Abstract
Olfactory receptors (ORs) comprise more than half of the large class I G protein-coupled receptor (GPCR) superfamily. Although cloned over a decade ago, little is known about their properties because wild-type ORs do not efficiently reach the cell surface following heterologous expression. Receptor–receptor interactions strongly influence surface trafficking of other GPCRs, and we examined whether a similar mechanism might be involved in OR surface expression. Olfactory neurons are known to express β-adrenergic receptors (ARs), and we found that coexpression with β2-ARs, but not any other AR subtypes, dramatically increased mouse 71 (M71) OR surface expression in human embryonic kidney 293 cells. A persistent physical interaction between M71 ORs and β2-ARs was shown by coimmunoprecipitation and by cointernalization of the two receptors in response to their specific ligands. Also, coexpression of wild-type M71 ORs with β2-ARs resulted in cAMP responses to the M71 ligand acetophenone. Finally, in situ hybridization studies showed extensive colocalization of M71 OR and β2-AR expression in mouse olfactory epithelium. These data demonstrate the successful heterologous surface expression of a functional wild-type OR and reveal that persistent physical association with other GPCRs can control OR surface expression.
Perception of smell begins with stimulation of olfactory receptors (ORs) on neurons within the olfactory epithelium, leading to excitation and propagation of currents to the main olfactory bulb (1, 2). ORs are class I G protein-coupled receptors (GPCRs) that signal through stimulation of Gαolf, which leads to activation of type III adenylyl cyclase and opening of cAMP-gated cation channels (3). Since the completion of the human and mouse genome sequencing projects, ≈350 receptors in humans (4) and ≈1,000 receptors in mice (5) have been identified, presumably to aid in the selective recognition of >100,000 different odors. However, the mechanism by which the olfactory system selectively recognizes specific odors remains unclear. It was initially hypothesized that each olfactory neuron expresses a single OR and that the axons of olfactory neurons expressing the same OR then converge in the main olfactory bulb (6, 7). However, increasing evidence suggests that detection is substantially more complex than previously thought. For example, olfactory neurons are not restricted to expression of a single OR subtype (8). In addition to ORs, olfactory neurons can express many other receptors, which facilitate modulation of olfactory responses by hormones and neurotransmitters. For example, epinephrine stimulation of endogenous β-adrenergic receptors (ARs) modifies the signaling of coexpressed ORs within olfactory neurons (9). Furthermore, multiple OR subtypes can respond to the same ligand, a single OR can respond to multiple ligands (10–12), and structurally similar odorant ligands can act as either agonists or antagonists (13). Thus, as the complexity of the olfactory system becomes increasingly clear, the need to develop simple assays to allow mass screening of ligand–receptor interactions becomes increasingly important.
To date, the primary problem preventing the characterization of the OR family has been the inability to obtain significant surface expression of wild-type receptors in heterologous systems (7). Upon heterologous transfection, essentially all ORs remain trapped within the endoplasmic reticulum, where they are unable to respond to agonist. Receptor mutations, such as C-terminal transmembrane truncation, N-terminal addition of rhodopsin sequences, N-terminal addition of epitope tags, or construction of OR/β2-AR chimeras (10, 11, 14–18) have been required to obtain OR surface expression. Although these techniques have proven useful for specific applications, the inability to examine wild-type ORs limits their applicability.
Like ORs, other class I GPCRs, such as α1D-ARs (19–21), α2C-ARs (22), adenosine 2b (23), and bitter-taste receptors (24), are known to be largely intracellular when expressed heterologously. Previously, we showed that α1B-ARs promote surface expression of intracellular α1D-ARs through direct physical association after cotransfection in human embryonic kidney (HEK) 293 cells (25, 26). Mutation and truncation studies suggested that this did not involve signaling pathways or the soluble N- or C-terminal extensions, but only the hydrophobic core and/or associated loops. Because ORs consist almost exclusively of such a hydrophobic core and associated loops (27), we explored the possibility that receptor–receptor interactions might influence OR trafficking. Olfactory neurons are known to express ARs (9), so we specifically examined whether ORs might physically associate with ARs to facilitate surface expression. We used the mouse 71 (M71) OR because it is one of the few ORs with a known ligand (12). By using a variety of techniques, we found that coexpression with β2-ARs results in a profound translocation of functional M71 ORs to the cell surface in HEK293 cells. We also found evidence for persistent physical association of the two receptors on the cell surface by coimmunoprecipitation and cointernalization in response to receptor-specific ligands and colocalization of M71 OR and β2-AR mRNA in mouse olfactory epithelium.
