Abstract
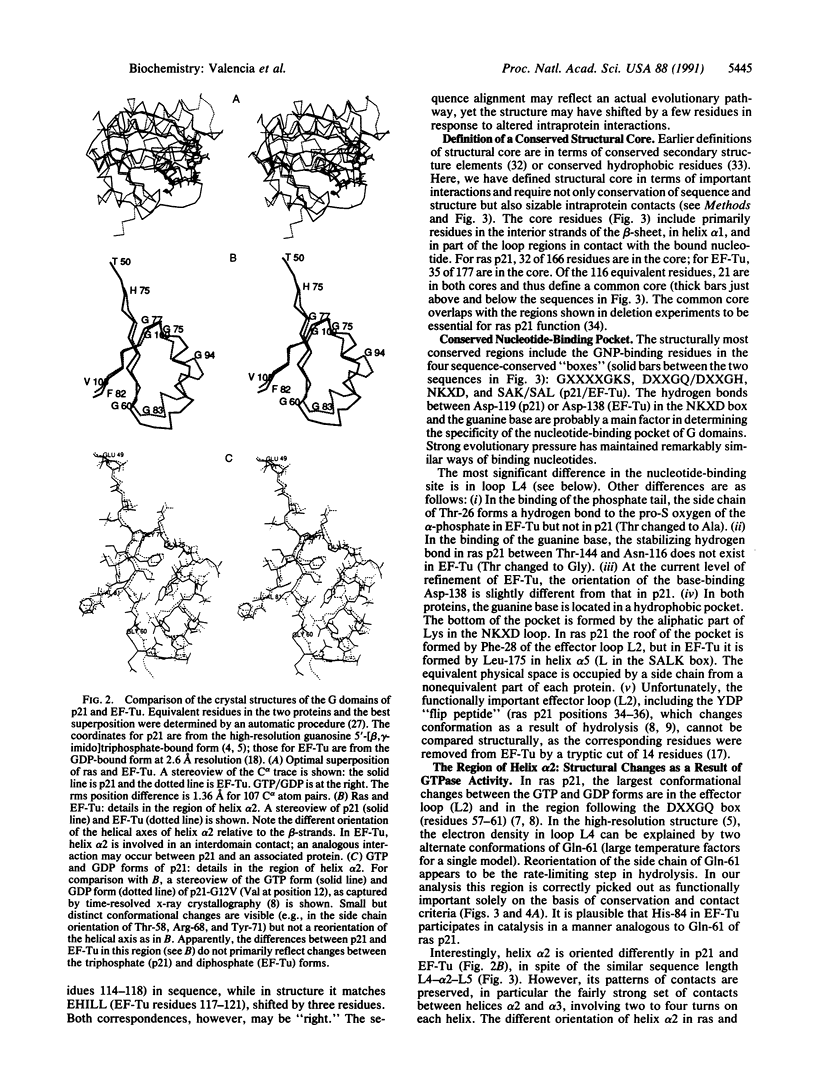
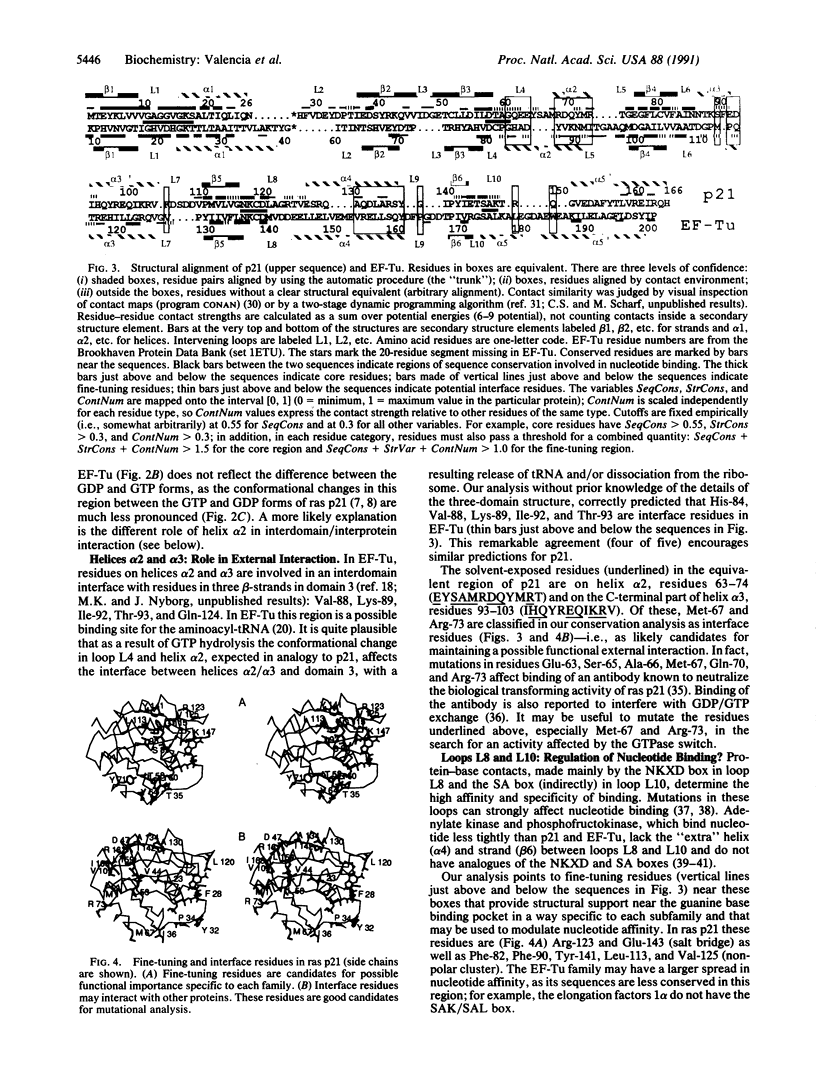
GTPase domains are functional and structural units employed as molecular switches in a variety of important cellular functions, such as growth control, protein biosynthesis, and membrane traffic. Amino acid sequences of more than 100 members of different subfamilies are known, but crystal structures of only mammalian ras p21 and bacterial elongation factor Tu have been determined. After optimal superposition of these remarkably similar structures, careful multiple sequence alignment, and calculation of residue-residue interactions, we analyzed the two subfamilies in terms of structural conservation, sequence conservation, and residue contact strength. There are three main results. (i) A structure-based alignment of p21 and elongation factor Tu. (ii) The definition of a common conserved structural core that may be useful as the basis of model building by homology of the three-dimensional structure of any GTPase domain. (iii) Identification of sequence regions, other than the effector loop and the nucleotide binding site, that may be involved in the functional cycle: they are loop L4, known to change conformation after GTP hydrolysis; helix alpha 2, especially Arg-73 and Met-67 in ras p21; loops L8 and L10, including ras p21 Arg-123, Lys-147, and Leu-120; and residues located spatially near the N and C termini. These regions are candidate sites for interaction either with the GTP/GDP exchange factor, with a GTPase-affected function, or with a molecule delivered to a destination site with the aid of the GTPase domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Chen J. M., Lee G., Brandt-Rauf P. W., Murphy R. B., Gibson K. D., Scheraga H. A., Rackovsky S., Pincus M. R. Conformations of the central transforming region (Ile 55-Met 67) of the p21 protein and their relationship to activation of the protein. Int J Pept Protein Res. 1990 Sep;36(3):247–254. doi: 10.1111/j.1399-3011.1990.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Chen J. M., Lee G., Murphy R. B., Brandt-Rauf P. W., Pincus M. R. Comparisons between the three-dimensional structures of the chemotactic protein CheY and the normal Gly 12-p21 protein. Int J Pept Protein Res. 1990 Jul;36(1):1–6. doi: 10.1111/j.1399-3011.1990.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Chen J. M., Lee G., Murphy R. B., Carty R. P., Brandt-Rauf P. W., Friedman E., Pincus M. R. Comparison of the computed structures for the phosphate-binding loop of the p21 protein containing the oncogenic site Gly 12 with the X-ray crystallographic structures for this region in the p21 protein and EFtu. A model for the structure of the p21 protein in its oncogenic form. J Biomol Struct Dyn. 1989 Apr;6(5):859–875. doi: 10.1080/07391102.1989.10506518. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton D. J., Hattori S., Shih T. Y. Mutations of the ras gene product p21 that abolish guanine nucleotide binding. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5076–5080. doi: 10.1073/pnas.83.14.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. F., Kjeldgaard M., la Cour T. F., Thirup S., Nyborg J. Structural determination of the functional sites of E. coli elongation factor Tu. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):203–208. doi: 10.1016/0167-4781(90)90167-z. [DOI] [PubMed] [Google Scholar]
