Abstract
The aim of this systematic review is to assess whether metformin could change the concentration of serum homocysteine (Hcy) with and without simultaneous supplementation of B-group vitamins or folic acid. A literature search was conducted in PubMed, EmBase, and Cochrane Central Register of Controlled Trials (CENTRAL) to identify randomized controlled trials (RCTs) reporting the concentration of serum Hcy in metformin-treated adults. Meta-analysis was applied to assess the association between metformin and the changes of Hcy concentration. Twelve publications were included in this study. In the overall analysis, metformin administration was not statistically associated with the change of Hcy when compared with the control treatment (mean difference (MD), 0.40 μmol/L; 95% confidence interval (CI), −0.07~0.87 μmol/L, p = 0.10). In the subgroup analysis, metformin was significantly associated with an increased concentration of Hcy in the absence of exogenous supplementation of folic acid or B-group vitamins (MD, 2.02 μmol/L; 95% CI, 1.37~2.67 μmol/L, p < 0.00001), but with a decreased concentration of serum Hcy in the presence of these exogenous supplementations (MD, −0.74 μmol/L; 95% CI, −1.19~−0.30 μmol/L, p = 0.001). Therefore, although the overall effect of metformin on the concentration of serum Hcy was neutral, our results suggested that metformin could increase the concentration of Hcy when exogenous B-group vitamins or folic acid supplementation was not given.
Keywords: metformin, homocysteine, vitamin B12, folic acid, systematic review, meta-analysis
1. Introduction
Metformin, a first-line drug for type 2 diabetes mellitus (T2DM) recommended by most guidelines of diabetes, is also widely used in patients with polycystic ovary syndrome (PCOS), pre-diabetes, and other diseases involving insulin resistance [1,2]. However, Vitamin B12 deficiency was noted to be a potential disadvantage of metformin by the latest American Diabetes Association (ADA) guidelines [1]. A previous meta-analysis demonstrated that metformin treatment was associated with a decreased concentration of serum Vitamin B12 in a dose-dependent manner [3].
Homocysteine (Hcy) is a key component in the one-carbon pathway of methionine metabolism, which plays a dominant role in DNA methylation. The accumulation of Hcy, known as hyperhomocysteinemia (HHcy), is often resulted from Vitamin B12 deficiency [4], and is associated with an increased risk of cardiovascular diseases, cognitive impairment, cancer, chronic renal failure and other chronic diseases [4,5,6,7,8,9,10,11]. However, no consensus was reached on whether metformin could induce Hcy elevation. This systematic review aimed to assess the association between metformin administration and the changes of serum Hcy concentration with and without simultaneous supplementation of B-group vitamins or folic acid.
2. Materials and Methods
2.1. Literature Search and Study Selection
This systematic review was conducted and reported according to the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. Literature search was conducted systematically in PubMed, EmBase, and Cochrane Central Register of Controlled Trials (CENTRAL) until January, 2016 by two authors (Q.Z. and S.L.). ‘Metformin’ and ‘homocysteine’ were used as keywords in the literature search. The references of original studies were also screened to ensure that potentially eligible publications were included. A detailed search strategy was presented in Supplementary Materials Figure S1.
We included studies which met the following criteria: (1) metformin was given as intervention while non-biguanide agents as control; (2) reporting serum Hcy concentrations as one of the outcomes; (3) designed as randomized controlled trials (RCTs). Studies with inadequate outcome data of interest, published in non-English languages, and duplicated reports were excluded. Two authors (Q.Z. and S.L.) reviewed all searched papers independently. Disagreements were settled by consensus between the two reviewers or by discussion with a third author (J.L.). The detailed inclusion and exclusion criteria were presented in Supplementary Materials Table S1.
2.2. Data Extraction and Quality Assessment
Data were obtained by two authors (Q.Z. and S.L.) independently from each included study using a predefined form. Disagreements were resolved by discussion with a third author (J.L.). The following information was extracted: title, date of publication time, author names, participant characteristics, intervention strategy, treatment received before study, background treatment (used in both groups together with the intervention), study outcomes, and method for Hcy assay. The risk of bias for each included RCT was assessed using the Cochrane Handbook for Systematic Reviews of Interventions [12].
2.3. Statistical Methods
A meta-analysis was conducted to assess the association between metformin and the changes of Hcy concentration. Considering the significant clinical heterogeneity, a random effects model was used. Subgroup analyses were conducted based on pre-defined parameters: gender, disease type, dosage of metformin, background treatment, pre-study treatment, control medication, duration of follow-up, and test method of Hcy. Pooled mean differences (MDs) and their 95% confidence intervals (CIs) were used for all continuous data. All absolute values and changes of serum Hcy concentration were unified and recorded as μmol/L. Funnel graph was also presented to evaluate the publication bias. Review Manager (RevMan 5.3 from the Cochrane Collaboration, Oxford, UK) was used for statistical analysis.
