Abstract
Several of the best-studied sex differences in the mammalian brain are ascribed to the hormonal control of cell death. This conclusion is based primarily on correlations between pyknotic cell counts in development and counts of mature neurons in adulthood; the molecular mechanisms of hormone-regulated, sexually dimorphic cell death are unknown. We asked whether Bax, a member of the Bcl-2 family of proteins that is required for cell death in many developing neurons, might be essential for sex differences in neuron number. We compared Bax knockout mice and their WT siblings, focusing on two regions of the mouse forebrain that show opposite patterns of sexual differentiation: the principal nucleus of the bed nucleus of the stria terminalis, in which males have more neurons than do females, and the anteroventral periventricular nucleus (AVPV), where females have more neurons overall and many more dopaminergic neurons than do males. Testosterone, or its metabolites, is responsible for the sex differences in both nuclei. A null mutation of the Bax gene completely eliminated sex differences in overall cell number in both the principal nucleus of the bed nucleus of the stria terminalis and AVPV. Thus, Bax-dependent cell death is required for sexual differentiation of cell number, regardless of whether testosterone decreases or increases cell death. In contrast, the sex difference in AVPV dopaminergic cell number, as measured by tyrosine hydroxylase immunohistochemistry, was not affected by Bax gene deletion, demonstrating heterogeneity of mechanisms controlling cell number within a single nucleus.
Cell death is a fundamental process in brain development capable of dictating functional relationships between populations of neurons. It is thought to play a particularly important role in the differentiation of areas involved in sexually dimorphic functions, as several of the best-known sex differences in the mammalian nervous system involve differences in neuron number. For example, male rats have many more neurons than do females in the sexually dimorphic nucleus of the preoptic area, the principal nucleus of the bed nucleus of the stria terminalis (BNSTp), and the spinal nucleus of the bulbocavernosus (1–4). Conversely, in the anteroventral periventricular nucleus (AVPV), a nucleus at the rostral extreme of the third ventricle implicated in the control of gonadotrophin secretion, overall volume, cell density, and the number of dopaminergic neurons are greater in females than in males (5–7). These sex differences can be reversed by manipulating testosterone, or its hormonal metabolites, during perinatal life. In principle, such sex differences could be caused by the differential birth, migration, phenotypic differentiation, and/or death of neurons in males and females. Evidence thus far, however, favors the hormonal regulation of cell death in each case.
In support, females have more cells displaying the morphological characteristics of apoptosis than do males in the sexually dimorphic nucleus of the preoptic area, BNSTp, and spinal nucleus of the bulbocavernosus during perinatal life (8–10). Conversely, males have more dying (pyknotic) cells than do females in the AVPV (11), and these patterns of developmental cell death are reversed by the same hormonal treatments that reverse sex differences in adult cell number (7–11). Although this comprises very convincing circumstantial evidence that cell death is a major factor in sexual differentiation of cell number, the mechanisms underlying sexually dimorphic, hormone-regulated cell death have been virtually unexplored.
Likely players in this process are proteins of the Bcl-2 family, which are potent regulators of death in many cell types. Some members of this family (e.g., Bcl-2 and Bcl-xL) inhibit cell death, whereas other family members (e.g., Bax) promote death (12). Although the precise mechanisms whereby Bcl-2 family members control apoptosis are still not clear, much of the available evidence supports the so-called “rheostat model,” in which the balance between death-promoting and death-repressing family members constitutes a critical checkpoint that determines whether a cell will execute an apoptotic program (13, 14). Specifically, a critical level of Bax oligomers at the mitochondrial membrane activates downstream effector molecules such as the caspases (see ref. 15 for review); death antagonists, such as Bcl-2 and Bcl-xL, may prevent cell death primarily by preventing the oligomerization of Bax (16). If this model is correct, then Bax is a singularly important member of the Bcl-2 family. Indeed, a null mutation of the Bax gene dramatically reduces or eliminates developmental cell death in peripheral ganglia, spinal and brainstem motoneurons, the retina, and some areas of the cerebellum and hippocampus (17). On the other hand, Bax deletion does not appear to prevent the death of several other neuronal subtypes (17–19). Thus, many neural areas depend on Bax for cell death, and in those cells in which Bax is involved the requirement is profound. Gonadal steroid hormones can regulate the expression of Bax and other Bcl-2 family proteins (20–25), suggesting a mechanism for sexually dimorphic cell death. Nonetheless, sexually dimorphic brain areas have not been examined in Bax knockout mice.
In the present study we evaluated neuron number in the BNSTp and AVPV of mice lacking the Bax gene. These interconnected cell groups exhibit opposite patterns of sexual differentiation in rats (26). Although sex differences in overall neuron number in the BNSTp have not yet been reported for mice, the cluster of androgen receptor-expressing neurons is larger in male mice than in females (27). In AVPV, overall cell density is greater in female mice than in males (5, 25) and, as in rats, female mice possess about three times as many dopaminergic neurons in AVPV as do males (28). We reasoned that if Bax is essential for cell death in the BNSTp and AVPV, then sex differences in neuron number might be reduced or eliminated in Bax knockout mice. We now report that deletion of the Bax gene abolishes sex differences in overall neuron number in both the BNSTp and AVPV, but does not affect the sex difference in AVPV dopaminergic cell number.