Experimental Procedures
Constructs. The M71 OR in pcDNA3.1 was amplified by PCR using specific primers containing XbaI and KpnI restriction sequences for insertion into pEGFP-N3. Hemagglutinin (HA)-tagged β1- and β2-ARs in pcDNA3.1 were obtained from H. Kurose (Kyushu University, Hakozaki, Japan), HA-tagged β3-ARs from S. Collins (Duke University Medical Center), and HA-tagged α2-ARs from L. Limbird (Vanderbilt University, Nashville, TN). HA-tagged α1A-, α1B-, and α1D-ARs were created earlier (25, 28).
Cell Culture and Transfection. HEK293 cells were propagated in DMEM with sodium pyruvate containing 10% heat-inactivated FBS, 100 μg/ml streptomycin, and 100 units/ml penicillin at 37°C in a humidified atmosphere with 5% CO2. Confluent plates were subcultured at a ratio of 1:5 for transfection. HEK293 cells were transfected with 3 μg of DNA of each construct for 12 h by using Lipofectamine 2000, and cells were used for experimentation 48–72 h after transfection.
Luminometer-Based Surface Expression. HEK293 cells transiently transfected with FLAG-tagged M71 ORs with and without HA-tagged AR subtypes were split into poly d-lysine-coated 35-mm dishes and grown overnight at 37°C. Cells were rinsed three times with PBS, fixed with 2% paraformaldehyde in PBS for 30 min, and rinsed three times with PBS. Cells were then incubated in blocking buffer (2% nonfat milk in PBS, pH 7.4) for 30 min and were then incubated with horseradish peroxidase-conjugated M2-anti-FLAG antibody in blocking buffer for1hat room temperature. Cells were washed three times with blocking buffer, once with PBS, and then incubated with enhanced chemiluminescence reagent (Pierce) for 15 s. Luminescence was determined by using a TD20/20 luminometer (Turner Designs, Sunnyvale, CA). Mean values ± SEM were calculated as percent absorbance in arbitrary units and were statistically compared by using a one-way ANOVA and post hoc comparison using Dunnett's test, with P <0.01 being considered significant.
Confocal Microscopy. Cells transiently transfected with HA- or GFP-tagged constructs were grown on sterile coverslips, were fixed for 30 min with 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, and were rinsed three times with PBS containing 0.5% normal horse serum (PBS+). For anti-HA immunostaining, fixed coverslips were blocked for 1 h in blocking buffer (PBS containing 1% BSA, 5% normal horse serum) containing 0.01% Triton X-100 to permeabilize cells. Anti-HA antibody was added to coverslips overnight at 4°C at 1:500 dilution in blocking buffer, washed three times with PBS+ and incubated with Rhodamine red-conjugated anti-rabbit IgG secondary antibody for 1 h at room temperature at 1:500 dilution in blocking buffer. Coverslips were washed three times with PBS+ and mounted onto slides by using Vectashield mounting medium. Cells were scanned with a Zeiss LSM 510 laser scanning confocal microscope as described (26). For detecting GFP, fluorescein isothiocyanate fluorescence was excited by using an argon laser at a wavelength of 488 nm, and the absorbed wavelength was detected for 510–520 nm for GFP. For detecting Rhodamine red, rhodamine fluorescence was excited by using a helium–neon laser at a wavelength of 522 nm.
Immunoprecipitation/Immunoblotting. HEK293 cells expressing FLAG-M71-GFP ORs with and without HA-tagged ARs were harvested by scraping in ice-cold PBS and were washed by repeated centrifugation and homogenization. Cell lysates were solubilized, immunoprecipitated with anti-FLAG M2 affinity resin, and probed by using anti-FLAG M2 or anti-HA monoclonal antibodies as described (29).
