Abstract
Vitamin K-dependent (VKD) proteins require carboxylation for diverse functions that include hemostasis, apoptosis, and Ca2+ homeostasis, yet the mechanism of carboxylation is not well understood. Combined biochemical and chemical studies have led to a long-standing model in which a carboxylase Cys catalytic base deprotonates vitamin K hydroquinone (KH2), leading to KH2 oxygenation and Glu carboxylation. We previously identified human carboxylase Cys-99 and Cys-450 as catalytic base candidates: Both were modified by N-ethylmaleimide (NEM) and Ser-substituted mutants retained partial activity, suggesting that the catalytic base is activated for increased basicity. Mutants with Cys-99 or Cys-450 substituted by Ala, which cannot ionize to function as a catalytic base, were therefore analyzed. Both single and double mutants had activity, indicating that Cys-99 and Cys-450 do not deprotonate KH2. [14C]NEM modification of C99A/C450A revealed one additional reactive group; however, Ser-substituted mutants of each of the eight remaining Cys retained substantial activity. To unequivocally test, then, whether any Cys or Cys combination acts as the catalytic base, a mutant with all 10 Cys substituted by Ala was generated. This mutant showed 7% wild-type activity that depended on factor IX coexpression, indicating a VKD protein effect on carboxylase maturation. NEM and diethyl pyrocarbonate inhibition suggested that the catalytic base is an activated His. These results change the paradigm for VKD protein carboxylation. The identity of the catalytic base is critical to understanding carboxylase mechanism and this work will therefore impact both reinterpretation of previous studies and future ones that define how this important enzyme functions.
The vitamin K-dependent (VKD) carboxylase uses the energy of vitamin K hydroquinone (KH2) oxygenation to catalyze the conversion of Glu residues in VKD proteins to γ-carboxylated Glu (Gla) (1). All VKD proteins are targeted to the carboxylase by a high-affinity carboxylase-binding site that is usually an N-terminal propeptide. This carboxylase recognition sequence is adjacent to the VKD Gla domains that contain clusters of Glu residues, and propeptide-mediated binding allows the processive modification of VKD proteins so that the multiple Glu residues are all converted to Gla residues (2, 3). Full carboxylation results in Ca2+-binding by the Gla domains and renders the VKD proteins active in several different functions that include hemostasis, apoptosis, phagocytosis, signal transduction, and Ca2+ homeostasis. During carboxylation, the KH2 cofactor is converted to a vitamin K epoxide product, and the carboxylase is also an epoxidase. Recycling of vitamin K epoxide back to the hydroquinone form is necessary for continual carboxylation in vivo, and this cycle is inhibited by coumarin-based oral anticoagulants like warfarin. Depletion of the KH2 supply leads to undercarboxylation of VKD proteins and consequent impaired function; for example, undercarboxylated hemostatic proteins cannot properly bind to the site of vascular injury to support coagulation. Therefore, understanding how VKD proteins are modified by the carboxylase to become active has important implications in a broad range of physiologies.
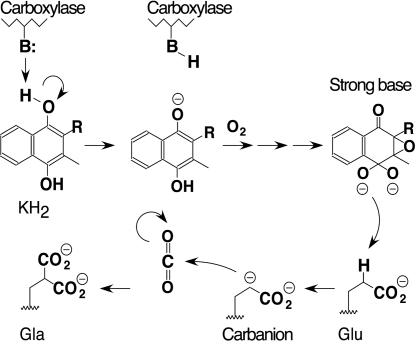
Defining the mechanism for VKD protein carboxylation has proven to be difficult. Both the epoxidation of KH2 and γ-carboxylation of Glu are unique reactions in biochemistry, leaving no precedents for the enzymology of the carboxylase. In addition, the carboxylase is an integral membrane protein, and structural determinations, such as x-ray crystallography or NMR, have not been reported for this enzyme. An explanation for the relationship between the epoxidation and carboxylation reactions was proposed by Dowd et al. (4, 5) on the basis of chemical modeling studies. Those studies revealed that reaction of KH2 with O2 leads to the formation of a strong vitamin K base and that protonation of this base causes collapse to the vitamin K epoxide product. The generation of a strong vitamin K base was of interest with regard to biochemical experiments that had previously suggested that the Glu substrate is converted to an intermediate carbanion that subsequently becomes carboxylated by CO2 to form the Gla product. For example, tritium exchange into Glu substrates was detected in the presence of T2O (6) and fluoride elimination from (2R,3S)-3-fluoroglutamate was observed (7). The conversion of Glu to a carbanion would require a strong base with a pKa of ≈25–28, and so the formation of a strong vitamin K base from KH2 and O2 observed by Dowd et al. (5) could explain the role of KH2 in the enzyme reaction. To form the strong vitamin K base, however, KH2 would first require deprotonation for reaction with O2. This requirement led Dowd et al. (5) to propose that one critical role of the carboxylase is to provide a weak base for KH2 deprotonation. This model, in which a weak carboxylase active site base is used to generate a strong vitamin K base from KH2, was termed the “base amplification mechanism” (Fig. 1).
Fig. 1.
The base amplification model for carboxylation. The model proposes that a weak base (B:) in the carboxylase active site deprotonates KH2, resulting in KH2 oxygenation and formation of a strong base intermediate. The strong base deprotonates the γ-carbon of a substrate Glu side chain to generate a carbanion intermediate, which subsequently reacts with CO2 to form the Gla product. Protonation of the strong vitamin K base results in formation of a vitamin K epoxide product, which is not shown.
