Abstract
Bartonella species are fastidious, Gram-negative human pathogens that can persist in the host bloodstream for years and bind to and invade several types of host cells. For many pathogens, adhesion to host cells and extracellular matrix (ECM) components is a critical virulence determinant. Bacteria often vary expression of surface adhesins by phase or antigenic variation to subvert the host immune response and permit adaptive interaction with different host structures. We developed a macaque animal model for Bartonella quintana infection to detect changes in bacterial outer-membrane proteins (OMP) during prolonged bloodstream infection. We identified a gene family encoding four highly conserved, 100-kDa, variably expressed OMP (Vomp), two of which function as adhesins. The variable expression of Vomp family members appears to be mediated by deletion of one or more vomp genes during chronic bloodstream infection. vomp deletion was observed also in isolates from humans with chronic B. quintana infection. The Vomp are closely related to the afimbrial adhesin, YadA, a virulence factor of Yersinia enterocolitica. The surface-expressed Vomp contain conserved structural features of YadA, including collagen-binding motifs. We demonstrate that the B. quintana Vomp are multifunctional OMP involved in binding to collagen and autoaggregation: VompC confers the ability to bind collagen IV, and VompA is necessary and sufficient for autoaggregation. The B. quintana Vomp are members of the newly recognized family of YadA-like trimeric autotransporters; the Vomp constitute a multigene family, they are variably expressed, and different virulence properties are attributable to individual Vomp family members.
Keywords: phase variation, multifunctional adhesin, collagen binding, bloodstream infection, YadA homolog
Bartonella species are Gram-negative bacteria that are arthropod-borne and extremely fastidious. The three major Bartonella pathogens for humans are Bartonella quintana, Bartonella henselae, and Bartonella bacilliformis. B. quintana is transmitted by the human body louse and causes relapsing fever (“trench fever”), endocarditis, and bacillary angiomatosis (1). B. henselae is transmitted by the cat flea or a cat scratch, causing cat-scratch disease and bacillary angiomatosis (1). Prolonged infection can occur, and unsuspected bloodstream infection with Bartonella can be detected in 5–14% (2, 3) of individuals in certain regions. In immunocompromised individuals (with cancer, transplanted organs, or HIV infection), Bartonella infection can cause debilitating, even fatal, illness. Bartonellae must adapt to markedly different environments, adhere to many cell types, and evade the host immune response during persistent bloodstream infection (even >1 year). Because most Bartonella infections and species were recognized only recently, little is known about Bartonella virulence strategies.
The mechanism by which Bartonella species persist in the host bloodstream is unknown, but successful evasion of the host immune response is critical. Phase and antigenic variation are among the most effective immune evasion strategies exploited by microbial pathogens to maintain persistent host infection (4, 5). Antigenic and phase variation involve a relatively high frequency change in immunodominant surface proteins during infection through alteration of the amino acid composition or by turning on and off expression of a protein (4). Many bacteria, such as Borrelia, Neisseria, and Campylobacter, use variation mechanisms (5–8). Virtually all of these antigenic- or phase-variable structures (e.g., adhesins) are virulence factors that are essential for colonization or persistence of the pathogen in the host (8). Variation of surface appendages also can direct tissue tropism and facilitate adaptation to environmental changes, such as those encountered in the transition between vector and host (5). Another Bartonella persistence strategy is invasion of and residence in the intracellular compartment of nonphagocytic host cells (9, 10). Binding of Bartonella to different host-cell types likely involves adhesins that are variably expressed, but the Bartonella adhesins that are involved have not been identified definitively.
Neither antigenic nor phase variation have been documented during Bartonella infection in vivo. We sought to identify outer-membrane proteins (OMP) of B. quintana that are variably expressed during prolonged bloodstream infection and that interact with host cellular structures. By analyzing sequential bloodstream isolates from an animal model, we identified and characterized a family of B. quintana variably expressed OMP (Vomp) adhesins that confer the two following important virulence phenotypes on B. quintana: autoaggregation and collagen binding.
Materials and Methods
Bacterial Strains and Growth Conditions. B. quintana strains (Table 1, which is published as supporting information on the PNAS web site) were streaked onto chocolate agar plates, incubated at 35°C in candle jars, and harvested after 5–7 days (11). Escherichia coli strains (Table 1) were grown in LB medium at 37°C. When required, kanamycin, ampicillin, carbenicillin, or nalidixic acid was added to growth medium at concentrations of 50, 100, 100, and 20 μg/ml, respectively. To induce expression of recombinant Vomp, E. coli overnight cultures were diluted 1:100 with fresh LB medium and the appropriate antibiotics. Isopropyl β-d-thiogalactoside (IPTG) was added for 1.5 h to a final concentration of 0.5–1 μg/ml when the OD at 600 nm reached 0.8.
