Lipid vacuoles in cardiomyocytes of the AdCMV-β-gal-treated ACS-transgenic mice are marked by arrows. None are noted in the other two groups. (* marks the lumen of the heart). (Bar, 500 nm.)
Extracardiac Mechanisms of Prevention of Cardiac Lipotoxicity. Because the lipotoxicity of ACS overexpression is largely caused by increased import of long-chain fatty acids synthesized elsewhere, rather than by increased lipogenesis and/or decreased oxidation in the cardiomyocytes themselves, we measured plasma TG and FFA. Seven days after treatment, the mean plasma TG and FFA levels of the hyperleptinemic mice were less than half of both normoleptinemic control groups (Table 2); at 8 weeks the mean FFA level was only 0.15 ± 0.03 mM, compared to 0.54 ± 0.05 mM in the ACS-transgenic controls. The rapid leptin-induced disappearance of TG from adipocytes, described in detail in previous reports (11, 12), seemed to have deprived the liver of a source of FFA required for TG production. Indeed, the expression profile of relevant liver enzymes, transcription factors, and nuclear receptors involved in fatty acid synthesis and oxidation did not suggest a major change in hepatic fatty acid metabolism, other than a 60% decline in acetyl CoA carboxylase -2 mRNA, and a 1.8-fold increase in acyl CoA oxidase mRNA (Tables 3 and 4), nor was there an increase in phosphorylated AMPK. However, there was a 6-fold increase in hepatic expression of uncoupling protein-2, which could augment uncoupled oxidation, and a 2.5-fold in expression of the “longevity gene” SIRT1, which triggers fat loss by repressing peroxisome proliferator-activated receptor-γ (13). The statistically or biologically significant changes in hepatic mRNA appear in Table 3.
Table 2. Plasma TG (mg per 100 ml) and FFA (mM) in ACS-transgenic mice before and at 7 days and 8 weeks after treatment with AdCMV-leptin or AdCMV-β-gal and in age-matched untreated wild-type controls.
| Time after baseline
|
|||||
|---|---|---|---|---|---|
| Genotype | Treatment | TG/FFA | Baseline | 7 days | 8 weeks |
| ACS-transgenic (n = 6) | AdCMV-β-gal | TG | 105 ± 10.5 | 100 ± 12.1 | 92 ± 19.3 |
| FFA | 0.80 ± 0.16 | 0.78 ± 0.12 | 0.54 ± 0.05 | ||
| AdCMV-leptin | TG | 98 ± 6.5 | 39 ± 3.1 | 37 ± 2.8 | |
| FFA | 0.82 ± 0.05 | 0.35 ± 0.07 | 0.15 ± 0.03 | ||
| Wildtype (n = 6) | None | TG | 100 ± 5.2 | 103 ± 7.2 | 83 ± 8.4 |
| FFA | 1.00 ± 0.10 | 1.07 ± 0.13 | 1.45 ± 0.07 | ||
Table 3. mRNA of relevant genes in the liver of ACS-transgenic mice 7 days after treatment with AdCMV-β-gal or AdCMV-leptin and in untreated wild type.
