Abstract
Chrysomeline larvae respond to disturbance and attack by everting dorsal glandular reservoirs, which release defensive secretions. The ancestral defense is based on the de novo synthesis of monoterpene iridoids. The catabolization of the host-plant O-glucoside salicin into salicylaldehyde is a character state that evolved later in two distinct lineages, which specialized on Salicaceae. By using two species producing monoterpenes (Hydrothassa marginella and Phratora laticollis) and two sequestering species (Chrysomela populi and Phratora vitellinae), we studied the molecular basis of sequestration by feeding the larvae structurally different thioglucosides resembling natural O-glucosides. Their accumulation in the defensive systems demonstrated that the larvae possess transport systems, which are evolutionarily adapted to the glycosides of their host plants. Minor structural modifications in the aglycon result in drastically reduced transport rates of the test compounds. Moreover, the ancestral iridoid-producing leaf beetles already possess a fully functional import system for an early precursor of the iridoid defenses. Our data confirm an evolutionary scenario in which, after a host-plant change, the transport system of the leaf beetles may play a pivotal role in the adaptation on new hosts by selecting plant-derived glucosides that can be channeled to the defensive system.
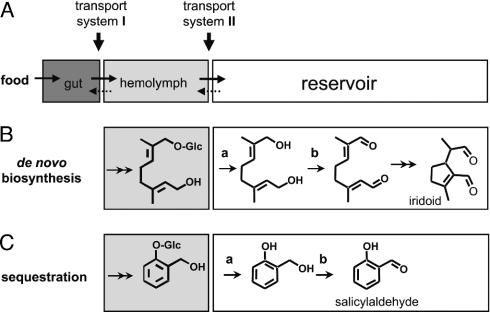
Plant-derived secondary metabolites play an important role in the coevolution of host plants and herbivorous insects. Several insects have overcome the chemical barrier of noxious plants and, thereby, have successfully colonized a niche that is not readily available to nonadapted competitors. Further advanced strategies additionally exploit the plant-derived toxins for the insects' own antipredator defense. This use of plant-derived secondary metabolites requires a complex scenario of adaptation, which is unlikely to occur frequently. The insects have to develop (i) a selective import system that selects for the appropriate compound(s) from the plethora of chemicals present in the food and (ii) a mode of hemolymph transport and storage capabilities for the toxic compounds that excludes negative interferences with the insects own physiological processes (Fig. 1A). The successful uptake and use of highly toxic pyrrolizidines (1), cardenolides (2), iridoid glycosides (3), cyanogenic glycosides (4), and glucosinolates (5) by Lepidopterans provide several examples for such successful adaptations. Although these examples clearly document the flow of the compounds from plants to the insect, almost nothing is known about the molecular basis of the import by and transport within the insect. Chrysomelid larvae provide a well suited system for study of the mechanistic basis of sequestration at different levels of evolutionary adaptation. Leaf beetles (Coleoptera: Chrysomelidae) constitute a very large group of species (6), each of which feeds on a restricted number of plant taxa. In many species, the adults and/or the larvae (especially in the subtribe Chrysomelina) protect themselves against predation by releasing defensive secretions from serial dorsal reservoirs (7). The defensive secretions may contain a single compound or a few compounds, or they may constitute a rather complex blend, covering different types of compound classes from different biosynthetic pathways, as shown in Scheme 1.
Fig. 1.
Principal route for plant-derived metabolites into the defensive gland. (A) At least two transport systems, (i) from the gut to hemolymph and (ii) from hemolymph to the reservoir, are necessary for sequestration. (B) De novo production of the iridoid monoterpene chrysomelidial in the basal group (H. marginella and P. laticollis). (C) Sequestration of salicin and subsequent conversion into salicylaldehyde (C. populi and P. vitellinae). Although the different toxins vary in both structure and biosynthetic origin, the reservoir enzymes involved (a, glucosidase; b, oxidase) are the same (7). Double arrows indicate multistep enzymatic reactions.
Scheme 1.
A basal group of chrysomelid larvae that specialized on plant families, such as Salicaceae, Brassicaceae, or Ranunculaceae, produces iridoid monoterpenes de novo (7, 8). The early biosynthetic steps toward, e.g., chrysomelidial, lead from mevalonic acid to the O-glucoside of 8-hydroxygeraniol (9), whereas the final transformations into the active compounds are carried out in the reservoir (Fig. 1B) (10). Further advanced species use defensive products whose synthesis depends partly or exclusively on secondary metabolites from the plant host. Interestingly, a subset of the enzymes (e.g., glucosidase and oxidase) needed for iridoid biosynthesis is also required in Chrysomela species that secrete salicylaldehyde, derived from phenol glycosides of the food plants (Fig. 1C) (11). The most evolved species (e.g., Chrysomela interna) sequester several plant-derived precursors simultaneously; e.g., glucosides of (3Z)-hex-3-en-1-ol and phenylethanol. After cleavage in the reservoir, the free aglycons are esterified with isobutyric acid and 2-methylbutyric acid derived from the insects' internal pools of amino acids (12, 13).