- Créchet J. B., Poullet P., Camonis J., Jacquet M., Parmeggiani A. Different kinetic properties of the two mutants, RAS2Ile152 and RAS2Val19, that suppress the CDC25 requirement in RAS/adenylate cyclase pathway in Saccharomyces cerevisiae. J Biol Chem. 1990 Jan 25;265(3):1563–1568. [PubMed] [Google Scholar]
- Créchet J. B., Poullet P., Mistou M. Y., Parmeggiani A., Camonis J., Boy-Marcotte E., Damak F., Jacquet M. Enhancement of the GDP-GTP exchange of RAS proteins by the carboxyl-terminal domain of SCD25. Science. 1990 May 18;248(4957):866–868. doi: 10.1126/science.2188363. [DOI] [PubMed] [Google Scholar]
- Downward J., Riehl R., Wu L., Weinberg R. A. Identification of a nucleotide exchange-promoting activity for p21ras. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5998–6002. doi: 10.1073/pnas.87.15.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Crechet J. B., De Vendittis E., Zahn R., Feger G., Vitelli A., Parmeggiani A. Yeast mutants temperature-sensitive for growth after random mutagenesis of the chromosomal RAS2 gene and deletion of the RAS1 gene. EMBO J. 1988 Nov;7(11):3375–3383. doi: 10.1002/j.1460-2075.1988.tb03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein J., Goody R. S., Wittinghofer A. Preparation and characterization of nucleotide-free and metal ion-free p21 "apoprotein". J Biol Chem. 1987 Jun 25;262(18):8455–8458. [PubMed] [Google Scholar]
- Hattori S., Clanton D. J., Satoh T., Nakamura S., Kaziro Y., Kawakita M., Shih T. Y. Neutralizing monoclonal antibody against ras oncogene product p21 which impairs guanine nucleotide exchange. Mol Cell Biol. 1987 May;7(5):1999–2002. doi: 10.1128/mcb.7.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. K., Kung H. F., Kamata T. Purification of a factor capable of stimulating the guanine nucleotide exchange reaction of ras proteins and its effect on ras-related small molecular mass G proteins. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8008–8012. doi: 10.1073/pnas.87.20.8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard T. J., Blundell T. L. Comparison of solvent-inaccessible cores of homologous proteins: definitions useful for protein modelling. Protein Eng. 1987 Jun;1(3):159–171. doi: 10.1093/protein/1.3.159. [DOI] [PubMed] [Google Scholar]
- Hwang Y. W., Sanchez A., Miller D. L. Mutagenesis of bacterial elongation factor Tu at lysine 136. A conserved amino acid in GTP regulatory proteins. J Biol Chem. 1989 May 15;264(14):8304–8309. [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Krengel U., Schlichting I., Scherer A., Schumann R., Frech M., John J., Kabsch W., Pai E. F., Wittinghofer A. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990 Aug 10;62(3):539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- McCormick F., Adari H., Trahey M., Halenbeck R., Koths K., Martin G. A., Crosier W. J., Watt K., Rubinfeld B., Wong G. Interaction of ras p21 proteins with GTPase activating protein. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):849–854. doi: 10.1101/sqb.1988.053.01.097. [DOI] [PubMed] [Google Scholar]