Development of an Animal Model for Human B. quintana Infection. We developed an animal model of B. quintana infection in rhesus macaques (Macaca mulatta) that reproduces the prolonged, high-titer bacteremia that is observed in humans (unpublished data). Animals were inoculated intradermally with inoculum derived from multiple colonies of B. quintana strain JK-31 isolated from a patient and passaged three times to generate sufficient inoculum. To study sequential B. quintana isolates from experimentally infected animals, blood was removed from animals twice weekly for 1 month after inoculation, then weekly for 2 months. Blood was drawn into 2-ml EDTA tubes (Becton Dickinson), centrifuged, and plated onto fresh chocolate agar. The B. quintana colony-forming units (cfu) per ml of blood were quantified, and at least five single colonies from sequential isolates were selected for evaluation of OMP profiles. Preimmune and postimmune sera were drawn simultaneously with cultures for the analysis of differentially expressed proteins.
Separation and Immunoblotting of B. quintana Cytosolic and Total OMP (TOMP) Fractions. Fractionation of B. quintana proteins was performed as described (12). The B. quintana TOMP fractions were analyzed by 1D and 2D SDS/PAGE. For immunoblots, the TOMP fractions were separated by 10% SDS/PAGE, and proteins were transferred to a nitrocellulose membrane, blocked, and blotted with either a 1:50 dilution of serum from the experimentally infected macaque or a 1:400 dilution of rabbit antiserum (Animal Pharm Services, Healdsburg, CA) against the N-terminal 15 aa of the Vomp (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
Identification and Sequencing of the vomp Gene Family from JK-31 and B. quintana Final Isolate at Day 70 (BQ2-D70). Three Vomp detected in the JK-31 TOMP preparation by using 2D SDS/PAGE were transferred to a polyvinylidene difluoride membrane, and the N-terminal sequence of each protein was determined (Kendrick Labs, Madison, WI). Additional internal-peptide sequences were determined by digesting the Vomp with endoproteinase Lys-C (Roche Molecular Biochemicals) and analyzing the digests by matrix-assisted laser desorption ionization MS. Several peaks representing internal-peptide fragments present in all three Vomp were chosen for further sequencing. The N-terminal and internal amino acid sequences were used to synthesize degenerate oligonucleotide primers, 100kd5Deg and 100kd3Deg (Table 2, which is published as supporting information on the PNAS web site), for library screening. The vomp family gene sequences of JK-31 and BQ2-D70 were obtained by screening a B. quintana JK-31 genomic library and by PCR amplification (see Supporting Materials and Methods).
Southern Blotting with B. quintana Genomic DNA from Patient and Sequential Animal Isolates. DNA was extracted from a population of simultaneously isolated colonies (from the bloodstream of animal BQ2) or from a single colony isolated after direct plating of blood (from chronically infected patients). DNA was subjected to Southern blotting (13) and probed with a PCR-amplified, 645-bp fragment located within the conserved region of the genes vompA, vompB, and vompC (primers: gene1consF and gene1consR; see Table 2).
Surface Expression of the Vomp on Live JK-31, BQ2-D70, and Recombinant E. coli Strains. An indirect fluorescent Ab (IFA) technique (14) was used to demonstrate Vomp surface expression on live B. quintana and on vomp recombinant E. coli strains (for construction of recombinant strains, see Supporting Materials and Methods). B. quintana and IPTG-induced E. coli vomp recombinant strains were incubated in anti-Vomp N-terminal Ab (1:50) and then FITC-labeled secondary Ab (1:100) (Zymed). After incubation, cells were washed, resuspended, spotted onto a glass slide, air dried, and mounted. Bacteria were visualized by using an Nikon Eclipse E600 epifluorescence microscope (Nikon).
Autoaggregation Assay of B. quintana Strains JK-31 and BQ2-D70, and Recombinant E. coli Strains Expressing Vomp. Autoaggregation was assayed by a modification of the method described by Laird and Cavanaugh (15). B. quintana were harvested, washed once, and resuspended with M199 supplemented with 1 μM glutamine and 1 μM sodium pyruvate. We added 3 ml of each B. quintana suspension (OD600 = 1.0–1.2; ≈108-109 cells per ml) to a plastic test tube and incubated them in a CO2-enriched atmosphere at 35°C. The IPTG-induced recombinant E. coli strains were washed and resuspended in 3 ml of LB/carbenicillin supplemented with 68 μg/ml chloramphenicol (to an OD600 = 1.0–1.2; ≈107-108 cells per ml) and incubated at 37°C. To quantify the autoaggregation, 50 μl was taken from the top of each culture tube from time 0 until 8 or 10 h, and OD600 was measured immediately. Statistical analysis was performed as described (see Supporting Materials and Methods).