| 7 days of hyperleptinemia
|
|||||
|---|---|---|---|---|---|
| ACS-transgenic mice
|
|||||
| mRNA | Wild type (n = 4) | P | AdCMV-β-gal (n = 4) | P | AdCMV-leptin (n = 4) |
| ACC1 | 1 ± 0.32 | 1.33 ± 0.06 | 1.82 ± 0.22 | ||
| ACC2 | 1 ± 0.15 | 1.06 ± 0.06 | <0.01 | 0.43 ± 0.04 | |
| ACO | 1 ± 0.04 | <0.001 | 0.33 ± 0.01 | <0.05 | 0.59 ± 0.07 |
| CPT-1 | 1 ± 0.16 | 1.11 ± 0.32 | 1.34 ± 0.29 | ||
| FAS | 1 ± 0.02 | <0.05 | 1.66 ± 0.05 | <0.01 | 0.70 ± 0.09 |
| GPAT | 1 ± 0.14 | <0.05 | 1.53 ± 0.25 | <0.05 | 0.91 ± 0.04 |
| PPARα | 1 ± 0.23 | 0.83 ± 0.20 | 0.73 ± 0.17 | ||
| PGC-1α | 1 ± 0.16 | 2.80 ± 0.14 | 2.01 ± 0.54 | ||
| SREBP-1c | 1 ± 0.17 | 0.87 ± 0.03 | 0.77 ± 0.10 | ||
| CD36 | 1 ± 0.11 | 2.09 ± 0.20 | <0.01 | 5.87 ± 0.61 | |
| MCD | 1 ± 0.15 | <0.05 | 0.38 ± 0.07 | 0.50 ± 0.06 | |
| SCD-1 | 1 ± 0.26 | <0.05 | 0.13 ± 0.01 | 0.42 ± 0.01 | |
| SIRT1 | 1 ± 0.20 | 1.00 ± 0.01 | <0.01 | 2.50 ± 0.11 | |
| UCP-1 | 1 ± 0.40 | 2.00 ± 0.03 | 1.50 ± 0.20 | ||
| UCP-2 | 1 ± 0.30 | 1.00 ± 0.30 | <0.05 | 6.00 ± 1.30 | |
mRNA of each gene listed in Table 1 was measured by real-time PCR using 18S as the invariant control in all. mRNAs that were unchanged or that were not biologically relevant are not shown. Data are expressed as fold change from the level in wild-type control. Each value represents the mean ± SEM of four mice. Abbreviations are as indicated in Table 1.
Table 4. mRNA of relevant genes in the heart of ACS-transgenic mice 7 days after treatment with AdCMV-β-gal or AdCMV-leptin and in untreated wild type.
| 7 days of hyperleptinemia
|
|||||
|---|---|---|---|---|---|
| ACS-transgenic mice
|
|||||
| mRNA | Wild type (n = 4) | P | AdCMV-β-gal (n = 4) | P | AdCMV-leptin (n = 4) |
| ACC1 | 1 ± 0.06 | 2.15 ± 0.68 | 1.48 ± 0.32 | ||
| ACC2 | 1 ± 0.02 | 1.10 ± 0.22 | 0.79 ± 0.10 | ||
| ACO | 1 ± 0.20 | 2.00 ± 0.87 | 1.67 ± 0.43 | ||
| CPT-1 | 1 ± 0.02 | 1.30 ± 0.03 | 1.30 ± 0.08 | ||
| FAS | 1 ± 0.09 | <0.05 | 3.24 ± 0.09 | <0.05 | 1.11 ± 0.12 |
| GPAT | 1 ± 0.14 | <0.05 | 1.57 ± 0.07 | <0.01 | 0.87 ± 0.14 |
| PPARα | 1 ± 0.25 | 1.10 ± 0.26 | <0.01 | 0.59 ± 0.18 | |
| PPARγ | 1 ± 0.14 | 1.10 ± 0.32 | 1.03 ± 0.17 | ||
| SPT | 1 ± 0.19 | 1.73 ± 0.51 | 1.07 ± 0.22 | ||
| PGC-1α | 1 ± 0.21 | 2.75 ± 0.17 | 1.69 ± 0.18 | ||
| SREBP-1c | 1 ± 0.40 | 1.00 ± 0.40 | 1.25 ± 0.15 | ||
| CD36 | 1 ± 0.64 | 0.86 ± 0.30 | 1.04 ± 0.30 | ||
| MCD | 1 ± 0.46 | <0.05 | 1.87 ± 0.21 | <0.05 | 0.93 ± 0.13 |
| SCD-1 | 1 ± 0.10 | 2.98 ± 1.01 | 3.14 ± 1.07 | ||
| SCD-4 | 1 ± 0.30 | 3.00 ± 1.80 | 3.00 ± 1.50 | ||
| Bax | 1 ± 0.12 | 1.89 ± 0.82 | 0.92 ± 0.51 | ||
| Bcl2 | 1 ± 0.33 | 1.41 ± 0.10 | 3.00 ± 1.50 | ||
| SIRT1 | 1 ± 0.33 | 1.43 ± 0.44 | 1.11 ± 0.10 | ||
| UCP-1 | 1 ± 0.29 | 1.20 ± 0.65 | 0.77 ± 0.31 | ||
| UCP-2 | 1 ± 0.24 | 1.20 ± 0.01 | 1.35 ± 0.24 | ||
| UCP-3 | 1 ± 0.04 | 1.39 ± 0.42 | 0.66 ± 0.22 | ||
mRNA of each gene listed in Table 1 was measured by real-time PCR using 18S as the invariant control in all. mRNAs that were unchanged or that were not biologically relevant are not shown. Data are expressed as fold change from the level in wild-type control. Each value represents the mean ± SEM of four mice. Abbreviations are as indicated in Table 1.