Materials and Methods
Animals. Male and female mice heterozygous for the Bax gene deletion (Bax+/–) on a C57BL/6 background were obtained from The Jackson Laboratory. These animals were derived from mice originally produced by Korsmeyer and colleagues (29). WT (Bax+/+) and knockout (Bax–/–) offspring were identified by PCR amplification of tail DNA using published primer sequences (17). Animals were housed three to four per cage in a 12:12 light/dark cycle and held at 22°C. In adulthood (≈85 days of age), the mice were overdosed with chloropent, blood was collected by cardiac puncture, and animals were perfused intracardially with saline followed by buffered formalin. Brains were removed from the skull, postfixed in formalin, frozen-sectioned at 30 μm, and stained with thionin. Weight of the testes, seminal vesicles, and bulbocavernosus muscles, as well as anogenital distance (the length between the phallus and the anus), were measured in males after perfusion.
As reported (29), we observed a reduction in testis size in adult Bax–/– males (210 ± 26 mg versus 61 ± 2 mg; P < 0.005). This finding could be a concern, given the crucial role of testicular androgens in sexual differentiation. However, the phenotype results from the failure of a wave of germinal cell death that takes place just before puberty (30), i.e., after the perinatal sexual differentiation period. In addition, the defect appears confined to germ cells of the seminiferous tubules (29); there is no evidence that the androgen-producing Leydig cells are affected by Bax deletion. In confirmation, we found no effect of Bax deletion on anogenital distance (P > 0.7), a sexually dimorphic measure that is sensitive to perinatal androgens, or on weight of the seminal vesicles or bulbocavernosus muscles, which are indirect measures of recent androgen exposure (P > 0.7 and P > 0.9, respectively; data not shown). Finally, RIAs revealed the expected main effect of sex on serum testosterone levels (male > female; P < 0.02), but no effect of genotype (P = 0.9) and no sex-by-genotype interaction (P = 0.9; data not shown). Thus, we found no suggestion of altered testosterone levels in Bax–/– males.
Volume of Cell Groups, Neuronal Cell Counts, and Cell Size. All measurements were made on slides coded to conceal the sex and genotype of the animals. Nuclear volume measurements and stereological cell counts were performed by using stereoinvestigator software (MicroBrightfield, Williston, VT). Outlines of the BNSTp and AVPV were traced in each section, and volume was estimated by multiplying summed cross-sectional areas by 30 μm (the section thickness before histological processing). The optical disector method was used for cell counts. This method provides an unbiased estimate of overall cell number that is not affected by the size, orientation, or packing density of cells (31). Counting was ipsilateral within a 20 × 20-μm2 counting frame that was moved in a random-systematic fashion through each cell group. Cells were recorded that exhibited the morphological characteristics of neurons and had clearly visible nucleoli that fell within the counting frame. Parameters were adjusted so that counts were made in ≈100–200 locations within each nucleus. For the BNSTp, alternate sections were sampled, and the spacing between counting frames was 10,000 μm2 (i.e., a sampling grid of 100 × 100 μm2). For the much smaller AVPV, every section was counted and a 60 × 60-μm2 sampling grid was used. Upper and lower guard heights of 1 μm were used for each nucleus, to avoid edge artifacts or the counting of split cells. Mean cell size in each nucleus was determined by tracing the outlines of at least 50 neurons within the BNSTp and 25 neurons in AVPV of each animal. Cells for tracing were chosen by randomly placing a grid over three sections through each nucleus. The sections were selected to span the rostrocaudal extent of each nucleus and all cells that fell within a specified area of the grid were traced to avoid bias in cell selection.
Tyrosine Hydroxylase (TH) Immunohistochemistry. To determine whether Bax gene deletion influences the number of dopaminergic neurons in AVPV, we performed immunohistochemistry for TH in brains harvested from a separate cohort of mice. Animals were perfused with 4% paraformaldehyde in borate buffer, pH 9.5, and 20-μm coronal sections were cut into three series on a freezing microtome. One series of free-floating sections was double-stained for TH and estrogen receptor α (ERα) (as in ref. 28) by using mouse anti-TH antiserum (1:1,000; DiaSorin, Stillwater MN) and rabbit anti-ERα (1:40,000, Upstate, Waltham, MA). Secondary antibodies were goat anti-mouse Alexa 488 and Alexa 568 (both at 1:200; Molecular Probes) for TH and ER, respectively. ER-immunoreactive cells were used to select sections containing AVPV and identify the borders of the AVPV in each animal. TH-immunoreactive (TH-ir) cells with clearly visible nuclei were then counted in all sections containing the AVPV.