This shift from autogenous biosynthesis in the basal group to a combinatorial biosynthesis modifying sequestered compounds with autogenous components in the highest evolved species is believed to originate from host-plant changes and by subsequent adaptation of the enzymes. According to phylogenetic analyses, this shift from de novo synthesis to the host-derived defense has evolved independently at least twice (14, 15). However, the molecular mechanisms facilitating the transitions among these different types of chemical defenses are only partially known. Because the basal species and the more advanced sequestering species share not only the principal architecture of their defensive system but also several enzymatic activities (see Fig. 1), the shift from de novo to host-derived defense may require only small changes in the specificity of enzymes present in the glands and reservoir (11, 16). Certainly, also the ability to acquire “unfamiliar” plant metabolites from food plays a pivotal role because the import system is the first molecular mechanism that selects for appropriate molecules that are delivered to the glandular system.
We investigated possible mechanisms for this adaptation by comparing the glycoside transport in two iridoid-producing species and in two salicin-sequestering leaf beetles, in particular the genus Chrysomela, the major taxon in which plant-derived defense has evolved. We first established a molecular phylogeny, which included the genera known to secrete iridoids and those sequestering salicin. From this phylogenetic analysis, we selected for transport experiments species that branched at the basis of each of the two groups. As representatives of iridoid-producing leaf beetles, we chose Hydrothassa marginella and Phratora laticollis, and we chose Chrysomela populi and Phratora vitellinae for the salicylaldehyde-secreting species. The latter independently evolved the ability to sequester salicin within the group of iridoid-producing species. By providing a rigorous structural proof for the molecular basis of transport in combination with a selection of evolutionary relevant species, we offer mechanistic and stereochemical insights into the process of sequestration and information concerning transport phenomena and their impact on the evolution of leaf-beetle defenses.
Materials and Methods
Phylogenetic Analysis. The phylogenetic analyses are based on nucleotide sequences from the 12S mitochondrial rRNA (12S) and the 16S mitochondrial rRNA (16S). Sequences reported in this article were deposited in the GenBank database (15). Genomic DNA of the Hydrothassa species was extracted from ethanol-preserved single adults by using standard protocols. The primers used to amplify the mitochondrial fragments were SR-N-14759 and SR-J-14233 for the 12S fragment and LR-J-12883 and LR-N-13398 for the 16S segment (17). The program soap (18) was used to produce one alignment for each of the 25 different sets of alignment parameters (weighted matrix, gap-opening penalties from 11 to 19 by steps of two, and extension penalties from 3 to 11 by steps of two). Any positions at which the alignments differed were excluded. All maximum-parsimony analyses were performed with paup* with heuristic searches. We checked whether “simple,” “closest,” and “random” stepwise-addition sequences yielded identical trees. Other settings were as follows: tree-bisection-reconnection (TBR) branch-swapping, multiple parsimonious trees (MULPARS), and zero-length branches collapsed. To avoid local optima, we performed 104 replicates both with random starting trees and with starting trees obtained by random stepwise addition. All characters were first weighted equally. We estimated the reliability of the various inferred clades by bootstrapping (400 replicates). The outgroup was represented by Oreina elongata and Chrysolina america (15).
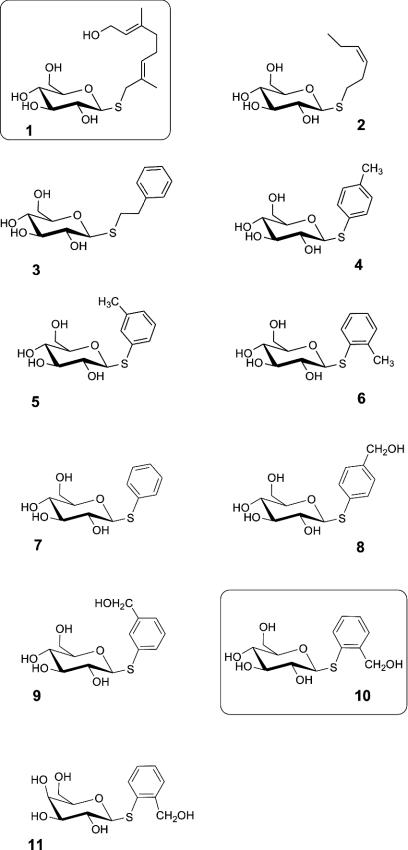
Chemicals. The thioglucosides 1 and 10 (Scheme 2) represent hydrolysis-resistant, synthetic S-analogs of natural O-glucosides involved in the biosynthesis of chrysomelidial (Fig. 1B) and salicin (Fig. 1C). The synthesis of 1 has been described (19).