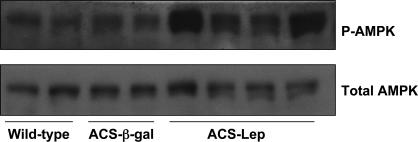
Intracardiac Mechanisms of Prevention of Lipotoxicity. In addition to the foregoing extracardiac findings, we found evidence for the effects of hyperleptinemia on cardiac metabolism. First, the phosphorylation state of cardiac AMPK (14) was examined for evidence of increased activity, as had been demonstrated in skeletal muscle (15) and adipose tissue (12) in response to leptin. Seven days after the injection of AdCMV-leptin, phosphorylation of AMPK was increased in the heart (Fig. 5). This have may have contributed to the reduced TG content by inactivating acetyl CoA carboxylase and reducing malonyl CoA (14), thereby decreasing fatty acid synthesis and increasing fatty acid oxidation (16). Phosphorylated AMPK had returned to baseline levels by 8 weeks after treatment (data not shown). No changes in mRNA of enzymes of fatty acid oxidation were observed (Table 4). Second, the lipogenic enzymes, fatty acid synthase, and glycerolphosphate acyl transferase mRNAs were significantly lower in hyperleptinemic ACS-transgenic mice at 7 days after treatment compared with AdCMV-β-gal-treated mice. This finding is consistent with the dramatic decrease in cardiac TG content. Third, in the steatotic hearts of normoleptinemic ACS-transgenic mice, the mRNA of malonyl CoA decarboxylase (MCD) was increased compared to wild-type controls, possibly representing a compensatory response to up-regulate fatty acid oxidation. MCD mRNA was returned to wild-type levels by hyperleptinemia (Table 4). Fourth, based on previous studies showing that cardiomyocyte loss was, at least in part, the result of lipid-induced programmed cell death (10), we measured mRNA of the antiapoptotic factor, Bcl2, and the proapoptotic factor, Bax, in the hearts of all groups. Although not statistically significant, Bcl2 mRNA was increased >2-fold in the hearts of hyperleptinemic ACS-transgenic mice, whereas Bax mRNA was 50% below the control ACS-transgenic mice (Table 4).
Fig. 5.
Effect of hyperleptinemia on total and phosphorylated AMPK. Western analysis of lystates from hearts of wild-type, ACS-β-gal, and ACS-leptin hearts was probed with antibodies that recognize phosphorylated AMPK (P-AMPK) or total AMPK.
Effect of Caloric Restriction on Cardiac Function of ACS-Transgenic Mice. To exclude reduction in caloric intake as a significant factor in the prevention of the cardiac phenotype by hyperleptinemia, we matched the food intake of six ACS-transgenic mice to that of the hyperleptinemic mice and studied their cardiac function 8 weeks later. Echocardiograms in the diet-matched mice did not differ from those of untreated ACS-transgenic mice on an ad libitum diet (data not shown).
