Abstract
Objective. Alkaptonuria (AKU) is a rare autosomal recessive disease resulting from a single enzyme deficiency in tyrosine metabolism. As a result, homogentisic acid cannot be metabolized, causing systemic increases. Over time, homogentisic acid polymerizes and deposits in collagenous tissues, leading to ochronosis. Typically, this occurs in joint cartilages, leading to an early onset, rapidly progressing osteoarthropathy. The aim of this study was to examine tissue turnover in cartilage affected by ochronosis and its role in disease initiation and progression.
Methods. With informed patient consent, hip and knee cartilages were obtained at surgery for arthropathy due to AKU (n = 6; 2 knees/4 hips) and OA (n = 12; 5 knees/7 hips); healthy non-arthritic (non-OA n = 6; 1 knee/5 hips) cartilages were obtained as waste from trauma surgery. We measured cartilage concentrations (normalized to dry weight) of racemized aspartate, GAG, COMP and deamidated COMP (D-COMP). Unpaired AKU, OA and non-OA samples were compared by non-parametric Mann–Whitney U test.
Results. Despite more extractable total protein being obtained from AKU cartilage than from OA or non-OA cartilage, there was significantly less extractable GAG, COMP and D-COMP in AKU samples compared with OA and non-OA comparators. Racemized Asx (aspartate and asparagine) was significantly enriched in AKU cartilage compared with in OA cartilage.
Conclusions. These novel data represent the first examination of cartilage matrix components in a sample of patients with AKU, representing almost 10% of the known UK alkaptonuric population. Compared with OA and non-OA, AKU cartilage demonstrates a very low turnover state and has low levels of extractable matrix proteins.
Keywords: alkaptonuria, osteoarthritis, biomarkers, ageing, glycosaminoglycan, cartilage oligomeric matrix protein, racemization, ochronosis
Rheumatology key messages
Alkaptonuria cartilage demonstrates an older profile of ageing biomarkers compared with OA and non-OA comparators.
Alkaptonuria cartilage shows a low turnover profile compared with cartilage from OA and normal comparators.
Anabolic therapy may be beneficial for patients with alkaptonuria.
Introduction
Alkaptonuria (AKU), a rare autosomal recessive condition, is classified as a rare form of OA [1]. AKU results from deficiency of a single enzyme, homogentisate 1,2-dioxygenase, responsible for opening the benzene ring of homogentisic acid (HGA), an intermediate in tyrosine metabolism. The absence or lack of homogentisate 1,2-dioxygenase function results in HGA accumulation in tissues. HGA is excreted in the urine, but renal excretion is insufficient to completely clear the burden of HGA in AKU [2]. Elevated HGA concentrations in urine, plasma and body tissues result in a classic triad of clinical presentations. These include the earliest presentation, from birth, of urine darkening upon standing or exposure to alkaline conditions [3, 4]. Over many years of HGA exposure, the second feature occurs—termed ochronosis or darkening of collagenous tissues—often seen in the ear pinna, eye sclerae and other less uniformly and readily examined areas [5, 6]. The process of ochronosis is not completely understood; however, it is suggested that HGA undergoes polymerization to a benzoquinone intermediate before assuming its final polymeric form in collagenous tissues [7]. The final presenting feature of the disease, namely early age onset of severe and rapidly progressing OA, results from ochronotic pigment deposition in articular cartilages, the presence of which alters cartilage biomechanical and biochemical properties [8]. The presence of this pigment appears to induce large-scale aggressive resorption of the subchondral bone plate and articular calcified cartilage due to stress shielding in AKU joints [8].
A novel ultrastructural bone turnover mechanism has been identified in both AKU and OA joint tissues, providing further evidence to support the similarity between the diseases [1, 2, 8]. In both cases, novel structures result from uncoupled bone turnover, deviating from the widely accepted Frostian bone turnover model. These structures also appear to be attached to the existing trabecular stock, without prior preparation of the trabecular surface by osteoclasts [8, 9]. The presence of ochronotic pigment in hyaline cartilage makes it impervious to enzymatic degradation by Tergazyme (a bacterial alkaline pronase); this may prevent the type of catabolism normally observed in OA cartilage [8]. As shown by ultrastructural analyses, pigment associates closely with collagen fibres in joint tissues, specifically in ligamentous capsule [10] and articular cartilage [11]. Along with ultrastructural evidence of a disorder of collagen fibres, solid state NMR demonstrates that there is disorder of extracellular proteins in ochronotic cartilage when compared with control cartilage [12]. However, little is known about the effects of ochronotic pigment on other matrix components, the biological ageing process and joint tissue turnover of these patients [8].