cAMP Assays. The protocol used to measure cAMP formation in HEK293 cells is a modification of a widely used prelabeling protocol (30). HEK293 cells were split into 24-well plates 24 h before experimentation. Because HEK293 cells do not easily take up 3H-adenine, 3H-adenosine was used to prelabel cells. Cells were prelabeled with 1 ml of fresh media containing 1 μCi (1 Ci = 37 GBq) of 3H-adenosine for 2 h. Cells were then washed once with 1 ml of Krebs buffer (120 mM NaCl/5.5 mM KCl/2.5 mM CaCl2/1.2 mM NaH2PO4/1.2 mM MgCl2/20 mM NaHCO3/11 mM glucose/0.029 mM Na2EDTA), and 1 ml of Krebs buffer at 37°C, pH 7.4, containing 200 μM 3-isobutyl-1-methylxanthine was added. Stock concentrations of acetophenone (Fisher) were dissolved in Krebs buffer containing 10% ethanol, such that final ethanol concentrations in cells were 0.1%. Isoproterenol was dissolved in Krebs buffer. Cells were incubated with drugs for 10 min, and reactions were stopped by addition of 77% trichloroacetic acid. A 50-μl aliquot of 10 mM cAMP was added as a carrier, and tubes were sonicated for 5 s and centrifuged for 5 min at 20,000 × g. Then 50-μl aliquots were removed to determine total radioactivity incorporated. 3H-cAMP formed was isolated by sequential Dowex (30) and alumina chromatography. Eluants from alumina columns were collected, 5 ml of scintillation fluid was added, and 3H-cAMP was quantified by using a liquid scintillation counter. Data are expressed as fold stimulation compared with vehicle-treated control and statistically compared by using an unpaired two-tailed t test, with P < 0.05 considered significant.
In Situ Hybridization. In situ hybridization was performed as described (31, 32). The M71 and β2-AR clones were linearized and antisense riboprobes were generated with SP6 RNA polymerase. Young (p8) mice were killed with deep anesthesia, and noses were rapidly dissected at 4°C and were then fresh-frozen on dry ice. Cryostat sections (30 μm) were placed on SuperFrost Plus slides, postfixed proteinase digested, and blocked. Overnight hybridizations of sections with 35S-UTP labeled riboprobes were performed at 52°C. After a stringent wash protocol, slides were apposed to autoradiography film (Kodak Maximum Resolution) and were digitally scanned at 2,400 dpi by using adobe photoshop.
Results
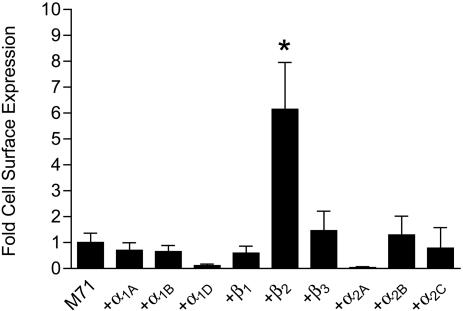
Coexpression with β2-ARs Results in Trafficking of M71 ORs to the Plasma Membrane. Earlier work (7) has demonstrated that essentially all ORs are sequestered at intracellular sites when heterologously expressed. To examine this issue for M71 ORs, we created M71 ORs containing N-terminal FLAG and C-terminal GFP epitopes to facilitate detection. By using a quantitative luminometer-based assay, we examined FLAG-M71-GFP OR cell-surface expression in unpermeabilized HEK293 cells. As shown in Fig. 1, minor M71 OR surface expression was detected when this construct was expressed alone. We then screened all nine AR subtypes (α1-, α2-, and β-ARs) for their ability to traffic M71 ORs to the surface. Remarkably, a 6- to 8-fold increase in M71 OR surface expression was observed on cotransfection with β2-ARs. However, none of the other eight AR subtypes increased M71 OR surface expression, suggesting that this interaction is highly specific. Interestingly, the specificity of this interaction was supported by the inability of β2-ARs to promote cell-surface expression of FLAG-tagged rat I7 or human 17–40 ORs (data not shown), suggesting that different ORs may require distinct partners to promote surface expression.
Fig. 1.
Specificity of M71 OR/β2-AR physical association. HEK293 cells were transiently cotransfected with FLAG-M71-GFP ORs and each of the nine AR subtypes. Cell-surface expression was determined by using a luminometer-based assay. The values are represented as fold surface expression over M71 OR alone. Only β2-ARs were found to significantly promote the surface expression of M71. Data are expressed as mean ± SEM of three to eight experiments (*, P < 0.01 compared with M71 alone).