3. Results
3.1. Search Results
Twelve papers, involving 1311 participants, 1156 of whom completed the studies, were included in the pooled analysis after screening of 1227 articles (Figure 1). Reasons for excluding each paper during full-text screening were presented in Supplementary Materials Table S2 [13,14,15,16,17,18,19,20,21,22,23,24,25]. Among included studies, 11 were parallel trials and two were 2 × 2 factorial designed trials. The mean age of participants ranged from 24.8 to 61.4 years. The mean duration of follow-up ranged from 6 to 224 weeks. Detailed characteristics of included studies were presented in Table 1 and Table 2. Additionally, primary and secondary outcomes of each included study and strategies of intervention were summarized in Supplementary Materials Tables S3 and S4.
Figure 1.
Flow diagram for study identification and inclusion.
Table 1.
Baseline characteristics of each included study.
| Study | Country | Patients, N (I/C) | BMI, (kg/m2) | Age (Years) | Participants | Women (%) |
|---|---|---|---|---|---|---|
| Carlsen 1997 [26] | Norway | 29/30 | NA | 53 | CHD without diabetes | All men |
| Carlsen 2007a [27] | Norway and Turkey | 31/32 | NA | NA | PCOS, infertile | All women |
| Carlsen 2007b [27] | Norway and Turkey | 16/18 | NA | NA | PCOS, pregnant | All women |
| de Jager 2010 * [28] Wulffele 2003 * [15] |
The Netherlands | 131/146 | 30 | 61.4 | Insulin-treated T2DM | 75.5 |
| Kilic 2011a [29] | Turkey | 24/25 | 29.6 | 28.9 | PCOS with IGT, BMI > 25 kg/m2 | All women |
| Kilic 2011b [29] | Turkey | 23/24 | 22.4 | 26.5 | PCOS with IGT, BMI < 25 kg/m2 | All women |
| Kilicdag 2005 [30] | Turkey | 15/15 | 27.7 | 24.8 | PCOS | All women |
| Sahin 2007 [31] | Turkey | 74/36 | 28.9 | 58.6 | Newly diagnosed T2DM | 58.2 |
| Schachter 2007a [32] | Israel | 28/23 | NA | NA | PCOS with IR | All women |
| Schachter 2007b [32] | Israel | 27/24 | NA | NA | PCOS with IR | All women |
| Derosa 2003 [33] | Italy | 49/53 | 25.0 | 53.6 | Drug naïve T2DM | 50.0 |
| Ghazeeri 2015 [34] | Lebanon | 18/19 | NA | 25.8 | PCOS | All women |
| Derosa 2004 [35] | Italy | 75/73 | 27.9 | NA | Newly diagnosed T2DM | 51.2 |
| Erem 2014 [36] | Turkey | 19/19 | 32.4 | 52.4 | Newly diagnosed T2DM | 71.1 |
| Hassan 2015 [37] | Egypt | 30/30 | 27.3 | NA | Newly diagnosed T2DM | All men |
I/C, intervention/control group; BMI, body mass index; CHD, coronary heart disease; PCOS, polycystic ovary syndrome; T2DM, type 2 diabetes mellitus; IGT, impaired glucose tolerance; NA, not available; IR, insulin resistance. * These two analyses were short-term and long-term outcomes of the same trial, respectively. The long-term follow-up data (de Jager, 2010 [28]) were included in the quantitative analysis.
Table 2.
Study characteristics of each included study.
| Study ID | Intervention | Control | Primary Treatment | Washout Period | Background Treatment | Follow-up | Assay Method of Hcy | B12 Supplement | Folic Acid Supplement |
|---|---|---|---|---|---|---|---|---|---|
| Carlsen 1997 [26] | Metformin (2000 mg/day) 1 | Blank | Coronary artery bypass surgery or angioplasty | Lifestyle intervention and lovastatin, 40 mg daily | Lovastatin, 40 mg daily | 40 weeks | HPLC | No | No |
| Carlsen 2007a 2 [27] | Metformin (2000 mg/day) | Placebo | NS | No | Lifestyle intervention, folic acid 0.4 mg/day and a daily multivitamin tablet | 16 weeks | HPLC | 1 μg/day | 0.4 mg/day |
| Carlsen 2007b 2 [27] | Metformin (1700 mg/day) | Placebo | NS | No | Lifestyle intervention, folate 1 mg/day and a daily multi-vitamin tablet | 16 weeks | HPLC | 1 μg/day | 1 mg/day |
| de Jager 2010 [28]/ Wulffele 2003 3 [15] |
Metformin (2550 mg/day) 4 | Placebo | Insulin | Insulin (12 weeks) | Insulin | 224 weeks | Chromsystems kit | No | No |
| Kilic 2011a 5 [29] | Metformin (1700 mg/day) | Oral contraceptive | NS | No | B-group vitamins | 24 weeks | CLI | 2000 mg/day | No |
| Kilic 2011b 5 [29] | Metformin (1700 mg/day) | Oral contraceptive | NS | No | B-group vitamins | 24 weeks | CLI | 2000 mg/day | No |
| Kilicdag 2005 [30] | Metformin (1700 mg/day) | Rosiglitazone (4 mg/day) | NS | No | No | 12 weeks | FPI | No | No |
