Abstract
Physical activity and the ingestion of dietary fiber are non-drug alternatives commonly used as adjuvants to glycemic control in diabetic individuals. Among these fibers, we can highlight beta-glucans. However, few studies have compared isolated and synergic effects of physical exercise and beta-glucan ingestion, especially in type 2 diabetic rats. Therefore, we evaluated the effects beta-glucan (Saccharomyces cerevisiae) consumption, associated or not to exercise, on metabolic parameters of diabetic Wistar rats. The diabetes mellitus (DM) was induced by high-fat diet (HFD) associated with a low dose of streptozotocin (STZ—35 mg/kg). Trained groups were submitted to eight weeks of exercise in aquatic environment. In the last 28 days of experiment, animals received 30 mg/kg/day of beta-glucan by gavage. Isolated use of beta-glucan decreased glucose levels in fasting, Glycated hemoglobin (HbA1c), triglycerides (TAG), total cholesterol (TC), low-density lipoprotein (LDL-C), the atherogenic index of plasma. Exercise alone also decreased blood glucose levels, HbA1c, and renal lesions. An additive effect for reducing the atherogenic index of plasma and renal lesions was observed when both treatments were combined. It was concluded that both beta-glucan and exercise improved metabolic parameters in type 2 (HFD/STZ) diabetic rats.
Keywords: dietary fibers, glycemic control, metabolic profile
1. Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia, caused by the absence or reduction in insulin production (type 1 DM) as well as the resistance to the action of this hormone, featuring type 2 DM [1]. About 90% of DM cases are of type 2, and this fact is associated with increased incidence of obesity and obesity in the general population, especially in developing nations [2,3]. In addition, DM may predispose to diseases, such as retinopathy, nephropathy, neuropathy and heart disease, further aggravating the health condition of patients [2,4].
Glycemic control in diabetic patients can be achieved through the use of exogenous insulin and/oral hypoglycemic drugs [5]. However, the interaction between medications could cause side effects, and does not prevent the diseases associated with DM, making necessary the search for non-pharmacological alternatives to assist in the maintenance of blood sugar levels [4,6]. In this sense, the practice of physical exercise and diet therapy has been recommended as a treatment or therapeutic adjuvant [7]. Physical exercise increases the uptake and utilization of circulating glucose and improves insulin sensitivity [8]. The ingestion of some dietary fibers has also been reported to show antihyperglycemic action—mainly by reducing the absorption of carbohydrates and lipids in the intestine. Among these fibers, we can highlight beta-glucans that are polysaccharides found in the composition of cereal, fungi, bacteria and some grass cell walls [9].
The chemical structure of beta-glucan varies according to its origin [10]. Beta-glucans found in plants and cereals are linear and have branchings with β-1,3/1,4-type glycosidic linkages (soluble with low molecular weight), while those found in yeasts and fungi have β-1,3/1,6-type glycosidic linkages (insoluble with high molecular weight) [11]. These conformations make beta-glucans exhibit distinct physicochemical characteristics, such as molecular mass and solubility [12,13]. Cereal beta-glucans are reported to show metabolic potential, while those from fungi and yeast increase immune response [10,14,15]. Although fungi beta-glucans are recognized to modulate the immune response [16], recent studies from our group have also demonstrated interesting metabolic effects of yeast beta-glucans (Saccharomyces cereviseae) [10,17,18].
Considering the previously known effects of both exercise and the beta-glucan on glycemic control and metabolism, it is necessary to investigate the concomitant action of these agents in the treatment of type DM. In addition, there is a shortage of studies evaluating such effects in type 2 diabetes model. Thus, the present study aimed to evaluate the effects of beta-glucan (Saccharomyces cerevisiae), associated or not to physical exercise, on the metabolic parameters of type 2 diabetic rats (HFD/STZ).
2. Materials and Methods
2.1. Animals
This study was approved by the Ethics Committee on Animal Use of Federal University of Lavras (CEUA protocol 002/2015). The animals were kept in accordance with the Guide to the Care and Use of Experimental Animals (1993). The number of animals per group was kept at a minimum for ethical reasons but still enough to reach statistical significance. Thus, a power calculation test was performed to determine the sample size. The sample size was determined to provide 80% power to recognize a significant difference of 20% among groups and a standard deviation of 15% with a 95% confidence interval (α = 0.05).
We used adult male Wistar rats (Rattus norvegicus albinos)—from the Animal Laboratory of the Federal University of Lavras (UFLA). Animals weighed 195.0 ± 15.7 g at the beginning of the study. Initially, rats were submitted to seven days of acclimatization in polypropylene boxes (dimensions 41 cm × 34 cm × 17.5 cm), containing wood shavings (for absorbing urine and water). Six animals were placed in each box. Throughout the experimental period, the rodents remained under controlled temperature (22 ± 2 °C), humidity (45% ± 15%) and luminosity (12–12 h light-dark cycle) conditions. High-fat diet and water were provided ad libitum throughout the experiment.