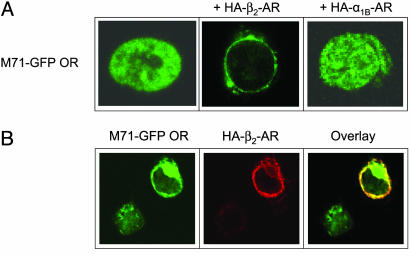
We also examined the intracellular localization of FLAG-M71-GFP ORs in HEK293 cells by using confocal microscopy. As shown for other ORs (7), M71 ORs were almost exclusively retained in intracellular compartments following heterologous expression (Fig. 2A Left). However, when coexpressed with β2-ARs containing N-terminal HA tags, M71 ORs were quantitatively translocated to the plasma membrane (Fig. 2A Center). In contrast, coexpression with HA-α1B-ARs (Fig. 2A Right) or HA-β1-ARs (data not shown) resulted in no change in M71 OR localization. By using rhodamine staining to identify HA-β2-AR localization, we found that HA-β2-ARs and M71 ORs exhibited almost complete colocalization (Fig. 2B) and that M71 OR surface expression did not occur in an adjacent cell that did not express β2-ARs. These data confirm and extend the above observations from the luminometer-based assay by using an independent technique.
Fig. 2.
Confocal imaging of M71 ORs in HEK293 cells reveals translocation to the plasma membrane upon coexpression with β2-ARs. (A) FITC fluorescence imaging of FLAG-M71-GFP OR alone (Left), + HA-β2-ARs (Center), or + HAα1B-ARs (Right). (B) Confocal imaging of HEK293 cells coexpressing FLAG-M71-GFP ORs and HA-β2-ARs by using FITC (488 nM) (Left) for GFP, rhodamine (522 nM) for anti-HA (Center), or overlay of both images (Right).
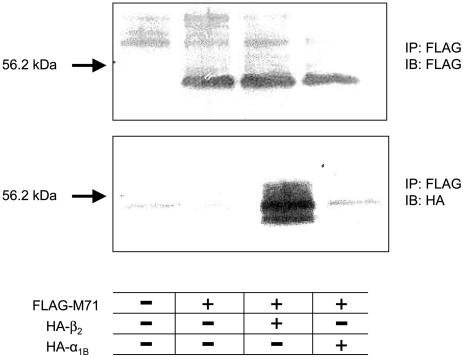
β2-ARs Physically Associate with M71 ORs to Promote Surface Localization. To determine whether translocation of M71 ORs to the cell surface was due to a direct physical interaction with β2-ARs in HEK293 cells, FLAG-M71-GFP ORs were coexpressed with either HA-β2-ARs or HA-α1B-ARs, solubilized, and immuno-precipitated with an anti-FLAG antibody. FLAG- and HA-tagged proteins were then detected by Western blotting. As shown in Fig. 3 Upper, FLAG-M71-GFP ORs were detected at ≈54 kDa in cells transfected with this construct. Membranes were stripped and reprobed by using anti-HA antibodies to detect AR subtypes (Fig. 3 Lower). Dense immunostaining was observed at ≈50 kDa in membranes cotransfected with FLAG-M71-GFP ORs and HA-β2-ARs, but not HA-α1B-ARs, demonstrating selective coimmunoprecipitation. Therefore, these data suggest that β2-ARs promote M71 OR cell-surface localization through a direct physical interaction.
Fig. 3.
Physical association between M71 ORs and β2-ARs. HEK293 cells were cotransfected with FLAG-M71-GFP OR alone or with HA-β2-ARs or HA-α1B-ARs. Cells were immunoprecipitated with anti-FLAG antibody and immunoblotted with anti-FLAG (Upper) or anti-HA (Lower) antibodies. A physical complex was found between M71 and β2-ARs but not between M71 and α1B-ARs.
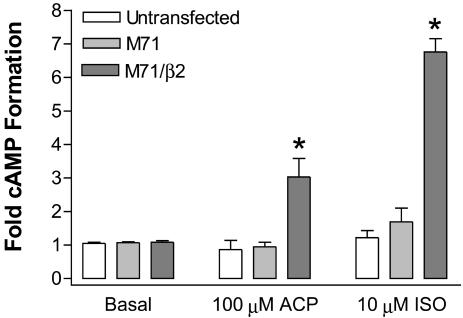
Wild-Type M71 ORs Are Functional upon Coexpression with β2-ARs. We next determined whether M71 ORs would initiate functional responses on trafficking to the cell surface by β2-ARs. Unlike previous studies that used chimeric or modified ORs to artificially induce surface expression, we used a wild-type M71 OR. cAMP accumulation was measured in HEK293 cells that were untransfected, transiently transfected with wild-type M71 ORs, or transiently cotransfected with HA-β2-ARs and wild-type M71 ORs. Fig. 4 shows that untransfected cells did not respond to either the M71 OR agonist acetophenone or the β-AR agonist isoproterenol. Cells expressing M71 ORs alone were also unresponsive to both agonists. However, cells expressing both M71 ORs and β2-ARs showed robust (3–7-fold) stimulation of cAMP formation by either acetophenone or isoproterenol (Fig. 4), demonstrating that surface localization of M71 ORs by coexpression with β2-ARs results in functional responses to M71 OR stimulation.
