Abstract
In the cyanobacterium Synechococcus elongatus PCC 7942, KaiA, KaiB, and KaiC are essential proteins for the generation of a circadian rhythm. KaiC is proposed as a negative regulator of the circadian expression of all genes in the genome, and its phosphorylation is regulated positively by KaiA and negatively by KaiB and shows a circadian rhythm in vivo. To study the functions of KaiC phosphorylation in the circadian clock system, we identified two autophosphorylation sites, Ser-431 and Thr-432, by using mass spectrometry (MS). We generated Synechococcus mutants in which these residues were substituted for alanine by using site-directed mutagenesis. Phosphorylation of KaiC was reduced in the single mutants and was completely abolished in the double mutant, indicating that KaiC is also phosphorylated at these sites in vivo. These mutants lost circadian rhythm, indicating that phosphorylation at each of the two sites is essential for the control of the circadian oscillation. Although the nonphosphorylatable mutant KaiC was able to form a hexamer in vitro, it failed to form a clock protein complex with KaiA, KaiB, and SasA in the Synechococcus cells. When nonphosphorylatable KaiC was overexpressed, the kaiBC promoter activity was only transiently repressed. These results suggest that KaiC phosphorylation regulates its transcriptional repression activity by controlling its binding affinity for other clock proteins.
Circadian rhythms, biological oscillations with a period length of ≈24 h, are found ubiquitously among eukaryotes and cyanobacteria. As an endogenous oscillator, the circadian clock regulates these rhythms to match various biological activities with daily environmental alterations (1). In the unicellular cyanobacterium Synechococcus elongatus PCC 7942 (2, 3), a gene cluster composed of kaiA, kaiB, and kaiC was cloned and shown to encode proteins essential for circadian rhythm generation (4). Mutations in these genes alter period length or cause arrhythmia (4). KaiC represses its own (kaiBC) expression, whereas KaiA enhances it (4). Thus, KaiC and KaiA likely act as negative and positive elements in the circadian feedback loop of kaiBC expression, respectively (4). In contrast to eukaryotes, in which many clock gene products primarily regulate cis-acting elements of clock genes in a promoter-specific manner (5), KaiC represses not only kaiBC but also the rhythmic expression of all of the other genes in the genome (6). Moreover, kaiBC genes driven by the Escherichia coli trc promoter are able to restore the complete circadian rhythm when induced at an appropriate level (6, 7). These results suggest that the KaiC protein primarily coordinates genome-wide circadian gene expression (6, 7).
To date, the following biochemical properties of Kai proteins have been revealed: (i) all three Kai proteins interact with each other to form protein complexes (8, 9); (ii) KaiC binds ATP to form a hexamer structure in vitro (10–12); (iii) KaiB and KaiC accumulate in a circadian fashion (13); (iv) KaiC undergoes circadian Ser/Thr-phosphorylation in vivo and has an autokinase/autophosphatase activity in vitro (7, 10, 14–17); and (v) KaiC phosphorylation is asserted and negated by KaiA and KaiB, respectively, both in vitro and in vivo (7, 14–17). In particular, KaiA-stimulated phosphorylation of KaiC seems to be a key process of the circadian scenario. For example, a long-period kaiA2 mutant, in which nonphosphorylated KaiC was highly accumulated throughout the circadian cycle, is suppressed by the kaiC15 mutation. In the kaiA2;kaiC15 mutant, both the long-period phenotype and the abnormal KaiC phosphorylation are suppressed (14).
Identification of the phosphorylation sites is crucial to study more precisely the function of KaiC phosphorylation. Here, we identified two autophosphorylation sites of KaiC (Ser-431 and Thr-432) by using MS, studied the biochemical and physiological functions of these sites by using site-directed mutagenesis, and confirmed that phosphorylation of both of these sites is indispensable for the cyanobacterial clock system.
Materials and Methods
Bacterial Strains and Plasmids. We used a PkaiBC reporter strain of Synechococcus, NUC42 (18), as a control strain for this study. NUC43 (18), in which kaiABC was replaced with a kanamycin resistance gene, was used as a host for the pCkaiABC targeting vector (4), which was used to reintroduce a kaiABC cluster into the original kai locus. E. coli DH5α and BL21 were used as hosts for plasmid construction and expression of GST-fusion proteins, respectively. pTS2KPtrc::kaiC (4) was used to introduce a construct to overexpress KaiC from the trc promoter into NSII (4).