2.2. Induction of Diabetes Mellitus
At the end of the acclimatization period, all animals were submitted to type 2 diabetes induction protocol as described by Wang et al. [19]. The animals received high-fat diet (HFD—25% fat, 48% carbohydrates and 20% protein) for 28 days. Then, a low dose of streptozotocin (dissolved in citrate buffer—pH = 4.5) was injected intraperitoneally (STZ—35 mg/kg). Blood glucose levels were measured 48 h after STZ injection. Rats with blood glucose levels above 200 mg/dL [19] were considered diabetic. This model mimic advanced stages of type 2 diabetes in humans [19,20]. Rats that did not reach these glucose values were excluded from the experiment. Glycemia was checked weekly to ensure that diabetes was not reversed.
After diabetes induction, animals were randomly divided into four groups containing six animals each. A completely randomized experimental design in a 2 × 2 factorial scheme was used: with or without exercise and with or without beta-glucan.
2.3. Physical Training
After an acclimatization period, an adaptation to the aquatic environment was performed. Animals undergoing physical training remained for two hours daily, during seven days, in a polyethylene tank with a total capacity of 300 L, containing five centimeters of water at a temperature of approximately 32 ± 2 °C. The purpose of this acclimatization was to reduce stress against the aquatic environment, without causing, however, changes arising from the physical training [21].
In the following week, animals were submitted to progressive swimming sessions with time increments. This phase consisted of swimming without load, in 50 cm of water (in order to avoid animal tail contact with the bottom of the tank), where the animals swam 10 min in the first day, increasing 10 min daily until the end of six days, when each animal was swimming for 60 uninterrupted minutes without load [22].
In the subsequent eight weeks, the animals swam for 60 min daily, five times a week with a load of 5% of their body weight. This load causes improvement in the animals’ endurance capacity, characterizing moderate intensity aerobic exercise [22]. After training sessions, we dried the animals with absorbent towels, before returning them to their cages [21].
2.4. Administration of Beta-Glucans
Simultaneously with training, in the last 28 days of the experiment, the animals in beta-glucan groups received a experimental solution and controls received saline—both by gavage. Beta-glucan solutions that contained 30 mg/kg of powder diluted in 0.3 mL saline solution prepared daily.
Beta-glucan used in the present study were derived from yeast Saccharomyces cerevisiae, with structural β-1,3/1,6 conformation. The beta-glucan powder presented the following composition: β-glucans—Min. 60.0%; Crude Protein—Max. 8.0%; pH (solution 2%) 4.0–7.0; Ash—Max. 10.0 g/100 g. Distribution of particle size: mean—41 μm; <20 μm 19%; 20–50 μm 43%; 50–100 μm 28%; 100–200 μm 10%; >200 μm 0%; Fluidity (seconds)—70.2; Angle of repose (degrees) 31.2; Compressibility 37%; Water retention capacity (mean) 7.4; and Solubility rate in water 7.9. The solutions were always administered daily in the morning. In animals under physical training, gavage was always performed with a minimum of 45 min before exercise, as described in previous studies [21,23].
2.5. Collection of Biological Material and Assessment of the Atherogenic Index of Plasma
At the end of the experimental period (eight weeks), the animals fasted for eight hours. Euthanasia was conducted by cardiac puncture under anesthesia (sodium thiopental 50 mg/kg ip). Glycated hemoglobin (HbA1c) and other blood biochemical parameters such as glucose, triacylglycerols (TAG), high density lipoprotein (HDL-C) and total cholesterol (TC) were determined using commercial kits (Labtest Diagnostica®, Belo Horizonte, Brazil and Gold analyzes diagnoses®, Belo Horizonte, Brazil) as described by Amr and Abeer [24]. The low-density lipoprotein (LDL) + very-low-density lipoprotein cholesterol (VLDL-C) levels of each animal were obtained by using the following equation: total cholesterol − HDL-C = LDL + VLDL-C [25]. Additionally, the animals’ atherogenic index of plasma was calculated using the equation: log (TG)/(HDL-C), which is used as a significant predictor of atherosclerosis [26]. This index was used because type 2 diabetes increases one’s chances of developing atherosclerosis [27].
2.6. Lee Index Assessment and Chemical Composition of the Body
The Lee index was calculated dividing the cubic root of body weight (grams) by the naso-anal length (cm) [18,28]. Internal organs, skin, head, feet and tail were removed from the animals and the clean carcasses were weighed and processed. Percentages of water, protein, fat and mineral matter present in the carcasses were evaluated by the meat FoodScan™ NIR analyzer (near-infra-red) (Foss, Warrington, UK) as performed by Vickers et al. [29]. This evaluation method of carcass composition has been considered as the gold standard [29].
2.7. Histological Analysis
Fragments of the right kidney and liver were fixed in 10% buffered formalin for 48 h, and then processed routinely for preparation of histological slices, which were then colored with hematoxylin-eosin [30]. An experienced veterinary pathologist conducted all histopathologic analysis (blind about experimental treatments). Tissue integrity, as well as the presence of alterations, were considered in the evaluations. Liver tissue ratings were assigned according to the presence and/or degree of steatosis as follows: no change—1; discreet—2; light—3; moderate—4; and severe—5. Steatosis was classified according to the presence of vacuoles in hepatocytes. Staining was performed with Periodic acid-Schiff (PAS) indicating accumulation of lipids or glycogen.