Adhesion of B. quintana and Recombinant E. coli Strains Expressing Vomp to Collagen IV. Bacterial adherence to collagen IV (human placenta; BD Biosciences) was tested by a modification of the method of Eberhard et al. (16). Glass coverslips in 24-well plates were coated overnight at 4°C with 300 μl of 20 μg/ml collagen IV (in coating buffer of 0.25 M NaHCO3/0.25 M Na2CO3, pH 9.6). Coverslips were washed with PBS containing 0.05% Tween 20 (PBST), blocked with 1% BSA for 2 h at room temperature, and then washed three times in PBS. Bartonella and IPTG-induced E. coli vomp recombinant strains were harvested, washed with PBS, and resuspended in PBS to OD600 ≈1.5 (≈107-109 cells per ml). For E. coli strains, mannose was added to a final concentration of 1% to block background binding (17). We added 1 ml of bacterial suspension to each well, and the plates were incubated at room temperature for 2 h with gentle shaking. After washing with PBST, bound bacteria were stained with a 1:500 dilution of Syto-9 (Molecular Probes) for 15 min. Coverslips were washed and mounted, and the binding of bacteria was observed by epifluorescence microscopy. Quantification and statistical analysis of the bound cells (Supporting Materials and Methods) were performed by counting three random fields of each slide for three independent experiments under ×400 and ×1,000 magnification for E. coli and B. quintana, respectively.
Results
An Animal Model to Identify Changes in B. quintana Proteins During Prolonged Bloodstream Infection. We established a rhesus macaque animal model that reproduces the manifestations of B. quintana infections in humans (unpublished data). Fig. 1A shows the B. quintana cfu/ml values for blood isolated from a representative infected rhesus macaque (BQ2), drawn after experimental inoculation with 6.6 × 107 cfu/ml. Inoculum strain JK-31, day 12 isolate (BQ2-D12), and day 70 isolate (BQ2-D70) were used for further analyses in this study. The inoculum consisted of multiple colonies that were isolated directly from a patient and in which the BQ2-D70 genotype was not detectable. Analysis of isolates from another animal that simultaneously received the same inoculum did not identify a BQ2-D70 genotype (data not shown).
Fig. 1.
Development of a rhesus macaque model of prolonged bloodstream infection leads to identification of an OMP present in the inoculum strain that is not detectable in a late isolate by immunoblotting. (A) A representative animal (BQ2) was inoculated with B. quintana JK-31 at time 0, and blood cultures were performed at regular intervals for 98 days. cfu of B. quintana bacteria per ml of blood are shown on the y axis. Bloodstream infection was detected on day 12 after experimental inoculation, and a relapsing pattern of bacteremia was observed, with the last isolate recovered on day 70. (B) Immunoblot analysis demonstrates that a 100-kDa protein (arrow in II) is present in JK-31 but absent in BQ2-D70. TOMP from JK-31 (lanes 2 and 4) and BQ2-D70 (lanes 3 and 5) were immunoblotted with preimmune serum (Panel I); or Day 70 postimmune serum (Panel II). Lane 1 shows protein standards.
Immunoblotting and 2D SDS/PAGE Identified a Family of 100-kDa OMP That Disappear During Infection. To screen for differences in OMP expression during prolonged bacteremia in the macaque, whole-cell lysates from sequentially isolated strains were fractionated into TOMP and cytosolic proteins. Direct comparison of JK-31 and BQ2-D70 TOMP by 1D SDS/PAGE did not reveal obvious differences (data not shown). Next, we immunoblotted the sequential isolate TOMP with preimmune serum and day 70 postimmune serum from the animal (Fig. 1B). With postimmune serum, a protein of ≈100 kDa was detected in JK-31 TOMP but not in BQ2-D70 (Fig. 1B). Abs to the 100-kDa protein were elicited in all other experimentally infected animals (data not shown), implying that this protein is an immunodominant antigen. Analysis by 2D SDS/PAGE revealed an OMP of ≈100 kDa that appeared to be present in three isoforms in JK-31, each migrating with a slightly different pI and apparent molecular mass (Fig. 2A). However, for BQ2-D70, none of these isoforms was visible (Fig. 2B).
Fig. 2.
A family of 100-kDa proteins identified in JK-31 by 2D SDS/PAGE are absent in BQ2-D70. TOMP fractions were subjected to 2D SDS/PAGE and visualized by silver staining. (A) TOMP profile from JK-31. (B) TOMP profile from BQ2-D70. The bracket and arrow indicate three 100-kDa proteins visible in JK-31 but not BQ2-D70.