Bacterial Expression and Purification of Kai Proteins. The kaiA and kaiC ORFs were cloned into the pGEX-6P-1 vector (Amersham Pharmacia) and then introduced to E. coli BL21. GST-fusion proteins were produced in E. coli as described in ref. 8. Cells expressing KaiA were collected and resuspended in extraction buffer (50 mM Tris, pH 8.0/150 mM NaCl/1 mM DTT) and homogenized by sonication. The homogenate was centrifuged at 24,000 × g, and the supernatant was applied to a glutathione Sepharose 4B column (Amersham Pharmacia). The column was washed with five-column volumes of the buffer. PreScission Protease (Amersham Pharmacia) was then applied to the column to remove the GST-tag, according to the manufacturer's protocol. KaiA was eluted with two-column volumes of 50 mM Tris, pH 8.0/300 mM NaCl, and the eluent was diluted to apply to a Resource Q column (Amersham Pharmacia). KaiA was then eluted with a 90–450 mM NaCl gradient. Protein concentration was determined by the Bradford method by using the Bio-Rad protein assay kit with BSA (Sigma) as a standard. The purity of KaiA was >95% as determined by SDS/PAGE. WT and mutant KaiCs were purified as described above, except with buffers containing 0.5 mM ATP and 5 mM MgCl2 throughout the purification steps. The final purity of KaiC was >95%.
In Vitro Autophosphorylation–Autodephosphorylation of KaiC. A protein phosphorylation–dephosphorylation assay was performed as described (14) with some modifications. In the presence (0.05 μg/μl) or absence of KaiA, KaiC proteins (0.2 μg/μl) were incubated in reaction buffer (50 mM Tris, pH 8.0/150 mM NaCl/1 mM MgCl2/1 mM ATP/1 mM DTT) at 30°C. A 5-μl aliquot of reaction mixture was withdrawn and subjected to SDS/PAGE (7.5%) followed by Coomassie brilliant blue staining.
Nanoflow Liquid Chromatography Electrospray Ionization Tandem MS (MS/MS). The protein digests were separated by using an Ultimate nano-LC system (Dionex) running at a column flow rate of 200 nl/min on a 75-μm i.d. × 150-mm column (C18-PepMap, Dionex). The peptides were eluted by using a linear gradient (solution A, 0.05% formic acid/water, versus solution B, 0.05% formic acid/acetonitrile), which started at 5% solution B and rose to 20% solution B in 80 min. The column effluent was monitored at 214 and 280 nm by using a scanning UV-VIS absorbance detector (Dionex) equipped with a 3-nl flow cell and was continuously introduced into a nanoflow electrospray ion source of a quadrupole/time-of-flight (Q-TOF) mass spectrometer (Micromass, Manchester, U.K.). The liquid chromatography/electrospray ionization–MS/MS spectra were recorded in the data-dependent mode, in which up to four precursor ions above a threshold (15 cps) were selected for MS/MS by each survey scan, which was controlled with a masslynx software system (Micromass). For MS/MS studies, the MS1 was used to select the precursor ions, which were subsequently fragmented in a collision cell by using argon as the collision gas and an appropriate collision energy (20–35 eV). MS/MS data were further processed by a maximum entropy data enhancement program, maxent 3 (Micromass), which is capable of deconvoluting a spectrum with peaks in a variety of charge states, thus producing a simplified spectrum consisting only of monoisotopic peaks in a single charge state. The resultant spectra were interpreted by seqms, a software aid for de novo sequencing by MS/MS (www.protein.osaka-u.ac.jp/rcsfp/profiling/indexEnglish.htm) (19).
Site-Directed Mutagenesis. KaiC ORF in pCkaiABC and pTS2KPtrc::kaiC was mutagenized by using the overlap extension method by PCR as previously described (10) to change a Ser at position 431 to Ala (S431A) and/or a Thr at 432 to Ala (T432A) mutation. The mutated pCkaiABC and pTS2KPtrc::kaiC were introduced to NUC43 and NUC42, respectively. Primers used for mutagenesis were as follows: 5′-TCCCATATCGCAACAATTACGGAT-3′ and 5′-ATCCGTAATTGTTGCGATATGGGA-3′ for the S431A mutation; 5′-CATATCTCAGCAATTACGGATACG-3′ and 5′-CGTATCCGTAATTGCTGCTGAGATATG-3′ for the T432A mutation; and 5′-TCCCATATCGCAGCAATTACGGATACG-3′ and 5′-CGTATCCGTAATTGCTGCGATATGGGA-3′ for the S431A;T432A double mutations.
Assay of Bioluminescence. After 12 h of darkness to entrain the circadian clock, the bioluminescence profile from the PkaiBC reporter was monitored under continuous light (LL) conditions at 30°C with a photomultiplier-based quantitative system as described in ref. 4.