Similarly, the presence of renal lesions was scored as: no change—1; mild degeneration—2; low degeneration—3; moderate degeneration—4; marked degeneration 5. We observed the presence of alterations in the proximal and distal convoluted tubules, and the presence of calcifications in the glomerulus.
2.8. Statistical Analysis
Data were subjected to analysis of variance (two-way ANOVA) and means were compared by Tukey test (p < 0.05). Nonparametric data of liver and kidney damage scores were analyzed by the Kruskal–Wallis test (p < 0.05). We performed all analyses using statistical program Sisvar (version 5.3, Universidade Federal de Lavras, Lavras, Minas Gerais, Brazil) [31].
3. Results
Animals submitted to physical training, or consuming beta-glucan isolated and in association, presented lower fast blood glucose and HbA1c levels than diabetic animals (Table 1). Serum levels of TAG, TC and LDL-C were significantly reduced in animals consuming beta-glucan, independently of physical training. In addition, HDL-C levels were higher in animals treated with beta-glucan. Exercise did not significantly alter this parameter (Table 1). The atherogenic index of plasma in animals treated with beta-glucan was lower in comparison to without treatment. An additive effect of beta-glucan and physical exercise was observed for the atherogenic index of plasma. Blood parameters and atherogenic index of plasma means and standard deviations are presented in Table 1.
Table 1.
Biochemical parameters and atherogenic index of plasma in type 2 diabetic rats (high-fat diet/streptozotocin) submitted to physical training and treated beta-glucan (30 mg/kg/day).
| Beta-Glucan | Physical Training | ||
|---|---|---|---|
| Without | With | ||
| Glucose (mg/dL) | Without | 371.0 (±21.4) A,a | 335.0 (±10.4) b |
| With | 311.0 (±25.0) b | 327.5 (±42.6) | |
| HbA1c (mg/dL) | Without | 9.4 (±0.4) A,a | 8.8 (±0.3) B |
| With | 8.33 (±0.1) b | 8.8 (±0.4) | |
| Triacylglycerols (mg/dL) | Without | 105.8 (±11.1) a | 99.7 (±2.2) a |
| With | 71.5 (±7.2) b | 57.5 (±12.8) b | |
| Total cholesterol (mg/dL) | Without | 88.8 (±22.9) a | 85.4 (±9.4) a |
| With | 65.1 (±3.3) b | 63.8 (±7.2) b | |
| HDL-C (mg/dL) | Without | 34.33 (±3.8) a | 37.7 (±6.9) |
| With | 42.66 (±5.2) b | 44.26 (±4.0) | |
| LDL-C (mg/dL) | Without | 34.6 (±20.1) a | 33.4 (±5.8) a |
| With | 10.95 (±3.4) b | 19.6 (±4.9) b | |
| Atherogenic index of plasma | Without | 1.6 (±0.6) a | 1.3 (±0.2) a |
| With | 0.6 (±0.1) A,b | 0.4 (±0.1) B,b | |
a,b Means followed by different letters in columns indicate significant differences between groups with and without beta-glucan treatment (p < 0.05); A,B Means followed by different letters in lines indicate significant difference between groups with and without physical training (p < 0.05).
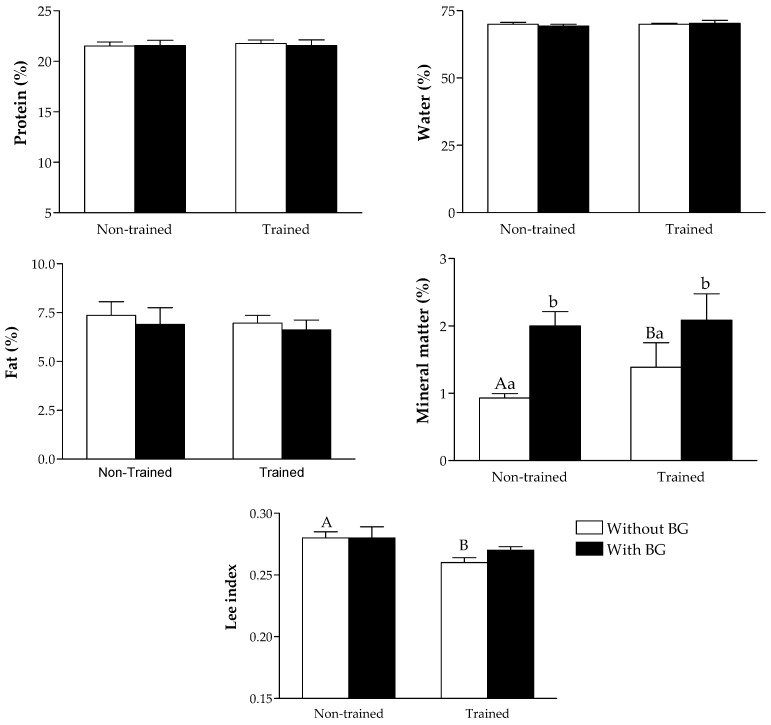
All treatments promoted similar results in the percentage of protein, fat and water in animals’ carcasses. An increase in the percentage of mineral matter was observed in groups under physical training and beta-glucan consumption, with an extra increase when both treatments were associated. Exercise promoted a decrease in the Lee index compared to controls, with similar results among the other groups (Figure 1).
