Abstract
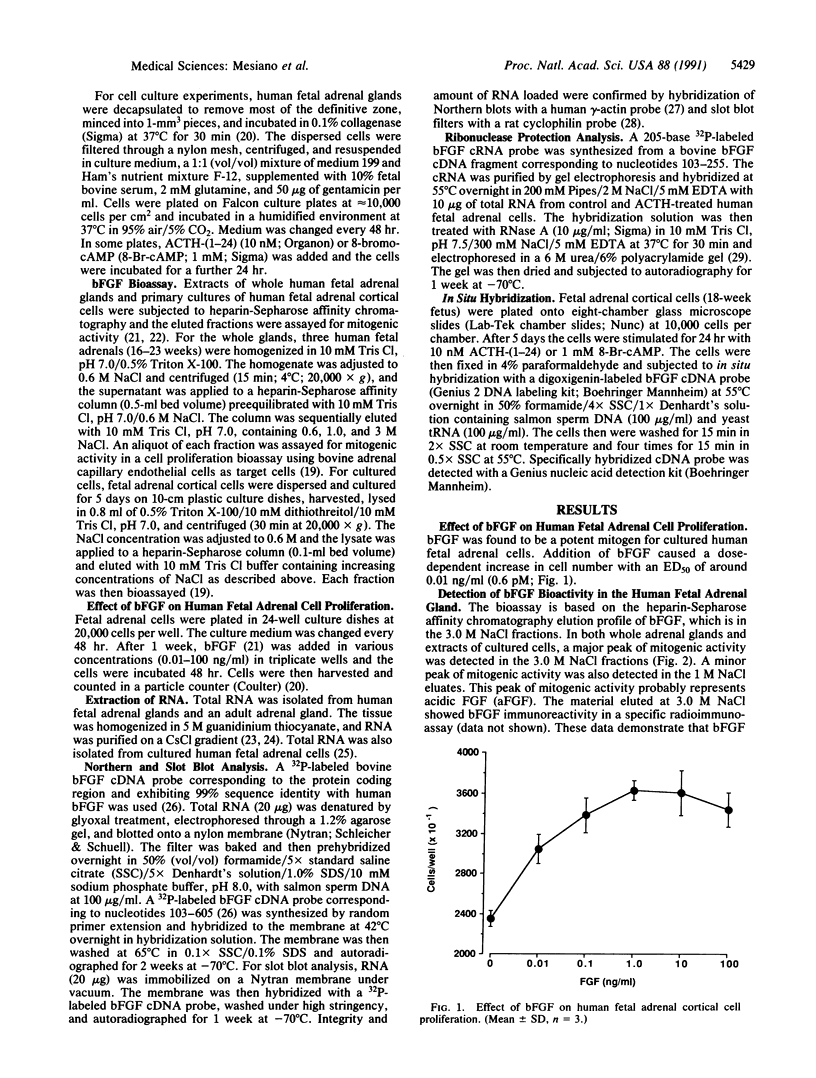
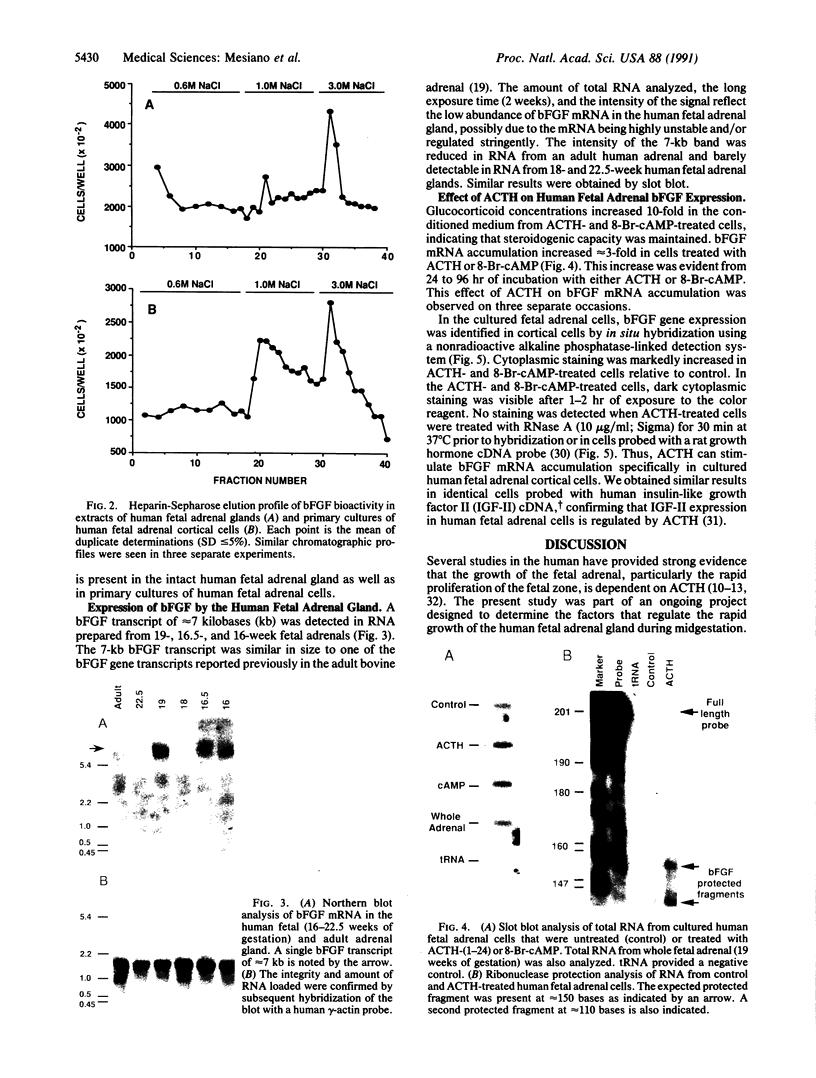
Human fetal adrenal growth after midgestation is very rapid and appears to be dependent upon pituitary adrenocorticotropin (ACTH) in vivo. We hypothesized that the regulation of fetal adrenal growth by ACTH is mediated by ACTH-stimulated local growth factor production. As we have found basic fibroblast growth factor (bFGF) to be a potent mitogen for human fetal adrenal cells in culture, we conducted studies to determine whether bFGF is synthesized by the human fetal adrenal gland and whether bFGF gene expression in primary cultures of human fetal adrenal cells is regulated by ACTH. Bioassayable bFGF-like activity was detected in extracts of whole human fetal adrenal glands and primary cultures of human fetal adrenal cells. Northern blot analysis of total RNA from whole human fetal adrenal glands revealed a characteristic 7-kilobase bFGF mRNA, indicating that the fetal adrenal bFGF bioactivity was most likely due to local synthesis. Slot blot and ribonuclease protection analysis showed that bFGF mRNA was present in very low amounts in total RNA from primary cultures of unstimulated human fetal adrenal cells but was increased 2- to 3-fold in cells exposed to 10 nM ACTH-(1-24) or 1 mM 8-bromoadenosine 3',5'-cyclic monophosphate for 24 hr. bFGF mRNA was localized to adrenocortical cells and not fibroblasts by in situ hybridization. bFGF mRNA was barely detectable in unstimulated cells, whereas it was markedly increased in cells exposed to either ACTH or 8-bromoadenosine 3',5'-cyclic monophosphate. These data support our hypothesis that the regulation of human fetal adrenal growth by ACTH at midgestation may be mediated by the stimulation of local growth factor production, and we suggest that bFGF may play a major role in this process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- BENIRSCHKE K. Adrenals in anencephaly and hydrocephaly. Obstet Gynecol. 1956 Oct;8(4):412–425. [PubMed] [Google Scholar]
- Böhlen P., Esch F., Baird A., Gospodarowicz D. Acidic fibroblast growth factor (FGF) from bovine brain: amino-terminal sequence and comparison with basic FGF. EMBO J. 1985 Aug;4(8):1951–1956. doi: 10.1002/j.1460-2075.1985.tb03876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coulter C. L., Young I. R., Browne C. A., McMillen I. C. Different roles for the pituitary and adrenal cortex in the control of enkephalin peptide localization and cortico-medullary interaction in the sheep adrenal during development. Neuroendocrinology. 1991 Mar;53(3):281–286. doi: 10.1159/000125730. [DOI] [PubMed] [Google Scholar]