Discussion
Congenitally unleptinized Zucker diabetic fatty rats with a loss-of-function mutation in their leptin receptors (17) exhibit severe steatosis of liver, pancreatic islets, heart, and skeletal muscles resulting from a generalized increase in lipogenesis and decrease in fatty acid oxidation (5–7). The resulting lipotoxicity can be reversed by restoring leptin action (3, 4, 18). This led to the hypothesis that the function of obesity-induced hyperleptinemia during overnutrition is to protect overnourished animals from lipotoxicity by increasing fatty acid oxidation in their nonadipose tissues (9), effectively partitioning the surplus lipids to the adipose tissues. This enables the accumulation of caloric stockpiles sufficient to prolong survival through famine without causing metabolic trauma and functional impairment in lean tissues.
The present study supports the protective role of leptin against a completely different form of lipotoxicity. In non-obese normally leptinized ACS-transgenic mice, lipotoxicity was caused, not by abnormalities in fatty acid synthesis or oxidation in cardiomyocytes, but rather by increased fatty acid import via the overexpressed ACS. Nonetheless, raising plasma leptin levels of these lean mice to the range of rodents with diet-induced obesity completely prevented dilated lipotoxic cardiomyopathy, abnormal echocardiographic patterns, elevated cardiac TG content, cardiomyocyte hypertrophy, fat droplets, and interstitial fibrosis on histologic examination.
Because in the ACS-transgenic model of lipotoxicity the underlying abnormality is excessive import of long-chain fatty acids into the heart, rather than increased myocardial lipogenesis or decreased oxidation, the profound reduction of the source of imported fatty acids, plasma TG, and FFA may have been the major factor in decreasing cardiac TG. Earlier studies of hyperleptinemia have demonstrated the rapid nearly total depletion of adipocyte TG within 7 days (11, 15) through intraadipocyte oxidation (9, 12, 19). The reduced FFA for very low-density lipoprotein (VLDL) formation lowered plasma TG, depriving the ACS-overexpressing heart of sufficient substrate for lipotoxicity.
Hyperleptinemia may also have had local antilipotoxic effects on the myocardium. In our model, phosphorylation of AMPK in myocardium was increased at 7 days after AdCMV-leptin treatment, suggesting that cardiomyocellular effects of leptin may have contributed to TG lowering through malonyl CoA reduction (16, 20). Leptin can also increase myocardial fatty acid oxidation without AMPK activation (21). Finally, local antiapoptotic action of leptin may have spared cardiomyocytes, as reflected by the increase in myocardial Bcl2 mRNA and the decrease in Bax mRNA (22).
Because elevated cardiomyocyte TG with left ventricular dysfunction may be common in overweight individuals (23), this study raises the possibility that reducing serum TG levels, even when normal, may have the unexpected benefit of preventing lipid overaccumulation in the myocardium and reducing the risk of myocardial lipotoxicity.
Acknowledgments
We thank Cai Li, Ph.D.; Christopher Newgard, Ph.D.; Neil B. Ruderman, M.D.; and Daniel W. Foster, M.D., for critical reading of the manuscript. This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 002700, the Department of Veterans Affairs Merit Review, the Jensen Charitable Lead Trust (R.H.U.), and the American Diabetes Association (J.E.S.).
Abbreviations: β-gal, β-galactosidase; AMPK, AMP-activated protein kinase; ACS, acyl CoA synthase; FFA, free fatty acids; TG, triglycerides; AdCMV-leptin, adenovirus containing the leptin cDNA; AdCMV-β-gal, adenovirus containing the β-gal cDNA.