Figure 1.
Chemical body composition (water, protein, fat and mineral matter) and Lee index of type 2 diabetic rats (high-fat diet/streptozotocin) submitted to physical training and treated with beta-glucan (30 mg/kg/day). A,B Significant difference between trained and non-trained groups; a,b Significant difference between groups with and without beta-glucans.
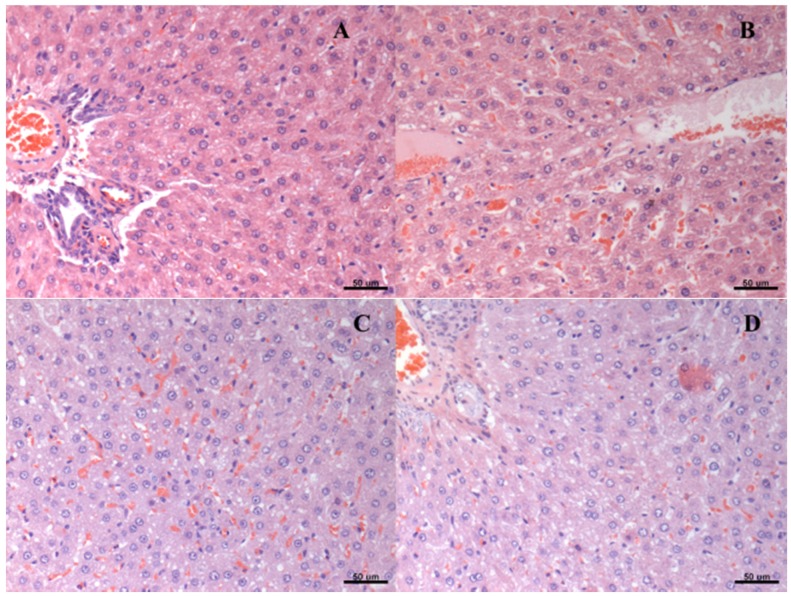
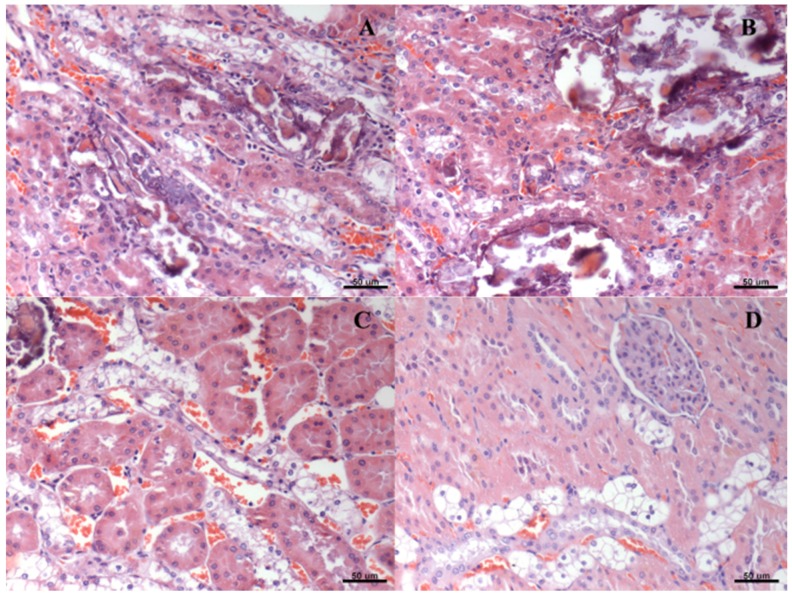
Liver histopathology slices revealed similar signs of steatosis in all groups (Table 2). Likewise, hydropic degeneration was found in the renal tissue from all groups. The degree of these lesions was attenuated by both physical exercise and beta-glucan ingestion (Table 3). Figure 2 and Figure 3 represent, respectively, hepatic steatosis and renal degeneration in the different experimental groups.
Table 2.
Degree of hepatic steatosis in type 2 diabetic rats (HFD/STZ) submitted to physical training and/or treated with beta-glucans (30 mg/kg/day).
| Group | Score of Steatosis | ||||
|---|---|---|---|---|---|
| * | ** | *** | **** | ***** | |
| A | 0 | 3 | 0 | 3 | 0 |
| B | 1 | 3 | 2 | 0 | 0 |
| C | 2 | 3 | 0 | 1 | 0 |
| D | 1 | 5 | 0 | 0 | 0 |
* No change. ** Discreet Degeneration; *** Mild degeneration; **** Moderate degeneration; ***** Marked degeneration; A: diabetes mellitus; B: diabetes mellitus + beta-glucan; C: diabetes mellitus + exercise; D: diabetes mellitus + beta-glucan + exercise.
Table 3.