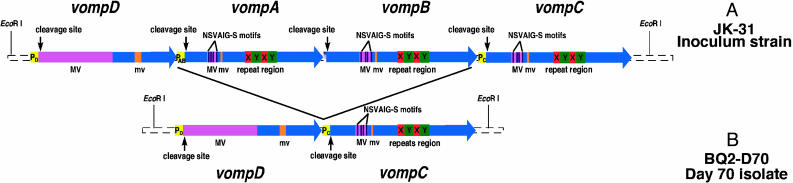
The Vomp Are Encoded by a Family of Four Highly Conserved, Tandemly Arranged Genes and Have Features Suggesting Surface Localization. We identified four tandemly arranged, closely related vomp genes, vompD, vompA, vompB, and vompC (named in order of identification), located on an ≈12.8-kb region of JK-31 genomic DNA (Fig. 3A). There is a 43-bp intergenic region between vompA and vompB and three putative promoter regions (Fig. 3A;PD,PAB, and PC). There also is a 267-bp vomp gene fragment located 914-bp downstream of vompC. The predicted molecular mass of the Vomp ranges from 95,943 to 100,516 Da (see Table 3, which is published as supporting information on the PNAS web site), with an acidic pI (4.77–5.08). Overall, there is 47–90% amino acid identity among the four Vomp (see Table 4, which is published as supporting information on the PNAS web site); VompA, VompB, and VompC are more closely related to each other (84–90% amino acid identity). The C-terminal VompD segment of ≈350 aa has 87–89% identity with the C-terminal regions of the other Vomp, but the N-terminal 448 aa of VompD has no homology to VompA, VompB, and VompC. Each Vomp has major variable (MV), minor variable, and extensively conserved regions (Fig. 3A). In VompA, VompB, and VompC, there also are two tandemly repeated regions at the C terminus, in an X-Y—X-Y pattern (X is composed of 33–45 aa, and Y is composed of 61 aa; Table 3). Each B. quintana Vomp is predicted to have a cleavable leader sequence (signal p program; ref. 18), which was confirmed for VompA, VompB, and VompC by N-terminal sequencing of the mature proteins. Interestingly, no Vomp has an Arg–Gly–Asp (RGD) motif that might mediate host-cell binding (19).
Fig. 3.
Schematic representation of the four vomp genes of JK-31 and the two genes of BQ2-D70. (A) Arrows indicate ORF and their orientations. Promoter regions (PD,PAB, and PC) are shown in yellow. Based on the deduced amino acid sequences, highly conserved regions among the four genes are shown in blue, MV regions are shown in light purple, and minor variable (mv) regions are shown in light orange. The putative collagen-binding motifs (NSVAIG-S) are indicated by dark purple vertical lines within the MV regions of VompA, VompB, and VompC. Two tandemly repeated regions are shown in dark orange (x) and green (y). Vertical arrows indicate the predicted leader-peptidase cleavage sites. (B) The vomp locus in BQ2-D70 has only two genes, vompD and vompC; vompA and vompB have been deleted during the course of infection.
Vomp Family Members Have Conserved Motifs and Domains Present in OMP Adhesins of Other Gram-Negative Pathogens. VompA, VompB, and VompC were found to be most closely related to a group of newly recognized afimbrial adhesins of Gram-negative bacteria, including the well characterized YadA from Yersinia enterocolitica (20), UspA from Moraxella catarrhalis (21), and NadA from Neisseria meningitidis (22). There also is homology with putative adhesins from E. coli O157:H7, Xylella fastidiosa, and Salmonella typhimurium. YadA, a multifunctional OMP, confers a number of critical virulence properties, including adherence to epithelial cells (23) and professional phagocytes (24), serum resistance (25), autoaggregation (26), and binding to extracellular matrix (ECM) proteins, including collagen (27). Of note, YadA is about half the size of Vomp, but the YadA N- and C-terminal halves share homology with the corresponding regions of VompA, VompB, and VompC (Fig. 7, which is published as supporting information on the PNAS web site).
The predicted secondary structure of Vomp revealed that the lollipop-shaped surface structure of YadA is likely also present in VompA, VompB, and VompC (Fig. 7). Like YadA, the C-terminal region of all four Vomp is predicted to consist of a membrane anchor of four transmembrane β-strands and an α-helical internal region with a propensity to form coiled-coils (20). Most importantly, four repeats of the NSVAIG-S collagen-binding motifs in YadA (28, 29) are found within the N-terminal half in VompA, VompB, and VompC (Figs. 3A and 7), followed by a conserved neck and stalk region. No NSVAIG-S collagen-binding motifs were identified in VompD.
Genomic Rearrangement of the vomp Locus, with Deletion of vompA and vompB, Occurs During Chronic Bloodstream Infection. Southern blot analysis (see Fig. 8, which is published as supporting information on the PNAS web site) and sequencing of the vomp locus in BQ2-D70 revealed that the locus is only 6.3 kb and that only vompD and vompC are present, with their putative promoters intact (Fig. 3B). Absence of vompA and vompB in BQ2-D70 indicates that gene rearrangement and/or deletion occurred in vivo during the course of B. quintana bloodstream infection. We found that progressive genomic rearrangement occurred during infection (see Fig. 8). Hybridization of probe to multiple sized fragments of genomic DNA from a population of isolates from day 12 is likely to represent a heterogeneous population of B. quintana in which some blood-stream isolates have no genes deleted, some have one gene deleted, and some have two genes deleted. By day 70 after inoculation, the population of bloodstream isolates has become more homogeneous, and the probe predominantly hybridizes to a single fragment.
vomp Gene Deletion and Heterogeneity in the vomp Locus Occur in Patient B. quintana Isolates. To identify whether heterogeneity occurs within the vomp locus of B. quintana isolated from the bloodstream of chronically infected patients, Southern blotting was performed with genomic DNA from B. quintana human isolates. Six different patterns of probe binding were identified (Fig. 4), presumably resulting from differences among the patient isolates in the number of vomp genes; in the JK-5, JK-7, and JK-56 isolates, a gene in the vomp locus appears to be deleted. In other human isolates, all vomp genes are likely to be present, but the regions flanking the vomp locus apparently differ in size. Restriction-fragment polymorphisms among strains may also be involved in pattern changes.