AKU is iconic in medicine as the first disease, demonstrated by Archibald Garrod in 1902, to conform to Mendelian inheritance [3]. The symptoms [1, 2], gene responsible [13, 14] and enzyme [15] are all known and well documented; even with this knowledge, a viable treatment is yet to be available to patients. Trials on a potential therapy, Nitisinone, were undertaken, but the outcome was deemed not beneficial [16, 17]. Nitisinone can inhibit ochronosis in a mouse model [18, 19]. However, Nitisinone is not without its side effects, including elevated plasma tyrosine [20]. Moreover, Nitisinone is not likely to benefit individuals with established pigmentation and tissue impregnation with a large burden of cross-linked HGA. A clearer understanding of the molecular changes occurring in cartilage could provide more options for treating this disease and shed new light on our understanding of the disease pathogenesis and progression.
Ageing leads to non-enzymatic post-translational modification of proteins, including racemization and isomerization [21, 22, 23]. Racemization refers to the time-dependent conversion of amino acids in proteins from the L form to the D form (only L amino acids are incorporated into mammalian proteins). Cartilage, a tissue with low turnover due to its aneural and avascular nature, undergoes quantifiable racemization of some amino acids during the lifespan [24, 25]. Recent advances in the field of biomarkers of ageing and turnover have demonstrated that COMP is a useful determinant of differential turnover between hip and knee, with knee cartilage showing a greater turnover response than hip cartilage [21]. In AKU, where cartilage and its constituent matrix components become bound by HGA polymeric pigment, we considered it likely that these biomarkers of ageing and turnover would provide novel insights into the pathophysiology of this iconic disease. Given that pigmented cartilage appears to be impervious to enzymatic degradation, we hypothesized that cartilage turnover of AKU specimens will be reduced compared with osteoarthritic and non-osteoarthritic comparators.
The aim of this study was to investigate the impact of ochronotic pigment on cartilage matrix turnover in joint samples from patients with AKU. Through investigation of aspartate racemization, GAG and COMP, we characterized the turnover of tissue relative to the amount of pigmentation and compared the results with those obtained using non-pigmented tissue from non-OA and OA samples.
Methods
Cartilage sample collection
Alkaptonuric knees (n = 2, mean age 58.5 years, range 58–59 years) and hips (n = 4, mean age 61.0 years, range 54–70 years) were obtained at the time of surgery from individuals undergoing total joint arthroplasty to alleviate symptoms associated with ochronotic osteoarthropathy. Alkaptonuric cartilage samples were acquired from macroscopically ochronotic areas of tissue. All alkaptonuric joint specimens showed extensive pigmentation of all zones of cartilage. One specimen showed complete pigmentation of all the cartilage and absence of subchondral bone in many areas. Osteophytes were present in one of the samples where cartilage absence had exposed underlying osseous tissues. Pigmentation of connective tissues, including ligaments, was seen in all samples. All AKU samples were obtained with informed patient consent according to the Declaration of Helsinki and under ethical approval from the Liverpool Research Ethics Committee. Waste articular cartilage specimens were obtained from randomly selected total knee (n = 5, mean age 68.6 years, range 54–88 years) and total hip (n = 7, mean age 81.9 years, range 72–90 years) arthroplasties performed at Duke University Medical Center to alleviate symptoms of OA. From each arthritic joint, cartilage was harvested from around the lesion (lesion cartilage). Non-arthritic control cartilage samples from knee (n = 1, mean age 36 years) and hip (n = 5, mean age 63.4 years, range 23–82 years) joints were obtained at the time of reconstructive surgery for trauma from patients without evidence of OA as determined by macroscopic inspection of the specimens by the surgeon. All samples from Duke University were collected under Duke Institutional Review Board approval as waste surgical specimens and according to the Declaration of Helsinki. Due to the early onset of the arthropathy in AKU, we were unable to directly age-match samples. Instead, we utilized a matching strategy to ensure that OA and non-OA samples encompassed the AKU sample ages. All samples in each group were from different donors.
Sample preparation
Soluble and insoluble cartilage proteins were prepared as previously described with minor modifications [21]. Cartilage was pulverized under liquid nitrogen and extracted for 24 h at 4°C with gentle mixing in 4M Guanidine-HCl (Gu-HCl) in sodium acetate buffer pH 4.0 with protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) at a ratio of 2 ml of Gu-HCL extraction buffer per 1 g of wet-weight cartilage. Following centrifugation and collection of the supernatant, the remaining cartilage was extracted a second time with Gu-HCl extraction buffer for a further 24 h. Both first and second Gu-HCl extractions were combined and stored at −80°C until needed. A 200-µl aliquot of extract was buffer exchanged on an AKTA purifier 10 (GE Healthcare, Pittsburgh, PA, USA) using a HiTrap Desalting Column (GE, Pittsburgh, PA, USA) into PBS and stored at −80°C, constituting the soluble protein fraction. The residual Gu-HCl insoluble protein fraction was washed a further five times in nanopure water over a 24 h period at 4°C with rotation mixing to remove any residual Gu-HCl and extracted proteins. Excess water was removed and the samples stored at −80°C until required for hydrolysis and HPLC analysis.