Degree of renal degeneration in type 2 diabetic rats (HFD/STZ) submitted to physical training and/or treated with beta-glucans (30 mg/kg/day).
| Score of Renal Degeneration | |||||
|---|---|---|---|---|---|
| Group | * | ** | *** | **** | ***** |
| A | 0 | 0 | 0 | 2 | 4 |
| B | 0 | 0 | 0 | 6 | 0 |
| C | 0 | 0 | 1 | 5 | 0 |
| D # | 0 | 0 | 1 | 5 | 0 |
* No change; ** Discreet Degeneration; *** Mild degeneration; **** Moderate degeneration; ***** Marked degeneration; # Difference compared to the DM group; A: diabetes mellitus; B: diabetes mellitus + beta-glucan; C: diabetes mellitus + exercise; D: diabetes mellitus + beta-glucan + exercise.
Figure 2.
Histological representation (hematoxylin and eosin—20×) of degrees of hepatic steatosis in type 2 diabetic rats (HFD/STZ) submitted to physical training and/or treated with beta-glucans (30 mg/kg/day). (A) diabetes mellitus; (B) diabetes mellitus + beta-glucan; (C) diabetes mellitus + exercise; (D) diabetes mellitus + beta-glucan + exercise.
Figure 3.
Histological representation (hematoxylin and eosin—20×) of degrees of renal degeneration in type 2 diabetic rats (HFD/STZ) submitted to physical training and/or treated with beta-glucans (30 mg/kg/day). (A) diabetes mellitus; (B) diabetes mellitus + beta-glucan; (C) diabetes mellitus + exercise; (D) diabetes mellitus + beta-glucan + exercise.
4. Discussion
The main findings of this study were related to improved glycemic control and reduced predisposition to atherosclerosis in animals subjected to both exercise beta-glucan consumption. Moreover, circulating lipoproteins levels, such as total cholesterol, LDL-C, and HDL-C, were improved in animals consuming beta-glucan, independently of physical exercise.
The effects of physical exercise on the improvement of glycemic control in diabetic patients (decrease in HbA1c and fasting glucose) are frequently reported [32,33,34]. Generally, this effect is due to the increased glucose uptake by skeletal muscle during exercise and increased insulin sensitivity for some hours after physical activity [35]. A beneficial effect in the glycemic control, in our study, was also observed after beta-glucan ingestion, as reported elsewhere in both animal [10] and in human studies [36]. Blood glucose control by beta-glucan consumption is probably due to the fact that these fibers form a gelatinous barrier in the intestinal lumen, hindering the absorption of carbohydrates and lipids by enterocytes [10,37,38]. In this sense, the same mechanism can be used to justify a reduction in circulating levels of total cholesterol, LDL-C and TAG found in groups treated with beta-glucan, with and without exercise. The improvement of the lipid profile, despite the consumption of beta-glucan, was a feature also observed in previous studies from our group [10,17]. The lower lipid absorption in the intestine favors the use of excessive cholesterol to the formation of bile salts in the liver, causing decreased blood concentrations of total cholesterol and LDL-C [39]. This mechanism is generally used to explain the anti-hypercholesterolemic effect of dietary fibers [39].
Among the possible beta-glucan’s action, we can highlight the stimulation of intestinal motility, as well as changes in the microbiota and modulation of hormones secretion in the intestine [27,40,41]. Intestinal motility can be stimulated by the increase in the viscosity of the digesta in the lumen, due to the formation of a gelatinous layer [42,43]. In addition, beta-glucan decreases carbohydrates and lipid absorption [42], and consequently decreasing constipation problems [11]. High molecular weight beta-glucans (1,3/1,6) can also serve as a substrate for symbiotic microorganisms present in the intestine, increasing IgA and lysozyme secretion, and, as a consequence, immune resistance [40]. Another mechanism related to the functional effect of beta-glucans is satiety, mediated by gastrointestinal hormones [41]. Beta-glucans modulate the secretion of ghrelin and peptide YY, in order to inhibit hunger, acting indirectly in glycemic and lipidemic control [44].
In this study, we did not observe reduction of circulating lipids in trained animals. This may be related to the training time or duration of exercise sessions. Moura et al. [45] also found similar levels of HDL-C, LDL-C and TAG in diabetic rats (induced by alloxan) and subjected to 44 days of training, compared to sedentary diabetic rats. Another study demonstrated that twelve weeks of aquatic training decreased cholesterol levels and TAG in diabetic Zucker rats [46]. However, in the present study, no significant differences were observed in circulating lipids in animals submitted to physical training, and the atherosclerotic plasma index was reduced when there was an association of exercise and beta-glucan consumption. This additive effect may be related to improvement of the lipid profile provided by the dietary fiber [47], and to the recognized cardiovascular benefits of exercise [48].
Liver and renal lesions observed in all groups are consistent with those observed in type 2 diabetes, where circulating lipid levels promote increased fat deposition both in the liver and kidney [49,50]. However, even with the benefits observed with beta-glucan consumption or exercise, no changes were found in the degree of steatosis in any treatment. Beta-glucan consumption did not significantly alter steatosis either in a recent study of our group that investigated the effects of these fibers in rats submitted to high-fat diet [18]. On the other hand, it was observed that, in Sprague–Dawley rats, hepatic steatosis was reversed after eight weeks of treadmill exercise associated with restrictive diet (low-fat) [51]. Thus, it is possible that, in this study, steatosis was not attenuated because the animals were consuming a high-fat diet throughout the experimental period.