- Crickard K., Ill C. R., Jaffe R. B. Control of proliferation of human fetal adrenal cells in vitro. J Clin Endocrinol Metab. 1981 Oct;53(4):790–796. doi: 10.1210/jcem-53-4-790. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Di Blasio A. M., Fujii D. K., Yamamoto M., Martin M. C., Jaffe R. B. Maintenance of cell proliferation and steroidogenesis in cultured human fetal adrenal cells chronically exposed to adrenocorticotropic hormone: rationalization of in vitro and in vivo findings. Biol Reprod. 1990 Apr;42(4):683–691. doi: 10.1095/biolreprod42.4.683. [DOI] [PubMed] [Google Scholar]
- Duperray A., Chambaz E. M. Effect of prostaglandin E1 and ACTH on proliferation and steroidogenic activity of bovine adreno-cortical cells in primary culture. J Steroid Biochem. 1980 Nov;13(11):1359–1364. doi: 10.1016/0022-4731(80)90098-9. [DOI] [PubMed] [Google Scholar]
- Easterling W. E., Jr, Simmer H. H., Dignam W. J., Frankland M. V., Naftolin F. Neutral C19-steroids and steroid sulfates in human pregnancy. II. Dehydroepiandrosterone sulfate, 16-alpha-hydroxydehydroepiandrosterone, and 16-alpha-hydroxydehydroepiandrosterone sulfate in maternal and fetal blood of pregnancies with anencephalic and normal fetuses. Steroids. 1966 Aug;8(2):157–178. doi: 10.1016/0039-128x(66)90090-0. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Ill C. R., Watson R. K. Kinetics of cAMP inhibition of DNA synthesis in bovine adrenocortical cells. Mol Cell Endocrinol. 1980 Jul;19(1):113–122. doi: 10.1016/0303-7207(80)90035-0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Esch F., Bohlen P. Corpus luteum angiogenic factor is related to fibroblast growth factor. Endocrinology. 1985 Dec;117(6):2383–2391. doi: 10.1210/endo-117-6-2383. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Hornsby P. J., Gill G. N. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology. 1977 Apr;100(4):1080–1089. doi: 10.1210/endo-100-4-1080. [DOI] [PubMed] [Google Scholar]
- Gray E. S., Abramovich D. R. Morphologic features of the anencephalic adrenal gland in early pregnancy. Am J Obstet Gynecol. 1980 Jun 15;137(4):491–495. doi: 10.1016/0002-9378(80)91134-5. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han V. K., D'Ercole A. J., Lund P. K. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science. 1987 Apr 10;236(4798):193–197. doi: 10.1126/science.3563497. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Smith R. G., Zhou H., Mansson P. E., Malark M. Androgens and glucocorticoids modulate heparin-binding growth factor I mRNA accumulation in DDT1 cells as analyzed by in situ hybridization. Mol Endocrinol. 1989 Nov;3(11):1839–1844. doi: 10.1210/mend-3-11-1839. [DOI] [PubMed] [Google Scholar]