Biochemical modification studies from several laboratories led to the conclusion that Cys is the catalytic base that deprotonates KH2 (8–13). Thus, the activity of carboxylase was abolished by thiol-specific inhibitors, such as N-ethylmaleimide (NEM), which reacts ≈103-fold faster with Cys than other amino acids at neutral pH (14), or mercurials like p-hydroxymercuribenzoate, which are also highly specific for sulfhydryls (15, 16). Consequently, models for carboxylation have incorporated a role for one or possibly two Cys residues in the mechanism (1, 13, 17–22) and in the base amplification model Dowd et al. (5) proposed that Cys is the catalytic base that deprotonates KH2 to initiate carboxylation.
To identify the active site Cys residues, we performed biochemical mapping on [14C]NEM-modified carboxylase. Carboxylase that was fully active and therefore in native conformation was obtained by isolating a factor IX–carboxylase complex, separating the carboxylase from factor IX, and then immediately performing NEM modification (23). Amino acid analysis of [14C]NEM-carboxylase revealed 1.8–2.3 reactive amino acids per carboxylase molecule and nearly all (93%) of the incorporated [14C]NEM was found in peptides containing Cys-99 or Cys-450. Mutants with each of these Cys residues substituted by Ser showed a large (≈100-fold) decrease in epoxidation and carboxylation, but both mutants clearly retained some activity (23).
The retention of activity suggested that the carboxylase contains an activation motif to initiate the reaction. To act as the catalytic base in KH2 deprotonation, Cys would require ionization, as would Ser performing the same role. However, the difference in pKa values for Cys and Ser [≈8 or 13, respectively (14)] would predict that a Cys to Ser substitution in the catalytic base would result in a much larger decrease in activity than we observed for a mechanism using general base catalysis. Our data therefore suggested that the carboxylase activates the catalytic base, for example, as in the catalytic triads of Cys or Ser proteases, which contain a nucleophilic Cys or Ser that becomes activated by association with His and Asp or Glu. Mutational analyses of a large number of proteases that use a Cys or Ser catalytic base show that interconversion of Cys and Ser can result in partial activity retention (24–28), and thus a catalytic triad mechanism in the carboxylase could explain our previous mutagenesis results. Such a mechanism was also attractive in explaining why KH2 epoxidation is barely detectable in the absence of the Glu substrate (29): If the VKD substrate itself contributes the Glu member of the catalytic triad, then, in the absence of substrate, the Cys catalytic base would not be activated for KH2 deprotonation, thereby regulating carboxylation to avoid unfettered production of an undesirable highly reactive vitamin K intermediate. To test the hypothesis that the carboxylase uses a catalytic triad mechanism, a series of mutational studies was performed. As described here, these experiments led to the surprising discovery that the catalytic base that deprotonates KH2 is not, in fact, a Cys. Instead, our data suggest that the carboxylase uses an activation motif that contains a His acting as the catalytic base.
Materials and Methods
Supporting Information. Fig. 4, Tables 5 and 6, and Supporting Materials and Methods are published as supporting information on the PNAS web site. The following are described in Supporting Materials and Methods: (i) construction of baculovirus-containing carboxylase mutants and their expression in infected insect cells, (ii) preparation of solubilized microsomes, (iii) activity and Western analysis of the carboxylase, and (iv) inhibition studies using NEM and diethyl pyrocarbonate (DEPC).
[14C]NEM Incorporation into Wild-Type and Mutant C99A/C450A Carboxylase. Microsomes (500 μl, 2 mg of total protein) from insect cells infected with baculoviruses containing either wild-type or C99A/C450A carboxylases were solubilized (as described in Supporting Materials and Methods) in the presence of propeptide (5 μM), and the supernatants were absorbed on affinity-purified rabbit anti-C-terminal carboxylase peptide antibody coupled to Sepharose (100 μl of resin, 200 μg of antibody against amino acids 744–758 of the human carboxylase). Solubilized microsomes from uninfected insect cells were also processed in parallel. The samples were rocked overnight, then transferred to columns and rinsed with 50 column volumes of HNCP [22 mM Hepes/0.22% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/0.22% phosphatidylcholine/500 mM NaCl/5 μM factor X propeptide, pH 7.4] at 4°C. Carboxylase was then eluted at room temperature for 1 h with HNCP containing C-terminal carboxylase peptide (final concentration, 200 μM). Immediately after purification, carboxylase was adjusted to pH 6.9 with 1 M N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid, pH 6.7, and then incubated at room temperature for 30 min with NEM or 34.2 mCi/mmol (1 Ci = 37 GBq) [14C]NEM (NEN), both at a final concentration of 1.8 mM. Unreacted NEM or [14C]NEM was then quenched by the addition of DTT to 10 mM. The nonradioactive NEM samples were assayed for activity (as described in Supporting Materials and Methods) along with untreated carboxylase to measure the extent of inactivation. Aliquots of the [14C]NEM-treated samples were subjected to SDS/PAGE, and the gels were then rinsed several times in Western transfer buffer (186 mM Gly/25 mM Tris base/20% methanol) to remove free [14C]NEM. The proteins were then transferred to nitrocellulose, followed by PhosphorImager analysis to determine the extent of [14C]NEM incorporation and by quantitative Western analysis (described below) to measure the amount of carboxylase protein present.