Regarding the effects of exercise, with or without the beta-glucan on the attenuation of renal lesions, it can be considered two mechanisms. The first one involves the reduction in lipotoxicity against moderate exercise [52], since the oxidative stress observed in diabetic patients is one of the factors that predispose to kidney damage [53]. The second one, more likely to explain the results of the present study, is the fact that exercise in moderate intensity promotes improvement in glycemic control, which consequently reduces the generation of advanced glycation-end products (AGE) [54]. Thus, the higher the blood glucose levels, the higher the formation of AGE that attack the kidney tissue and cause diabetic nephropathy [55].
Results of the present research show very promising effects of beta-glucan ingestion for glucose control. Complimentary studies are encouraged, evaluating insulin/leptin levels and inflammatory and cardiovascular parameters as well. The improvement of metabolic parameters in animals that consumed beta-glucans may be related to a decrease in the absorption of nutrients that increase plasma levels of glucose and lipids [37,39]. These changes were not as evident in animals subjected to exercise, possibly due to high-fat diet maintenance for the entire period.
5. Conclusions
Both exercise and beta-glucan consumption alone improved glycemic control in diabetic rats. In the present study, the combination of exercise and beta-glucans improved the atherosclerotic index and decreased renal lesions when compared to the isolated use of the treatments.
Acknowledgments
The authors thank the Coordination of Improvement of Higher Education Personnel (CAPES), the Research Foundation of the State of Minas Gerais (FAPEMIG), the National Council for Research and Technological Development (CNPq), and the Federal University of Lavras (UFLA), Brazil.
Author Contributions
E.F.A., L.J.P. and M.G.Z. conceptualized the study. E.F.A., A.R.V.L., P.N.G. and I.E.N. conducted the experiments. D.R.O. performed histopathologic analysis. M.G.Z. contributed to statistical analysis. E.F.A., F.H.F.A., and L.J.P. were involved in writing and editing the manuscript. All authors participated in the design of the study, study supervision, data interpretation, and revision and approval of the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 3.Kahn S.E., Cooper M.E., del Prato S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet (Lond. Engl.) 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standards of medical care in diabetes—2015 abridged for primary care providers. Clin. Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teixeira-Lemos E., Nunes S., Teixeira F., Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: Focus on its antioxidant and anti-inflammatory properties. Cardiovasc. Diabetol. 2011;10:12. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens J.W., Khunti K., Harvey R., Johnson M., Preston L., Woods H.B., Davies M., Goyder E. Preventing the progression to type 2 diabetes mellitus in adults at high risk: A systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res. Clin. Pract. 2015;107:320–331. doi: 10.1016/j.diabres.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Thompson D., Karpe F., Lafontan M., Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012;92:157–191. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- 9.Samuelsen A.B.C., Schrezenmeir J., Knutsen S.H. Effects of orally administered yeast-derived beta-glucans: A review. Mol. Nutr. Food Res. 2014;58:183–193. doi: 10.1002/mnfr.201300338. [DOI] [PubMed] [Google Scholar]
- 10.De Oliveira Silva V., Lobato R.V., Andrade E.F., de Macedo C.G., Napimoga J.T.C., Napimoga M.H., Messora M.R., Murata R.M., Pereira L.J. β-Glucans (Saccharomyces cereviseae) Reduce Glucose Levels and Attenuate Alveolar Bone Loss in Diabetic Rats with Periodontal Disease. PLoS ONE. 2015;10:e0134742. doi: 10.1371/journal.pone.0134742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahar S., Swami G., Nagpal N., Nagpal M.A., Singh G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011;2:94–103. doi: 10.4103/2231-4040.82953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonck E., Stuyven E., Goddeeris B., Cox E. The effect of beta-glucans on porcine leukocytes. Vet. Immunol. Immunopathol. 2010;135:199–207. doi: 10.1016/j.vetimm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani M.S., Bellini M.F., Angeli J.P.F., Oliveira R.J., Silva A.F., Ribeiro L.R. Beta-Glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. 2008;658:154–161. doi: 10.1016/j.mrrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Andrade E.F., Lobato R.V., Araújo T.V., Zangerônimo M.G., Sousa R.V., Pereira L.J. Effect of beta-glucans in the control of blood glucose levels of diabetic patients: A systematic review. Nutr. Hosp. 2014;31:170–177. doi: 10.3305/nh.2015.31.1.7597. [DOI] [PubMed] [Google Scholar]
- 15.Akramiene D., Kondrotas A., Didziapetriene J., Kevelaitis E. Effects of beta-glucans on the immune system. Medicina (Kaunas) 2007;43:597–606. [PubMed] [Google Scholar]