| Sahin 2007 [31] | Metformin (1700 mg/day) | Blank | Lifestyle intervention | Lifestyle intervention (4 weeks) | Lifestyle intervention | 6 weeks | CLI | No | No |
| Schachter 2007a 6 [32] | Metformin (1700 mg/day) | Blank | NS | No | Infertility treatment and folic acid 0.4 mg daily | Three cycles of treatment 7 | FPI | No | 0.4 mg/day |
| Schachter 2007b 6 [32] | Metformin (1700 mg/day) | Blank | NS | No | Infertility treatment and B-group vitamins | Three cycles of treatment 7 | FPI | 0.5 mg/day | 0.4 mg/day |
| Derosa 2003 [33] | Metformin (1500–2500 mg/day) 8 | Repaglinide (2–4 mg/day) 8 | NS | Placebo | Lifestyle intervention | 60 weeks | HPLC | No | No |
| Ghazeeri 2015 [34] | Metformin (1700 mg/day) | placebo | NS | 3 months of rosuvastatin (10 mg/day) | Rosuvastatin (10 mg/day) | 24 weeks | NA | No | No |
| Derosa 2004 [35] | Metformin (1000–3000 mg/day) 9 | Glimepiride (1–4 mg/day) 9 | NS | No | Lifestyle intervention | 56 weeks | HPLC and fluorescence detection | No | No |
| Erem 2014 [36] | Metformin (2000 mg/day) | Pioglitazone (15–45 mg/day) 10 | No | No | Lifestyle intervention | 12 months | ELISA | No | No |
| Hassan 2015 [37] | Metformin (1000 mg/day) | moderately calorie-restricted diet and an active lifestyle | No | No | No | 3 months | enzyme-linked immunoassay and an automated fluorescence polarization analyzer | No | No |
HPLC, High pressure liquid chromatography; NS, Not significant; CLI, Chemiluminescence immunoassay; FPI, Fluorescence polarization immunoassay. 1 The average daily intake of metformin was 1707 mg at week 4, 1759 mg at week 12, and 1741 mg at week 40; 2 This article included two independent RCTs; 3 These two analyses were short-term and long-term outcomes of the same trial, respectively. The long-term follow-up data (de Jager, 2010 [28]) were included in the quantitative analysis; 4 Each patient in this group was given his or her maximally tolerated daily dose (one, two, or three tablets of 850 mg) during the trial. The actual mean dose in the metformin-treated group was 2050 mg/day; 5,6 These are 2 × 2 factorial designed trials with four treatment arms in each trial; 7 This study did not report the exact duration of follow-up; 8 The average daily intake of metformin was 2000 mg, and that of repaglinide was 3 mg; 9 The average daily intake of metformin was 2500 mg, and that of glimepiride was 3 mg; 10 Each patient in this group was given his or her maximally tolerated daily dose during the trial (15 mg/day in six patients, 30 mg/day in twelve patients, and 45 mg/day in one patient).
3.2. Quality Assessment
The risk of bias of the included studies was demonstrated in Supplementary Materials Figures S2 and S3. The procedures of random sequence generation in seven studies [26,27,31,33,35,36,37], of allocation concealment in nine studies [26,27,28,31,33,34,35,36,37], and of blinding of participants and personnel in five studies [30,32,33,34,36] were not clearly described. Selective reporting risk was unclear in one study [32]. One study was at high risk of bias in allocation concealment [32], and one study provided incomplete outcome data [32]. Quality assessment of each included study was shown in Supplementary Materials Table S5.
Potential publication bias was suspected from the funnel graph analyses, which was presented in Supplementary Materials Figure S4.
3.3. Metformin and Homocysteine
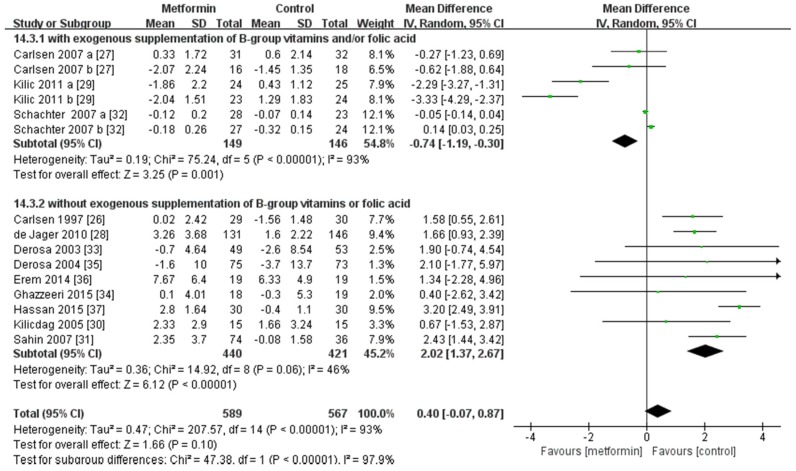
The results of the overall analysis showed that metformin did not have a statistically significant effect on the concentration of Hcy when compared with the control treatment (MD, 0.40 μmol/L; 95% CI, −0.07~0.87 μmol/L, p = 0.10; Figure 2). Subgroup analyses according to whether the patients received folic acid or B-group vitamins supplementation showed that administration of metformin was associated with a decreased concentration of serum Hcy in the patients receiving regular folic acid or B-group vitamins (MD, −0.74 μmol/L; 95% CI, −1.19~−0.30 μmol/L, p = 0.001), while with an elevated concentration of serum Hcy in the patients without any supplementation (MD, 2.02 μmol/L; 95% CI, 1.37~2.67 μmol/L, p < 0.00001).
