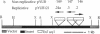Abstract
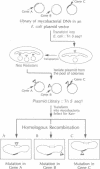
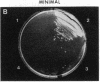
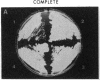
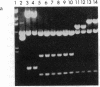
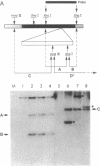
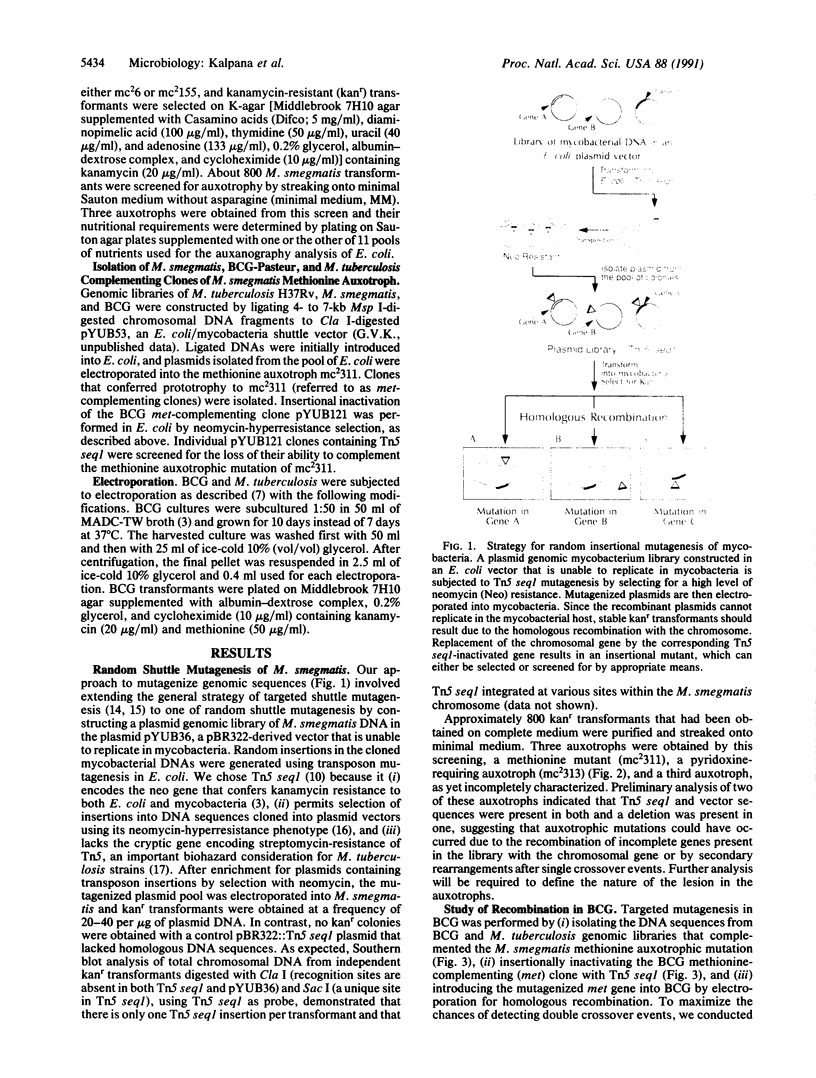
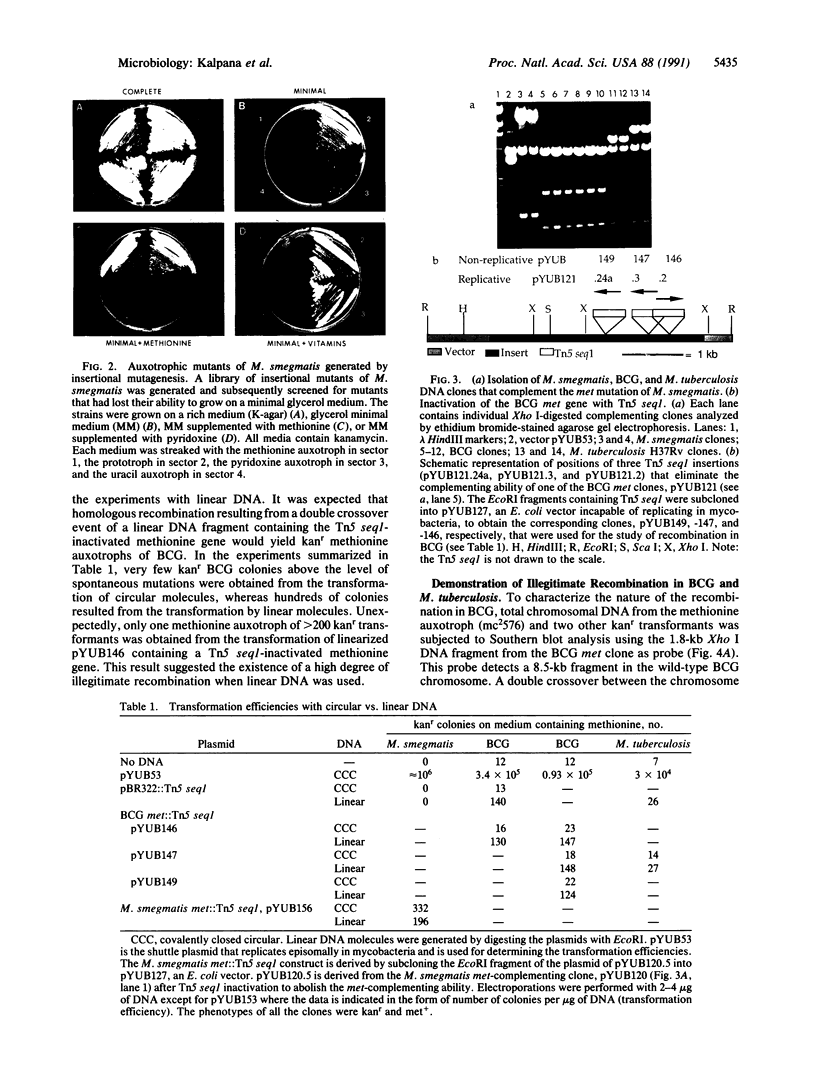
Mycobacteria, particularly Mycobacterium tuberculosis, Mycobacterium leprae, and Mycobacterium avium, are major pathogens of man. Although insertional mutagenesis has been an invaluable genetic tool for analyzing the mechanisms of microbial pathogenesis, it has not yet been possible to apply it to the mycobacteria. To overcome intrinsic difficulties in directly manipulating the genetics of slow-growing mycobacteria, including M. tuberculosis and bacille Calmette-Guérin (BCG) vaccine strains, we developed a system for random shuttle mutagenesis. A genomic library of Mycobacterium smegmatis was subjected to transposon mutagenesis with Tn5 seq1, a derivative of Tn5, in Escherichia coli and these transposon-containing recombinant plasmids were reintroduced into mycobacterial chromosomes by homologous recombination. This system has allowed us to isolate several random auxotrophic mutants of M. smegmatis. To extend this strategy to M. tuberculosis and BCG, targeted mutagenesis was performed using a cloned BCG methionine gene that was subjected to Tn5 seq1 mutagenesis in E. coli and reintroduced into the mycobacteria. Surprisingly for prokaryotes, both BCG and M. tuberculosis were found to incorporate linear DNA fragments into illegitimate sites throughout the mycobacterial genomes at a frequency of 10(-5) to 10(-4) relative to the number of transformants obtained with autonomously replicating vectors. Thus the efficient illegitimate recombination of linear DNA fragments provides the basis for an insertional mutagenesis system for M. tuberculosis and BCG.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Schmandt M. A., Lowe J. B. Specificity of transposon Tn5 insertion. Genetics. 1983 Dec;105(4):813–828. doi: 10.1093/genetics/105.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. Genetic analysis of bacterial virulence determinants in Bordetella pertussis and the pathogenetic Yersinia. Curr Top Microbiol Immunol. 1985;118:1–11. doi: 10.1007/978-3-642-70586-1_1. [DOI] [PubMed] [Google Scholar]