Fig. 4.
Coexpression with β2-ARs results in wild-type M71 OR coupling to cAMP responses. HEK293 cells were transiently transfected with M71 ORs alone or in combination with HA-β2-ARs. Cells were prelabeled with 3H-adenosine for 2 h and stimulated for 10 min with 10 μM isoproterenol (ISO) or 100 μM acetophenone (ACP). Data are mean ± SEM of six to eight experiments (*, P < 0.01 compared with basal).
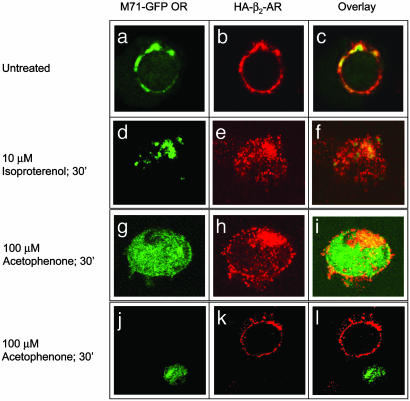
Selective Agonist Stimulation Results in Cointernalization of M71-GFP ORs and HA-β2-ARs. After agonist stimulation, β2-ARs are rapidly desensitized through phosphorylation by β-AR kinases and the subsequent binding of β-arrestins, leading to their internalization into clathrin-coated vesicles (33). To determine whether β2-ARs and M71 ORs remain physically associated throughout this process, we determined whether chronic exposure to selective agonists would cause cointernalization of the two receptors. As shown in Fig. 5, stimulation of HEK293 cells coexpressing FLAG-M71-GFP ORs and HA-β2-ARs with 10 μM isoproterenol for 30 min (Fig. 5 d–f) resulted in significant internalization of both β2-ARs and M71 ORs. Similarly, stimulation with 100 μM acetophenone for 30 min resulted in robust internalization of both receptors (g–i). However, acetophenone stimulation did not promote internalization of β2-ARs in cells that did not express M71 ORs (j–l). These studies suggest that β2-ARs and M71 ORs persistently associate on the cell surface, as well as during the endocytic process that follows agonist stimulation.
Fig. 5.
Cointernalization of M71 ORs and β2-ARs. HEK293 cells were cotransfected with FLAG-M71-GFP ORs and HA-β2-ARs and grown on sterile coverslips (a–c). Cells were stimulated with either 10 μM isoproterenol (d–f) or 100 μM acetophenone (g–l) for 30 min, fixed, immunostained, and visualized with confocal microscopy by using FITC (488 nm) to observe GFP fluorescence (Left) or rhodamine (522 nm) to observe anti-HA fluorescence (Center). (Right) An overlay of GFP and rhodamine fluorescence is shown. Stimulation with acetophenone induced robust internalization of not only M71 but also β2-AR when the two receptors were coexpressed (g–i). In contrast, acetophenone had no effect on β2-AR subcellular localization when M71 was not present, as shown in the top cell in j–l. Similarly, isoproterenol induced cointernalization of the two receptors when they were expressed together (d–f).
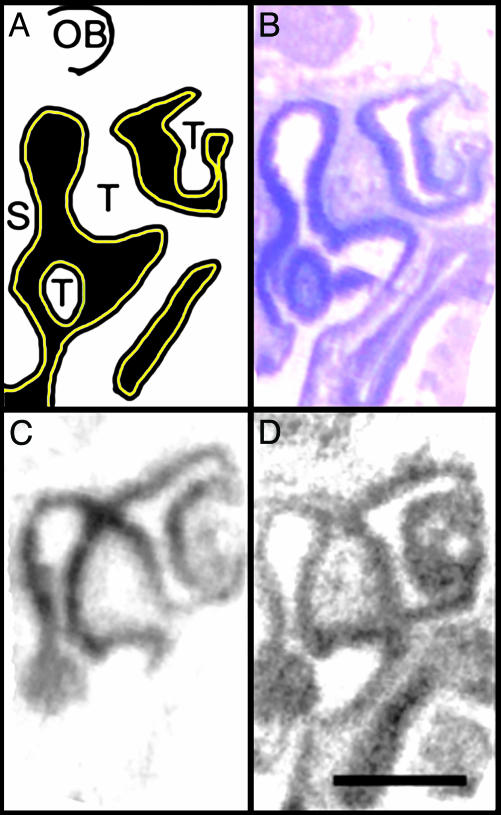
M71 ORs and β2-ARs Colocalize in Mouse Olfactory Epithelium. To determine whether M71 ORs and β2-ARs are coexpressed in olfactory sensory neurons, in situ hybridization was performed on freshly isolated mouse nasal cavity sections by using specific riboprobes (Fig. 6). M71 ORs and β2-AR mRNAs were selectively expressed with a high degree of colocalization in the dorso-medial receptor zone. Interestingly, β2-ARs were more widely expressed than M71 ORs, consistent with previous functional data demonstrating the widespread existence of β-ARs in olfactory neurons (9).