Fig. 4.
Southern blot analysis of chromosomal DNA from nine human B. quintana isolates reveals heterogeneity in vomp gene copy number. Genomic DNA from the B. quintana human (JK-31 and JK-5 to JK-63) and macaque (BQ2-D70) isolates were digested with EcoRV and probed with a fragment of the conserved 5′ region of vompA, vompB, and vompC. The probe binds to the two fragments of BQ2-D70 DNA flanking the vomp EcoRV site, indicating the presence of vompC, and to four bands in JK-31, indicating the presence of vompA, vompB, and vompC. In isolates JK-5, JK-7, and JK-56, only three bands are detected, suggesting the presence of only two copies of the conserved region. In other human isolates, all three genes are present, but the region flanking the vomp genes differs. There are a total of six different patterns observed (I–VI, shown above the respective lanes). The probe control is shown in the far right lane.
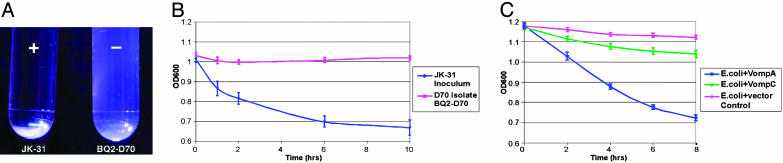
Vomp Mediates B. quintana Autoaggregation, and VompA, not VompC, Is the Major Determinant of the Autoaggregation Phenotype. We demonstrated by IFA that Vomp is expressed on the surface of B. quintana JK-31 and vomp recombinant E. coli strains but not on BQ2-D70 (see Fig. 9, which is published as supporting information on the PNAS web site). We next assayed for an autoagglutination phenotype, and we observed that JK-31 began to sediment within the first hour, resulting in a significant decrease in OD relative to BQ2-D70, which remained suspended (Fig. 5B; P < 0.05). After 36 h of incubation, JK-31 formed a flocculate pellet at the bottom of the tube, but BQ2-D70 remained in suspension (Fig. 5A). This result suggests that the VompA and/or VompB expressed in JK-31 but not in BQ2-D70 mediates the autoaggregation phenotype.
Fig. 5.
Autoaggregation profile of JK-31, BQ2-D70, and E. coli recombinant strains expressing VompA or VompC. (A) B. quintana strains were suspended and allowed to settle undisturbed for 36 h. JK-31 formed a pellet at the bottom of the tube (+ tube). BQ2-D70 did not autoaggregate, and remained suspended (– tube). (B) For quantitation, B. quintana strains were suspended in media, and the OD600 was determined. The OD600 decreased with JK-31 but not BQ2-D70 (P < 0.05). (C) E. coli strains were suspended in media, and the OD600 was determined. Expression of VompA, but not VompC, in E. coli resulted in a significant decrease in the OD600 compared with the E. coli control (P < 0.05). Data points represent the mean ± SEM of three independent assays done in duplicate (B. quintana) or triplicate (E. coli).
We then assayed for ability of Vomp expression to confer gain-of-function on nonaggregative E. coli. Quantitative analysis by fluorescence-activated cell-sorting demonstrated that surface expression of VompA and VompC are equivalent on the induced, recombinant E. coli strains (data not shown). Sedimentation profiles in Fig. 5C show that the sedimentation rate of the vompA, but not vompC, recombinant was significantly greater than the E. coli control (P < 0.05). This assay demonstrates that, of VompA and VompC, VompA is the major determinant of the autoaggregation phenotype of B. quintana. This result is consistent with the finding that JK-31 (vompA+), but not BQ2-D70 (vompA–), autoagglutinates.
VompC and VompA are Collagen-Binding Adhesins with Different Binding Efficiencies. Because the YadA NSVAIG-S collagen-binding motifs are present in B. quintana Vomp (Fig. 3), we tested JK-31 and BQ2-D70 for the ability to bind to ECM components, and we found that both bind to collagen IV, a basement-membrane component (data not shown). Because Vomp is not detected on the surface of BQ2-D70, binding could be due to other adhesins present on B. quintana that confer redundant collagen-binding ability (redundant adhesins are present in many bacteria) (30). To characterize the contribution of individual Vomp, we tested whether expression of VompA or VompC in E. coli could confer binding to collagen IV. Both conferred the ability to bind collagen IV (Fig. 6A), but quantification revealed different collagen IV binding efficiencies, with 6.6-fold greater binding by VompC than by VompA (Fig. 6B; P < 0.05). Thus, VompA and VompC are both adhesins, and expression of either is sufficient to mediate binding to the basement-membrane component of host cells, but with different efficiencies.
Fig. 6.
