Abstract
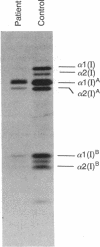
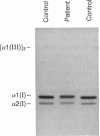
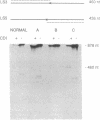
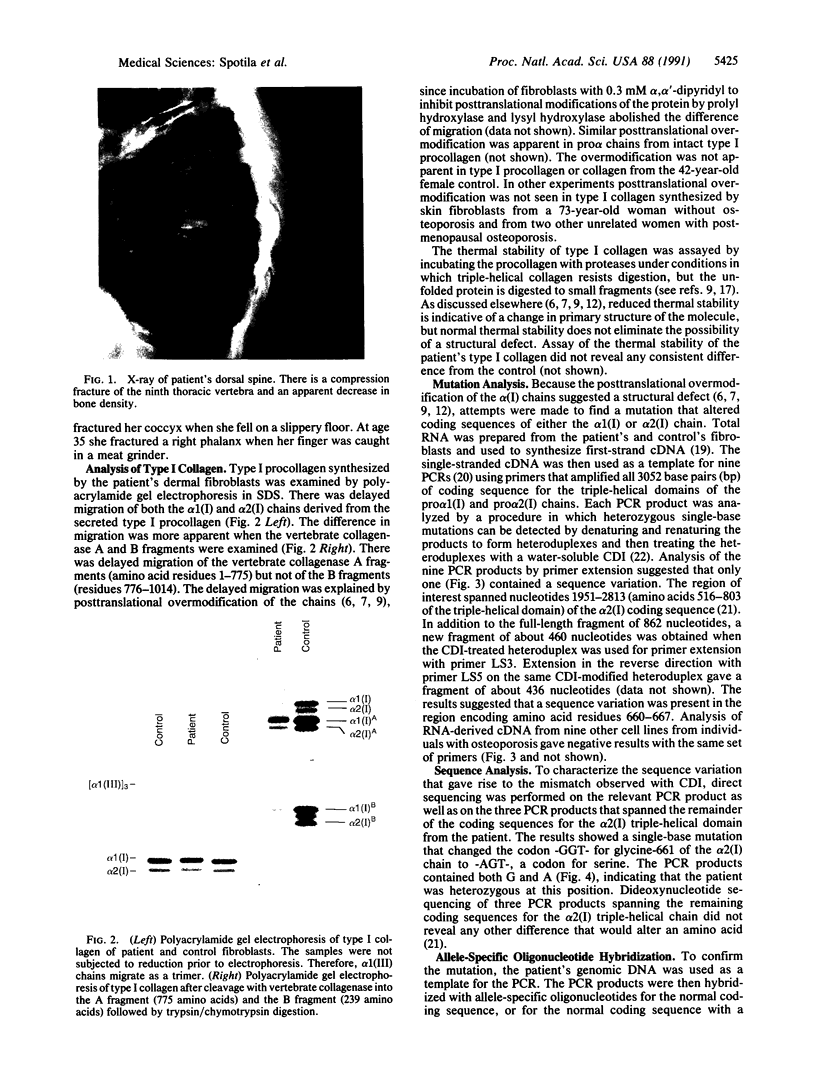
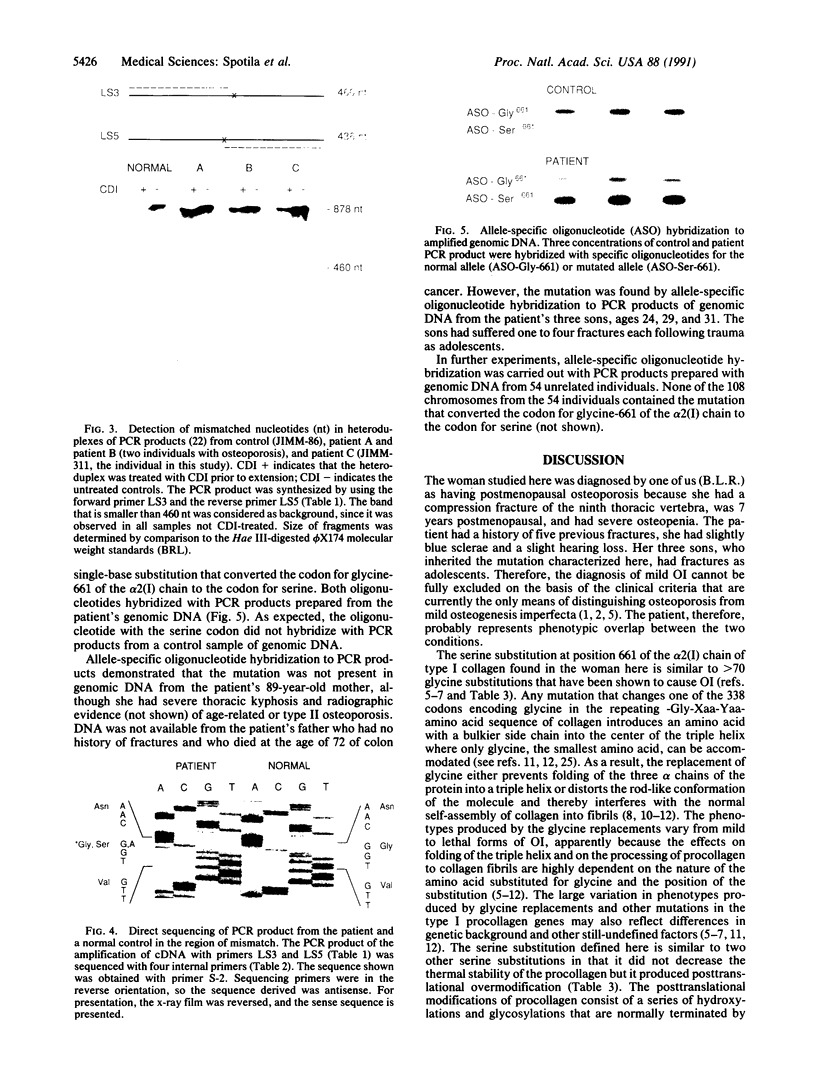
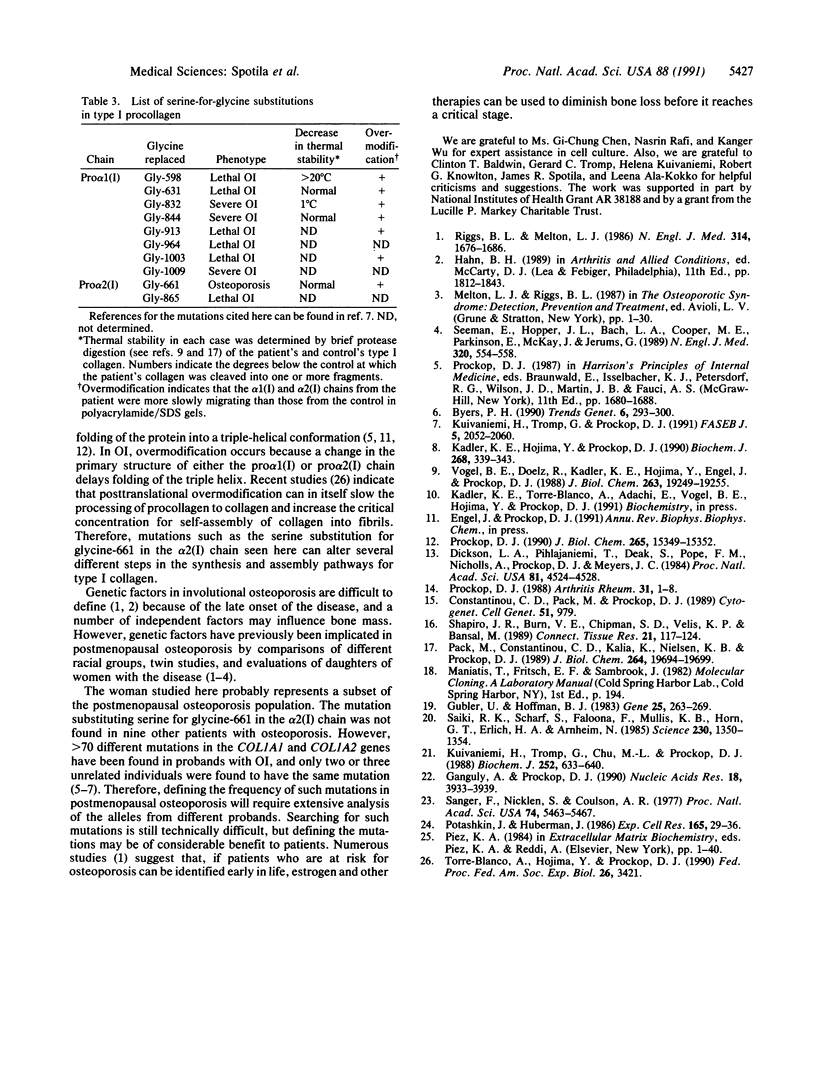
Mutations in the two genes for type I collagen (COL1A1 or COL1A2) cause osteogenesis imperfecta (OI), a heritable disease characterized by moderate to extreme brittleness of bone early in life. Here we show that a 52-year-old postmenopausal woman with severe osteopenia and a compression fracture of a thoracic vertebra had a mutation in the gene for the alpha 2(I) chain of type I collagen (COL1A2) similar to mutations that cause OI. cDNA was prepared from the woman's skin fibroblast RNA and assayed for the presence of a mutation by treating DNA heteroduplexes with carbodiimide. The results indicated a sequence variation in the region encoding amino acid residues 660-667 of the alpha 2(I) chain. Further analysis demonstrated a single-base mutation that caused a serine-for-glycine substitution at position 661 of the alpha 2(I) triple-helical domain. The substitution produced posttranslational overmodification of the collagen triple helix, as is seen with most glycine substitutions that cause OI. The patient had a history of five previous fractures, slightly blue sclerae, and slight hearing loss. Therefore, the results suggest that there may be phenotypic and genotypic