Data Analysis. The effects of Bax gene deletion on overall cell number, TH-ir cell number, nuclear volume, and cell size were evaluated by two-way ANOVAs (sex-by-genotype). Planned comparisons were performed by using a Fisher's test if significant main effects or interactions were found. Cell counts in AVPV did not pass a homogeneity of variance test (Bartlett-Box test, P = 0.015), because of greater variances in Bax knockouts than in WT animals. Data for this dependent variable were therefore analyzed in two ways: two-way ANOVA followed by planned comparisons using separate variances (Systat, Evanston, IL) and the Kruskal-Wallis nonparametric test. Both analyses gave the identical pattern of outcomes; probabilities from the ANOVA are reported here.
Results
BNSTp. Subdivisions of the BNST have been given various names by different investigators. We followed the conventions of Dong and Swanson (32) in defining the “principal nucleus” (also known as the “encapsulated” region of the BNST, or the medial part of the posteromedial subdivision of the BNST; Fig. 1).
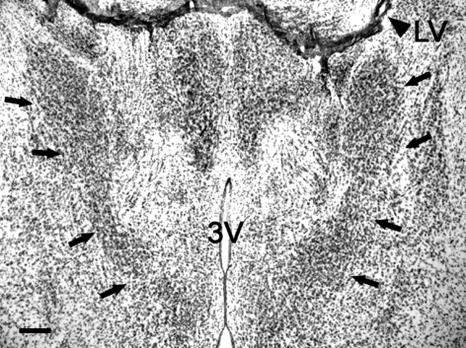
Fig. 1.
Photomicrograph of the BNSTp (arrows) in a male Bax+/+ mouse. LV, lateral ventricle; 3V, third ventricle. (Scale bar = 200 μm.)
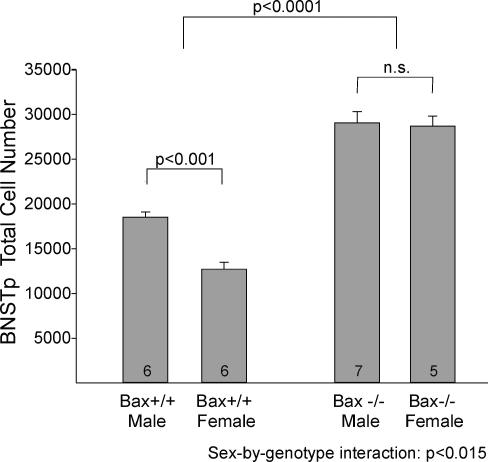
Deletion of the Bax gene eliminated a sex difference in BNSTp cell number (Fig. 2). WT males had ≈45% more cells in the BNSTp than did WT females, with no overlap between the sexes (P < 0.001). Bax deletion increased cell number in both sexes, but more in females than in males, which abolished the sex difference in cell number in Bax–/– animals (P > 0.8). Statistically, these findings were reflected in a main effect of sex (P < 0.01), a main effect of genotype (P < 0.0001), and a sex-by-genotype interaction (P < 0.015) in the two-way ANOVA.
Fig. 2.
Cell number in the BNSTp of WT (Bax+/+) and Bax knockout (Bax–/–) mice. Cell number in the BNSTp is greater in WT males than in females. Deletion of the Bax gene increases cell number overall and eliminates the sex difference. The number of animals per group is indicated at the base of each bar. n.s., Not significant.
The sex difference in BNSTp volume previously found in rats was also confirmed in C57BL/6 mice, with BNSTp volumes ≈75% greater in WT males than in WT females (P < 0.0005; Table 1). There was a main effect of genotype on BNSTp volume, with greater volumes in Bax knockout animals (P < 0.0005), and a sex-by-genotype interaction that approached, but did not reach significance (P = 0.06). Although Bax gene deletion reduced the sex difference in BNSTp volumes, volumes remained ≈25% larger in Bax–/– males compared with Bax–/– females (P < 0.01; Table 1).
Table 1. Nucleus volume, nucleus length, and cell size in the BNSTp and AVPV of WT and Bax knockout (Bax KO) mice.
| Cell group | Volume, × 106 μ3 | Length, μm | Soma size, μm2 |
|---|---|---|---|
| BNSTp | |||
| WT male (n = 6) | 46.8 ± 3.2* | 445 ± 56† | 136 ± 5* |
| WT female (n = 6) | 26.8 ± 1.9 | 413 ± 52 | 123 ± 2 |
| Bax KO male (n = 7) | 59.0 ± 1.0* | 467 ± 26 | 120 ± 3* |
| Bax KO female (n = 5) | 47.7 ± 2.3 | 462 ± 44 | 108 ± 2 |
| AVPV | |||
| WT male (n = 4) | 8.4 ± 0.3† | 233 ± 14* | 74 ± 4 n.s. |
| WT female (n = 6) | 11.3 ± 0.7 | 290 ± 10 | 77 ± 4 |
| Bax KO male (n = 6) | 12.0 ± 1.2 | 300 ± 15 n.s. | 58 ± 4 n.s. |
| Bax KO female (n = 5) | 11.4 ± 1.2 | 309 ± 22 | 66 ± 4 |
n.s., Not different from females of the same genotype.