Western Analyses. Synechococcus cell extracts were prepared by using one of the following buffers: 10 mM TES–NaOH, pH 7.0/10 mM NaCl/5 mM EDTA to detect multiple KaiC bands (see Fig. 3); 50 mM Tris, pH 8.0/100 mM KCl/5 mM MgCl2/0.2% glycerol/0.1 mM EDTA/1% Triton X-100, supplemented with Complete EDTA-free protease inhibitor mixture (Roche Diagnostics) according to the manufacturer's protocol for the immunoprecipitation analysis (see Fig. 4); or 62.5 mM Tris, pH 6.8/10% glycerol/2% SDS for Western analysis (see Fig. 5B). Cells were collected and resuspended in one of the above buffers, followed by disruption using a MultiBeads Shocker (Yasui Kikai, Osaka) for 5 min at 30-s intervals at 2°C. Protein concentration was determined by the BCA method using BSA as a standard. Cell extracts were supplemented with 4× SDS sample buffer (see Figs. 3 and 4) or 5% 2-mercaptoethanol and 0.1% bromophenol blue (see Fig. 5B), subjected to SDS/PAGE, and transferred to nitrocellulose membranes, followed by incubation with appropriate antibodies: anti-KaiA, anti-KaiB, anti-KaiC, and anti-SasA antisera at a 1:500, 1:2,000, 1:2,000, and 1:2,000 dilutions, respectively (see Figs. 4 and 5), and anti-KaiC antiserum at a 1:500 dilution (see Fig. 3). After the immunoblots were washed three times with 0.3% Tween 20 in PBS for 10 min, they were treated as described below. The membranes were incubated with alkaline phosphatase-conjugated anti-rabbit Ig at a 1:1,000 dilution (Sigma), and then protein bands were detected with BCIP/NBT Combo (Invitrogen) with the manufacturer's instructions (see Fig. 3). Alternatively, blots were incubated with horseradish peroxidase-conjugated anti-rabbit Ig (Amersham Pharmacia) at a 1:2,000 dilution as a secondary antibody, and the signals were detected with ECL Western blotting detection reagents (Amersham Pharmacia; see Figs. 4 and 5).
Fig. 3.
The S431A and T432A mutations impair KaiC phosphorylation in vivo and circadian rhythm in Synechococcus.(A) Proteins were extracted from WT and mutant strains at LL4 and LL16 and subjected to immunoblot analysis to examine the phosphorylation state of KaiC. The upper four bands of KaiC from the WT strain are phosphorylated, and the lower two are nonphosphorylated. Only two of the phosphorylated bands were detected from mutant strains containing either S431A or T432A mutations. In contrast, in the double mutant (S431A;T432A), the phosphorylated forms of KaiC completely disappeared, indicating that these sites were also phosphorylated in vivo. KaiC was not detected from a kaiABC-depleted strain (Δkai). (B) Bioluminescence profile to monitor the PkaiBC promoter activity in the mutant strains. A robust circadian rhythm was observed in WT strains, whereas the rhythm was abolished in the kaiABC-deficient strain. Strains with mutations at the phosphorylation sites also showed an arrhythmic phenotype.
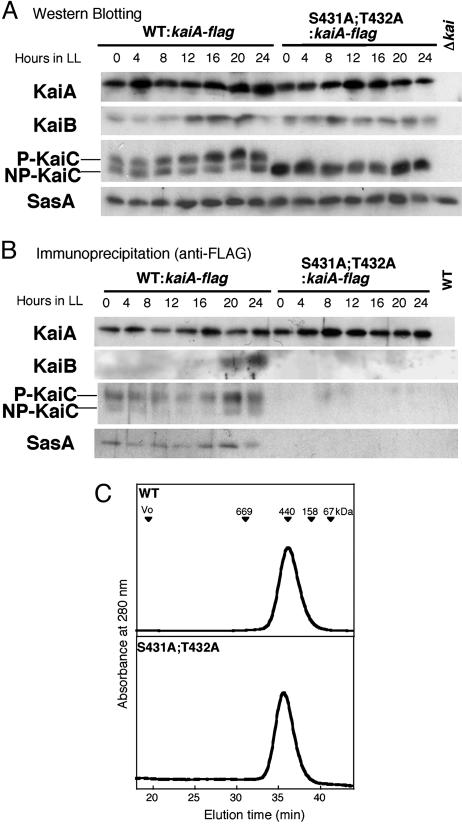
Fig. 4.
Effects of KaiC phosphorylation on the clock protein complex formation. The kaiA gene was mutagenized to introduce a FLAG-epitope tag to the C terminus of KaiA in WT and S431A;T432A Synechococcus strains, named WT:kaiA-flag and S431A;T432A:kaiA-flag, respectively. Δkai indicates a kaiABC-depleted strain. (A) Proteins were extracted from the WT and mutant cells collected at the indicated time and subjected to SDS/PAGE and immunoblotting analysis by using anti-KaiA, anti-KaiB, anti-KaiC, and anti-SasA antisera. (B) Cell extracts prepared at the indicated time were subjected to immunoprecipitation by using anti-FLAG antibody, followed by immunoblot analysis using the antibodies mentioned above. From WT strain, clock proteins were coimmunoprecipitated with KaiA, whereas KaiA failed to form a protein complex with KaiB, KaiC, and SasA in the double mutant. WT indicates a wild-type strain without FLAG-epitope tag. (C) Chromatographic profiles of recombinant WT-KaiC and KaiC[S431A;T432A] by using Superose 6 gel filtration chromatography. Proteins were monitored by absorbance at 280 nm. Positions of the molecular mass standards are indicated by arrowheads.
Fig. 5.
The effect of overexpression of KaiC and KaiC[S431A;T432A] on the activity of the kaiBC promoter (PkaiBC). (A) Bioluminescence from PkaiBC reporter strains carrying a Ptrc::kaiC or a Ptrc::kaiC[S431A;T432A] construct was monitored. Cells were treated with IPTG or water at the time indicated by the bar. (B) Accumulation of overexpressed KaiC and KaiC[S431A;T432A] in Synechococcus. Proteins were extracted from cells at LL24 with or without 24 h of IPTG addition and subjected to immunoblot analysis.