Figure 2.
Overall analysis on Hcy concentration and subgroup analysis on Hcy concentration of patients with or without folic acid or B-group vitamins supplementation. The changes from baseline (Mean ± SD) between the two groups were compared. SD, standard deviation; CI, confidence interval; IV, inverse variance.
Subgroup analyses were conducted according to gender, disease type, dosage of metformin, background treatment, pre-study treatment, control treatment, duration of follow-up, change of Vitamin B12 concentration, and assay method of Hcy. Detailed results were presented in Supplementary Materials Table S6. It was demonstrated that the administration of metformin was associated with a significant reduction of serum Hcy among young female patients with PCOS. In addition, subgroup analysis based on the dosage of metformin showed that higher dosages of metformin (≥2000 mg daily) were associated with an elevation of serum Hcy, when compared with dosages less than 2000 mg daily (MD, 1.07 μmol/L; 95% CI, −0.17~2.30 μmol/L, p = 0.09).
3.4. Adverse Events
Five studies reported that more patients in the metformin group suffered gastrointestinal side effects when compared with those in the control [27,28,30,33,35]. Also, five papers did not provide any information about adverse events [26,29,31,32,34]. No death was reported. Details were shown in Supplementary Materials Table S7.
4. Discussion
Our study did not find significant association between metformin treatment and the change of serum Hcy concentration in the overall population. However, the subgroup analyses noted that metformin administration was associated with elevation of Hcy in the patients without supplementation of folic acid or B-group vitamins, which indicated that metformin might induce HHcy in the absence of exogenous folic acid or B-group vitamins supplementation.
Hcy is a sulfur amino acid with a free sulfhydryl group as the final metabolite of methionine and Vitamin B12 serves as a cofactor in the degeneration of Hcy to methionine. The insufficiency in Vitamin B12 results in the accumulation of Hcy, which is known as HHcy. HHcy is a well-established risk factor for cardiovascular diseases, cognitive impairment, and chronic renal failure [4,5,6,7,8,9], moreover, Hcy has been found to be an independent predictor of all-cause and vascular mortality [38,39]. Metformin has been demonstrated to be associated with reduction of serum Vitamin B12 concentration [3,40]. It has been shown that metformin could induce Vitamin B12 malabsorption by enhancing bacterial overgrowth, altering bacterial flora in enteric canal, and binding to the Vitamin B12-intrinsic factor (IF). This malabsorption ultimately leads to a reduction of serum Vitamin B12 [41,42,43,44,45]. Hence, some researchers were calling attention to the monitoring of Vitamin B12 concentration in the diabetic patients treated with metformin, and suggested Vitamin B12 supplementation could be considered in patients with Vitamin B12 deficiency [3,46,47], although some authors still doubt the clinical significance of this reduction [48,49]. A recent clinical trial suggested that the metformin-associated reduction of the serum Vitamin B12 was due to the increased transportation and utility of Vitamin B12 by cells stimulated by metformin [50]. One of our subgroup analyses showed that metformin raised serum Hcy in the patients without folic acid or Vitamin B12 supplementation, but reduced Hcy when folic acid or Vitamin B12 was supplemented, indicating that metformin-associated Vitamin B12 reduction might be responsible for Hcy elevation, and exogenous folic acid and Vitamin B12 may rescue the methionine metabolic disturbance in metformin-treated patients. Considering Hcy as an important biomarker of a series of diseases and the few adverse effects of folic acid and Vitamin B12, exogenous supplementation of these two vitamins could be necessary for metformin-treated patients, which is consistent with the recommendation of regular Vitamin B12 supplementation in the current American Association of Clinical Endocrinologists (AACE) guideline [51]. However, this recommendation had not yet been supported by well-designed randomized trials.
Our subgroup analyses also demonstrated that, the administration of metformin might cause a significant reduction of serum Hcy in young women with PCOS. However, it must be noted that, most of the young women enrolled received exogenous folic acid or B-group vitamins supplementation, and hence the observed Hcy reduction might be partially caused by the effects of exogenous folic acid or B-group vitamins. Meanwhile, estrogen, progestin, and age may also have some effects on the concentration of Hcy [5,8]. Experimental studies are required to further explain this difference.