Significantly different from females of the same genotype.
ANOVA not significant, post hocs not performed.
A sex difference in cell size may explain why Bax gene deletion did not eliminate the sex difference in BNSTp volume, despite equal numbers of BNSTp cells in Bax–/– males and females. Soma size of BNSTp neurons was larger in males than in females of both genotypes (P = 0.001 for main effect of sex, and P < 0.005 for planned comparisons between males and females within each genotype; Table 1). As has been reported for other neural cell groups in Bax knockout mice (12), Bax gene deletion led to a small (≈10%), but reliable reduction in mean cell size in the BNSTp (P < 0.0005), with no interaction between sex and genotype on this measure.
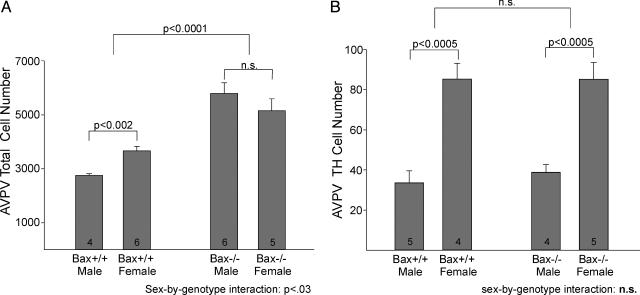
AVPV. Total cell counts have not previously been reported for AVPV of rats or mice, but cell density and volume estimates (both higher in females) have led to the assumption that females have more neurons in the AVPV than do males. Stereological cell counts confirmed this prediction: cell number was 33% higher in the AVPV of WT females than in WT males (P < 0.005). Although the sex difference is not large, it appears to be quite consistent as we found no overlap in cell counts between the sexes. In keeping with the hypothesis that Bax is required for the development of this dimorphism, a null mutation of the Bax gene eliminated the sex difference in AVPV neuron number (Fig. 3A). The elimination of Bax more than doubled cell number in males (P < 0.001; Figs. 3 and 4) while increasing cell number ≈40% in females (P < 0.03). As a result, there was no sex difference in AVPV cell number in Bax–/– animals (P > 0.30). In fact, in contrast to the pattern in WT animals, Bax–/– males had slightly (nonsignificantly) more neurons in AVPV than did Bax–/– females (Fig. 3A). This latter observation likely accounts for why we did not find a significant main effect of sex on AVPV cell number (P > 0.6); we found, however, a main effect of genotype (P < 0.0005) and a sex-by-genotype interaction (P < 0.05) in the two-way ANOVA.
Fig. 3.
Total cell number (A) and TH-ir cell number (B) in the AVPV of WT and Bax knockout mice. Counts of total cells and TH-ir cells are greater in WT females than in WT males. Bax deletion eliminates the sex difference in total cell number, but not in TH cell number. n.s., Not significant.
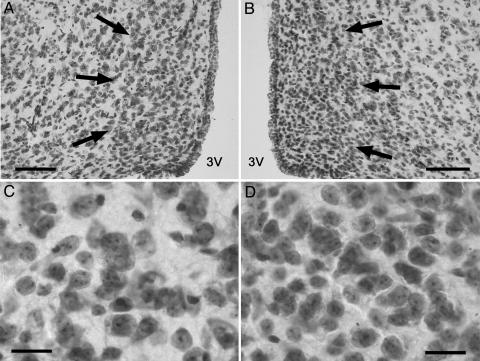
Fig. 4.
AVPV cell number is increased, and mean cell size is reduced by Bax gene deletion. (A and B) Low-power photomicrographs through AVPV of a Bax+/+ male (A) and Bax–/– male (B). (C and D) High-magnification views of the sections shown in A and B: Bax+/+ male (C) and Bax–/– male (D). (Scale bars = 100 μm for A and B; 20 μm for C and D.)
Bax gene deletion also eliminated a sex difference in the length of AVPV. Volumetric sex differences of AVPV have previously been attributed primarily to a sex difference (female > male) in the rostro-caudal length of the nucleus (5). In confirmation, AVPV was longer in Bax+/+ females than in Bax+/+ males in the present sample (P < 0.01). Bax deletion increased nucleus length (P = 0.015 for main effect of genotype), but the increase was significant only for males (P < 0.05 and P > 0.6 in post hoc tests for males and females, respectively), and there was no longer a sex difference in AVPV length among Bax–/– animals (P > 0.3). Although the pattern of results was similar for AVPV volumes, ANOVA indicated no significant main effects and no interaction of sex and genotype on this measure (Table 1).
As in the BNSTp, mean cell size in AVPV was smaller in Bax–/– animals than in WT controls (P < 0.005; Table 1). However, there was no main effect of sex and no sex-by-genotype interaction on AVPV cell size.