Fig. 6.
Coexpression of M71 ORs and β2-AR mRNAs in olfactory epithelium. (A) A schematic diagram of half of a mouse nasal cavity, demonstrating the septum (S) in the middle and the olfactory turbinates (T), which extend from the walls into the air space into which odorants flow (black). The entire cavity is covered with olfactory epithelium containing olfactory sensory neurons (yellow stripe). The anterior portion of the olfactory bulb (OB) lies above the cribiform plate superior to the nasal cavity. (B) Cresyl violet staining demonstrates mature olfactory epithelium as dense purple staining lining the cavity. (C) In situ hybridization demonstrates M71 ORs (dark signal) are selectively expressed in the dorso-medial receptor zone. (D) In situ hybridization demonstrates β2-ARs are also expressed in mature olfactory sensory neurons. Notably, β2-ARs are colocalized with M71 in addition to the other ventrolateral expression zones. (Bar, 1 mm.)
Discussion
The inability to obtain heterologous OR expression has directly hindered the characterization of this very large and important family of class I GPCRs for over a decade. In this study, we demonstrate that a mouse OR can be translocated to the cell surface in a functional manner through persistent physical association with the β2-AR. Thus, it seems that ORs can be added to the growing list of intracellular GPCRs that require specific GPCR partners for chaperoning to the cell surface. This phenomenon was first reported for class III GABAB receptors, which require assembly of two distinct seven-transmembrane proteins to form a single functional receptor (34). A second and more complex example is the T1R1 family of taste receptors (35). This family of class III GPCRs contains three subtypes (T1R1, T1R2, and T1R3) that require obligate assembly of two distinct subunits to form a single functional receptor. Interestingly, one complex (T1R1/T1R3) forms an amino acid umami receptor (36), whereas another (T1R2/T1R3) forms a sweet-taste receptor with a completely distinct pharmacology (35). Similar complexes occur with other members of the small class III family of GPCRs, including the metabotropic glutamate receptors (37). However, until recently, this phenomenon has been restricted to the class III GPCR subfamily. Increasing evidence now supports the concept of physical interactions between the much larger class I (rhodopsin) family of GPCRs (38). For example, we recently showed that α1B-ARs promote surface expression of normally intracellular α1D-ARs through direct physical association (25, 26). Interestingly, a recent report suggests that β2-ARs must first form multimeric complexes in the endoplasmic reticulum for surface expression (39). This result raises the possibility that many, if not all, GPCRs must form multiprotein complexes to facilitate surface expression. For some receptors, such as the β2-AR, homomeric associations may be sufficient to allow for trafficking to the plasma membrane, whereas for other receptors, such as ORs, interactions with other specific receptor types may be required for surface expression.
The observation that ORs can interact with β2-ARs may help explain previous findings that inactivation of β-AR kinase-2 and β-arrestin (40) or knockout of β-AR kinase-2 (41) results in increased OR stimulation of cAMP formation. Because β-AR kinase-2 and β-arrestin have not been found to directly interact with ORs, the ability of these proteins to regulate olfactory signaling may depend on physical interactions between ORs and ARs. An important role for OR–AR interactions in vivo is also supported by previous studies in which OR stimulation was found to be attenuated by β-AR antagonists (42, 43) and by stimulation of β-ARs in olfactory neurons (9). Although we have shown that β2-ARs and M71 ORs are coexpressed in olfactory epithelium, further studies are required to determine whether β-AR regulation of olfactory responses in vivo depends on the direct physical association between β2-ARs and ORs that we have described here.