The increase of serum Hcy concentration in the metformin-treated patients was confirmed by a series of observational studies [16,17,18,20,25,52,53,54,55,56]. These studies indicated that metformin was associated with an elevated concentration of serum Hcy compared with control treatment. Moreover, in Yilmaz’s trial [20], where all the included patients were young women with PCOS but without Vitamin B12 or folic acid supplementation, Hcy was found to be elevated, while Vitamin B12 was reduced in the metformin-treated patients. In addition, Carlsen and colleagues [27] noticed that Hcy was reduced in pregnant women but not in infertile women. No explanation has been established currently, but further investigations on the pseudo reduction of Vitamin B12 during pregnancy and the effect of estrogen and progestin on the concentration of Hcy might help us better understand the underlying mechanism. In Carlsen’s and Kilic’s trials [27,29], all participants received exogenous folic acid or B-group vitamins and, interestingly, metformin-treated patients had a lower concentration of Hcy compared with controls. A possible explanation was that exogenous folic acid or B-group vitamins might counteract the reduction of the Vitamin B12 absorption caused by metformin [46]. In Schachter’s trial [32], no matter whether the patients were treated with metformin or not, the reduction of Hcy in the patients receiving both Vitamin B12 and folic acid was greater than that in the patients receiving folic acid only (metformin-treated: −0.18 versus −0.12 μmol/L; metformin-untreated: −0.32 versus −0.07 μmol/L). It indicated that Vitamin B12 was critical in reducing serum Hcy, which could be explained by the vital role of Vitamin B12 in the metabolism of methionine [5].
Our study has several limitations. Firstly, the heterogeneity among the included studies was significant. Although subgroup analyses were conducted to explore possible sources of heterogeneity, factors such as weight, age, gender, and race might still influence the results of our study. Particularly, the dosage and the follow-up duration of included studies varied largely, although subgroup analyses did not find significant effects of these factors on the results. Secondly, in our subgroup analysis concerning exogenous B-group vitamins or folic acid supplementation, most patients receiving exogenous B-group vitamins or folic acid were diagnosed with PCOS or infertility, which could induce some potential biases. Further studies are required to demonstrate the interaction between metformin and B-group vitamins in patients with PCOS or infertility. Thirdly, long-term outcomes such as mortality and cardiovascular events were not studied in our analysis. Finally, the strength of the pooled results was restricted by the generally high risk of bias of included studies.
5. Conclusions
Although there is no significant change of the concentration serum Hcy between metformin-treated and non-biguanide-treated patients in the overall pooled analysis, our subgroup analysis suggested that metformin may induce an elevation of serum Hcy concentration in the absence of B-group vitamins or folic acid supplementation. Nevertheless, given the supplementation of B-group vitamins or folic acid, metformin could even be associated with reduced concentration of serum Hcy. Since HHcy is a risk factor for a series of adverse clinical outcomes, supplementation of B-group vitamins or folic acid might be necessary in metformin-treated patients, regardless of the background diseases. However, further investigations are still required to demonstrate the effects and long-term outcomes of Vitamin B12 or folic acid supplementation in the metformin-treated patients.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (Grant No. 81400811 and 21534008), the National Basic Research Program of China (2015CB942800), the Scientific Research Project of Health and Family Planning Commission of Sichuan Province (Grant No. 130029 and 150149) and the Scientific and Technical Supporting Program of Sichuan Province (Grant No. 2014FZ0048).
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/8/12/798/s1; Table S1: Detailed inclusion and exclusion criteria of each included studies in the meta-analysis, Table S2: Rationale for excluding studies after full-text screening, Table S3: Primary and secondary outcomes of each included study, Table S4: Intervention strategy of each included study, Table S5: Rationale of quality assessment for each included study, Table S6: Summary of subgroup analysis, Table S7: Adverse events, Figure S1: Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies, Figure S2: Risk of bias summary: review authors’ judgements about each risk of bias item for each included study, Figure S3: Funnel plot of comparisons.
Author Contributions
S.L. and J.L. designed this study; Q.Z., Q.L. and K.R. extracted the data; X.S. and L.L. are both biostatisticians from the Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, China; X.S. provided methodological guidance of this manuscript as well as reviewed the statistics; L.L. and Q.Z. performed the data analysis and statistical procedure; Q.Z. and S.L. drafted the manuscript and all authors reviewed and revised the manuscript; J.L. is responsible for the integrity of the study as a whole.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 2.Global Guideline for Type 2 Diabetes—2012. [(accessed on 2 January 2016)]. Available online: http://www.idf.org/new-global-guideline-type-2-diabetes-out-now-0.