- 16.Stier H., Ebbeskotte V., Gruenwald J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-d-glucan. Nutr. J. 2014;13:38. doi: 10.1186/1475-2891-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobato R.V., de Silva V.O., Andrade E.F., Orlando D.R., Zangeronimo M.G., de Sousa R.V., Pereira L.J. Metabolic effects of β-glucans (Saccharomyces cerevisae) per os administration in rats with streptozotocin-induced diabetes. Nutr. Hosp. 2015;32:256–264. doi: 10.3305/nh.2015.32.1.9013. [DOI] [PubMed] [Google Scholar]
- 18.De Araújo T.V., Andrade E.F., Lobato R.V., Orlando D.R., Gomes N.F., de Sousa R.V., Zangeronimo M.G., Pereira L.J. Effects of beta-glucans ingestion (Saccharomyces cerevisiae) on metabolism of rats receiving high-fat diet. J. Anim. Physiol. Anim. Nutr. (Berl) 2016 doi: 10.1111/jpn.12452. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., Duan G., Lu Y., Pang S., Huang X., Jiang Q., Dang N. The effect of simvastatin on glucose homeostasis in streptozotocin induced type 2 diabetic rats. J. Diabetes Res. 2013;2013:274986. doi: 10.1155/2013/274986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skovsø S. Modeling type 2 diabetes in rats using high-fat diet and streptozotocin. J. Diabetes Investig. 2014;5:349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rambo L.M., Ribeiro L.R., Oliveira M.S., Furian A.F., Lima F.D., Souza M.A., Silva L.F.A., Retamoso L.T., Corte C.L.D., Puntel G.O., et al. Additive anticonvulsant effects of creatine supplementation and physical exercise against pentylenetetrazol-induced seizures. Neurochem. Int. 2009;55:333–340. doi: 10.1016/j.neuint.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Gobatto C.A., de Mello M.A., Sibuya C.Y., de Azevedo J.R., dos Santos L.A., Kokubun E. Maximal lactate steady state in rats submitted to swimming exercise. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;130:21–27. doi: 10.1016/S1095-6433(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 23.Andrade E.F., Lobato R.V., de Araújo T.V., Orlando D.R., da Costa D.V., de Oliveira Silva V., Rogatto G.P., Zangeronimo M.G., Rosa P.V., Pereira L.J. Adaptation to physical training in rats orally supplemented with glycerol. Can. J. Physiol. Pharmacol. 2015;93:63–69. doi: 10.1139/cjpp-2014-0312. [DOI] [PubMed] [Google Scholar]
- 24.Amr A.R., Abeer E.E.-K. Hypolipideimic and Hypocholestermic Effect of Pine Nuts in Rats Fed High Fat, Cholesterol-Diet. World Appl. Sci. J. 2011;15:1667–1677. [Google Scholar]
- 25.Martinez-flores H.E., Kil Y. Effect of high fiber products on blood lipids and lipoproteins in hamsters. Nutr. Res. 2004;24:85–93. doi: 10.1016/j.nutres.2003.08.016. [DOI] [Google Scholar]
- 26.Hemmati M., Zohoori E., Mehrpour O., Karamian M., Asghari S., Zarban A., Nasouti R. Anti-atherogenic potential of jujube, saffron and barberry: Anti-diabetic and antioxidant actions. EXCLI J. 2015;14:908–915. doi: 10.17179/excli2015-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleissner C.A., Galkina E., Nadler J.L., Ley K. Mechanisms by which diabetes increases cardiovascular disease. Drug Discov. Today Dis. Mech. 2007;4:131–140. doi: 10.1016/j.ddmec.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M.O. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am. J. Physiol. 1929;89:24–33. [Google Scholar]
- 29.Vickers S.P., Cheetham S., Headland K., Dickinson K., Grempler R., Mayoux E., Mark M., Klein T. Combination of the sodium-glucose cotransporter-2 inhibitor empagliflozin with orlistat or sibutramine further improves the body-weight reduction and glucose homeostasis of obese rats fed a cafeteria diet. Diabetes Metab. Syndr. Obes. Targets Ther. 2014;7:265–275. doi: 10.2147/DMSO.S58786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade E.F., Lobato R.V., Araújo T.V., Orlando D.R., Gomes N.F., Alvarenga R.R., Rogatto G.P., Zangeronimo M.G., Pereira L.J. Metabolic effects of glycerol supplementation and aerobic physical training on Wistar rats. Can. J. Physiol. Pharmacol. 2014;92:744–751. doi: 10.1139/cjpp-2014-0187. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotec. 2011;35:1039–1042. [Google Scholar]
- 32.Hall K.E., McDonald M.W., Grisé K.N., Campos O.A., Noble E.G., Melling C.W.J. The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced Type 1 diabetic rodents. Metabolism. 2013;62:1485–1494. doi: 10.1016/j.metabol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Ghiasi R., Ghadiri Soufi F., Somi M.H., Mohaddes G., Mirzaie Bavil F., Naderi R., Alipour M.R. Swim Training Improves HOMA-IR in Type 2 Diabetes Induced by High Fat Diet and Low Dose of Streptozotocin in Male Rats. Adv. Pharm. Bull. 2015;5:379–384. doi: 10.15171/apb.2015.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silveira A.P.S., Bentes C.M., Costa P.B., Simão R., Silva F.C., Silva R.P., Novaes J.S. Acute effects of different intensities of resistance training on glycemic fluctuations in patients with type 1 diabetes mellitus. Res. Sports Med. 2014;22:75–87. doi: 10.1080/15438627.2013.852096. [DOI] [PubMed] [Google Scholar]