Heterologous expression of VompC or VompA in E. coli confers the ability to bind collagen IV. (A) Coverslips were coated with collagen IV (Right) or BSA control (Left). Expression of VompC in E. coli significantly increases adhesion to collagen IV. Expression of VompA shows a moderate number of E. coli adhering to collagen IV but significantly less than the E. coli expressing VompC. (B) Quantitative analysis of the adherence of vomp recombinant E. coli strains to collagen IV. Data represent the mean ± SEM of three independent assays done in triplicate. *, P < 0.05, compared with E. coli control; **, P < 0.05, compared with both E. coli control and E. coli expressing VompA.
Discussion
Virulence properties of Bartonella species include the ability to persist in remarkably disparate hosts and niches and to adhere to many different host-cell types (e.g., erythrocytes in the mammalian bloodstream and epithelial cells in the arthropod gastrointestinal tract). In the bloodstream of mammals, B. quintana can cause prolonged, relapsing fever similar to Borrelia species (5). Relapsing fever occurs when Borrelia surface proteins undergo antigenic variation to circumvent the host immune attack. To test our hypothesis that Bartonella persists by modifying critical surface proteins during chronic infection, we first developed an animal model to compare the repertoire of proteins expressed in sequential bloodstream isolates. We identified a family of four 100-kDa Vomp that are immunodominant and variably expressed on the surface of B. quintana during prolonged bloodstream infection. Sequencing of the late isolate BQ2-D70 revealed that vompA and vompB had been deleted. Analysis of other B. quintana strains that were recovered from humans revealed that vomp genes are deleted and/or rearranged, implicating phase variation as a strategy used by B. quintana to facilitate chronic human infection.
Although phase variation usually implies reversibility, it can be irreversible, as reviewed by Henderson et al. (6). However, it is critical that bacteria maintain the ability to express essential virulence genes, such as adhesins. There are several mechanisms by which functional vomp genes could be restored in the setting of gene deletion, including gene duplication or recombination either with exogenously acquired B. quintana DNA or with vomp gene fragments at other locations on the chromosomal DNA. The newly released genome sequence for the B. quintana strain Toulouse reveals vomp gene fragments downstream of vompC: one that is tandemly arranged (similar to the fragment that we have identified in JK-31) and one that is inverted (31). In addition, this strain has only three complete vomp genes: vompD, vompC, and between these two genes, a gene that is a mosaic of vompA, vompB, and vompC, potentially representing some recombination event(s). All of our isolates have at least two vomp genes, leaving the potential for duplication or recombination between the remaining genes in the locus or with fragments located at other sites on the chromosome (that were not detected by the probes used in our Southern blot analyses). The mechanisms of deletion and recombination of vomp genes require further investigation.
The Vomp have extensive N- and C-terminal amino acid and secondary-structure homology with the Y. enterocolitica adhesin YadA, including conservation of many of the YadA functional domains. For YadA (20) and Vomp, the conserved C-terminal domain is hydrophobic, has four transmembrane β-strands, and is predicted to serve as an anchor within the cell membrane (20). Although VompD differs at the N terminus, it shares many structural features with VompA, VompB, and VompC, including the conserved C-terminal membrane anchor. The extensively conserved region just upstream of the predicted membrane anchor domain of the Vomp is probably responsible for the maintenance of a functional molecular structure, as with YadA (20). The two M. catarrhalis OMP adhesions, UspA1 and UspA2, have a secondary structural arrangement that is remarkably similar to Vomp. UspA1 has head, neck, stalk, and membrane anchor regions, as in Bartonella VompA, VompB and VompC; UspA2 has the membrane anchor region but not the N-terminal domains, similar to VompD (20).
Within the putative stalk domain of the C-terminal conserved region of VompA, VompB, and VompC, there are two long, tandemly arranged, repeated regions, in an X-Y—X-Y configuration. The function of these repeated regions is currently unknown, but these regions could facilitate deletion of single or multiple vomp genes (6). These tandem repeats also could be involved in oligomerization of Vomp monomers, similar to the YadA homooligomers resembling “lollipops” on the surface of Yersinia (20). Analysis of the vomp locus in isolates from additional humans and animals will provide insight into the function of these regions in Bartonella.
Homology between Vomp and YadA led us to investigate the adhesive properties of the B. quintana Vomp. YadA is the prototype of a recently recognized class of proteobacterial afimbrial adhesins that also includes the N. meningitidis adhesin, NadA. In vivo, both function as virulence determinants essential for establishing infection in animal models (22, 32). In vitro, YadA confers the ability to bind epithelial cells (23), ECM constituents of the host, including collagen (27), and other Y. enterocolitica bacilli (autoaggregation; (26)). Autoaggregation, which facilitates intraspecies communication and biofilm formation, is involved in pathogenesis pathways of Gram-negative bacteria (33, 34). We observed autoaggregation of JK-31, but not of BQ2-D70. We also demonstrated that surface expression of VompA, but not VompC, on B. quintana and E. coli is necessary and sufficient for autoaggregation. The YadA autoaggregation determinants have been mapped to the surface-exposed N terminus (35) that is conserved in VompA, VompB and VompC. Each Vomp differs in the MV region of the N terminus, and this MV region diversity may determine the Vomp autoagglutination phenotype. There are several potential roles for autoaggregation in Bartonella pathogenesis. First, during invasion of endothelial cells, bartonellae form a tightly aggregated bacterial mass or “invasome” on the surface of the endothelial cell (9). Second, the close approximation of bartonellae could facilitate communication among bacilli in the focal tissue infection of bacillary angiomatosis, where Bartonella forms microcolonies that are associated with angiogenesis in the extracellular collagen (36).
