Aphysiological black box is to a biologist what an ornately decorated package is to a small child: a mysterious treasure that promises delightful toys within. With fitting élan, a small community of scientists has ripped open the packaging of the cyanobacterial circadian clock, compiled the parts list, examined the gears, and begun to piece together the mechanism. Over the past 2 years, the 3D molecular structures have been solved for the core components of the cyanobacterial circadian clock: KaiA, KaiB, and KaiC (1–6). In a surprisingly literal analogy to mechanical timepieces, the protein that seems to be at the heart of the clock mechanism, KaiC, forms a hexameric ring that even looks like a cog: the escape wheel, perhaps (5, 7, 8). Previous work has shown that KaiC has an autophosphorylation activity, and that the presence of KaiA and KaiB modulates the extent to which KaiC is phosphorylated (6, 9). In this issue of PNAS, Nishiwaki et al. (10) biochemically identify two amino acid residues on KaiC to which phosphoryl groups covalently attach, and show the necessity in vivo of a phosphorylation-competent residue at these positions. By searching the crystal structure for evidence of phosphorylated sites, Xu et al. (11) pinpoint a third residue that may “borrow” the phosphoryl group dynamically. Together, their work contributes richly to our understanding of what makes the gears mesh and turn to crank out a 24-h timing circuit.
The emerging model of the cyanobacterial circadian oscillator differs markedly from those that have been proposed for various eukaryotic systems (12, 13). In the latter schemes, many of the central components are either transcription factors that directly stimulate the genes encoding other clock components, or antagonists of the transcription factors that block their ability to carry out such stimulation. This basic yin–yang of mutual negative regulation is embellished by kinases that affect the stability of factors, heterodimer formation, movement of dimers from the cytoplasm to the nucleus, and exchange of partners inside the nucleus. Central to the models is the resulting undulation of transcription from clock component genes. However, the underlying timing mechanism in cyanobacteria does not depend on transcription of the kai genes by specific factors, because heterologous promoters can substitute admirably for expression of KaiC (14, 15). In addition, expression of the three known clock components oscillates in a shared phase, precluding their function as a teeter–totter-style interlocked transcription feedback loop, although overexpression of KaiA or KaiC stimulates or represses, respectively, kaiBC expression. The cyanobacterial clock, as described for the model organism Synechococcus elongatus, does have some features reminiscent of the posttranslational aspects of eukaryotic clocks: key components oscillate in abundance with one cycle per day, and the phosphorylation state of a crucial factor changes rhythmically as well.
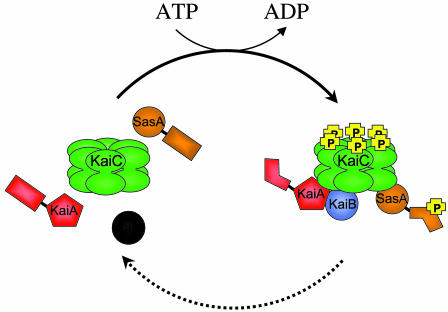
The cyanobacterial clock assembles and disassembles during the course of a day, defining the circadian period.
What, then, is the fundamental biochemical event that takes a day to complete in cyanobacteria? Unlike the tag-team relay of eukaryotic clock parts, the cyanobacterial clock components engage in a group hug (13). Analysis of the sizes of complexes in which KaiA, KaiB, KaiC, and a closely associated kinase, SasA, are found throughout the day shows that late at night they are all in a very high molecular-weight complex, presumably a shared complex (16). Because each of these components (at minimum) is a dimer, KaiC is known to be a hexamer, and other proteins may be present as well, the cyanobacterial clock can be thought of as an organelle unto itself: a “periodosome” that assembles and disassembles during the course of a day, defining the circadian period.
It is the assembly of the periodosome that is likely to be influenced by the phosphorylation state of KaiC. Nishiwaki et al. (10) identified serine 431 and threonine 432 as the residues that become phosphorylated when KaiC is incubated in vitro with ATP. Xu et al. (11) recognized the same residues based on density differences between phosphorylated and nonphosphorylated KaiC subunits and reasoned that threonine 426, which faces serine 431, is near enough to hydrogen bond with the phosphoryl group and perhaps serve as an alternate ligand. Mutation of any of these three residues to alanine, which is not a substrate for phosphorylation, abolished the circadian rhythms of gene expression in S. elongatus. However, the hexamerization of KaiC was not affected, even in a double or triple mutant that shows no phosphorylation of KaiC in vivo.
An important difference in the S431A:T432A double mutant relative to wild type is the complete inability to coimmunoprecipitate, with KaiC, the other proteins that are usually part of the periodosome (10) (Fig. 1). KaiA and KaiB are known to directly interact with KaiC, to be part of the high molecular-weight complex that forms during the night, and to affect the phosphorylation rate of KaiC (positively and negatively, respectively) (13). The new data indicate that specific phosphorylation of KaiC is necessary to allow the other Kai proteins, in turn, to exert their influence. The extent to which KaiC is phosphorylated varies during the circadian cycle, as may the ratios of particular phosphorylated states. It is easy to imagine that each phosphoryl modification tweaks the conformation, shifting the KaiC landscape to enhance or exclude access to a heterologous partner; the heterotypic interaction, reciprocally, changes the receptivity of KaiC ligands to phosphorylation. Some phosphorylation-dependent changes in the hexamer itself can be inferred from the crystal structure (11). A glimpse into the conformational changes that Kai heterotypic interactions impart is evident in the rotation of the C-terminal dimeric domain of KaiA when it binds a specific peptide of KaiC (4).