Scheme 2.
The thioglucosides of (3Z)-hex-3-en-1-ol 2 and phenylethanol 3 were obtained by Mitsunobu-coupling of the free alcohols with 2,3,4,6-tetra-O-acetyl-β-d-thioglucopyranose and deprotection with methoxide in methanol (20). 2 and 3 served as hydrolysis-resistant analogs of O-glucosides found in Salicaceae and Betulaceae (12). Aromatic thioglucosides 4→7 were obtained by means of a Königs–Knorr protocol using the thiocresols and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (19). An analogous procedure afforded the isomeric o-, m-, and p-mercaptobenzoates 8, 9, 10, starting from the isomers of methyl mercaptobenzoates and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide. The thiogalactoside 11 was obtained from methyl o-mercaptobenzoate and galactopyranosyl bromide. Ester and acetate groups were reduced by lithium aluminum hydride to the thioglucosides 8→11 (21). All compounds were purified by high-speed CounterCurrent chromatography with the ternary solvent mixture chloroform/methanol/water (8:13:7, vol/vol/vol). Crytallographic data for thiosalicin 10 are available from the Cambridge Crystallographic Data Centre (accession no. CCDC-246856). Crystallographic data of salicin were reported in ref. 22.
Leaf Beetles. Larvae of H. marginella were collected near Treignes (Belgium) and reared in the laboratory (20°C and 16:8 light/dark period) on leaves of Ranunculus repens. Larvae of C. populi were collected near Torino (Italy), and larvae from P. laticollis and P. vitellinae were collected near Brussels and reared on leaves of Populus canadensis. Third-instar larvae (9–12 d) were used for feeding experiments, which were conducted under rearing conditions by using leaves of the typical host plants.
Feeding Experiments. For all experiments, the upper surfaces of leaves were impregnated with solutions of the test compounds 1→11 in methanol (0.5 μmol·cm–2 leaf). The solvent was allowed to evaporate before larval feeding. The experimental design was validated by dual-choice experiments in which C. populi was fed on leaves treated with the test solutions or the pure solvents. Because no feeding preference was found, the experimental procedure did not affect the choice or feeding behavior. The leaf areas that were used in experiments were chosen to correspond to the amount of larval feeding. In kinetic experiments (n = 6) with C. populi, samples were collected at 2, 4, 8, 16, 32, and 48 h after the start of feeding (each replicate: 25 larvae feeding on ≈225-cm2 leaves; thioglucoside 10 solution applied to the leaves; four individuals were sampled at each time point). Generally, samples were taken 48 h after start of feeding (see Fig. 6). For each replication, five to seven larvae were fed on 25- to 35-cm2 leaves. C. populi was fed with compounds 1→11 (n = 3–10). Compounds 1-3, 6, 8, and 10 were examined on P. vitellinae (n = 5–11). H. marginella was fed with compounds 1→10 (n = 2–5). Compounds 1-3, 6, 8, 10, and 11 were tested on P. laticollis (n = 3–7).
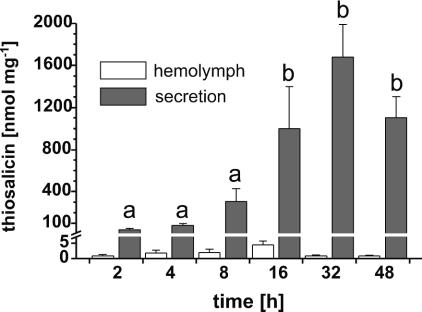
Fig. 6.
Mean abundance and standard error of concentration in secretion and hemolymph of C. populi after feeding on compound 10 for various time intervals. The concentration of 10 was found to be time-dependent in secretion samples. Differences are indicated by different letters (P < 0.05).