Quantitative Western Analysis. Solubilized microsomes or purified carboxylase were processed in parallel with a carboxylase standard curve, which showed a linear response over the range of analysis (50–200 ng). Purified carboxylase (100–200 ng) was first treated with 4,000 units of endoglycosidase Hf, by using the recommended conditions (New England Biolabs), to separate carboxylase from the small amount of antibody that leached from the resin during affinity purification. After gel electrophoresis and transfer to nitrocellulose, the blots were probed with affinity-purified anti-C-terminal carboxylase peptide antibody (0.04 μg/ml) and then doubly purified anti-rabbit alkaline phosphatase conjugate (Bio-Rad). Fluorescent signal was produced by using AttoPhos substrate according to the manufacturer's instructions (Promega), followed by scanning on a StormImager. Carboxylase levels in each set of samples were determined by comparison with the accompanying standards.
Results
C99S and C450S Mutants Show Decreased Activity That Is Propeptide-Dependent. Cys-99 and Cys-450 were previously shown to react with the carboxylase inhibitor NEM, and C99S and C450S had specific activities 2% or 19% that of wild-type enzyme, respectively (Table 1). These mutants, as well as all others described in these studies, were expressed in insect cells that did not contain endogenous carboxylase. Assay optimization revealed that the presence of propeptide during solubilization increased the specific activities of C99S and C450S. The carboxylase normally exists in vivo as a complex with VKD proteins, and the VKD propeptide mediates binding and therefore had the potential to stabilize carboxylase activity. When C99S and C450S were solubilized in the presence of propeptide, 7-fold and 2-fold respective increases in specific activity were observed (Table 1). Wild-type carboxylase activity was unaffected by the presence of propeptide during solubilization. The analyses show, then, that C99S and C450S retain substantial carboxylase activity.
Table 1. C99S and C450S retain carboxylase activity that is propeptide-dependent.
| Specific activity, %
|
||
|---|---|---|
| Carboxylase variant | Propeptide absent | Propeptide present |
| Wild type | 100 | 100 |
| C99S | 2 | 14 |
| C450S | 19 | 40 |
| Mock | 0 | 0 |
Microsomes from viral- or mock-infected cells were solubilized in the absence or presence of propeptide (5 μM), and all of the preparations were then assayed for carboxylase activity in the presence of propeptide. Quadruplicate samples (duplicate aliquots of 10 or 20 μl) were assayed for 30 min. Background levels were determined by assaying a sample containing water instead of microsomes, which gave a value identical to that of the mock sample. The ratio of signal to background ranged between 150 (for wild-type enzyme) and 3 (for C99S). Microsomal aliquots were also subjected to quantitative Western blot analysis to determine the amount of carboxylase protein present and consequent specific activity. The variation in specific activities was <10%, and the entire experiment was performed twice. The values for cells solubilized in the absence of propeptide are higher than our previously determined values (23) because of the optimization of assay and isolation conditions.
Mutations in Cys-99 and Cys-450 That Block Potential Ionization Do Not Eliminate Carboxylase Activity. C99S or C450S activity retention could be due to an activation mechanism that normally ionizes the catalytic base and, to a lesser extent, the Ser-substituted Cys (see Introduction). To determine whether either Cys-99 or Cys-450 acted as the catalytic base, then, these residues were mutated to Ala, which cannot ionize and would therefore not be able to function as the catalytic base. The Ala-substituted mutants gave similar results to the corresponding Ser mutants: C99A retained 31% activity versus 14% for C99S, and C450A had 34% activity versus 40% for C450S (Table 1, propeptide present, and Table 2, single mutants). These values were obtained with microsomes solubilized in the presence of propeptide, and C99A and C450A showed a similar response to propeptide as that observed with the cognate Ser mutants (Table 1 and data not shown). C99A and C450A (Table 2, single mutants) and C99S and C450S (data not shown) all had ratios of epoxidation to carboxylation similar to that of wild-type carboxylase (i.e., 1:1), indicating that coupling of KH2 oxygenation to the conversion of Glu to Gla was unaffected in these mutants.
Table 2. Carboxylase mutants with Ala substituted for Cys99 and/or Cys450 retain significant activity.
| Carboxylase variant | Carboxylase specific activity, pmol/ng·h-1 (%) | Epoxidase specific activity, pmol/ng·h-1 (%) |
|---|---|---|
| Single mutants | ||
| Wild type | 26.5 (100) | 27.8 (100) |
| C99A | 8.3 (31) | 9.4 (34) |
| C450A | 8.9 (34) | 11.1 (40) |
| Mock | 0 (0) | 0 (0) |
| Double mutants | ||
| Wild type | 18.0 (100) | 18.7 (100) |
| C99S/C450S | 3.2 (18) | 2.9 (16) |
| C99A/C450A | 8.2 (46) | 7.4 (40) |
| Mock | 0 (0) | 0 (0) |
Microsomes from baculoviral- or mock-infected insect cells were solubilized in the presence of propeptide, and carboxylase specific activities were determined by carboxylase assay (by using a specific activity of 40 cpm/pmol for 14CO2) and quantitative Western blots as described in the legend to Table 1. Aliquots from the same preparations were also assayed on the same day for epoxidase activity. The sets of solubilized microsomes in the single and double mutant groups were analyzed on separate days, which accounts for the difference in the wild-type specific activities.