overlap between mild osteogenesis imperfecta and postmenopausal osteoporosis, and that a subset of women with postmenopausal osteoporosis may have mutations in the genes for type I procollagen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Dickson L. A., Pihlajaniemi T., Deak S., Pope F. M., Nicholls A., Prockop D. J., Myers J. C. Nuclease S1 mapping of a homozygous mutation in the carboxyl-propeptide-coding region of the pro alpha 2(I) collagen gene in a patient with osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4524–4528. doi: 10.1073/pnas.81.14.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Prockop D. J. Detection of single-base mutations by reaction of DNA heteroduplexes with a water-soluble carbodiimide followed by primer extension: application to products from the polymerase chain reaction. Nucleic Acids Res. 1990 Jul 11;18(13):3933–3939. doi: 10.1093/nar/18.13.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Collagen fibrils in vitro grow from pointed tips in the C- to N-terminal direction. Biochem J. 1990 Jun 1;268(2):339–343. doi: 10.1042/bj2680339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Chu M. L., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988 Jun 15;252(3):633–640. doi: 10.1042/bj2520633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Pack M., Constantinou C. D., Kalia K., Nielsen K. B., Prockop D. J. Substitution of serine for alpha 1(I)-glycine 844 in a severe variant of osteogenesis imperfecta minimally destabilizes the triple helix of type I procollagen. The effects of glycine substitutions on thermal stability are either position of amino acid specific. J Biol Chem. 1989 Nov 25;264(33):19694–19699. [PubMed] [Google Scholar]
- Potashkin J. A., Huberman J. A. Characterization of DNA sequences associated with residual nuclei of Saccharomyces cerevisiae. Exp Cell Res. 1986 Jul;165(1):29–40. doi: 10.1016/0014-4827(86)90530-6. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Prockop D. J. Osteogenesis imperfecta. A model for genetic causes of osteoporosis and perhaps several other common diseases of connective tissue. Arthritis Rheum. 1988 Jan;31(1):1–8. doi: 10.1002/art.1780310101. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Melton L. J., 3rd Involutional osteoporosis. N Engl J Med. 1986 Jun 26;314(26):1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E., Hopper J. L., Bach L. A., Cooper M. E., Parkinson E., McKay J., Jerums G. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989 Mar 2;320(9):554–558. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- Shapiro J. R., Burn V. E., Chipman S. D., Velis K. P., Bansal M. Osteoporosis and familial idiopathic scoliosis: association with an abnormal alpha 2(I) collagen. Connect Tissue Res. 1989;21(1-4):117–124. doi: 10.3109/03008208909050002. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]