Immunoprecipitation. Immunoprecipitation was performed as described in ref. 9 with some modifications. Briefly, Synechococcus cell lysates were prepared as described above, and 360-μl aliquots containing 500 μg of total protein were incubated with 20 μl of ANTI-FLAG M2 affinity gel (Sigma) at 4°C for 2 h. The beads were resuspended in 80 μl of SDS sample buffer without reducing reagents, and the proteins were eluted by boiling. The supernatant was supplemented with 0.1% 2-mercaptoethanol and 0.1% bromophenol blue, followed by SDS/PAGE.
Gel Filtration Chromatography. WT and KaiC[S431A;T432A] were applied to Superose 6 10/300 GL columns (Amersham Pharmacia) equilibrated with 150 mM NaCl, 20 mM Tris·HCl, (pH 8.0), 0.5 mM EDTA, 0.5 mM ATP, and 5 mM MgCl2. Molecular mass standards for the gel filtration analysis were thyroglobulin (669 kDa), ferritin (440 kDa), aldorase (158 kDa), and albumin (67 kDa).
Overexpression of KaiC. The PkaiBC reporter strain NUC42 was transformed with plasmids that overexpress normal or mutated KaiC as described above. Conditions for overexpression were the same as described previously (4), except that 15, 100, or 1,000 μM isopropyl β-d-thiogalactopyranoside (IPTG) was administered to induce overexpression.
Results
KaiC Is Autophosphorylated at Ser-431 and Thr-432. We expressed and purified recombinant KaiC from E. coli and subjected the protein to an autophosphorylation reaction in vitro. KaiC purified in the presence of ATP appeared as three bands on SDS/PAGE; the lower band was nonphosphorylated KaiC, and the upper two were the phosphorylated forms (14). When KaiC was incubated for 8 h with KaiA, phosphorylation of KaiC was enhanced as previously reported (7, 14–17). To elucidate the autophosphorylation sites of KaiC, these gel bands of the phosphorylated forms were excised, subjected to in situ digestion by using trypsin and Asp-N, and analyzed by nanoflow liquid chromatography/electrospray ionization–MS/MS. The peptides, which corresponded to 78% of the KaiC sequence, were detected in a single run. In addition, the peptide (amino acids 427–434) was found to be a mixture of nonphosphorylated, singly phosphorylated, and doubly phosphorylated forms, based on the observed molecular masses and the difference in mass (80 Da), which corresponded to a phosphate group (Fig. 1A). It is noteworthy that two species of monophosphorylated peptides, observed at the same m/z, were obtained at different retention times (20.88 and 27.06 min), suggesting the presence of the peptides with different phosphorylation sites in the same sequence (Fig. 1 A). The MS/MS analyses from the doubly charged ions of the phosphorylated peptides revealed the phosphorylation sites to be Thr-432 (Fig. 1B) or Ser-431 (Fig. 1C) for the singly phosphorylated species, and to be Ser-431 and Thr-432 for the doubly phosphorylated species (data not shown). The relative abundances of these molecular species were estimated from their peak intensities in the reconstituted ion chromatography to be 8.5% (nonphosphorylated), 46.6% (Thr-432-phosphorylated), 11.9% (Ser-431-phosphorylated), and 33.0% (Ser-431-, Thr-432-phosphorylated) (Fig. 1 A).
Fig. 1.
Identification of the phosphorylation sites of the KaiC by nanoflow liquid chromatography/electrospray ionization–MS/MS (see Materials and Methods). (A) The ion chromatogram of KaiC digested with trypsin and AspN was reconstituted on the basis of the molecular masses of the nonphosphorylated (MH+, 873.4), singly phosphorylated (MH+, 953.4), and doubly phosphorylated (MH+, 1033.4) peptide (Asp-427-Ser-His-Ile-Ser-Thr-Ile-Thr) from a single run. Those precursor ions were also automatically subjected to MS/MS analysis by the data-dependent mode of the quadrupole/time-of-flight (Q-TOF) (see Materials and Methods). The MS/MS spectra of the peptides, singly phosphorylated at Thr-432 (B) and Ser-431 (C), which had been deconvoluted with maxent 3, were interpreted by using seqms (see Materials and Methods). The signals marked by asterisks in C were assigned to the sequence ions of the peptide (Asp-334-Phe-Glu-Glu-Met-Glu-Arg; MH+, 955.4), which was coeluted with the Ser-431-phosphorylated peptide. The arrows (→) show the sequences from the N terminus based on bn ions, where n denotes the arbitrary positions counted from the N terminus, which were produced by cleavage of peptide bonds during MS/MS. Amino acids in three letters in the spectra denote immonium ions. The nomenclature of these ions is in accordance with previous work (35).