Chemical Analyses. Glandular secretions and hemolymph samples were collected with glass capillaries (19). Samples were weighed and diluted in 40 μl of methanol/water (1:1, vol/vol). After centrifugation, the supernatant was analyzed by a combination of RP-HPLC and MS. The analyses were carried out with a Thermoquest LCQ (Egelsbach, Germany) in the atmospheric pressure chemical ionization (APCI) mode (vaporizer temperature, 560°C) connected to an Agilent HP1100 HPLC system. Thioglucosides 1→10 from C. populi and H. marginella were separated on a Grom-Sil ODS-3 CP (125 × 2 mm) as described by Feld et al. (19). Samples from P. vitellinae and P. laticollis, and the thiogalactoside 11 were analyzed on a Grom-Sil 80 ODS-7 pH column (125 × 2 mm) (19). Thioglucoside 1 was quantified by integration of the ion [M + H-H2O]+ (m/z, 331). Thioglucoside 2 was quantified by the quasimolecular ion [M + H2O]+ (m/z, 296). The same ion was used to quantify 3 (m/z, 318), 4→6 (m/z, 304), 7 (m/z, 290), and 8→11 (m/z, 320). Standard curves were recorded for 1→11 and used for calibration (see Figs. 4 and 5). Abundances were expressed as nmol of compound per mg–1 of sample (undiluted secretion or hemolymph). The threshold of detection was set at 1 nmol·mg–1 secretion. Compounds were separated by gradient elution at 0.25 ml·min–1 (solvent A, water plus 0.5% acetic acid; solvent B, acetonitrile plus 0.5% acetic acid) according to the following protocol: starting with 2% solvent B, raising within 20 min to 100% solvent B, and maintaining for 8 min before recycling.
Fig. 4.
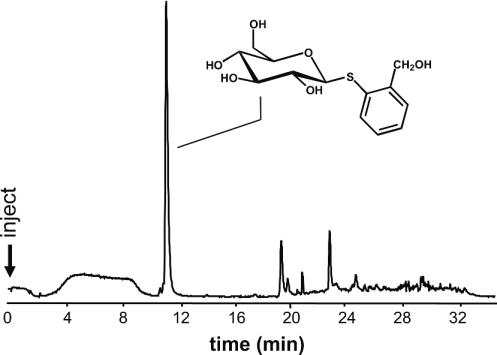
RP-HPLC separation of components from the secretion of P. vitellinae after 48 h of feeding on leaves of P. canadensis impregnated with thiosalicin 10.
Fig. 5.
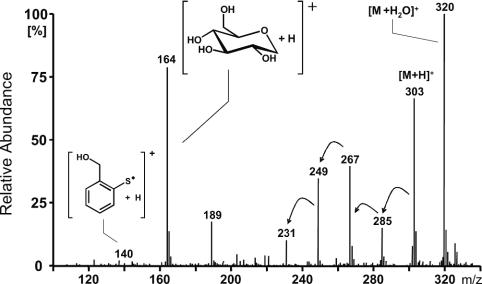
Mass spectrum of thiosalicin 10. Analyses were carried out in the atmospheric pressure chemical ionization (APCI) mode. All thioglucosides displayed comparable spectra with strong quasimolecular ions [M + H2O]+ and [M + H]+.
Statistics. To identify differences in transport efficacy, data were statistically analyzed (23) by ANOVA, correcting for different sample sizes (SPSS). To satisfy assumptions of normality and homogeneity of variance (tested by Levenes' goodness-of-fit test), each variable was first transformed logarithmically. In all experiments, a one-way ANOVA followed by a Student–Newman–Keuls t test isolated differences within secretion samples. The hemolymph samples were not treated statistically because of the low concentrations at the limit of the analytical approach.
Results
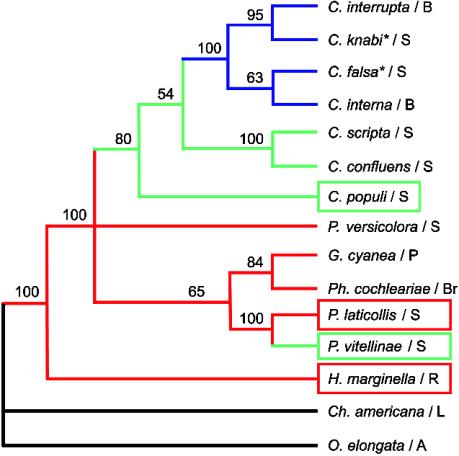
The mitochondrial DNA phylogeny presented in Fig. 2 is fully consistent with previous analyses (15). Secretion of autogenous iridoid monoterpenes is an ancestral character. Secretion of host-derived salicylaldehyde later evolved independently twice in the genus Chrysomela and in P. vitellinae, both specialized on Salicaceae. The ability to produce leaf-alcohol esters evolved latest and led to the chemical defense of the Chrysomela species that shifted to Betulaceae. H. marginella emerged at the basis of the whole Chrysomelina clade, and P. laticollis belongs to the same genus as P. vitellinae; therefore, they were the best candidates for investigating possible ancestral conditions.