Most bacteria assemble several different adhesins on their surface, and each adhesin may bind to one or more host cell receptors and ECM components, depending on environmental conditions. For intracellular bacteria, including Yersinia, adherence to host ECM components such as collagen is a crucial initial step in reaching the intracellular compartment (37). Bartonella species adhere to and invade endothelial cells and red blood cells of the mammalian host (38), and also adhere to the intestinal epithelium of the arthropod vector (39). The four different but related B. quintana Vomp create the potential for adaptation to these different environments. The closely related XadA adhesin of the plant pathogen X. fastidiosa is hypothesized to be involved in binding to the insect vector foregut but not the plant host (40). One or more B. quintana Vomp may be expressed only in the arthropod vector (e.g., VompD, which is least homologous to the other Vomp, lacks collagen-binding motifs, and is most homologous with XadA). To effect collagen binding in the primate host, collagen-binding motifs are present in the MV region of VompA, VompB, and VompC, but they differ in sequence and combination for each Vomp. Thus the binding specificity can be unique to each Vomp. Indeed, we found that expression of VompA or VompC in E. coli was sufficient to confer collagen-binding properties, but VompA and VompC differed significantly in their binding ability. Adhering to different host substrates and directing tissue tropism by expression of different vomp genes is an attractive potential pathogenesis strategy for Bartonella. Binding specificity of the Vomp adhesin could be further refined by reorganization of the Vomp into different combinations of heterooligomers, in addition to controlling expression by gene rearrangement.
In summary, we have identified a family of novel B. quintana OMP adhesins that undergo phase variation in vivo during prolonged bloodstream infection. We have demonstrated that the multifunctional B. quintana Vomp are associated with important virulence traits including autoaggregation and adhesion to collagen IV, a host-cell ECM component. The specificity of substrate binding differs between VompA and VompC, which may be determined by unique motifs in each MV region. Further studies will be important in determining how expression of different vomp family members affects the structure and binding specificity of the Vomp adhesin and in defining the role of Vomp in specific steps of Bartonella pathogenesis.
Supplementary Material
Acknowledgments
We thank Rickie W. Kasten and the Primate Center staff at the University of California (Davis), Manuel Amieva for constructive comments, and Mary Ann Gawinowicz (Columbia University, New York) for N-terminal sequencing and matrix-assisted laser desorption ionization MS. J.E.K. was supported by National Institutes of Health Grants R01AI43703 and AI52813, a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, and the University of California (San Francisco) Academic Senate and University-wide AIDS Research Program.
Abbreviations: cfu, colony-forming units; OMP, outer-membrane protein(s); Vomp, variably expressed OMP; ECM, extracellular matrix; MV; major variable; TOMP, total OMP; IPTG, isopropyl β-d-thiogalactoside.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY618465 and AY618466).
References
- 1.Koehler, J. E. (2000) in Persistent Bacterial Infections, eds. Nataro, J. P., Blaser, M. J. & Cunningham-Rundles, S. (ASM Press, Washington, D.C.), pp. 339–353.