In striking contrast to our observations on nucleus length and overall cell number, Bax deletion did not affect the number of dopaminergic cells in AVPV. A small subset of AVPV neurons is dopaminergic, as identified by the presence of TH, the rate-limiting enzyme for catecholamine synthesis, and absence of dopamine-β-hydroxylase, the enzyme required for conversion of dopamine to norepinephrine (6). In confirmation of previous reports (25, 28), the number of TH-ir neurons in AVPV was much greater in female mice than in males (P < 0.0005; Fig. 3B). However, there was no effect of Bax gene deletion and no sex-by-genotype interaction on TH cell number (P > 0.7 in both cases).
Discussion
The present results demonstrate that sex differences in total cell number are absent in two forebrain regions in adult mice lacking Bax. The most likely explanation for our findings is that Bax deletion rescued neurons in the BNSTp and AVPV that would otherwise die during the perinatal cell death period. If so, this substantiates the role of cell death in sexual differentiation of the forebrain and identifies Bax as an essential protein in that process.
Sex differences in nuclear volumes were also reduced by Bax gene deletion. One possible limitation of these volumetric findings is that we examined adult, gonadally intact animals. Circulating levels of gonadal steroid hormones in adulthood affect neuronal size and/or the volume of cell groups in several neural regions (33–35) and could have affected volume measures here. However, we are not aware of any reports of circulating hormones influencing AVPV volume, and a sex difference in BNSTp volume of guinea pigs is not altered by adult gonadectomy (36). More importantly, cell counts, which form the basis for our inferences about cell death, are not affected by this consideration because the stereological counting techniques used here are not influenced by the orientation, packing density, or size of cells.
We previously reported that overexpression of the cell death antagonist, Bcl-2, blunts sex differences in cell density in AVPV and motoneuron number in the spinal nucleus of the bulbocavernosus (25). Effects of Bcl-2 overexpression were relatively modest, however. In addition, it is not possible in an overexpression study to address the physiological relevance of a given protein. Indeed, the forced overexpression of Bcl-2 saves many embryonic neurons from cell death, yet Bcl-2 gene deletion has only subtle effects on developmental neuronal death in vivo (37, 38). The gene deletion approach used here allows for the demonstration that Bcl-2 family proteins are indispensable for the formation of sex differences in overall cell number in the BNSTp and AVPV. Because all animals were gonadally intact in the current study, Bax status apparently overrides endogenous hormonal signals in determining cell number of sexually dimorphic forebrain nuclei. As a follow-up, we predict that perinatal testosterone administration would reduce cell number in the AVPV of WT females but not in their Bax knockout sisters, and that neonatal gonadectomy would be similarly ineffective in reducing cell number in the BNSTp of Bax–/– males.
Cell death is a widespread, if not ubiquitous, process during neural development (39). Our finding that a Bax gene knockout increases overall cell number in the AVPV and BNSTp of both sexes is consistent with observations that both males and females exhibit pyknotic cells in these nuclei during perinatal life (10, 11), and suggests that gonadal steroids create sex differences in neuron number by modulating a baseline rate of cell death. If Bax deletion prevents essentially all cell death in the BNSTp and AVPV, as it does in other Bax-dependent neural regions (17), then cell counts in Bax–/– mice should reflect the number of cells present before the onset of naturally occurring cell death. The inference from our data (Fig. 2) is that perinatal mice presumably start out with ≈30,000 neurons in the BNSTp; of these, ≈35% are lost in males and 55% are lost in females. In AVPV (Fig. 3) males may lose 52%, and females 29%, of the ≈5,400 neurons present in both sexes before cell death. Thus, our results support the hypothesis that opposite patterns of cell death are responsible for sex differences in the BNSTp and AVPV.
Although the total number of cells in AVPV was increased in Bax–/– mice, the subset of TH-ir neurons was not affected by the gene knockout. Similarly, Bcl-2 overexpression had no effect on TH cell number in AVPV in our previous study (25). Thus, different mechanisms control the sexual differentiation of overall cell number and dopaminergic cell number in AVPV. It is possible that the marked sex difference in TH cell number is not caused by cell death. For example, testosterone (after conversion to estradiol; ref. 28) may act on a pluripotent set of neurons in the perinatal AVPV to inhibit differentiation of the dopaminergic phenotype, leading to fewer TH-ir neurons in males. A similar explanation has been put forward to explain sex differences in vasopressinergic neuron number in the BNSTp (26).
Our results, however, do not exclude a role for cell death in the differentiation of dopaminergic neurons in AVPV. In fact, exogenous estrogens reduce the number of TH-ir cells in explants of neonatal rat AVPV, and this effect can be blocked by caspase inhibitors (E.M.W. and R.B.S., unpublished work). Thus, the sex difference in TH-ir cell number may be caused by a cell death program independent of Bax. In many cell types Bax is redundant with the proapoptotic Bcl-2 family member, Bak, and in such cases the genes for both proteins must be deleted to prevent cell death (40, 41). Full-length Bak is reported to be markedly reduced or absent in developing neurons (42), which might account for the singular importance of Bax in neuronal cell death. The pattern of results seen here could be explained if most AVPV neurons express only Bax, but presumptive dopaminergic neurons in AVPV express full-length Bak as well as Bax. Finally, there are multiple apoptotic pathways (43), and it is possible that TH-ir cell number is regulated by a cell death mechanism that is independent of all Bcl-2 family proteins. Whatever the case may be, our observations indicate that the control of cell number varies not only from region to region, but also among subtypes of neurons within a single region.