Cys-99 and Cys-450 reacted with NEM (23), yet C99A and C450A retained activity (Table 2, single mutants). One possibility that could reconcile these observations is if both Cys residues act together to deprotonate KH2. Mutation of an individual Cys to Ala would then not abolish activity, because the remaining Cys would still be able to deprotonate KH2 to some extent. To test for this possibility, double mutants with both Cys residues substituted by Ser or Ala were generated, and the specific activities were determined. In each case, the specific activity of the double mutant was not reduced any further from that of the corresponding Cys-99 mutant. Thus, the carboxylase specific activities of C99S/C450S and C99S were 18% or 14%, respectively, of wild-type activity, and the specific activity of C99A/C450A was 46% of wild-type carboxylase versus 31% for C99A (Table 1, propeptide present, and Table 2). As with the singly substituted mutants, the epoxidation to carboxylation ratio was unaffected in the double mutants (Table 2, double mutants). The observation that the double mutants did not exhibit any further loss of activity thus rules out a model in which Cys-99 and Cys-450 act together to deprotonate KH2.
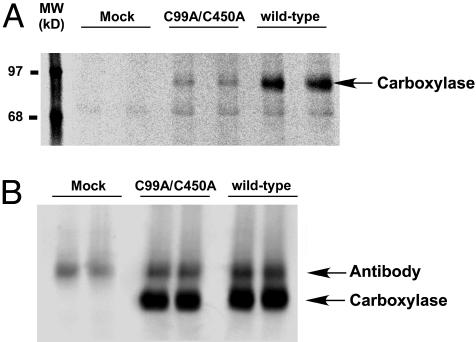
NEM Reactivity with C99A/C450A Reveals an Additional Site of Modification Besides Cys-99 and Cys-450. Activity retention by C99S/C450S and C99A/C450A raised the question of whether NEM abolished activity through the modification of another residue that serves as the catalytic base. The C99A/C450A mutant was an excellent tool to determine whether an additional NEM-modified residue existed, because the two known reactive amino acids were replaced by Ala, which cannot react with NEM. Incorporation of [14C]NEM into the C99A/C450A mutant was therefore tested in parallel with wild-type enzyme. The experiments were performed using conditions selective for thiol-reactivity, i.e., neutral pH and low NEM concentrations. Quantitation of [14C]NEM incorporation by PhosphorImager analysis and of carboxylase protein by quantitative Western analysis (Fig. 2) showed a decrease in the ratio of mole [14C]NEM incorporated per mole carboxylase from 3.5 (for wild-type carboxylase) to 1 (for C99A/C450A). This decrease was consistent with the loss of two NEM-reactive groups (Cys-99 and Cys-450) but also showed the presence of an additional carboxylase residue that was modified by [14C]NEM.
Fig. 2.
C99A/C450A carboxylase incorporates reduced but residual amounts of [14C]NEM. Wild-type and C99A/C450A carboxylases were purified in the presence of propeptide (to stabilize activity) and in the absence of reducing agents, and the proteins were incubated with [14C]NEM immediately after purification (described in Materials and Methods). Aliquots were then analyzed by SDS/PAGE and PhosphorImager to determine the amount of [14C]NEM incorporation (A) or by quantitative Western blots (B). The samples subjected to quantitative Western blot analysis were treated with endoglycosidase H before SDS/PAGE to separate carboxylase from antibody that leached from the resin during affinity purification.
The effect of NEM on carboxylase activity was also determined by using aliquots from the same purified enzyme preparations (see Materials and Methods). This experiment revealed that, like wild-type enzyme, the activity of C99A/C450A was inhibited by NEM (data not shown). These data therefore raised the question of whether one of the eight remaining Cys in the carboxylase was the catalytic base whose activity was being inhibited by NEM modification.
Mutants with Individual Cys to Ser Substitutions Retain Carboxylase Activity. Mutants with individual Cys to Ser substitutions were tested for activity to determine whether any of the remaining Cys was a candidate for the catalytic base. Mutation to Ser was chosen because Ser is the most conservative substitution and therefore less likely to destabilize protein structure, which could confound analysis, and because Ser substitution for the Cys catalytic bases can result in large decreases in activity (24–28). We previously generated several of these mutants [C99S, C139S, C288S, C311S, C343S, and C450S (23, 30)], and the remainder were generated in these studies (Supporting Materials and Methods). The specific activities were determined by carboxylase assay and quantitative Western analysis of microsomes. The analyses were performed on microsomes rather than purified enzymes to rule out potential differences in activity that might occur during isolation, as in the inactivation that occurred during the purification of C99S and C450S (data not shown). Microsomes were solubilized in the presence or absence of propeptide to determine whether any mutant other than C99S and C450S showed propeptide dependence, and all of the eight remaining mutants exhibited the same specific activity independent of propeptide (data not shown). As seen in Table 3 for the specific activities determined in the absence of propeptide, most of the mutants retained substantial activity, and C99S and C450S showed the largest losses in activity.
Table 3. Individual Cys-substituted carboxylase mutants retain activity.
| Carboxylase variant | Carboxylase, pmol/ng·h-1 | Specific activity, % |
|---|---|---|
| Wild type | 9.7 | 100 |
| C99S | 0.2 | 2 |
| C134S | 10.1 | 104 |
| C139S | 11.3 | 116 |
| C288S | 10.9 | 112 |
| C311S | 7.7 | 79 |
| C323S | 9.6 | 99 |
| C343S | 6.1 | 63 |
| C343A | 5.9 | 61 |
| C450S | 1.8 | 19 |
| C598S | 7.4 | 76 |
| C700S | 6.0 | 62 |
| Mock | <0.1 | 0 |
Microsomes from baculoviral- or mock-infected insect cells were solubilized in the absence of propeptide and then assayed for carboxylase specific activity as described in the legend to Table 1.