Next, we substituted Ser-431 and/or Thr-432 for Ala (S431A, T432A, and S431A;T432A) by site-directed mutagenesis and then expressed the mutant KaiC in E. coli. Purified mutant proteins were subjected to SDS/PAGE and Coomassie brilliant blue staining to examine the phosphorylation state. As noted previously (14), two phosphorylated bands were found in the WT KaiC (Fig. 2, WT). In contrast, a single phosphorylated band was detected in the KaiC with single mutation (KaiC[S431A] and KaiC[T432A]) (Fig. 2, S431A and T432A) but no phosphorylated band was detected in the double mutant KaiC (KaiC[S431A;T432A]) (Fig. 2, S431A;T432A). These results indicate that Ser-431 and Thr-432 are major autophosphorylation sites of KaiC and suggest that no additional phosphorylation site is present.
Fig. 2.
Phosphorylation state of KaiC protein mutated at autophosphorylation sites. WT and mutant KaiC proteins containing S431A, T432A, or S431A;T432A mutations were incubated with ATP and MgCl2 for 2, 4, 8, and 24 h in the presence (+) or absence (–) of KaiA followed by separation of phosphorylated (P) and nonphosphorylated (NP) KaiC by SDS/PAGE on 7.5% acrylamide gels.
Phosphorylation of KaiC is enhanced by KaiA (7, 14–17), and phosphorylated KaiC is gradually dephosphorylated in the absence of KaiA (7, 15). To characterize the phosphorylation–dephosphorylation reactions of KaiC mutants, WT or mutated KaiC was incubated at 30°C in the presence or absence of KaiA. Prepared samples (incubation time 0) appeared as phosphorylated and nonphosphorylated bands (Fig. 2). In the absence of KaiA, WT KaiC, KaiC[S431A], and KaiC[T432A] proteins were almost completely dephosphorylated after 8 h of incubation (Fig. 2, WT, S431A, and T432A), suggesting that dephosphorylation of KaiC in the absence of KaiA is not site-dependent. After 8 h of incubation with KaiA, >90% of WT and KaiC[S431A] were converted to the phosphorylated forms (Fig. 2, WT and S431A). Phosphorylation of KaiC[T432A] was also enhanced by KaiA; however, even after 8 or 24 h of incubation, about half of the KaiC remained in the nonphosphorylated form (Fig. 2, T432A). As expected, no phosphor ylation was detected for KaiC[S431A;T432A] even after 8 h of incubation with KaiA (Fig. 2, S431A;T432A). These results suggest that the KaiC phosphorylation state equilibrium differs between Ser-431 and Thr-432 in the presence of KaiA; that is, Thr-432 is more liable to be phosphorylated than is Ser-431.
Ser-431 and Thr-432 Are Phosphorylated in Synechococcus Cells. To examine KaiC phosphorylation in vivo, S431A, T432A, and S431A;T432A mutations were introduced into the kaiC locus of the Synechococcus genome. Proteins extracted from these mutants were subjected to SDS/PAGE followed by immunoblotting analysis using anti-KaiC antiserum to examine the phosphorylation state of KaiC. KaiC from the WT strain was detected as six bands (Fig. 3A), and the upper four bands were identified as the phosphorylated form by lambda protein phosphatase digestion (data not shown). In each single mutant (S431A or T432A strains), only two phosphorylated bands of KaiC were detected, whereas all of the four phosphorylated bands were completely abolished in the double mutant (S431A;T432A strain). These observations indicate that KaiC is also phosphorylated at these sites in vivo (Fig. 3A). Bioluminescence profiles of these mutant strains under continuous light conditions are shown in Fig. 3B. Even the single mutation completely abolished the circadian rhythm of the PkaiBC activity, indicating that KaiC phosphorylation at each of the two sites is essential for circadian oscillation in Synechococcus.
Phosphorylation of KaiC Is Required for the Clock Protein Complex Formation. Kai proteins and SasA, a His kinase that modulates circadian rhythm (20), form a heteromultimeric protein complex of 400–600 kDa during the subjective night (9). We examined whether phosphorylation affected the interactions of KaiC with other clock proteins. In this experiment, we fused the FLAG epitope to the C terminus of KaiA as a tag to immunoprecipitate KaiA. This addition of FLAG did not affect the bioluminescence rhythm of the WT strain (data not shown). At first, intracellular accumulation of these proteins was assayed in the kaiA-flag strains (WT:KaiA-flag and S431A;T432A:KaiA-flag strains). As shown in Fig. 4A, no obvious differences in the cellular levels of KaiA, KaiB, KaiC, or SasA were observed between these strains, except that phosphor ylated KaiC was missing in the S431A;T432A:KaiA-flag mutant.