- Jacobs W. R., Docherty M. A., Curtiss R., 3rd, Clark-Curtiss J. E. Expression of Mycobacterium leprae genes from a Streptococcus mutans promoter in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1926–1930. doi: 10.1073/pnas.83.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Tuckman M., Bloom B. R. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature. 1987 Jun 11;327(6122):532–535. doi: 10.1038/327532a0. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Skarnes W. C., Rossant J. Production of a mutation in mouse En-2 gene by homologous recombination in embryonic stem cells. Nature. 1989 Mar 9;338(6211):153–156. doi: 10.1038/338153a0. [DOI] [PubMed] [Google Scholar]
- Martin C., Timm J., Rauzier J., Gomez-Lus R., Davies J., Gicquel B. Transposition of an antibiotic resistance element in mycobacteria. Nature. 1990 Jun 21;345(6277):739–743. doi: 10.1038/345739a0. [DOI] [PubMed] [Google Scholar]
- Murray C. J., Styblo K., Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990 Mar;65(1):6–24. [PubMed] [Google Scholar]
- Nag D. K., Huang H. V., Berg D. E. Bidirectional chain-termination nucleotide sequencing: transposon Tn5seq1 as a mobile source of primer sites. Gene. 1988 Apr 15;64(1):135–145. doi: 10.1016/0378-1119(88)90487-8. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Snapper S. B., Lugosi L., Jekkel A., Melton R. E., Kieser T., Bloom B. R., Jacobs W. R., Jr Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6987–6991. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990 Nov;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Manoil C., Mekalanos J. J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989 Apr;171(4):1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Holden D. W., Leong S. A. Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci U S A. 1988 Feb;85(3):865–869. doi: 10.1073/pnas.85.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]