One of the Cys residues, Cys-343, was further analyzed by Ala substitution. Cys-343 lies within a region of the carboxylase that binds VKD proteins (30), and substitution by Ser caused partial loss of activity (Table 3). However, the same specific activity was observed when Cys-343 was substituted by a nonionizable Ala, ruling out the possibility of this Cys serving as the catalytic base. Thus, these mutagenesis analyses did not reveal an obvious candidate for the catalytic base.
A Mutant Lacking All Cys Residues Retains Carboxylase Activity. Our mutagenesis experiments were difficult to reconcile with a model in which a carboxylase Cys acts as the catalytic base; however, there were two remaining possibilities for Cys involvement in KH2 deprotonation. One possibility is that one of the seven Cys that was tested for a Ser, but not an Ala, substitution (Tables 2 and 3) is the catalytic base and that highly efficient activation in the substituted Ser mutant yielded more activity than usually observed when other enzymes with Cys catalytic bases are mutated. The second possibility is that some unknown combination of Cys residues acts together to deprotonate KH2. To test both possibilities in one stroke, a carboxylase mutant with all 10 Cys residues replaced by Ala was generated and analyzed. This mutant, called C(all 10)A (for C99A/C134A/C139A/C288A/C311A/C323A/C343A/C450A/C598A/C700A), was shown to have activity that was, interestingly, dramatically affected by coexpression with the VKD protein factor IX. Thus, when C(all 10)A was initially expressed like the other mutants, i.e., by infection of insect cells with a carboxylase-containing baculovirus, carboxylase activity was barely detected (Table 4, single infections). However, when C(all 10)A-containing baculovirus was coinfected with baculovirus containing factor IX, a 30- to 60-fold increase in carboxylase activity was observed in the cell extracts (Table 4, coinfections). Cells infected with only factor IX-containing baculovirus did not contain carboxylase activity (Table 4, single infections), showing that the activity in coinfected cells was due to the C(all 10)A carboxylase mutant. The experiment presented in Table 4 was performed by using small-scale lysates because of the number of infection permutations that were tested. In subsequent studies, the specific activity of C(all 10)A was determined by using solubilized microsomes prepared from insect cells coinfected with factor IX and either C(all 10)A or wild-type carboxylase, which showed that the specific activity of C(all 10)A was 7% that of wild-type carboxylase (data not shown). The ratio of epoxidase to carboxylase activity in C(all 10)A was the same as wild-type enzyme (i.e., 1:1), indicating that global Cys mutation did not disrupt coupling of KH2 oxygenation and Glu to Gla conversion. These data unequivocally demonstrate that Cys does not act as the catalytic base in the carboxylase.
Table 4. Expression of a carboxylase mutant with all Cys residues substituted by Ala shows retention of activity that depends on factor IX coexpression.
| Baculovirus contents | Carboxylase activity, cpm × 10-3 |
|---|---|
| Single infections | |
| Wild-type carboxylase | 238.4 |
| C(all 10)A carboxylase-1 | 0.2 |
| C(all 10)A carboxylase-2 | 0.1 |
| Mock | 0.0 |
| Factor IX | 0.0 |
| Coinfections | |
| Wild-type carboxylase and factor IX | 152.4 |
| C(all 10)A carboxylase-1 and factor IX | 6.0 |
| C(all 10)A carboxylase-2 and factor IX | 6.3 |
Insect cells were either singly infected with baculovirus-containing carboxylase or factor IX or coinfected with baculoviruses containing both factor IX and carboxylase, and lysates were prepared and assayed for carboxylase activity. All of the permutations shown were tested in a single experiment, and two independently generated C(all 10)A viruses were analyzed. Lysates (100 μl) were assayed in duplicate for 1 h. Background was determined by assaying a sample containing water instead of lysate and gave a value identical to the mock and factor IX samples of the single infections (i.e., ≈500 cpm). The variation in duplicate values was <10%, and the experiment was performed twice.
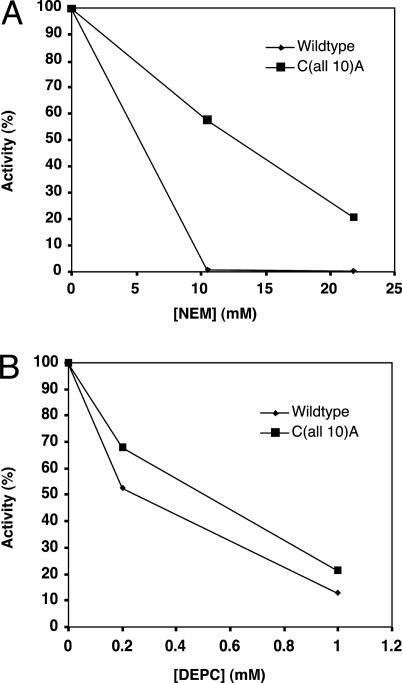
C(all 10)A Carboxylase Activity Is Inhibited by NEM and DEPC. The C(all 10)A mutant was tested for NEM-reactivity to try to resolve why C99A/C450A activity was inhibited by NEM, even though all Cys mutants other than Cys-99 or Cys-450 showed substantial activity (Table 3). One possibility is that Cys modification by NEM causes greater perturbation of carboxylase structure (and, therefore, activity) than Ser substitution; however, it is also possible that NEM inhibition is due to modification of an amino acid other than Cys. To try to distinguish these possibilities, then, wild-type and C(all 10)A carboxylase were both incubated with NEM, which resulted in inhibition of both carboxylases (Fig. 3A). Inhibition of the C(all 10)A mutant therefore indicates that modification of a residue other than Cys is responsible for at least part of the inhibition of the carboxylase by NEM.
Fig. 3.