Fig. 2.
Maximum-parsimony reconstruction of the chemical defensive strategies on the maximum-parsimony strict consensus tree. Bootstrap values are given above the branches. Red, autogenous monoterpene iridoids; green, host-derived defense based on salicylaldehyde derived from salicin; blue, mixed metabolism (esterification of de novo carboxylic acids by plant alcohols). Asterisks indicate taxa that have a dual defense, combining the host-derived metabolism and the mixed metabolism. O, Oreina; Ch, Chrysolina; H, Hydrothassa; P, Phratora; Ph, Phaedon; G, Gonioctena; P, Plagiodera; and C, Chrysomela. Boxes indicate the species chosen to study the transport mechanisms. A, feeding on Asteraceae; B feeding on Betulaceae; Br, feeding on Brassicaceae; L, feeding on Lamiaceae; P, feeding on Polygonaceae; R, feeding on Ranunculaceae; and S, feeding on Salicaceae.
Two different transport mechanisms for O-glucosides can be envisioned. Because the gut of these insects often contains highly active glucosidases (24), the O-glucosides could be cleaved, allowing the less polar aglycons to cross membranes by diffusion and finally reach the defensive system.
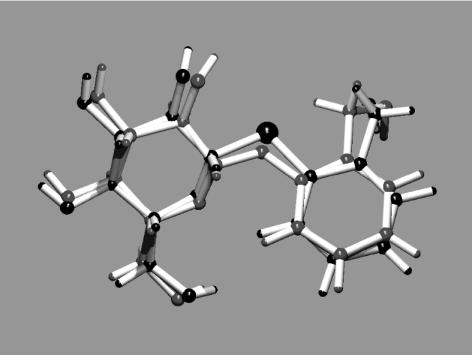
Polar compounds, such as O-glycosides (Fig. 1 B and C and Scheme 2), are not able to pass membranes per se but require a transport system to cross the gut membrane and to enter the defensive system (Fig. 1 A). Because O-glucosides may be cleaved in the gut, we chose the corresponding S-glucosides, which are resistant to hydrolysis. S-glucosides are structurally highly similar to their O-analogs (Fig. 3) and may adopt almost identical bioactive conformations (25). A superposition of the crystal structures of the O-glucoside of salicin and that of the corresponding S-glucoside demonstrates the perfect structural match of the two compounds (Fig. 3).
Fig. 3.
Superposition of the molecular structures of salicin and thiosalicin. Data were obtained by x-ray crystallography (22). The C—S bonds are longer than the C—O analogs. The angle of the glycosidic bond in salicin (C—O—C, 117.7°) is significantly larger than in thiosalicin 10 (C—S—C, 102.4°).
A typical feeding experiment is shown in Fig. 4. Compounds were separated by RP-HPLC and detected by MS (see Materials and Methods). Thiosalicin 10 elutes as a single compound and displays intense [M + H2O]+ and [M + H]+ quasimolecular ions (Fig. 5).
An active-transport mechanism for the thiosalicin 10 is operative in C. populi, judging from kinetic experiments (Fig. 6) that show a time-dependent accumulation of 10 in the glandular reservoirs (df = 5, P < 0.001, F = 16.23). Saturation occurred 16 h after the start of feeding and remained fairly constant for the next 30 h. Although the reservoir contains a highly active glucosidase (24), thiosalicin 10 is obviously not cleaved (10). Hemolymph samples contained only very low quantities of 10, but the loading pattern largely reflected the pattern of accumulation in the reservoir. The level was, on average, ≈2–4% of that of the reservoir concentration, corresponding to 250- to 500-fold enrichment in the latter. However, hemolymph levels should be viewed skeptically because the amounts were close to the detection limits of the HPLC-MS (26).
Because maximum accumulation of thiosalicin in the reservoir of C. populi was reached after 16 h, all other samples were collected 48 h after the start of the feeding. When larvae of the iridoid-producing group (H. marginella and P. laticollis) were exposed to the thioglucoside 1, both reached the same level of 1 in their reservoirs (H. marginella:df = 9, P < 0.001, F = 27.95; P. laticollis: df = 6, P < 0.001, F = 18.79), coincident with the final level of 10 in the sequestering species C. populi (df = 10, P < 0.001, F = 30.70). A much lower level of 10 (Fig. 7) was found in the reservoirs of P. vitellinae (df = 5, P < 0.001, F = 23.86). Accordingly, both the ancestral iridoid producers and the more advanced salicin-sequestering species possess the ability to select and transport plant-derived glucosides.