- 3.Liu Q., Li S., Quan H., Li J. Vitamin B12 status in metformin treated patients: Systematic review. PLoS ONE. 2014;9:e100379. doi: 10.1371/journal.pone.0100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon L.R. Disorders of cobalamin (vitamin B12) metabolism: Emerging concepts in pathophysiology, diagnosis and treatment. Blood Rev. 2007;21:113–130. doi: 10.1016/j.blre.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Selhub J. 5-homocysteine metabolism. Annu. Rev. Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Mandaviya P.R., Stolk L., Heil S.G. Homocysteine and DNA methylation: A review of animal and human literature. Mol. Genet. Metab. 2014;113:243–252. doi: 10.1016/j.ymgme.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Strain J.J., Dowey L., Ward M., Pentieva K., McNulty H. B-vitamins, homocysteine metabolism and CVD. Proc. Nutr. Soc. 2007;63:597–603. doi: 10.1079/PNS2004390. [DOI] [PubMed] [Google Scholar]
- 8.Miller J.W. Encyclopedia of Human Nutrition. 3rd ed. Elsevier; Amsterdam, The Netherlands: 2013. Homocysteine; pp. 424–430. [Google Scholar]
- 9.Durand P.P.M., Loreau N., Lussier-Cacan S., Blache D. Impaired homocysteine metabolism and atherothrombotic disease. Lab. Investig. 2011;81:645–672. doi: 10.1038/labinvest.3780275. [DOI] [PubMed] [Google Scholar]
- 10.Debreceni B., Debreceni L. The role of homocysteine-lowering B-vitamins in the primary prevention of cardiovascular disease. Cardiovasc. Ther. 2014;32:130–138. doi: 10.1111/1755-5922.12064. [DOI] [PubMed] [Google Scholar]
- 11.Yang B., Fan S., Zhi X., Wang Y., Wang Y., Zheng Q., Sun G. Prevalence of hyperhomocysteinemia in China: A systematic review and meta-analysis. Nutrients. 2015;7:74–90. doi: 10.3390/nu7010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochrane Handbook for Systematic Reviews of Interventions. [(accessed on 25 December 2015)]. Available online: http://handbook.cochrane.org/
- 13.Gatford K.L., Houda C.M., Lu Z.X., Coat S., Baghurst P.A., Owens J.A., Sikaris K., Rowan J.A., Hague W.M. Vitamin B12 and homocysteine status during pregnancy in the metformin in gestational diabetes trial: Responses to maternal metformin compared with insulin treatment. Diabetes Obes. Metab. 2013;15:660–667. doi: 10.1111/dom.12080. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan D., Forder P., Simes J., Whiting M., Kritharides L., Merrifield A., Donoghoe M., Colman P.G., Graham N., Haapamaki H., et al. Associations between the use of metformin, sulphonylureas, or diet alone and cardiovascular outcomes in 6005 people with type 2 diabetes in the field study. Diabetes Res. Clin. Pract. 2011;94:284–290. doi: 10.1016/j.diabres.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Wulffele M.G., Kooy A., Lehert P., Bets D., Ogterop J.C., Borger van der Burg B., Donker A.J., Stehouwer C.D. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: A randomized, placebo-controlled trial. J. Intern. Med. 2003;254:455–463. doi: 10.1046/j.1365-2796.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- 16.Ham A.C., Enneman A.W., van Dijk S.C., Oliai Araghi S., Swart K.M., Sohl E., van Wijngaarden J.P., van der Zwaluw N.L., Brouwer-Brolsma E.M., Dhonukshe-Rutten R.A., et al. Associations between medication use and homocysteine levels in an older population, and potential mediation by vitamin B12 and folate: Data from the B-PROOF study. Drugs Aging. 2014;31:611–621. doi: 10.1007/s40266-014-0192-2. [DOI] [PubMed] [Google Scholar]
- 17.Sahin Y., Unluhizarci K., Yilmazsoy A., Yikilmaz A., Aygen E., Kelestimur F. The effects of metformin on metabolic and cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Clin. Endocrinol. 2007;67:904–908. doi: 10.1111/j.1365-2265.2007.02985.x. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopal G., Reddy A.P., Venkata Harinarayan C., Suresh V., Bitla A., Rao S.P.V.L.N., Sachan A. Effect of lifestyle modification and metformin therapy on emerging cardiovascular risk factors in overweight indian women with polycystic ovary syndrome. Metab. Syndr. Relat. Disord. 2012;10:273–279. doi: 10.1089/met.2011.0127. [DOI] [PubMed] [Google Scholar]
- 19.Russo G.T., Giandalia A., Romeo E.L., Marotta M., Alibrandi A., de Francesco C., Horvath K.V., Asztalos B., Cucinotta D. Lipid and non-lipid cardiovascular risk factors in postmenopausal type 2 diabetic women with and without coronary heart disease. J. Endocrinol. Investig. 2014;37:261–268. doi: 10.1007/s40618-013-0023-z. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz M., Bukan N., Ayvaz G., Karakoc A., Toruner F., Cakir N., Arslan M. The effects of rosiglitazone and metformin on oxidative stress and homocysteine levels in lean patients with polycystic ovary syndrome. Hum. Reprod. 2005;20:3333–3340. doi: 10.1093/humrep/dei258. [DOI] [PubMed] [Google Scholar]