We then examined the interaction of clock proteins in the phosphorylation mutant of KaiC (S431A;T432A). Immunoprecipitation using anti-FLAG antibody (Fig. 4B) demonstrated that in the WT:kaiA-flag strain KaiA always interacted with KaiC and SasA, whereas KaiA associated with KaiB only at LL20 and LL24. These results are consistent with our previous observations that the large protein complex containing KaiA, KaiB, KaiC, and SasA is formed exclusively at late subjective night (9, 17). Note that KaiA preferably associates with phosphorylated KaiC in WT:kaiA-flag cells and that KaiA failed to associate with KaiC in the S431A;T432A:kaiA-flag mutant cells. Because the S431A;T432A mutation also negated the association of KaiA with KaiB or SasA, phosphorylation of KaiC must be important for the clock protein interactions in vivo. KaiA association with nonphosphorylated KaiC in WT:kaiA-flag cells is estimated by densitometry to be ≈50% of that to phosphorylated KaiC. This association can be explained if the KaiA bound to the KaiC hexamer consists of both phosphorylated and nonphosphorylated KaiC (see below).
It has been shown recently that KaiC associates to form a homo-hexamer in the presence of ATP in vitro (11, 12). We therefore examined the effect of phosphorylation on this hexamer formation using gel filtration chromatography. KaiC [S431A;T432A], as well as the WT strain, was eluted in a peak corresponding to the ≈440-kDa marker protein (Fig. 4C), which was the same elution pattern as reported previously (11, 12), indicating that KaiC[S431A;T432A] existed as a hexamer. These results suggest that phosphorylation is not necessary for the hexamer formation of KaiC.
Transcriptional Repression by KaiC Requires KaiC Phosphorylation. Previously, we reported that overexpression of KaiC repressed almost all genes in the genome including kaiBC (6). To examine the effect of KaiC phosphorylation on the gene repression activity of KaiC, WT kaiC or kaiC[S431A;T432A] was overexpressed under the control of the bacterial inducible promoter, Ptrc. As reported previously (4, 6), continuous overexpression of WT kaiC with 1 mM IPTG immediately nullified the bioluminescence of the PkaiBC reporter, and smaller doses of IPTG (15 and 100 μM) completely repressed the PkaiBC promoter after one and two cycles of the rhythm (Fig. 5A Left). In the strain overexpressing nonphosphorylatable KaiC, the bioluminescence of the PkaiBC reporter was similar to that in the WT KaiC-overexpressing strain for only 24 h after the induction; the PkaiBC reporter then gradually elevated to the peak level of the uninduced profile (Fig. 5A Right). Although the mechanism for this transient response is unknown, it is evident that KaiC phosphorylation is necessary for its transcriptional repression activity because the total amounts of both WT KaiC and KaiC[S431A;T432A] were similar, as confirmed by immunoblotting (Fig. 5B).
Discussion
In this study, we have identified two autophosphorylation sites of KaiC, Ser-431 and Thr-432, using MS. We also found that KaiC phosphorylation was abolished in the mutant cyanobacterium in which both of these residues were replaced by Ala, indicating that these residues are major phosphorylation sites in vivo. Thr-432 has been predicted as the most probable candidate phosphorylation site, based on three-dimensional molecular modeling of the KaiC hexamer (A.H. and M.G., unpublished data). In addition, Thr-432 and the flanking amino acid sequence match well with the consensus for the casein kinase II phosphorylation site. Both Ser-431 and Thr-432 are well conserved among KaiC proteins in various cyanobacterial species (A.H. and M.G., unpublished data).
Based on the peak intensities of reconstituted ion chromatography (Fig. 1 A), >90% of KaiC was phosphorylated within 8 h of incubation, and the relative abundances of the phosphorylated KaiC species were estimated to be 46.6%, 11.9%, and 33.0% for the Thr-432-phosphorylated, Ser-431-phosphorylated, and S431;T432-phosphorylated forms, respectively. These estimates are consistent with a previous phosphoamino acid analysis (14). It can therefore be presumed that the rate of Thr-432 phosphorylation is faster than that of Ser-431 phosphorylation, and/or that the rate of dephosphorylation at Thr-432 is slower than that at Ser-431. In vitro autophosphorylation analyses using KaiC[T432A] (in which Ser-431 is intact) and KaiC[S431A] (in which Thr-432 is intact; Fig. 2) showed that >90% of Thr-432 was phosphorylated within 8 h, but only about half of Ser-431 was phosphorylated after 8 or 24 h of incubation. These results are consistent with the ion intensities of phosphorylated peptides, in that Thr-432 is more liable to be phosphorylated than is Ser-431. It is possible that phosphorylation and/or dephosphorylation at these residues are interdependent processes because the doubly phosphorylated peptide is more abundant than the S431A-singly phosphorylated one. Further kinetic analyses are necessary to address this issue.
The functions of phosphorylated clock proteins have been intensively studied in Drosophila and Neurospora. Drosophila PER is phosphorylated by DBT, a homolog of vertebrate casein kinase Iε, and casein kinase II (21–23) and dephosphorylated by protein phosphatase 2A (24). TIM is phosphorylated by Shaggy, an ortholog of glycogen synthase kinase-3 (25). It has been demonstrated that phosphorylation of PER and TIM regulates crucial steps in the circadian feedback loop such as degradation by the ubiquitin–proteasome pathway (21, 26, 27) and nuclear translocation of the PER–TIM complex (25). Recently, Nawathean and Rosbash (28) demonstrated that phosphorylation of PER directly increases its transcriptional repression activity and suggested that nuclear localization of phosphorylated PER is an indirect consequence of PER–DNA association. In Neurospora, the FREQUENCY (FRQ) protein is phosphorylated by casein kinase II (29, 30) and dephosphorylated by PP1 and PP2A (31). Phosphorylation sites of FRQ were identified by systematic mutagenesis (32). FRQ phosphorylation assumed to regulate its stability (29, 30, 32) and its association with WHITE COLLAR proteins (29). Phosphorylated FRQ is ubiquitinated and degraded by the ubiquitin–proteasome pathway (33).