In conclusion, our data demonstrate that M71 ORs are expressed and functional at the cell surface through persistent physical association with β2-ARs, thereby providing a molecular mechanism by which ORs may be functionally expressed in olfactory neurons. Because stimulation of M71 ORs and β2-ARs results in receptor cointernalization, our studies also shed light on the mechanisms underlying the desensitization of olfactory responses as well as the mechanisms underlying adrenergic regulation of olfaction. Finally, coexpression of ORs with other GPCRs may serve as a general mechanism for obtaining OR surface expression and responsiveness in heterologous cells, allowing for more detailed analysis of this enormous and poorly understood GPCR family.
Acknowledgments
We thank Drs. John Scott, Howard Rees, and Allan Levey for helpful advice and assistance. This work was supported by the National Institutes of Health and the W. M. Keck Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OR, olfactory receptor; AR, adrenergic receptor; M 71, mouse 71; HA, hemagglutinin; GPCR, G protein-coupled receptor; HEK, human embryonic kidney.
References
- 1.Barber, R. D. & Ronnett, G. V. (2000) Mol. Neurobiol. 21, 161–173. [DOI] [PubMed] [Google Scholar]
- 2.Buck, L. B. (2000) Cell 100, 611–618. [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts, P. (2004) Nat. Rev. Neurosci. 5, 263–278. [DOI] [PubMed] [Google Scholar]
- 4.Malnic, B., Godfrey, P. A. & Buck, L. B. (2004) Proc. Natl. Acad. Sci. USA 101, 2584–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godfrey, P. A., Malnic, B. & Buck, L. B. (2004) Proc. Natl. Acad. Sci. USA 101, 2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chess, A., Simon, I., Cedar, H. & Axel, R. (1994) Cell 78, 823–834. [DOI] [PubMed] [Google Scholar]
- 7.McClintock, T. S. & Sammeta, N. (2003) NeuroReport 14, 1547–1552. [DOI] [PubMed] [Google Scholar]
- 8.Li, J., Ishii, T., Feinstein, P. & Mombaerts, P. (2004) Nature 428, 393–399. [DOI] [PubMed] [Google Scholar]
- 9.Kawai, F., Kurahashi, T. & Kaneko, A. (1999) Nat. Neurosci. 2, 133–138. [DOI] [PubMed] [Google Scholar]
- 10.Krautwurst, D., Yau, K. W. & Reed, R. R. (1998) Cell 95, 917–926. [DOI] [PubMed] [Google Scholar]
- 11.Kajiya, K., Inaki, K., Tanaka, M., Haga, T., Kataoka, H. & Touhara, K. (2001) J. Neurosci. 21, 6018–6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozza, T., Feinstein, P., Zheng, C. & Mombaerts, P. (2002) J. Neurosci. 22, 3033–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oka, Y., Omura, M., Kataoka, H. & Touhara, K. (2004) EMBO J. 23, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gantz, I., Schaffer, M., DelValle, J., Logsdon, C., Campbell, V., Uhler, M. & Yamada, T. (1991) Proc. Natl. Acad. Sci. USA 88, 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimelbrant, A. A., Stoss, T. D., Landers, T. M. & McClintock, T. S. (1999) J. Neurochem. 72, 2301–2311. [DOI] [PubMed] [Google Scholar]
- 16.Wetzel, C. H., Oles, M., Wellerdieck, C., Kuczkowiak, M., Gisselmann, G. & Hatt, H. (1999) J. Neurosci. 19, 7426–7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivic, L., Zhang, C., Zhang, X., Yoon, S. O. & Firestein, S. (2002) J. Neurobiol. 50, 56–68. [DOI] [PubMed] [Google Scholar]
- 18.Levasseur, G., Persuy, M. A., Grebert, D., Remy, J. J., Salesse, R. & Pajot-Augy, E. (2003) Eur. J. Biochem. 270, 2905–2912. [DOI] [PubMed] [Google Scholar]