- 21.Anderson J., Pena A.S., Sullivan T., Gent R., D’Arcy B., Olds T., Coppin B., Couper J. Does metformin improve vascular health in children with type 1 diabetes? Protocol for a one year, double blind, randomised, placebo controlled trial. BMC Pediatr. 2013;13:108. doi: 10.1186/1471-2431-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulffele M.G., Kooy A., Lehert P., Bets D., Donker A.J., Stehouwer C.D. Does metformin decrease blood pressure in patients with type 2 diabetes intensively treated with insulin? Diabet. Med. 2005;22:907–913. doi: 10.1111/j.1464-5491.2005.01554.x. [DOI] [PubMed] [Google Scholar]
- 23.Luque-Ramirez M., Mendieta-Azcona C., del Rey Sanchez J.M., Maties M., Escobar-Morreale H.F. Effects of an antiandrogenic oral contraceptive pill compared with metformin on blood coagulation tests and endothelial function in women with the polycystic ovary syndrome: Influence of obesity and smoking. Eur. J. Endocrinol. 2009;160:469–480. doi: 10.1530/EJE-08-0725. [DOI] [PubMed] [Google Scholar]
- 24.Luque-Ramirez M., Alvarez-Blasco F., Uriol Rivera M.G., Escobar-Morreale H.F. Serum uric acid concentration as non-classic cardiovascular risk factor in women with polycystic ovary syndrome: Effect of treatment with ethinyl-estradiol plus cyproterone acetate versus metformin. Hum. Reprod. 2008;23:1594–1601. doi: 10.1093/humrep/den095. [DOI] [PubMed] [Google Scholar]
- 25.Gharakhani M., Neghab N., Farimani M. Is reducing ovarian volume in polycystic ovarian syndrome patients after administration of metformin associated with improving cardiovascular risk factors? Int. J. Fertil. Steril. 2011;5:90–95. [PMC free article] [PubMed] [Google Scholar]
- 26.Carlsen S.M., Folling I., Grill V., Bjerve K.S., Schneede J., Refsum H. Metformin increases total serum homocysteine levels in non-diabetic male patients with coronary heart disease. Scand. J. Clin. Lab. Investig. 1997;57:521–527. doi: 10.3109/00365519709084603. [DOI] [PubMed] [Google Scholar]
- 27.Carlsen S.M., Kjotrod S., Vanky E., Romundstad P. Homocysteine levels are unaffected by metformin treatment in both nonpregnant and pregnant women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2007;86:145–150. doi: 10.1080/00016340600855946. [DOI] [PubMed] [Google Scholar]
- 28.De Jager J., Kooy A., Lehert P., Wulffele M.G., van der Kolk J., Bets D., Verburg J., Donker A.J., Stehouwer C.D. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: Randomised placebo controlled trial. BMJ. 2010;340:c2181. doi: 10.1136/bmj.c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilic S., Yilmaz N., Zulfikaroglu E., Erdogan G., Aydin M., Batioglu S. Inflammatory-metabolic parameters in obese and nonobese normoandrogenemic polycystic ovary syndrome during metformin and oral contraceptive treatment. Gynecol. Endocrinol. 2011;27:622–629. doi: 10.3109/09513590.2010.530706. [DOI] [PubMed] [Google Scholar]
- 30.Kilicdag E.B., Bagis T., Zeyneloglu H.B., Tarim E., Aslan E., Haydardedeoglu B., Erkanli S. Homocysteine levels in women with polycystic ovary syndrome treated with metformin versus rosiglitazone: A randomized study. Hum. Reprod. 2005;20:894–899. doi: 10.1093/humrep/deh700. [DOI] [PubMed] [Google Scholar]
- 31.Sahin M., Tutuncu N.B., Ertugrul D., Tanaci N., Guvener N.D. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2007;21:118–123. doi: 10.1016/j.jdiacomp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Schachter M., Raziel A., Strassburger D., Rotem C., Ron-El R., Friedler S. Prospective, randomized trial of metformin and vitamins for the reduction of plasma homocysteine in insulin-resistant polycystic ovary syndrome. Fertil. Steril. 2007;88:227–230. doi: 10.1016/j.fertnstert.2006.11.071. [DOI] [PubMed] [Google Scholar]
- 33.Derosa G., Mugellini A., Ciccarelli L., Crescenzi G., Fogari R. Comparison of glycaemic control and cardiovascular risk profile in patients with type 2 diabetes during treatment with either repaglinide or metformin. Diabetes Res. Clin. Pract. 2003;60:161–169. doi: 10.1016/S0168-8227(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 34.Ghazeeri G., Abbas H.A., Skaff B., Harajly S., Awwad J. Inadequacy of initiating rosuvastatin then metformin on biochemical profile of polycystic ovarian syndrome patients. J. Endocrinol. Investig. 2015;38:643–651. doi: 10.1007/s40618-015-0237-3. [DOI] [PubMed] [Google Scholar]
- 35.Derosa G., Franzetti I., Gadaleta G., Ciccarelli L., Fogari R. Metabolic variations with oral antidiabetic drugs in patients with type 2 diabetes: Comparison between glimepiride and metformin. Diabetes Nutr. Metab. 2004;17:143–150. [PubMed] [Google Scholar]
- 36.Erem C., Ozbas H.M., Nuhoglu I., Deger O., Civan N., Ersoz H.O. Comparison of effects of gliclazide, metformin and pioglitazone monotherapies on glycemic control and cardiovascular risk factors in patients with newly diagnosed uncontrolled type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes. 2014;122:295–302. doi: 10.1055/s-0034-1370989. [DOI] [PubMed] [Google Scholar]
- 37.Hassan M.H., Abd-Allah G.M. Effects of metformin plus gliclazide versus metformin plus glimepiride on cardiovascular risk factors in patients with type 2 diabetes mellitus. Pak. J. Pharm. Sci. 2015;28:1723–1730. [PubMed] [Google Scholar]