Because testosterone increases cell death in AVPV but decreases death in the BNSTp, the molecular control of cell survival in these two nuclei must differ. The present results indicate that the difference is upstream of the Bax checkpoint. One possibility is that different hormonal metabolites modulate cell death in the AVPV and BNSTp. Estradiol mimics effects of testosterone on cell death in AVPV (44), but the testosterone metabolites that regulate apoptosis in the BNSTp have not been examined. Steroid hormone receptors may also be expressed differentially in the two nuclei. The AVPV of adult mice expresses predominantly ERα, whereas both ERα and ERβ are found at high levels in the BNST (45). It is not known whether the same pattern of results holds for perinatal mice. However, because opposing actions have sometimes been ascribed to ERα and ERβ (46–48), the differential expression of steroid receptors could contribute to the profound difference in response to testosterone in the AVPV and BNSTp. Similarly, the complement of steroid receptor cofactors expressed in each nucleus could lead to differences in hormone-induced gene expression and cell survival in the AVPV and BNSTp (49).
Whether or not there are differences in the hormone metabolites, receptors, and/or cofactors mediating effects of testosterone on cell death in the AVPV and BNSTp, the present results suggest differential regulation of Bax activity in these two nuclei. The subcellular distribution and death-promoting activity of Bax is determined by interactions with other Bcl-2 family proteins (16, 50), and there is ample evidence that gonadal steroid hormones regulate the expression of Bax and other family members in peripheral tissues and cancer cell lines (e.g., refs. 20–22). Moreover, in different cell types a given hormone can have opposite effects on the expression of Bcl-2 family members and, consequently, opposing effects on survival (51, 52). Gonadal steroid hormones also regulate Bcl-2 family member expression in the nervous systems of adults (e.g., refs. 23 and 24), including in motoneurons of the sexually dimorphic spinal nucleus of the bulbocavernosus of adult rats (53). The results presented here establish Bax as a protein essential for sexual differentiation of the brain. A logical next step, therefore, is to examine the hormonal regulation of Bcl-2 family member expression in developing sexually dimorphic forebrain regions, with special emphasis on Bax and those proteins known to interact with Bax.
Our observations raise the question of whether sex differences in neuroendocrine function or behavior are affected by Bax gene deletion. For example, the ability of estrogens to activate gonadotrophin releasing-hormone neurons in the forebrain and trigger a luteinizing hormone surge is sexually differentiated in rodents, and the AVPV is critically implicated in this response (54, 55). Might the “feminization” of overall cell number in the AVPV allow Bax–/– males to mount a luteinizing hormone surge? With respect to behavior, no gross changes have been noted in Bax–/– animals by us or other investigators (29), and at this point, it is unlikely that more detailed behavioral observations would allow for meaningful conclusions about the functional consequences of sex differences in neuron number. Neurons rescued by Bax gene deletion in nonsexually dimorphic areas often are atrophic and may not be incorporated into functional circuits (12, 56). Consistent with these reports, we observed a reduction in mean cell size in both the AVPV and BNST of Bax–/– animals. Thus, we do not know whether the increased number of neurons in Bax–/– mice actually alters connectivity among sexually dimorphic brain regions. In addition, multiple brain areas are affected by the absence of Bax (both in sexually dimorphic and monomorphic areas) and, as shown here, the brains of Bax–/– mice are mosaic with respect to sexual differentiation. For example, Bax–/– females have a masculine cell number in the BNSTp and feminine cell number in AVPV. Moreover, within AVPV some cell types are affected by Bax deletion, whereas others (i.e., TH-ir cells) are not. For these reasons it is difficult to know what to predict regarding behavioral changes in Bax–/– mice or to ascribe any changes in behavior to particular brain regions or cell types. Our findings establish Bax–/– mice as a powerful model for testing the role of cell death in the development of neural sex differences and identifying the molecular bases of hormone-regulated cell death. Whether this model will prove equally useful in determining how sex differences in cell number contribute to sex differences in behavior or neuroendocrine responses will depend on our ability to characterize the cell types rescued within sexually dimorphic regions and establish whether rescued cells participate in functional neural circuits.
Acknowledgments
This work was supported by National Institutes of Health Grants HD33044 and MH68482 (to N.G.F.), MH47538 and MH01597 (to G.J.d.V.), and NS37952 (to R.B.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BNSTp, principal nucleus of the bed nucleus of the stria terminalis; AVPV anteroventral periventricular nucleus; TH, tyrosine hydroxylase; TH-ir, TH-immunoreactive; ER, estrogen receptor.