In cyanobacteria, phosphorylation of KaiC at each of Ser-431 and Thr-432 is essential for the clock system because a single mutation at any of these sites completely abolished the circadian rhythm. In contrast to the Drosophila and Neurospora system, KaiC could be phosphorylated by its autokinase activity because no phosphorylated KaiC was found in S431A;T432A mutant cells that was missing the autophosphorylation sites. Note that phosphorylated KaiC is more abundant in the S431A mutant, and nonphosphorylated KaiC is more abundant in the T432A mutant both in vitro (Fig. 2) and in vivo (Fig. 3). This correlation also implies that KaiC is phosphorylated by its autokinase activity in vivo. Moreover, unlike the case for eukaryotes, phosphorylation of KaiC is not responsible for its degradation because the cellular content of KaiC was not altered in the single and the double mutants (Fig. 3). Instead, KaiC phosphorylation would affect the clock protein complex formation (see below).
Whereas nonphosphorylated KaiC[S431A;T432A] assembles into a hexamer in vitro (Fig. 4C), KaiC phosphorylation is required for the in vivo formation of the Kai proteins–SasA complex, as KaiA failed to interact with other components of the complex in the S431A;T432A mutant (Fig. 4). The question is how KaiC phosphorylation contributes to the formation of this complex. We have previously identified two KaiA binding domains (CKABD1 and CKABD2) of KaiC at corresponding regions of its duplicated structure (34). Because Ser-431 and Thr-432 are located in CKABD2, KaiC phosphorylation–dephosphorylation at these residues would regulate its affinity for KaiA. Further structural studies on the Kai protein complex are necessary to address this issue.
These studies have revealed that KaiC phosphorylation regulates the formation of the Kai/SasA complex; however, the function of this complex remains unknown. Because it is formed in the late subjective night when kaiBC mRNA reaches its nadir, we suspect that this large complex negatively regulates gene expression in Synechococcus. In fact, as shown in Fig. 5, continuous overexpression of nonphosphorylatable KaiC failed to repress kaiBC promoter activity. Previously, we found, using a kaiA null mutant in which KaiC phosphorylation is absent, that KaiC overexpression did not inhibit kaiBC expression but gradually enhanced it (14). These results also suggest that KaiC phosphorylation is required for circadian transcriptional repression.
Acknowledgments
We thank Drs. Toru Hisabori (Tokyo Institute of Technology, Tokyo), Youji Sakagami (Nagoya University), Yoshihiro Suzuki (Nagoya University), and members of the Kondo laboratories for fruitful discussion and advice. Analysis of DNA sequencing was conducted with the aid of the CREST–Akita Plant Molecular Science Satellite Laboratory in Life Research Support Centre, instituted in Akita Prefectural University. This research was supported in part by Japanese Ministry of Education, Culture, Sports, Science, and Technology Grants-in-Aid 12208006 (to M.G.), 15GS0320 (to T.T.), and 15GS0308 and 14COEEA01 (to T.K.) and Japanese Society for Promotion of Science Grant 13680778 (to H.I.). H.K. and Y.K. were supported by Japanese Society for Promotion of Science Fellowships for Young Scientists 16005806 and 13001761, respectively.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LL, continuous light; MS/MS, tandem MS; IPTG, isopropyl β-d-thiogalactopyranoside.
See Commentary on page 13697.
References
- 1.Bunning, E. (1973) The Physiological Clock (Springer, New York), 3rd Ed.