NEM and DEPC inhibit carboxylase lacking Cys residues. Solubilized microsomes were prepared from insect cells coinfected with factor IX and either wild type or C(all 10)A and were then incubated in NEM for 10 min, followed by quenching of excess NEM with DTT (A), or in DEPC for 10 min, followed by quenching of excess DEPC with imidazole (B). The carboxylase activity of untreated, NEM-treated, or DEPC-treated samples was then determined. The details are described in Supporting Materials and Methods.
Biochemical modification experiments were performed to try to determine which amino acid is the catalytic base. These studies showed that both wild-type carboxylase and C(all 10)A were inhibited in a dose-dependent manner by DEPC (Fig. 3B). Inhibition was observed by using conditions in which DEPC shows good specificity for His (i.e., neutral pH) (14, 31), suggesting, then, that His is the nonthiol catalytic base.
Discussion
These studies require a revision of the model for how the VKD carboxylase deprotonates KH2 to initiate carboxylation. The current paradigm is that a Cys deprotonates KH2 to allow oxygenation and formation of a strong vitamin K base that deprotonates Glu, which results in Gla formation (Fig. 1). Our previous studies (23) implicated Cys-99 or Cys-450 as a carboxylase catalytic base candidate and suggested that the carboxylase may use an activation motif that ionizes Cys or a substituted Ser to enable KH2 deprotonation. Therefore, in the present studies Cys-99 and Cys-450 were converted to Ala, which does not ionize and consequently cannot function as a catalytic base. C99A, C450A, and the double mutant C99A/C450A all retained activity that was inhibitable by NEM. However, substitution of each of the remaining eight Cys residues by Ser resulted in carboxylase mutants with substantial activity. To test, then, whether Cys actually is the catalytic base that ionizes KH2, a mutant with all 10 Cys converted to Ala was generated. This mutant retained significant activity, showing definitively that the carboxylase catalytic base is not a Cys.
NEM modification of a non-Cys residue contributes an additional layer of complexity to interpreting experiments on carboxylase modification by NEM, which results in >99% loss of wild-type activity (Fig. 3A and ref. 8). The carboxylase is multiply modified by NEM, and inactivation could be due to some or all of the NEM modifications. In addition, inhibition could result from various mechanisms that include losing basicity, blocking the access of substrates/cofactors to the active site by the addition of a bulky group to the thiol side chain, or perturbing the structural integrity of the carboxylase. A final complexity is that the Glu substrate exposes an NEM-reactive group (19) that may regulate epoxidation (29), and our studies raise the question of whether this residue is a Cys or the nonthiol catalytic base. NEM modification of the catalytic base would abolish activity and is the most likely explanation for the inhibition of the C(all 10)A mutant by NEM (Fig. 3A). However, the increased resistance of C(all 10)A to NEM inhibition (Fig. 3A) suggests that NEM modification of at least one additional residue other than the catalytic base contributes to loss of activity in wild-type carboxylase. Decreased activity because of Cys-99 modification by NEM is a good possibility, because substitution of Cys-99 by Ser or Ala caused appreciable loss of activity (Tables 1 and 2). Vitamin K protects wild-type carboxylase against NEM inactivation (8, 23), suggesting that the sites of NEM modification are at or near the active site. Further studies should reveal whether vitamin K protects against [14C]NEM modification of all or only some residues as well as whether the nonthiol catalytic base is, in fact, the residue exposed by Glu substrate to allow NEM reactivity. Our previous NEM mapping studies did not detect the nonthiol catalytic base, and one explanation is that it was not exposed by the Glu substrate because NEM modification was performed on pure carboxylase.
Tie et al. (20) recently also performed NEM modification of the carboxylase but obtained results different from ours, which we attribute to differences in the structural integrity of the carboxylase molecules analyzed. Thus, although we identified Cys-99 and Cys-450 as NEM-reactive residues, Tie et al. (20) concluded that these two amino acids form a disulfide bond and that four other residues (Cys-139, Cys-311, Cys-323, and Cys-343) are modified by NEM. It is important to note that when NEM is used to probe thiol function in enzymes, it is critical that the enzyme is structurally intact to avoid artifacts like disulfide exchange or exposure of buried Cys residues during denaturation. For this reason, we performed NEM modification on carboxylase that was active and therefore in the native conformation: Carboxylase was isolated in a factor IX–carboxylase complex from mammalian cells, separated from factor IX, and then immediately modified with NEM. The activity was monitored to show that the carboxylase was fully active before modification. In contrast, Tie et al. (20) incubated the carboxylase overnight in an oxidizing buffer before NEM modification, did not monitor activity, and did not quench the NEM before exposure of the carboxylase and NEM to SDS. Consequently, we think the differences in NEM mapping are due to the analysis of structurally different carboxylases and that native carboxylase has three NEM-reactive residues, which include the free thiols Cys-99 and Cys-450 and a nonthiol residue. This interpretation is supported by the present studies that showed a decrease from 3.5 NEM-modified residues in wild-type carboxylase to 1 in C99A/C450A (Fig. 2). Three NEM-reactive residues in wild-type carboxylase is supported by previous studies by ourselves and others (19, 23) in which amino acid analysis of human carboxylase and quantitative Western analysis of bovine carboxylase revealed 2–3 NEM-modified residues per mole of carboxylase.