- 19.McCune, D. F., Edelmann, S. E., Olges, J. R., Post, G. R., Waldrop, B. A., Waugh, D. J., Perez, D. M. & Piascik, M. T. (2000) Mol. Pharmacol. 57, 659–666. [DOI] [PubMed] [Google Scholar]
- 20.Chalothorn, D., McCune, D. F., Edelmann, S. E., Garcia-Cazarin, M. L., Tsujimoto, G. & Piascik, M. T. (2002) Mol. Pharmacol. 61, 1008–1016. [DOI] [PubMed] [Google Scholar]
- 21.Hague, C., Chen, Z., Pupo, A. S., Schulte, N. A., Toews, M. L. & Minneman, K. P. (2004) J. Pharmacol. Exp. Ther. 309, 388–397. [DOI] [PubMed] [Google Scholar]
- 22.von Zastrow, M., Link, R., Daunt, D., Barsh, G. & Kobilka, B. (1993) J. Biol. Chem. 268, 763–766. [PubMed] [Google Scholar]
- 23.Sitaraman, S. V., Wang, L., Wong, M., Bruewer, M., Hobert, M., Yun, C. H., Merlin, D. & Madara, J. L. (2002) J. Biol. Chem. 277, 33188–33195. [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekar, J., Mueller, K. L., Hoon, M. A., Adler, E., Feng, L., Guo, W., Zuker, C. S. & Ryba, N. J. (2000) Cell 100, 703–711. [DOI] [PubMed] [Google Scholar]
- 25.Uberti, M. A., Hall, R. A. & Minneman, K. P. (2003) Mol. Pharmacol. 64, 1379–1390. [DOI] [PubMed] [Google Scholar]
- 26.Hague, C., Uberti, M. A., Chen, Z., Hall, R. A. & Minneman, K. P. (2004) J. Biol. Chem. 279, 15541–15549. [DOI] [PubMed] [Google Scholar]
- 27.Buck, L. & Axel, R. (1991) Cell 65, 175–187. [DOI] [PubMed] [Google Scholar]
- 28.Vicentic, A., Robeva, A., Rogge, G., Uberti, M. & Minneman, K. P. (2002) J. Pharmacol. Exp. Ther. 302, 58–65. [DOI] [PubMed] [Google Scholar]
- 29.Pupo, A. S., Uberti, M. A. & Minneman, K. P. (2003) Eur. J. Pharmacol. 462, 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero, S. W. & Minneman, K. P. (1999) J. Pharmacol. Exp. Ther. 290, 980–988. [PubMed] [Google Scholar]
- 31.Ressler, K. J., Sullivan, S. L. & Buck, L. B. (1993) Cell 73, 597–609. [DOI] [PubMed] [Google Scholar]
- 32.Ressler, K. J., Sullivan, S. L. & Buck, L. B. (1994) Cell 79, 1245–1255. [DOI] [PubMed] [Google Scholar]
- 33.Kohout, T. A. & Lefkowitz, R. J. (2003) Mol. Pharmacol. 63, 9–18. [DOI] [PubMed] [Google Scholar]
- 34.Marshall, F. H., Jones, K. A., Kaupmann, K. & Bettler, B. (1999) Trends Pharmacol. Sci. 20, 396–399. [DOI] [PubMed] [Google Scholar]
- 35.Nelson, G., Hoon, M. A., Chandrashekar, J., Zhang, Y., Ryba, N. J. & Zuker, C. S. (2001) Cell 106, 381–390. [DOI] [PubMed] [Google Scholar]
- 36.Nelson, G., Chandrashekar, J., Hoon, M. A., Feng, L., Zhao, G., Ryba, N. J. & Zuker, C. S. (2002) Nature 416, 199–202. [DOI] [PubMed] [Google Scholar]
- 37.Pin, J. P. & Acher, F. (2002) Curr. Drug Target CNS Neurol. Disord. 1, 297–317. [DOI] [PubMed] [Google Scholar]
- 38.Bouvier, M. (2001) Nat. Rev. Neurosci. 2, 274–286. [DOI] [PubMed] [Google Scholar]
- 39.Salahpour, A., Angers, S., Mercier, J. F., Lagace, M., Marullo, S. & Bouvier, M. (2004) J. Biol. Chem. 279, 33390–33397. [DOI] [PubMed] [Google Scholar]
- 40.Dawson, T. M., Arriza, J. L., Jaworsky, D. E., Borisy, F. F., Attramadal, H., Lefkowitz, R. J. & Ronnett, G. V. (1993) Science 259, 825–829. [DOI] [PubMed] [Google Scholar]
- 41.Peppel, K., Boekhoff, I., McDonald, P., Breer, H., Caron, M. G. & Lefkowitz, R. J. (1997) J. Biol. Chem. 272, 25425–25428. [DOI] [PubMed] [Google Scholar]
- 42.Firestein, S. & Shepherd, G. M. (1992) NeuroReport 3, 661–664. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd, G. M. & Firestein, S. (1991) J. Steroid Biochem. Mol. Biol. 39, 583–592. [DOI] [PubMed] [Google Scholar]