- 2.Golden, S. S., Ishiura, M., Johnson, C. H. & Kondo, T. (1997) Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 327–354. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki, H. & Kondo, T. (2000) Plant Cell Physiol. 41, 1013–1020. [DOI] [PubMed] [Google Scholar]
- 4.Ishiura, M., Kutsuna, S., Aoki, S., Iwasaki, H., Andersson, C. R., Tanabe, A., Golden, S. S., Johnson, C. H. & Kondo, T. (1998) Science 281, 1519–1523. [DOI] [PubMed] [Google Scholar]
- 5.Young, M. W. & Kay, S. A. (2001) Nat. Rev. Genet. 2, 702–715. [DOI] [PubMed] [Google Scholar]
- 6.Nakahira, Y., Katayama, M., Miyashita, H., Kutsuna, S., Iwasaki, H., Oyama, T. & Kondo, T. (2004) Proc. Natl. Acad. Sci. USA 101, 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu, Y., Mori, T. & Johnson, C. H. (2003) EMBO J. 22, 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki, H., Taniguchi, Y., Ishiura, M. & Kondo, T. (1999) EMBO J. 18, 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kageyama, H., Kondo, T. & Iwasaki, H. (2003) J. Biol. Chem. 278, 2388–2395. [DOI] [PubMed] [Google Scholar]
- 10.Nishiwaki, T., Iwasaki, H., Ishiura, M. & Kondo, T. (2000) Proc. Natl. Acad. Sci. USA 97, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori, T., Saveliev, S. V., Xu, Y., Stafford, W. F., Cox, M. M., Inman, R. B. & Johnson C. H. (2002) Proc. Natl. Acad. Sci. USA 99, 17203–17208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi, F., Suzuki, H., Iwase, R., Uzumaki, T., Miyake, A., Shen, J. R., Imada, K., Frukawa, Y., Yonemura, K., Namba, K. & Ishiura, M. (2003) Genes Cells 8, 287–296. [DOI] [PubMed] [Google Scholar]
- 13.Xu, Y., Mori, T. & Johnson, C. H. (2000) EMBO J. 19, 3349–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki, H., Nishiwaki, T., Kitayama, Y., Nakajima, M. & Kondo, T. (2002) Proc. Natl. Acad. Sci. USA 99, 15788–15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi, F., Ito, H., Fujita, M., Iwase, R., Uzumaki, T. & Ishiura, M. (2004) Biochem. Biophys. Res. Commun. 316, 195–202. [DOI] [PubMed] [Google Scholar]
- 16.Williams, S. B., Vakonakis, I., Golden, S. S. & LiWang, A. C. (2002) Proc. Natl. Acad. Sci. USA 99, 15357–15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitayama, Y., Iwasaki, H., Nishiwaki, T. & Kondo, T. (2003) EMBO J. 22, 2127–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura, H., Nakahira, Y., Imai, K., Tsuruhara, A., Kondo, H., Hayashi, H., Hirai, M., Saito, H. & Kondo, T. (2002) Microbiology 148, 2903–2909. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-de-Cossio, J., Gonzalez, J., Satomi, Y., Shima, T., Okumura, N., Besada, V., Betancourt, L., Padron, G., Shimonishi, Y. & Takao, T. (2000) Electrophoresis 21, 1694–1699. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki, H., Williams, S. B., Kitayama, Y., Ishiura, M., Golden, S. S. & Kondo, T. (2000) Cell 101, 223–233. [DOI] [PubMed] [Google Scholar]
- 21.Kloss, B., Price, J. L., Saez, L., Blau, J., Rothenfluh, A., Wesley, C. S. & Young, M. W. (1998) Cell 94, 97–107. [DOI] [PubMed] [Google Scholar]
- 22.Lin, J. M., Kilman, V. L., Keegan, K., Paddock, B., Emery-Le, M., Rosbash, M. & Allada, R. (2002) Nature 420, 816–820. [DOI] [PubMed] [Google Scholar]
- 23.Akten, B., Jauch, E., Henova, G. K., Kim, E. Y., Edery, I., Raabe, T. & Jackson, F. R. (2003) Nat. Neurosci. 6, 251–257. [DOI] [PubMed] [Google Scholar]
- 24.Sathyanarayanan, S., Zheng, X., Xiao, R. & Sehgal, A. (2004) Cell 116, 603–615. [DOI] [PubMed] [Google Scholar]
- 25.Martinek, S., Inonog, S., Manoukian, A. S. & Young, M. W. (2001) Cell 105, 769–779. [DOI] [PubMed] [Google Scholar]
- 26.Ko, H. W., Jiang, J. & Edery, I. (2002) Nature 420, 673–678. [DOI] [PubMed] [Google Scholar]
- 27.Grima, B., Lamouroux, A., Chelot, E., Papin, C., Limbourg-Bouchon, B. & Rouyer, F. (2002) Nature 420, 178–182. [DOI] [PubMed] [Google Scholar]
- 28.Nawathean, P. & Rosbash, M. (2004) Mol. Cell 13, 213–223. [DOI] [PubMed] [Google Scholar]
- 29.Yang, Y., Cheng, P. & Liu, Y. (2002) Genes. Dev. 16, 994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, Y., Cheng, P., He, Q., Wang, L. & Liu, Y. (2003) Mol. Cell. Biol. 23, 6221–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, Y., He, Q., Cheng, P., Yarden, O. & Liu, Y. (2004) Genes Dev. 18, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, Y., Loros, J. & Dunlap, J. C. (2000) Proc. Natl. Acad. Sci. USA 97, 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He, Q., Cheng, P., Yang, Y., He, Q., Yu, H. & Liu, Y. (2003) EMBO J. 22, 4421–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi, Y., Yamaguchi, A., Hijikata, A., Iwasaki, H., Kamagata, K., Ishiura, M., Go, M. & Kondo, T. (2001) FEBS Lett. 496, 86–90. [DOI] [PubMed] [Google Scholar]
- 35.Roepstorff, P. & Fohlman, J. (1984) Biomed. Mass Spectrom. 11, 601 (lett.). [DOI] [PubMed] [Google Scholar]