References
- 1.Dodson, R. E. & Gorski, R. A. (1992) J. Neurobiol. 24, 80–88. [DOI] [PubMed] [Google Scholar]
- 2.Guillamon, A., Segovia, S. & del Abril, A. (1988) Dev. Brain Res. 44, 281–290. [DOI] [PubMed] [Google Scholar]
- 3.Hines, M., Allen, L. S. & Gorski, R. A. (1992) Brain Res. 579, 321–326. [DOI] [PubMed] [Google Scholar]
- 4.Breedlove, S. M. & Arnold, A. P. (1983) J. Neurosci. 3, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleier, R., Byne, W. & Siggelkow, I. (1982) J. Comp. Neurol. 212, 118–130. [DOI] [PubMed] [Google Scholar]
- 6.Simerly, R. B., Swanson, L. W., Handa, R. J. & Gorski, R. A. (1985) Neuroendocrinology 40, 501–510. [DOI] [PubMed] [Google Scholar]
- 7.Sumida, H., Nishizuka, M., Kano, Y. & Arai, Y. (1993) Neurosci. Lett. 151, 41–44. [DOI] [PubMed] [Google Scholar]
- 8.Nordeen, E. J., Nordeen, K. W., Sengelaub, D. R. & Arnold, A. P. (1985) Science 229, 671–673. [DOI] [PubMed] [Google Scholar]
- 9.Davis, E. C., Popper, P. & Gorski, R. A. (1996) Brain Res. 734, 10–18. [PubMed] [Google Scholar]
- 10.Chung, W. C., Swaab, D. F. & de Vries, G. J. (2000) J. Neurobiol. 43, 234–243. [PubMed] [Google Scholar]
- 11.Arai, Y., Murakami, S. & Nishizuka, M. (1994) Horm. Behav. 28, 313–319. [DOI] [PubMed] [Google Scholar]
- 12.Merry, D. E. & Korsmeyer, S. J. (1997) Annu. Rev. Neurosci. 20, 245–267. [DOI] [PubMed] [Google Scholar]
- 13.Deckwerth, T. L., Elliot, J. L., Knudson, C. M., Johnson, E. M. J., Snider, W. D. & Korsmeyer, S. J. (1996) Neuron 17, 401–411. [DOI] [PubMed] [Google Scholar]
- 14.Gross, A., McDonnell, J. M. & Korsmeyer, S. J. (1999) Genes Dev. 13, 1899–1911. [DOI] [PubMed] [Google Scholar]
- 15.Desagher, S. & Martinou, J. C. (2000) Trends Cell Biol. 10, 369–377. [DOI] [PubMed] [Google Scholar]
- 16.Cheng, E. H.-Y. A., Wei, M. C., Weiler, S., Flavell, R. A., Mak, T. W., Lindsten, T. & Korsmeyer, S. J. (2001) Mol. Cell 8, 705–711. [DOI] [PubMed] [Google Scholar]
- 17.White, F. A., Keller-Peck, C. R., Knudson, C. M., Korsmeyer, S. J. & Snider, W. D. (1998) J. Neurosci. 18, 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan, H., Favero, M. & Vogel, M. W. (2001) J. Comp. Neurol. 436, 82–91. [PubMed] [Google Scholar]
- 19.Middleton, G. & Davies, A. M. (2001) Development (Cambridge, U.K.) 128, 4715–4728. [DOI] [PubMed] [Google Scholar]
- 20.Wang, T. T. & Phang, J. M. (1995) Cancer Res. 55, 2487–2489. [PubMed] [Google Scholar]
- 21.Kandouz, M., Siromachkova, M., Jacob, D., Chretien Marquet, B., Therwath, A. & Gompel, A. (1996) Int. J. Cancer 68, 120–125. [DOI] [PubMed] [Google Scholar]
- 22.Huang, Y., Ray, S., Reed, J. C., Ibrado, A. M., Tang, C., Nawabi, A. & Bhalla, K. (1997) Breast Cancer Res. Treat. 42, 73–81. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Segura, L. M., Cardona-Gomez, P., Naftolin, F. & Chowen, J. A. (1998) NeuroReport 9, 593–597. [DOI] [PubMed] [Google Scholar]
- 24.Dubal, D. B., Shughrue, P. J., Wilson, M. E., Merchenthaler, I. & Wise, P. M. (1998) J. Neurosci. 19, 6385–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zup, S. L., Carrier, H., Waters, E., Tabor, A., Bengston, L., Rosen, G., Simerly, R. & Forger, N. G. (2003) J. Neurosci. 23, 2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vries, G. J. & Simerly, R. B. (2002) in Hormones, Brain, and Behavior, eds. Pfaff, D. W., Arnold, A. P., Etgen, A. M., Fahrbach, S. E., Moss, R. L. & Rubin, R. T. (Academic, New York), Vol. 4, pp. 137–189. [Google Scholar]
- 27.Wersinger, S. R., Sannen, K., Villalba, C., Lubahn, D. B., Rissman, E. F. & de Vries, G. J. (1997) Horm. Behav. 32, 176–183. [DOI] [PubMed] [Google Scholar]