Fig. 1.
Phosphorylation of the KaiC hexamer is required for association with the other proteins of the periodosome. Late at night, when KaiC phosphorylation is maximal in the cyanobacterium, KaiA, KaiB, and KaiC, as well as the clock-associated kinase SasA, are recovered together in a high-molecular-weight complex: the periodosome. In wild-type cells, assembly and disassembly of the periodosome occur once per cycle, as does a wave of phosphorylation of KaiC. When key residues of KaiC have been mutated to preclude phosphorylation, the periodosome proteins do not associate, and the circadian rhythms of gene expression are abolished, even though KaiC still forms a hexamer. The figure depicts autophosphorylation of the KaiC hexamer (forward arrow, with ATP hydrolysis) as a prerequisite for association with the other proteins. Only one phosphoryl group is depicted per monomer, although two or more may be present on each subunit. Dissolution of the complex (dashed arrow) may accompany dephosphorylation, but the molecular details of these events have not yet been defined. Drawing by S. R. Canales.
Specific phosphorylation of KaiC is necessary to allow the other Kai proteins to exert their influence.
Clearly, the regulated phosphorylation of KaiC and the ability to form the periodosome comprise steps in the time delay that accumulates 24-h time in the cell. Less clear is what the complex does when it forms. Both groups show that overexpression of nonphosphorylating mutant KaiC variants causes suppression of expression from the kaiBC promoter, although the reports differ as to whether this is a transient effect. The mechanism of negative autoregulation of kaiC is not known, but it appears to be a global effect on gene expression rather than a specific feedback (14). Direct interaction of KaiC with the cyanobacterial chromosome has been proposed but not demonstrated.
Mutation to alter the residues that KaiC will autophosphorylate in vitro resulted in nonphosphorylated KaiC in vivo (10, 11). This result might suggest that there are no external kinases that act on KaiC. However, it is equally possible that autophosphorylation is a prerequisite to allow access to a KaiC kinase, just as it is needed for interaction with KaiA, KaiB, and SasA. Identification of other potential components of the periodosome, intracellular localization of the clock parts, and elucidation of other potential modifications all may yield gears that are required to smoothly tick away the time and ensure that daughter cells do not run fast or slow.
The cyanobacterial clock box, no longer black, is a chest filled with bioluminescence and attractive toys. Putting together the pieces to design a clock is a tedious task, but S. elongatus is a gracious host, and the guests at the party are hard at work.
References
- 1.Garces, R. G., Wu, N., Gillon, W. & Pai, E. F. (2004) EMBO J. 23, 1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattanayek, R., Wang, J., Mori, T., Xu, Y., Johnson, C. H. & Egli, M. (2004) Mol. Cell 15, 375–388. [DOI] [PubMed] [Google Scholar]
- 3.Uzumaki, T., Fujita, M., Nakatsu, T., Hayashi, F., Shibata, H., Itoh, N., Kato, H. & Ishiura, M. (2004) Nat. Struct. Mol. Biol. 11, 584–585. [DOI] [PubMed] [Google Scholar]
- 4.Vakonakis, I. & LiWang, A. C. (2004) Proc. Natl. Acad. Sci. USA 101, 10925–10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vakonakis, I., Sun, J., Wu, T., Holzenburg, A., Golden, S. S. & LiWang, A. C. (2004) Proc. Natl. Acad. Sci. USA 101, 1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams, S. B., Vakonakis, I., Golden, S. S. & LiWang, A. C. (2002) Proc. Natl. Acad. Sci. USA 99, 15357–15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori, T., Saveliev, S. V., Xu, Y., Stafford, W. F., Cox, M. M., Inman, R. B. & Johnson, C. H. (2002) Proc. Natl. Acad. Sci. USA 99, 17203–17208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi, F., Suzuki, H., Iwase, R., Uzumaki, T., Miyake, A., Shen, J. R., Imada, K., Furukawa, Y., Yonekura, K., Namba, K., et al. (2003) Genes Cells 8, 287–296. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki, H., Nishiwaki, T., Kitayama, Y., Nakajima, M. & Kondo, T. (2002) Proc. Natl. Acad. Sci. USA 99, 15788–15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiwaki, T., Satomi, Y., Nakajima, M., Lee, C., Kiyohara, R., Kageyama, H., Kitayama, Y., Temamoto, M., Yamaguchi, A., Hijikata, A., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 13927–13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu, Y., Mori, T., Pattanayek, R., Pattanayek, S., Egli, M. & Johnson, C. H. (2004) Proc. Natl. Acad. Sci. USA 101, 13933–13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panda, S., Hogenesch, J. B. & Kay, S. A. (2002) Nature 417, 329–335. [DOI] [PubMed] [Google Scholar]
- 13.Golden, S. S. & Canales, S. R. (2003) Nat. Rev. Microbiol. 1, 191–199. [DOI] [PubMed] [Google Scholar]
- 14.Nakahira, Y., Katayama, M., Miyashita, H., Kutsuna, S., Iwasaki, H., Oyama, T. & Kondo, T. (2004) Proc. Natl. Acad. Sci. USA 101, 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu, Y., Mori, T. & Johnson, C. H. (2003) EMBO J. 22, 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kageyama, H., Kondo, T. & Iwasaki, H. (2003) J. Biol. Chem. 278, 2388–2395. [DOI] [PubMed] [Google Scholar]