Our Cys-99 and Cys-450 substitution mutants showed appreciable activity (14–40%, Tables 1 and 2) whereas Tie et al. (20) were unable to obtain activity in mutants substituted in these two residues. Tie et al. (20) performed Cys mutagenesis similar to our analyses (Table 3 and refs. 23 and 30) and concluded that most Cys-substituted mutants retained substantial activity. Their activity measurements were performed on carboxylase purified in the absence of propeptide, whereas ours were on microsomes solubilized in the presence or absence of propeptide. We observed almost total inactivation of Ala- or Ser-substituted Cys-99 or Cys-450 when these mutants were purified in the absence (but not presence) of propeptide (data not shown), which likely accounts for the inability of Tie et al. (20) to detect activity in these two mutants.
The fact that Cys-99 and Cys-450 substitution mutants only show partial activity raises the question of their roles in epoxidation and carboxylation. These two amino acids and Cys-139 are the only Cys residues that are evolutionarily conserved in all carboxylase orthologs examined to date, which include mammals, Conus, and Drosophila (22, 32–34). We previously observed a 6- to 8-fold increase in the Km values for the Glu substrate Boc-Glu-Glu-Leu-OMe with C99S and C450S enzymes (23), suggesting that these residues are part of the active site. This interpretation is consistent with the observation that substitution of Cys-99 or Cys-450, but no other Cys residue, resulted in activities that depended on whether VKD propeptide was present during their isolation (Table 1 and data not shown). Response to propeptide may be related to conformational changes that are induced by propeptide binding: Free propeptide activates the carboxylase through a decrease in the Km for the Glu substrate (35) and propeptide covalently attached to Glu-containing peptides markedly decreases the Km values for both Glu substrate and KH2 cofactor (36, 37). The sum of these data, then, indicate that Cys-99 and Cys-450 are exposed, free thiols near the active site. We suggest that mutations in these free thiols perturb the active site and that the presence of propeptide helps rescue activity by inducing a conformational change that prevents structural deformation of the active site caused by the mutations.
C(all 10)A, like the Cys-99 and Cys-450 mutants, required the presence of a VKD protein sequence during isolation to retain activity; however, these sequences were needed at an earlier stage than microsomal solubilization. Thus, C(all 10)A activity was barely detectable unless this mutant was coexpressed with factor IX, which led to a dramatic increase (30- to 60-fold) in activity (Table 4). Western analysis showed that increased activity was due to a change in specific activity rather than increased synthesis, and the effect of factor IX could not be recapitulated by adding propeptide-containing full-length factor IX to cell extracts containing free C(all 10)A (data not shown). Thus, coinfection of factor IX with C(all 10)A, which allows potential interaction from the moment translation begins, somehow increases carboxylase specific activity. Wild-type carboxylase is active when expressed alone, and this difference from C(all 10)A may be due to differences in the efficiency of the step(s) affected by factor IX. The production of mature, active carboxylase involves multiple processes that could be affected by factor IX, for example protein folding that achieves proper membrane topology and consequent activity of the carboxylase, an integral membrane protein. Regardless of the mechanism, the observation that factor IX coexpression affects C(all 10)A specific activity has implications for how VKD proteins and carboxylase assemble in the endoplasmic reticulum, i.e., whether they undergo individual maturation before assembly or whether their association precedes the maturation steps that result in a productive complex.
Our experiments establishing that a Cys does not act as the catalytic base raise the obvious question of the identity of this base. Candidate bases include His, Lys, Asp, Glu, or Ser, although Ser is not likely because active carboxylase is routinely prepared in the presence of Ser protease inhibitors. We found that DEPC, a His-selective reagent, inhibits carboxylase activity (Fig. 3B), and His acting as the catalytic base would be consistent with the pH optimum for the carboxylase (≈7). The inhibition of C(all 10)A by DEPC is particularly significant in implicating His, because DEPC can also react with Cys, which is not present in this mutant. A His catalytic base can be reconciled with NEM inhibition, because NEM can react with amines at high pH and concentration, albeit at rates that are orders of magnitude slower than observed with Cys (14). The fact, then, that NEM significantly inhibited C(all 10)A carboxylase activity at neutral pH, at low NEM concentration, and after only 10 min (Fig. 3A) shows unusual NEM-reactivity that still suggests an activation mechanism for carboxylation, most likely by a Glu or Asp, but with an amine rather than Cys being activated. Further studies should identify which residue is the catalytic base, which will be critical to understanding the unique mechanism of carboxylation. Such an understanding is important because of the broad range of physiologies impacted by carboxylation and because a defined mechanism will advance strategies for therapeutic intervention of these physiologies.
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: VKD, vitamin K-dependent; KH2, vitamin K hydroquinone; Gla, γ-carboxylated Glu; NEM, N-ethylmaleimide; DEPC, diethyl pyrocarbonate.