References
- 1.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi, I. S., Matarese, G., Lord, G. M., Keogh, J. M., Lawrence, E., Agwu, C., Sanna, V., Jebb, S. A., Perna, F., Fontana, S., et al. (2002) J. Clin. Invest. 110, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimomura, I., Hammer, R. E., Richardson, J. A., Ikemoto, S., Bashmakov, Y., Goldstein, J. L. & Brown, M. S. (1998) Genes Dev. 12, 3182–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oral, E. A., Simha, V., Ruiz, E., Andewelt, A., Premkumar, A., Snell, P., Wagner, A. J., DePaoli, A. M., Reitman, M. L., Taylor, S. I., et al. (2002) N. Engl. J. Med. 346, 570–578. [DOI] [PubMed] [Google Scholar]
- 5.Lee, Y., Hirose, H., Ohneda, M., Johnson, J. H., McGarry, J. D. & Unger, R. H. (1994) Proc. Natl. Acad. Sci. USA 91, 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou, Y. T., Grayburn, P., Karim, A., Shimabukuro, M., Higa, M., Baetens, D., Orci, L. & Unger, R. H. (2000) Proc. Natl. Acad. Sci. USA 97, 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, Y., Wang, M. Y., Kakuma, T., Wang, Z. W., Babcock, E., McCorkle, K., Higa, M., Zhou, Y. T. & Unger, R. H. (2001) J. Biol. Chem. 276, 5629–5635. [DOI] [PubMed] [Google Scholar]
- 8.Halaas, J. L., Gajiwala, K. S., Maffei, M., Cohen, S. L., Chait, B. T., Rabinowitz, D., Lallone, R. L., Burley, S. K. & Friedman, J. M. (1995) Science 269, 543–546. [DOI] [PubMed] [Google Scholar]
- 9.Wang, M-Y., Lee, Y. & Unger, R. H. (1999) J. Biol. Chem. (1999) 274, 17451–17444. [DOI] [PubMed] [Google Scholar]
- 10.Chiu, H. C., Kovacs, A., Ford, D. A., Hsu, F. F., Garcia, R., Herrero, P., Saffitz, J. E. & Schaffer, J. E. (2001) J. Clin. Invest. 107, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, G., Koyama, K., Yuan, X., Lee, Y., Zhou, Y. T., O'Doherty, R., Newgard, C. B. & Unger, R. H. (1996) Proc. Natl. Acad. Sci. USA 93, 14795–14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orci, L., Cook, W. S., Ravazzola, M., Wang, M. Y., Park, B. H., Montesano, R. & Unger, R. H. (2004) Proc. Natl. Acad. Sci. USA 101, 2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado De Oliveira, R., Leid, M., McBurney, M. W. & Guarente, L. (2004) Nature 429, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie, D. G. & Carling, D. (1997) Eur. J. Biochem. 246, 259–273. [DOI] [PubMed] [Google Scholar]
- 15.Minokoshi, Y., Kim, Y. B., Peroni, O. D., Fryer, L. G., Muller, C., Carling, D. & Kahn, B. B. (2002) Nature 415, 339–343. [DOI] [PubMed] [Google Scholar]
- 16.McGarry, J. D., Leatherman, G. F. & Foster, D. W. (1978) J. Biol. Chem. 253, 4128–4136. [PubMed] [Google Scholar]
- 17.Iida, M., Murakami, T., Ishida, K., Mizuno, A., Kuwajima, M. & Shima, K. (1996) Biochem. Biophys. Res. Commun. 224, 597–604. [DOI] [PubMed] [Google Scholar]
- 18.Wang, M. Y., Koyama, K., Shimabukura, M., Newgard, C. B., Unger, R. H. (1998) Proc. Natl. Acad. Sci. USA 95, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimabukura, M., Koyama, K., Chen, G., Wang, M.-Y., Trieu, F., Newgard, C. B. & Unger, R. H. (1997) Proc. Natl. Acad. Sci. USA 94, 4637–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruderman, N. B., Saha, A. K., Vavvas, D. & Witters, L. A. (1999) Am. J. Physiol. 276, E1–E18. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson, L. L., Fischer, M. A. & Lopaschuk, G. D. (2002) J. Biol. Chem. 277, 29424–29430. [DOI] [PubMed] [Google Scholar]
- 22.Shimabukura M., Wang, M.-Y., Zhou, Y.-T., Newgard, C. B. & Unger, R. H. (1998) Proc. Natl. Acad. Sci. USA 95, 9558–9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szczepaniak, L. S., Dobbins, R. L., Metzger, G. J., Sartoni-D'Ambrosia, G., Arbique, D., Vongpatanasin, W., Unger, R. & Victor, R. G. (2003) Magn. Reson. Med. 49, 417–423. [DOI] [PubMed] [Google Scholar]