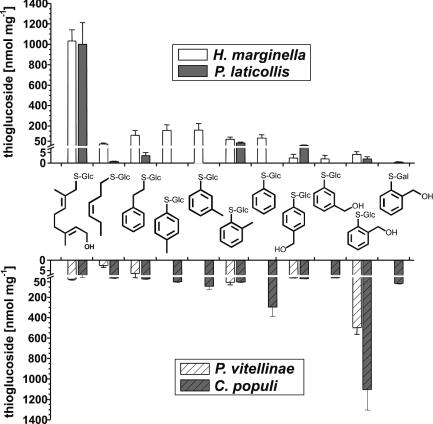
Fig. 7.
Survey of transport efficacies as determined for the two leaf-beetle groups of iridoid-producing (H. marginella and P. laticollis) and sequestering (C. populi and P. vitellinae) species fed with different thioglucosides 1–11.
Striking differences in the transport capabilities of the two insect groups became obvious on exposure of the larvae to leaves impregnated with thioglucosides not related to an O-glucoside precursor of their own defensive pathways. Larvae of H. marginella and P. laticollis were not able to accumulate thiosalicin 10 (Fig. 7). Conversely, the two sequestering species C. populi and P. vitellinae were not able to import the thioglucoside 1 most efficiently accumulated by H. marginella and P. laticollis. The impact of the stereochemistry of the thioglucosides was assessed by feeding the regioisomers 8 and 9. Unlike thiosalicin 10, larvae of C. populi and P. vitellinae did not accumulate 8 and 9 in their reservoir or in the hemolymph. The isomeric aromatic thioglucosides 4, 5, and 6 lacking the hydroxy group of the side chain are accumulated moderately in all species. Complete removal of the substituent from the aromatic ring leads to an increased transport (≈20% of the accumulation of 10) of 7 in C. populi but not in H. marginella. Thioglucosides 2 and 3, which represent analogs of natural O-glucosides from Betulaceae, were not enriched by P. laticollis, P. vitellinae, and C. populi. Only larvae of H. marginella showed a weakly enhanced level (≈10% of the accumulation of 1). The latter species also imported the glucoside of m-thiocresol 5, which reached a level of ≈20% of that of the optimum substrate 1 (Fig. 7). None of the tested species was able to accumulate the thiogalactoside 11 (≈10% of the level of thiosalicin in C. populi, see Fig. 7), demonstrating that the configuration of the sugar is essential for the transport process.
Discussion
The current study provides the first systematic analysis of the molecular and structural requirements for the sequestration of glycosidically bound plant metabolites by leaf-beetle larvae. The high accumulation of the thioglycosides 1 and 10 in their defensive glands proves the existence of a complex transport system. The first system has to be located in the gut membrane loading the glucosides into the epithelial cells followed by export to the hemolymph. Because the concentration of the glucosides is higher in the gut than in the hemolymph, this first transport must not operate against a gradient. A second shuttles the glucoside from the hemolymph to the reservoir and is able to enrich the glucoside up to 500-fold (Figs. 6 and 7). In the case of the natural O-glucoside, such an extreme accumulation does not occur, because the glucoside is rapidly cleaved and further transformed into iridoids (27). All transports systems display a very high selectivity and are obviously evolutionarily adapted to the class of compounds to be sequestered. The thioglucoside 1 resembling the O-glucoside precursor involved in the de novo biosynthesis of the iridoids in H. marginella and P. laticollis (Fig. 1B) is selectively accumulated only in these species. Larvae of P. vitellinae and C. populi, the two salicin-sequestering species, lack this ability. Moreover, larvae of Phaedon cochleariae and Gastrophysa viridula, two other iridoid-producing species, are also able to accumulate the thioglucoside 1 in their reservoir (19) (Fig. 1B). This finding seems paradoxical because 8-hydroxygeraniol or its glucosides are not abundant plant metabolites. On the other hand, recent studies on the localization of early terpenoid biosynthesis revealed that the autogenous synthesis of 8-hydroxygeraniol is localized in the fat body. Semiquantitative PCR revealed a high expression of hydroxymethylglutaryl coenzyme A reductase (28), and analysis by HPLC-MS showed rather high concentrations of the glucoside of 8-hydroxygeraniol in this tissue (unpublished data). This result requires for a hemolymph-based transport shuttling the glycoside between the fat body and the reservoir. The presence of a similar transport system in the gut may suggest a facultative use of this system if host plants provide appropriate precursors. A recent study with Rumex obtusifolius, the natural host plant of G. viridula, revealed that these larvae do not sequester terpenoid precursors from this plant (29). Within the group sequestering phenolglucosides, the selectivity of the transport system is comparably high. Larvae of C. populi and P. vitellinae are efficient in transporting the aromatic thioglucoside 10, corresponding to the natural