- 2.Weinman, D. & Pinkerton, H. (1937) Proc. Soc. Exp. Biol. Med. 37, 596–598. [Google Scholar]
- 3.Brouqui, P., Lascola, B., Roux, V. & Raoult, D. (1999) N. Engl. J. Med. 340, 184–189. [DOI] [PubMed] [Google Scholar]
- 4.Borst, P. (1991) Immunol. Today 12, A29–A33. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G. & Restrepo, B. I. (2000) Emerging Infect. Dis. 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson, I. R., Owen, P. & Nataro, J. P. (1999) Mol. Microbiol. 33, 919–932. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin, J. & Blaser, M. J. (1997) Proc. Natl. Acad. Sci. USA 94, 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson, B. D. & Meyer, T. F. (1992) Trends Genet. 8, 422–428. [DOI] [PubMed] [Google Scholar]
- 9.Dehio, C., Meyer, M., Berger, J., Schwarz, H. & Lanz, C. (1997) J. Cell Sci. 110, 2141–2154. [DOI] [PubMed] [Google Scholar]
- 10.Schulein, R., Seubert, A., Gille, C., Lanz, C., Hansmann, Y., Piemont, Y. & Dehio, C. (2001) J. Exp. Med. 193, 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park, S., Kelminson, K., Lee, A., P. Zhang, P., Warner, R., Rehkopf, D., Calderwood, S. & Koehler, J. (2001) J. Bacteriol. 183, 5751–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy, T. F., Dudas, K. C., Mylotte, J. M. & Apicella, M. A. (1983) J. Infect. Dis. 147, 838–846. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J., M. T., and Fritsch E. F. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 14.Koehler, J. E., Birkelund, S. & Stephens, R. S. (1992) Mol. Microbiol. 6, 1087–1094. [DOI] [PubMed] [Google Scholar]
- 15.Laird, W. J. & Cavanaugh, D. C. (1980) J. Clin. Microbiol. 11, 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberhard, T., Virkola, R., Korhonen, T. K., Kronvall, G. & Ullberg, M. (1998) Infect. Immun. 66, 1791–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapperud, G., Namork, E., Skurnik, M. & Nesbakken, T. (1987) Infect. Immun. 55, 2247–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen, H., Engelbrecht, J., Brunak, S. & von Heijne, G. (1997) Protein Eng. 10, 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi, Y., Relman, D. A. & Nishikawa, A. (2001) Microb. Pathog. 30, 279–288. [DOI] [PubMed] [Google Scholar]
- 20.Hoiczyk, E., Roggenkamp, A., Reichenbecher, M., Lupas, A. & Heesemann, J. (2000) EMBO J. 19, 5989–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cope, L. D., Lafontaine, E. R., Slaughter, C. A., Hasemann, J., C.A., Aebi, C., Henderson, F. W., McCracken, J., G.H. & Hansen, E. J. (1999) J. Bacteriol. 181, 4026–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comanducci, M., Bambini, S., Brunelli, B., Adu-Bobie, J., Arico, B., Capecchi, B., Giuliani, M. M., Masignani, V., Santini, L., Savino, S., et al. (2002) J. Exp. Med. 195, 1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heesemann, J. & Gruter, L. (1987) FEMS Microbiol. Lett. 40, 229–233. [Google Scholar]
- 24.Roggenkamp, A., Ruckdeschel, K., Leitritz, L., Schmitt, R. & Heesemann, J. (1996) Infect. Immun. 64, 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balligand, G., Laroche, Y. & Cornelis, G. (1985) Infect. Immun. 48, 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skurnik, M. (1984) J. Appl. Bacteriol. 56, 355–363. [DOI] [PubMed] [Google Scholar]
- 27.Emody, L., Heesemann, J., Wolf-Watz, H., Skurnik, M., Kapperud, G., O'Toole, P. & Wadstrom, T. (1989) J. Bacteriol. 171, 6674–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Tahir, Y., Kuusela, P. & Skurnick, M. (2000) Mol. Microbiol. 37, 192–206. [DOI] [PubMed] [Google Scholar]
- 29.Nummelin, H., Merckel, M. C., Leo, J. C., Lankinen, H., Skurnik, M. & Goldman, A. (2004) EMBO J. 23, 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hultgren, S. J., Abraham, S., Caparon, M., Falk, P., St. Geme III, J. W. & Normark, S. (1993) Cell 73, 887–901. [DOI] [PubMed] [Google Scholar]
- 31.Alsmark, C., Frank, A., Karlberg, E., Legault, B., Ardell, D., Canback, B., Eriksson, A., Naslund, A., Handley, S., Huvet, M., La Scola, B., Holmberg, M. & Andersson, S. (2004) Proc. Natl. Acad. Sci. USA 101, 9716–9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepe, J. C., Wachtel, M. R., Wagar, E. & Miller, V. L. (1995) Infect. Immun. 63, 4837–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danese, P. N., Pratt, L. A., Dove, S. L. & Kolter, R. (2000) Mol. Microbiol. 37, 424–432. [DOI] [PubMed] [Google Scholar]
- 34.Rainey, P. B. & Travisano, M. (1998) Nature 394, 69–72. [DOI] [PubMed] [Google Scholar]
- 35.Tamm, A., Tarkkanen, A., Korhonen, T., Kuusela, P., Toivanen, P. & Skurnik, M. (1993) Mol. Microbiol. 10, 995–1011. [DOI] [PubMed] [Google Scholar]
- 36.LeBoit, P. E., Berger, T. G., Egbert, B. M., Beckstead, J. H., Yen, T. S. B. & Stoler, M. H. (1989) Am. J. Surg. Pathol. 13, 909–920. [PubMed] [Google Scholar]
- 37.Ljungh, A. & Wadstrom, T. (1995) in Methods in Enzymology, eds. Doyle, R. J. & Ofek, I. (Academic, San Diego), Vol. 253, pp. 501–515.7476412 [Google Scholar]
- 38.Dehio, C. (2001) Trends Microbiol. 9, 279–285. [DOI] [PubMed] [Google Scholar]
- 39.Ito, S. & Vinson, J. W. (1965) J. Bacteriol. 89, 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambais, M. R., Goldman, M. H. S., Camargo, L. E. A. & Goldman, G. H. (2000) Curr. Opin. Microbiol. 3, 459–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.