References
- 1.Berkner, K. L. (2000) J. Nutr. 130, 1877–1880. [DOI] [PubMed] [Google Scholar]
- 2.Stenina, O., Pudota, B. N., McNally, B. A., Hommema, E. L. & Berkner, K. L. (2001) Biochemistry 40, 10301–10309. [DOI] [PubMed] [Google Scholar]
- 3.Morris, D. P., Stevens, R. D., Wright, D. J. & Stafford, D. W. (1995) J. Biol. Chem. 270, 30491–30498. [DOI] [PubMed] [Google Scholar]
- 4.Dowd, P. & Zheng, Z. B. (1995) Proc. Natl. Acad. Sci. USA 92, 8171–8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowd, P., Hershline, R., Ham, S. W. & Naganathan, S. (1995) Science 269, 1684–1691. [DOI] [PubMed] [Google Scholar]
- 6.Anton, D. L. & Friedman, P. A. (1983) J. Biol. Chem. 258, 14084–14087. [PubMed] [Google Scholar]
- 7.Vidal-Cros, A., Gaudry, M. & Marquet, A. (1990) Biochem. J. 266, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canfield, L. M. (1987) Biochem. Biophys. Res. Commun. 148, 184–191. [DOI] [PubMed] [Google Scholar]
- 9.Canfield, L. M., Sinsky, T. A. & Suttie, J. W. (1980) Arch. Biochem. Biophys. 202, 515–524. [DOI] [PubMed] [Google Scholar]
- 10.Suttie, J. W., Lehrman, S. R., Geweke, L. O., Hageman, J. M. & Rich, D. H. (1979) Biochem. Biophys. Res. Commun. 86, 500–507. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, P. A. & Shia, M. (1976) Biochem. Biophys. Res. Commun. 70, 647–654. [DOI] [PubMed] [Google Scholar]
- 12.Mack, D. O., Suen, E. T., Girardot, J. M., Miller, J. A., Delaney, R. & Johnson, B. C. (1976) J. Biol. Chem. 251, 3269–3276. [PubMed] [Google Scholar]
- 13.Morris, D. P., Soute, B. A., Vermeer, C. & Stafford, D. W. (1993) J. Biol. Chem. 268, 8735–8742. [PubMed] [Google Scholar]
- 14.Wong, S. S. (1993) in Chemistry of Protein Conjugation and Cross-Linking (CRC, Boca Raton, FL), pp. 7–48.
- 15.Smyth, D. G., Blumenfeld, O. O. & Konigsberg, W. (1964) Biochem. J. 91, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorin, G., Martic, P. A. & Doughty, G. (1966) Arch. Biochem. Biophys. 115, 593–597. [DOI] [PubMed] [Google Scholar]
- 17.Suttie, J. W. (1985) Annu. Rev. Biochem. 54, 459–477. [DOI] [PubMed] [Google Scholar]
- 18.Presnell, S. R. & Stafford, D. W. (2002) Thromb. Haemostasis 87, 937–946. [PubMed] [Google Scholar]
- 19.Bouchard, B. A., Furie, B. & Furie, B. C. (1999) Biochemistry 38, 9517–9523. [DOI] [PubMed] [Google Scholar]
- 20.Tie, J. K., Mutucumarana, V. P., Straight, D. L., Carrick, K. L., Pope, R. M. & Stafford, D. W. (2003) J. Biol. Chem. 278, 45468–45475. [DOI] [PubMed] [Google Scholar]
- 21.Tie, J., Wu, S. M., Jin, D., Nicchitta, C. V. & Stafford, D. W. (2000) Blood 96, 973–978. [PubMed] [Google Scholar]
- 22.Bandyopadhyay, P. K., Garrett, J. E., Shetty, R. P., Keate, T., Walker, C. S. & Olivera, B. M. (2002) Proc. Natl. Acad. Sci. USA 99, 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pudota, B. N., Miyagi, M., Hallgren, K. W., West, K. A., Crabb, J. W., Misono, K. S. & Berkner, K. L. (2000) Proc. Natl. Acad. Sci. USA 97, 13033–13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amara, A. A. & Rehm, B. H. A. (2003) Biochem. J. 374, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia, Y., Kappock, T. J., Frick, T., Sinskey, A. J. & Stubbe, J. (2000) Biochemistry 39, 3927–3936. [DOI] [PubMed] [Google Scholar]
- 26.Matsuka, Y. V., Pillai, S., Gubba, S., Musser, J. M. & Olmsted, S. B. (1999) Infect. Immun. 67, 4326–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson, M. A. & Semler, B. L. (1991) Proc. Natl. Acad. Sci. USA 88, 9919–9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rancourt, C., Tihanyi, K., Bourbonniere, M. & Weber, J. M. (1994) Proc. Natl. Acad. Sci. USA 91, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiura, I., Furie, B., Walsh, C. T. & Furie, B. C. (1997) Proc. Natl. Acad. Sci. USA 94, 9069–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pudota, B. N., Hommema, E. L., Hallgren, K. W., McNally, B. A., Lee, S. & Berkner, K. L. (2001) J. Biol. Chem. 276, 46878–46886. [DOI] [PubMed] [Google Scholar]
- 31.Lundblad, R. L. (1995) Techniques in Protein Modification (CRC, Boca Raton, FL).
- 32.Walker, C. S., Shetty, R. P., Clark, K. A., Kazuko, S. G., Letsou, A., Olivera, B. M. & Bandyopadhyay, P. K. (2001) J. Biol. Chem. 276, 7769–7774. [DOI] [PubMed] [Google Scholar]
- 33.Li, T., Yang, C. T., Jin, D. & Stafford, D. W. (2000) J. Biol. Chem. 275, 18291–18296. [DOI] [PubMed] [Google Scholar]
- 34.Czerwiec, E., Begley, G. S., Bronstein, M., Stenflo, J., Taylor, K., Furie, B. C. & Furie, B. (2002) Eur. J. Biochem. 269, 6162–6172. [DOI] [PubMed] [Google Scholar]
- 35.Knobloch, J. E. & Suttie, J. W. (1987) J. Biol. Chem. 262, 15334–15337. [PubMed] [Google Scholar]
- 36.Furie, B., Bouchard, B. A. & Furie, B. C. (1999) Blood 93, 1798–1808. [PubMed] [Google Scholar]
- 37.Soute, B. A., Ulrich, M. M., Watson, A. D., Maddison, J. E., Ebberink, R. H. & Vermeer, C. (1992) Thromb. Haemostasis 68, 521–525. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.