- 35.Borghouts L.B., Keizer H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000;21:1–12. doi: 10.1055/s-2000-8847. [DOI] [PubMed] [Google Scholar]
- 36.He L., Zhao J., Huang Y., Li Y. The difference between oats and beta-glucan extract intake in the management of HbA1c, fasting glucose and insulin sensitivity: A meta-analysis of randomized controlled trials. Food Funct. 2016;7:1413–1428. doi: 10.1039/C5FO01364J. [DOI] [PubMed] [Google Scholar]
- 37.Tappy L., Gügolz E., Würsch P. Effects of breakfast cereals containing various amounts of beta-glucan fibers on plasma glucose and insulin responses in NIDDM subjects. Diabetes Care. 1996;19:831–834. doi: 10.2337/diacare.19.8.831. [DOI] [PubMed] [Google Scholar]
- 38.Choi J.S., Kim H., Jung M.H., Hong S., Song J. Consumption of barley beta-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010;54:1004–1013. doi: 10.1002/mnfr.200900127. [DOI] [PubMed] [Google Scholar]
- 39.Rideout T.C., Harding S.V., Jones P.J., Fan M.Z. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: Current understandings and future research priorities. Vasc. Health Risk Manag. 2008;4:1023–1033. doi: 10.2147/VHRM.S3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raa J. Immune modulation by non-digestible and non-absorbable beta-1,3/1,6-glucan. Microb. Ecol. Health Dis. 2015;26:27824. doi: 10.3402/mehd.v26.27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rebello C.J., Burton J., Heiman M., Greenway F.L. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial. J. Diabetes Complicat. 2015;29:1272–1276. doi: 10.1016/j.jdiacomp.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyna N.Y., Cano C., Bermúdez V.J., Medina M.T., Souki A.J., Ambard M., Nuñez M., Ferrer M.A., Inglett G.E. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. Am. J. Ther. 2003;10:438–443. doi: 10.1097/00045391-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Battilana P., Ornstein K., Minehira K., Schwarz J.M., Acheson K., Schneiter P., Burri J., Jéquier E., Tappy L. Mechanisms of action of beta-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. 2001;55:327–333. doi: 10.1038/sj.ejcn.1601160. [DOI] [PubMed] [Google Scholar]
- 44.Vitaglione P., Lumaga R.B., Stanzione A., Scalfi L., Fogliano V. β-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite. 2009;53:338–344. doi: 10.1016/j.appet.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Moura L.P., Puga G.M., Beck W.R., Teixeira I.P., Ghezzi A.C., Silva G.A., Mello M.A.R. Exercise and spirulina control non-alcoholic hepatic steatosis and lipid profile in diabetic Wistar rats. Lipids Health Dis. 2011;10:2–7. doi: 10.1186/1476-511X-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Lemos E.T., Pinto R., Oliveira J., Garrido P., Sereno J., Mascarenhas-Melo F., Páscoa-Pinheiro J., Teixeira F., Reis F. Differential effects of acute (extenuating) and chronic (training) exercise on inflammation and oxidative stress status in an animal model of type 2 diabetes mellitus. Mediat. Inflamm. 2011;2011:253061. doi: 10.1155/2011/253061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bays H.E., Evans J.L., Maki K.C., Evans M., Maquet V., Cooper R., Anderson J.W. Chitin-glucan fiber effects on oxidized low-density lipoprotein: A randomized controlled trial. Eur. J. Clin. Nutr. 2013;67:2–7. doi: 10.1038/ejcn.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts C.K., Chen A.K., Barnard R.J. Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis. 2007;191:98–106. doi: 10.1016/j.atherosclerosis.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Alsaad K.O., Herzenberg A.M. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: An update. J. Clin. Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birkenfeld A.L., Shulman G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 Diabetes. Hepatology. 2014;59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauthier M.-S., Couturier K., Latour J.-G., Lavoie J.-M. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J. Appl. Physiol. 2003;94:2127–2134. doi: 10.1152/japplphysiol.01164.2002. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh S., Khazaei M., Moien-Afshari F., Ang L.S., Granville D.J., Verchere C.B., Dunn S.R., McCue P., Mizisin A., Sharma K., et al. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am. J. Physiol. Renal Physiol. 2009;296:700–708. doi: 10.1152/ajprenal.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka Y., Kume S., Araki S., Isshiki K., Chin-Kanasaki M., Sakaguchi M., Sugimoto T., Koya D., Haneda M., Kashiwagi A., et al. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int. 2011;79:871–882. doi: 10.1038/ki.2010.530. [DOI] [PubMed] [Google Scholar]
- 54.Boor P., Celec P., Behuliak M., Grančič P., Kebis A., Kukan M., Pronayová N., Liptaj T., Ostendorf T., Šebeková K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009;58:1669–1677. doi: 10.1016/j.metabol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 55.Yamagishi S.-I., Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid. Med. Cell. Longev. 2010;3:101–108. doi: 10.4161/oxim.3.2.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]