- 38.Anderson J.L., Muhlestein J.B., Horne B.D., Carlquist J.F., Bair T.L., Madsen T.E., Pearson R.R. Plasma homocysteine predicts mortality independently of traditional risk factors and C-reactive protein in patients with angiographically defined coronary artery disease. Circulation. 2000;102:1227–1232. doi: 10.1161/01.CIR.102.11.1227. [DOI] [PubMed] [Google Scholar]
- 39.Bates C.J., Mansoor M.A., Pentieva K.D., Hamer M., Mishra G.D. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: The national diet and nutrition survey of people aged 65 years and over. Br. J. Nutr. 2010;104:893–899. doi: 10.1017/S0007114510001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niafar M., Hai F., Porhomayon J., Nader N.D. The role of metformin on vitamin B12 deficiency: A meta-analysis review. Intern. Emerg. Med. 2015;10:93–102. doi: 10.1007/s11739-014-1157-5. [DOI] [PubMed] [Google Scholar]
- 41.Caspary W.F., Creutzfeldt W. Analysis of the inhibitory effect of biguanides on glucose absorption: Inhibition of active sugar transport. Diabetologia. 1971;7:379–385. doi: 10.1007/BF01219474. [DOI] [PubMed] [Google Scholar]
- 42.Caspary W.F., Zavada I., Reimold W., Deuticke U., Emrich D., Willms B. Alteration of bile acid metabolism and vitamin-B12-absorption in diabetics on biguanides. Diabetologia. 1977;13:187–193. doi: 10.1007/BF01219698. [DOI] [PubMed] [Google Scholar]
- 43.Scarpello J.H., Greaves M., Sladen G.E. Small intestinal transit in diabetics. Br. Med. J. 1976;2:1225–1226. doi: 10.1136/bmj.2.6046.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schafer G. Some new aspects on the interaction of hypoglycemia-producing biguanides with biological membranes. Biochem. Pharmacol. 1976;25:2015–2024. doi: 10.1016/0006-2952(76)90424-X. [DOI] [PubMed] [Google Scholar]
- 45.Carmel R., Rosenberg A.H., Lau K.S., Streiff R.R., Herbert V. Vitamin B12 uptake by human small bowel homogenate and its enhancement by intrinsic factor. Gastroenterology. 1969;56:548–555. [PubMed] [Google Scholar]
- 46.Mashavi M., Hanah R., Boaz M., Gavish D., Matas Z., Fux A., Shargorodsky M. Effect of homocysteine-lowering therapy on arterial elasticity and metabolic parameters in metformin-treated diabetic patients. Atherosclerosis. 2008;199:362–367. doi: 10.1016/j.atherosclerosis.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 47.Mazokopakis E.E., Starakis I.K. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res. Clin. Pract. 2012;97:359–367. doi: 10.1016/j.diabres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Filioussi K., Bonovas S., Katsaros T. Should we screen diabetic patients using biguanides for megaloblastic anaemia? Aust. Fam. Phys. 2003;32:383–384. [PubMed] [Google Scholar]
- 49.Moore E.M., Mander A.G., Ames D., Kotowicz M.A., Carne R.P., Brodaty H., Woodward M., Boundy K., Ellis K.A., Bush A.I., et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36:2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obeid R., Jung J., Falk J., Herrmann W., Geisel J., Friesenhahn-Ochs B., Lammert F., Fassbender K., Kostopoulos P. Serum vitamin B12 not reflecting vitamin B12 status in patients with type 2 diabetes. Biochimie. 2013;95:1056–1061. doi: 10.1016/j.biochi.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Garber A.J., Abrahamson M.J., Barzilay J.I., Blonde L., Bloomgarden Z.T., Bush M.A., Dagogo-Jack S., Davidson M.B., Einhorn D., Garvey W.T., et al. American association of clinical endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement. Endocr. Pract. 2013;19:536–557. doi: 10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yegnanarayan R., Suryavanshi M., Singh M., Desai S. A comparative study of the glycemic control of various antidiabetic agents and the role of homocysteine in the therapy of type 2 diabetes mellitus. J. Diabetes Complicat. 2008;22:104–111. doi: 10.1016/j.jdiacomp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Hoogeveen E.K., Kostense P.J., Jakobs C., Bouter L.M., Heine R.J., Stehouwer C.D. Does metformin increase the serum total homocysteine level in non-insulin-dependent diabetes mellitus? J. Intern. Med. 1997;242:389–394. doi: 10.1046/j.1365-2796.1997.00231.x. [DOI] [PubMed] [Google Scholar]
- 54.Krysiak R., Gilowski W., Okopien B. The effect of testosterone on cardiovascular risk factors in men with type 2 diabetes and late-onset hypogonadism treated with metformin or glimepiride. Pharmacol. Rep. 2016;68:75–79. doi: 10.1016/j.pharep.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Sato Y., Ouchi K., Funase Y., Yamauchi K., Aizawa T. Relationship between metformin use, vitamin B12 deficiency, hyperhomocysteinemia and vascular complications in patients with type 2 diabetes. Endocr. J. 2013;60:1275–1280. doi: 10.1507/endocrj.EJ13-0332. [DOI] [PubMed] [Google Scholar]
- 56.Wile D.J., Toth C. Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care. 2010;33:156–161. doi: 10.2337/dc09-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.