O-glucoside salicin (Fig. 1C). The corresponding p- and m-isomers of 10 accumulated in both species only in minor quantities. The glucose part of 10 seems to be essential because the thiogalactoside 11 was not significantly transported (Fig. 7). Considering that the transport system requires the glucose moiety for recognition thioglucosides 10 and 11 differ only in the configuration at C-4 of their sugar moieties (glucose versus galactose). Furthermore, efficient transportation requires the sugar moiety (glucose) orthopositioned to the benzylalcohol. The p-, m-, and o-thiocresolglucosides 4 to 6 and the phenyl derivative 7 lacking the hydroxyl group of the aglycon were accumulated less efficiently than 10 but more efficiently than the isomers 8 and 9. To account for the high selectivity of the transport processes, we postulate a network of hydrogen bonds between the transport protein and the hydroxyl groups of the glucose and the aglycon, which together seem to account for selection and transportation of 10. It is unknown whether the two complex transport systems in the gut and the glandular system are identical (30). Initial results with gut tissue from iridoid-producing insects (P. cochleariae and G. viridula) demonstrate a comparative selectivity for the uptake of 1 into the epithelial cells to that observed for the living insect (S. Discher and W.B., unpublished data).
Conclusions
Studies on the sequestration of plant-derived metabolites by leaf beetles have suggested that the transition from one chemical defense to another is supported by the development of a dual chemical sequestration or dual mode of biosynthesis (31). Such a dual-defense system certainly enhances the success rate of host-plant shifts because the chance to find a new host, which provides precursors that can be sequestered and subsequently optimized into a novel defense line, is higher if the transport capabilities of the insect are not limited to a single compound. The dualism of sequestration and de novo biosynthesis in the basal group of the leaf beetles (19) may represent such a productive situation from which new modes of chemical defenses, even the shift to an obligatory sequestration, may originate. Therefore, the highly specific transport of salicin in C. populi and P. vitellinae did not necessarily evolve de novo. Recruitment of the ancestral-transport mechanism for the O-glucoside of 8-hydroxygeraniol (Fig. 1B and 1C) and its subsequent adaptation to salicin may have evolved more easily. Thus, the shift from de novo biosynthesis of iridoids to plant host-derived salicylaldehyde, although a spectacular phenotypic innovation, requires only a few mutations of ancestral processes. This process is facilitated also by the observation that the first enzymes in the reservoirs (glucosidases) are generally unspecific. Subsequent adaptation of the more selective oxidases (16, 32) is likely to occur and does not require enzymatic “innovations.” The highest evolved leaf beetles (e.g., Chrysomela interrupta, see Fig. 2) do not focus on single compounds but, rather, sequester a large number of plant-derived glucosides. After transport to the reservoir, the sugar moieties are removed and the free alcohols are esterified to butyrates (12). In line with the above considerations, the chemical defense in these beetles, rather than appearing constrained by inherited biochemical pathways, appears to be particularly flexible in evolutionary time.
Acknowledgments
We thank Claes Johansson, Angelika Berg, and Anja Biedermann for technical assistance; Drs. Bernd Schneider and Renate Ellinger for NMR analyses; Janine Rattke for high-resolution MS analyses; Dr. Helmar Görls for x-ray crystallography (Friedrich-Schiller-Universität, Jena, Germany); the helpful personnel at the Botanical Gardens in Jena for providing leaves; and Claes Gustafsson (Botanical Institute, Göteborg University, Sweden) for species determination of P. canadensis. This work was supported by Belgian Fund for Joint Basic Research Grant 2.4543.03 (to J.M.P.).
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY027591–AY027628 and AY027695–AY027760).
References
- 1.Hartmann, T., Witte, L. (1995) in Alkaloids: Chemical and Biological Perspectives, ed. Pelletier, S. W. (Pergamon, Oxford), pp. 155–233.
- 2.Malcolm, S. B. & Brower, L. P. (1989) Experientia 45, 284–295. [Google Scholar]
- 3.Dyer, L. A. & Bowers, M. D. (1996) J. Chem. Ecol. 22, 1527–1539. [DOI] [PubMed] [Google Scholar]
- 4.Nahrstedt, A. (1996) in Phytochemical Diversity and Redundancy in Ecological Interactions, eds. Romeo, J. T., Saunders, J. A. & Barbosa, P. (Plenum, New York), pp. 217–230.