- McCormick F., Clark B. F., la Cour T. F., Kjeldgaard M., Norskov-Lauritsen L., Nyborg J. A model for the tertiary structure of p21, the product of the ras oncogene. Science. 1985 Oct 4;230(4721):78–82. doi: 10.1126/science.3898366. [DOI] [PubMed] [Google Scholar]
- Milburn M. V., Tong L., deVos A. M., Brünger A., Yamaizumi Z., Nishimura S., Kim S. H. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990 Feb 23;247(4945):939–945. doi: 10.1126/science.2406906. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Krengel U., Petsko G. A., Goody R. S., Kabsch W., Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990 Aug;9(8):2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panniers R., Rowlands A. G., Henshaw E. C. The effect of Mg2+ and guanine nucleotide exchange factor on the binding of guanine nucleotides to eukaryotic initiation factor 2. J Biol Chem. 1988 Apr 25;263(12):5519–5525. [PubMed] [Google Scholar]
- Parmeggiani A., Swart G. W., Mortensen K. K., Jensen M., Clark B. F., Dente L., Cortese R. Properties of a genetically engineered G domain of elongation factor Tu. Proc Natl Acad Sci U S A. 1987 May;84(10):3141–3145. doi: 10.1073/pnas.84.10.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinstein J., Vetter I. R., Schlichting I., Rösch P., Wittinghofer A., Goody R. S. Fluorescence and NMR investigations on the ligand binding properties of adenylate kinases. Biochemistry. 1990 Aug 14;29(32):7440–7450. doi: 10.1021/bi00484a013. [DOI] [PubMed] [Google Scholar]
- Riis B., Rattan S. I., Clark B. F., Merrick W. C. Eukaryotic protein elongation factors. Trends Biochem Sci. 1990 Nov;15(11):420–424. doi: 10.1016/0968-0004(90)90279-k. [DOI] [PubMed] [Google Scholar]
- Sander C., Schneider R. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins. 1991;9(1):56–68. doi: 10.1002/prot.340090107. [DOI] [PubMed] [Google Scholar]
- Schlichting I., Almo S. C., Rapp G., Wilson K., Petratos K., Lentfer A., Wittinghofer A., Kabsch W., Pai E. F., Petsko G. A. Time-resolved X-ray crystallographic study of the conformational change in Ha-Ras p21 protein on GTP hydrolysis. Nature. 1990 May 24;345(6273):309–315. doi: 10.1038/345309a0. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Scolnick E. M. Identification of effector residues and a neutralizing epitope of Ha-ras-encoded p21. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4725–4729. doi: 10.1073/pnas.83.13.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. R., Orengo C. A. Protein structure alignment. J Mol Biol. 1989 Jul 5;208(1):1–22. doi: 10.1016/0022-2836(89)90084-3. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Kung H. F., Bekesi E., Robins T., Johnsen M., Vass W. C., Lowy D. R. Mutational analysis of a ras catalytic domain. Mol Cell Biol. 1986 Jul;6(7):2646–2654. doi: 10.1128/mcb.6.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. A cytosolic protein catalyzes the release of GDP from p21ras. Science. 1990 Apr 6;248(4951):67–69. doi: 10.1126/science.2181667. [DOI] [PubMed] [Google Scholar]