- Honnebier W. J., Jöbsis A. C., Swaab D. F. The effect of hypophysial hormones and human chorionic gonadotrophin (HCG) on the anencephalic fetal adrenal cortex and on parturition in the human. J Obstet Gynaecol Br Commonw. 1974 Jun;81(6):423–438. doi: 10.1111/j.1471-0528.1974.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Jaffe R. B., Serón-Ferré M., Crickard K., Koritnik D., Mitchell B. F., Huhtaniemi I. T. Regulation and function of the primate fetal adrenal gland and gonad. Recent Prog Horm Res. 1981;37:41–103. doi: 10.1016/b978-0-12-571137-1.50007-4. [DOI] [PubMed] [Google Scholar]
- Jost A. Problems of fetal endocrinology: the adrenal glands. Recent Prog Horm Res. 1966;22:541–574. doi: 10.1016/b978-1-4831-9825-5.50017-8. [DOI] [PubMed] [Google Scholar]
- LANMAN J. T. The adrenal gland in the human fetus. An interpretation of its physiology and unusual developmental pattern. Pediatrics. 1961 Jan;27:140–158. [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S. H., Miller W. L. Extraadrenal steroid 21-hydroxylation is not mediated by P450c21. J Clin Invest. 1989 Nov;84(5):1497–1502. doi: 10.1172/JCI114325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S. H., Vaisse C. cAMP regulates P450scc gene expression by a cycloheximide-insensitive mechanism in cultured mouse Leydig MA-10 cells. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7775–7779. doi: 10.1073/pnas.86.20.7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menapace L., Armato U., Whitfield J. F. The effects of corticotrophin (ACTH1-24), cyclic AMP and TPA (12-O-tetradecanoyl phorbol-13-acetate) on DNA replication and proliferation of primary rabbit adrenocortical cells in a synthetic medium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1295–1303. doi: 10.1016/s0006-291x(87)80274-7. [DOI] [PubMed] [Google Scholar]
- Milner A. J. Cyclic AMP and the differentiation of adrenal cortical cells grown in tissue culture. J Endocrinol. 1972 Nov;55(2):405–413. doi: 10.1677/joe.0.0550405. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Sato R., Sato Y., Friesen H. G. Fibroblast growth factor messenger ribonucleic acid expression in a human astrocytoma cell line: regulation by serum and cell density. Mol Endocrinol. 1988 Jul;2(7):591–598. doi: 10.1210/mend-2-7-591. [DOI] [PubMed] [Google Scholar]