- 5.Nishida, R. (2002) Annu. Rev. Entomol. 47, 57–92. [DOI] [PubMed] [Google Scholar]
- 6.Jolivet, P. H. A. (1988) in Biology of Chrysomelidae, eds. Jolivet, P. H. A., Petitpierre, E. & Hsiao, T. H. (Kluwer, Dordrecht, The Netherlands), pp. 1–24.
- 7.Pasteels, J. M. & Rowell-Rahier, M. (1991) Entomol. Gener. 15, 227–235. [Google Scholar]
- 8.Pasteels, J. M., Braekman, J. C., Daloze, D. & Ottinger, R. (1982) Tetrahedron 38, 1891–1897. [Google Scholar]
- 9.Oldham, N. J., Veith, M., Boland, W. & Dettner, K. (1996) Naturwissenschaften 83, 470–473. [Google Scholar]
- 10.Daloze, D. & Pasteels, J. M. (1994) J. Chem. Ecol. 20, 2089–2097. [DOI] [PubMed] [Google Scholar]
- 11.Pasteels, J. M., Duffey, S. & Rowell-Rahier, M. (1990) J. Chem. Ecol. 16, 211–222. [DOI] [PubMed] [Google Scholar]
- 12.Hilker, M. & Schulz, S. (1994) J. Chem. Ecol. 20, 1075–1093. [DOI] [PubMed] [Google Scholar]
- 13.Termonia, A. & Pasteels, J. M. (1999) Chemoecology 9, 13–23. [Google Scholar]
- 14.Pasteels, J. M., Rowell-Rahier, M., Braekman, J. C. & Daloze, D. (1984) Biochem. Syst. Ecol. 12, 395–406. [Google Scholar]
- 15.Termonia, A., Hsiao, T. H., Pasteels, J. M. & Milinkovitch, M. C. (2001) Proc. Natl. Acad. Sci. USA 98, 3909–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veith, M., Oldham, N. J., Dettner, K., Pasteels, J. M. & Boland, W. (1997) J. Chem. Ecol. 23, 429–443. [Google Scholar]
- 17.Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H. & Flook, P. (1994) Ann. Entomol. Soc. Am. 87, 651–701. [Google Scholar]
- 18.Loytynoja, A. & Milinkovitch, M. C. (2001) Bioinformatics 17, 573–574. [DOI] [PubMed] [Google Scholar]
- 19.Feld, B. K., Pasteels, J. M. & Boland, W. (2001) Chemoecology 11, 191–198. [Google Scholar]
- 20.Falconer, R. A., Jablonkai, I. & Toth, I. (1999) Tetrahedron Lett. 40, 8663–8666. [Google Scholar]
- 21.Tsvetkov, Y. E., Byramova, N. E. & Backinowsky, L. V. (1983) Carbohydr. Res. 115, 254–258. [Google Scholar]
- 22.Ueno K. (1984) Acta Crystallogr. C 40, 1726–1728. [Google Scholar]
- 23.Sachs, L. (1999) Angewandte Statistik (Springer, Berlin).
- 24.Pasteels, J. M., Rowell-Rahier, M., Braekman, J. C. & Dupont, A. (1983) Physiol. Entomol. 8, 307–314. [Google Scholar]
- 25.Yuasa, H., Saotome, C. & Kanie, O. (2002) Trends Glycosci. Glycotechnol. 14, 231–251. [Google Scholar]
- 26.Taylor, J. R. (1997) An Introduction to Error Analysis (Univ. Sci. Books, Mill Valley, CA).
- 27.Weibel, D. B., Oldham, N. J., Feld, B., Glombitza, G., Dettner, K. & Boland, W. (2001) Insect Biochem. Mol. Biol. 31, 583–591. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein, J. L. & Brown, M. S. (1990) Nature 343, 425–430. [DOI] [PubMed] [Google Scholar]
- 29.Søe, A. R. B., Bartram, S., Gatto, N. & Boland, W. (2004) Isotopes Environ. Health Stud. 40, 177–182. [DOI] [PubMed] [Google Scholar]
- 30.Frick, C. & Wink, M. (1995) J. Chem. Ecol. 21, 557–575. [DOI] [PubMed] [Google Scholar]
- 31.Termonia, A., Pasteels, J. M., Windsor, D. M. & Milinkovitch, M. C. (2002) Proc. R. Soc. London Ser. B 269, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brueckmann, M., Termonia, A., Pasteels, J. M. & Hartmann, T. (2002) Insect Biochem. Mol. Biol. 32, 1517–1523. [DOI] [PubMed] [Google Scholar]