- 28.Simerly, R. B., Zee, M. C., Pendleton, J. W., Lubahn, D. B. & Korach, K. S. (1997) Proc. Natl. Acad. Sci. USA 94, 14077–14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudson, C. M., Tung, K. S., Tourtellotte, W. G., Brown, G. A. & Korsmeyer, S. J. (1995) Science 270, 96–99. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez, I., Ody, C., Araki, K., Garcia, I. & Vassalli, P. (1997) EMBO J. 16, 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West, M.J. (1999) Trends Neurosci. 22, 51–61. [DOI] [PubMed] [Google Scholar]
- 32.Dong, H. W. & Swanson, L. W. (2004) J. Comp. Neurol. 471, 396–433. [DOI] [PubMed] [Google Scholar]
- 33.Commins, D. & Yahr, P. (1984) J. Comp. Neurol. 224, 132–140. [DOI] [PubMed] [Google Scholar]
- 34.Bloch, G. J. & Gorski, R. A. (1988) J. Comp. Neurol. 275, 604–612. [DOI] [PubMed] [Google Scholar]
- 35.Cooke, B. M., Tabibnia, G. & Breedlove, S. M. (1999) Proc. Natl. Acad. Sci. USA 96, 7538–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hines, M., Davis, F. C., Conquelin, A., Goy, R. W. & Gorski, R. A. (1985) J. Neurosci. 5, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. (1993) Cell 75, 229–240. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama, K., Negishi, I., Kuida, K., Sawa, H. & Loh, D. Y. (1994) Proc. Natl. Acad. Sci. USA 91, 3700–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oppenheim, R. W. (1991) Annu. Rev. Neurosci. 14, 453–501. [DOI] [PubMed] [Google Scholar]
- 40.Lindsten, T., Ross, A. J., King, A., Zong, W.-X., Rathmell, J. C., Shiels, H. A., Ulrich, E., Waymire, K.G., Mahar, P., Frauwirth, K., et al. (2000) Mol. Cell 6, 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei, M. C., Zong, W.-X., Cheng, E. H.-Y., Lindsten, T., Panoutsakopoulou, V., Ross, A. J., Roth, K. A., MacGregor, G. R., Thompson, C. B. & Korsmeyer, S. J. (2001) Science 292, 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun, Y.-F., Yu, L.-Y., Saarma, M., Timmusk, T. & Arumae, U. (2001) J. Biol. Chem. 276, 16240–16247. [DOI] [PubMed] [Google Scholar]
- 43.Hengartner, M. O. (2000) Nature 407, 770–776. [DOI] [PubMed] [Google Scholar]
- 44.Arai, Y., Sekine, Y. & Murakami, S. (1996) Neurosci. Res. 25, 403–407. [DOI] [PubMed] [Google Scholar]
- 45.Mitra, S. W., Hoskin, E., Yudkovitz, J., Pear, L., Wilkinson, H. A., Hayashi, S., Pfaff, D. W., Ogawa, S., Rohrer, S. P., Schaeffer, J. M., et al. (2003) Endocrinology 114, 2055–2067. [DOI] [PubMed] [Google Scholar]
- 46.Nilsen, J., Mor, G. & Naftolin, F. (2000) J. Neurobiol. 43, 64–78. [DOI] [PubMed] [Google Scholar]
- 47.Matthews, J. & Gustafsson, J. A. (2003) Mol. Interv. 3, 281–292. [DOI] [PubMed] [Google Scholar]
- 48.Paruthiyil, S., Parmar, H., Kerekatte, V., Cunha, G. R., Firestone, G. L. & Leitman, D. C. (2004) Cancer Res. 64, 423–428. [DOI] [PubMed] [Google Scholar]
- 49.Shibata, H., Spencer, T. E., Onate, S. A., Jenster, G., Tsai, S. Y., Tsai, M. J. & O'Malley, B. W. (1997) Recent Prog. Horm. Res. 52, 141–164. [PubMed] [Google Scholar]
- 50.Eskes, R., Desagher, S., Antonsson, B. & Martinou, J.-C. (2000) Mol. Cell. Biol. 20, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buttyan, R., Shabsigh, A., Perlman, H. & Colombel, M. (1999) Trends Endocrinol. Metab. 10, 47–54. [DOI] [PubMed] [Google Scholar]
- 52.Toda, I., Sullivan, B. D., Wickham, L. A. & Sullivan, D. A. (1999) J. Steroid Biochem. Mol. Biol. 71, 49–61. [DOI] [PubMed] [Google Scholar]
- 53.Zup, S. L. & Forger, N. G. (2002) Brain Res. 950, 312–316. [DOI] [PubMed] [Google Scholar]
- 54.Wiegand, S. J. & Terasawa, E. (1982) Neuroendocrinology 34, 395–404. [DOI] [PubMed] [Google Scholar]
- 55.Petersen, S. L. & Barraclough, C. A. (1989) Brain Res. 484, 279–289. [DOI] [PubMed] [Google Scholar]
- 56.Sun, W., Gould, T. W., Vinsant, S., Prevette, D. & Oppenheim, R. W. (2003) J. Neurosci. 23, 7298–7310. [DOI] [PMC free article] [PubMed] [Google Scholar]