- New M. I., Dupont B., Pang S., Pollack M., Levine L. S. An update of congenital adrenal hyperplasia. Recent Prog Horm Res. 1981;37:105–181. doi: 10.1016/b978-0-12-571137-1.50008-6. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Neville A. M. Morphological responses to corticotrophin and cyclic AMP by adult rat adrenocortical cells in monolayer culture. J Endocrinol. 1973 Mar;56(3):529–536. doi: 10.1677/joe.0.0560529. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouët J., Gospodarowicz D. Transforming growth factor beta-1 positively modulates the bioactivity of fibroblast growth factor on corneal endothelial cells. J Cell Physiol. 1989 Nov;141(2):392–399. doi: 10.1002/jcp.1041410221. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. M., Comline R. S., Fowden A. L., Silver M. Adrenal cortex of fetal lamb: changes after hypophysectomy and effects of Synacthen on cytoarchitecture and secretory activity. Q J Exp Physiol. 1983 Jan;68(1):15–27. doi: 10.1113/expphysiol.1983.sp002697. [DOI] [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- STUDZINSKI G. P., HAY D. C., SYMINGTON T. Observations on the weight of the human adrenal gland and the effect of preparations of corticotropin of different purity on the weight and morphology of the human adrenal gland. J Clin Endocrinol Metab. 1963 Mar;23:248–254. doi: 10.1210/jcem-23-3-248. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Basic fibroblast growth factor: production and growth stimulation in cultured adrenal cortex cells. Endocrinology. 1987 Feb;120(2):796–800. doi: 10.1210/endo-120-2-796. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D., Magness R. R., Stone R., Waterman M. R., Simpson E. R. Angiotensin II inhibits luteinizing hormone-stimulated cholesterol side chain cleavage expression and stimulates basic fibroblast growth factor expression in bovine luteal cells in primary culture. J Biol Chem. 1990 Jan 5;265(1):5–8. [PubMed] [Google Scholar]
- Suyama A. T., Long J. A., Ramachandran J. Ultrastructural changes induced by ACTH in normal adrenocortical cells in culture. J Cell Biol. 1977 Mar;72(3):757–763. doi: 10.1083/jcb.72.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen R., Miller W. L. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc [corrected], in human steroidogenic tissues. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1590–1594. doi: 10.1073/pnas.84.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]