Determination of protein content
Total protein was determined using the commercially available BCATM Protein Assay Kit and performed according to the manufacturer’s instructions (Pierce, Rockford, IL, USA). The presence of HGA was assessed by addition of 10M NaOH to the sample extract. No colour change indicative of the presence of HGA was seen in any samples, assuring us of the lack of interference by HGA with the bicinchoninic acid (BCA) assay for protein determination.
Determination of GAG content
The GAG content of cartilage extracts was quantified by a dye-binding assay with dimethylmethylene blue (DMMB) (Sigma, St Louis, MO, USA), as previously described [26]. Our analyses demonstrated no confounding of the DMMB reaction by HGA (Taylor, unpublished results).
Quantification of racemized amino acids
Racemized Asx (aspartate and asparagine) concentrations in cartilage extracts were quantified using a previously described HPLC approach developed and validated in our laboratory [24]. Briefly, the insoluble and soluble cartilage proteins were acid hydrolyzed in 6M HCl at 105°C for 6 h. The resulting free D- and L-amino acids were derivatized to fluorescent compounds by addition of o-phthaldialdehyde and N-tert-butyloxycarbonyl-l-cysteine. The subsequent derivatives were separated by reversed-phase HPLC using a C18 column and mobile phases of 0.2M acetic acid adjusted to a pH of 6.0 and an acetonitrile gradient. The resulting peaks were quantified fluorometrically (ex340 nm, em440 nm) by comparison with commercially available pure D- and L-amino acids (Fluka Biochemicals).
COMP ELISA
Total COMP (T-COMP) concentrations of the Gu-HCl soluble protein fraction of cartilage were quantified by ELISA using the mAb 16F12 for capture and biotinylated mAb 17C10 for detection, as previously described [27] with the following minor modifications. Plates were blocked with 3% BSA/PBS/0.02% sodium azide for 2 h. Streptavidin–alkaline phosphatase conjugate (ExtrAvadin, Sigma, St Louis, MO, USA) was diluted 30 000 times in 0.01% BSA/PBS/0.02% sodium azide, and the phosphatase substrate (4-nitrophenyl phosphate disodium salt hexahydrate, Sigma, St Louis, MO, USA) was used as the detection agent. Plates were read at wavelength 405 nm after 20 min of incubation.
D-COMP sandwich ELISA
D-COMP concentrations of the Gu-HCl soluble protein fraction of cartilage were determined using the D-COMP sandwich ELISA, as previously described [21]. Briefly, 96-well plates were coated with the deamidated COMP-specific 6-1A12 D-COMP mAb in 0.02M sodium carbonate coating buffer, pH 9.6 overnight and incubated at 4°C [21]. Plates were blocked for at least 2 h with 5% w/v BSA in PBS, pH 7.4 at 37°C, prior to use. Samples or standard were diluted in 0.1% w/v BSA in PBS as required prior to incubation of 50 µl of sample or standard overnight at 4°C. Unbound sample was discarded and the plate washed with PBS-tween wash buffer before incubation with the biotinylated 17-C10 COMP detection mAb (a gift from Dr V. Vilim). Unbound 17-C10 was discarded and the plate washed. Due to the lower levels of D-COMP, the D-COMP ELISA sensitivity was increased using avidin poly-horseradish peroxidase (poly-HRP, Thermo Scientific, Rockford, IL, USA), added for 30 min at room temperature with gentle mixing. Excess avidin-HRP was discarded, the plate washed and signal detected using tetramethyl benzidine (TMB, Sigma-Aldrich, St Louis, MO, USA) reagent after stopping with 2M HCl and detection at 450 nm.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 5 software (La Jolla, CA, USA). All results were analysed as unpaired data using the non-parametric Mann–Whitney U test.
Histological analysis of alkaptonuric samples
Histological analysis of alkaptonuric samples was undertaken by fixing joint tissues at surgery in 10% PBFS for Hematoxylin & Eosin staining or by mounting with tissue-tek and freezing in liquid N2 for von Kossa; 5-µm and 10-µm sections were cut, respectively. Non-decalcified cryostat sections of AKU tissue were stained in aqueous 5% silver nitrate for 30 min under a bench lamp then washed with dH2O; a 5% solution of sodium thiosuphate was applied for 1 min to terminate the reaction. Sections were dehydrated through graded alcohols and mounted in DPX (Sigma Aldrich, UK).
Results
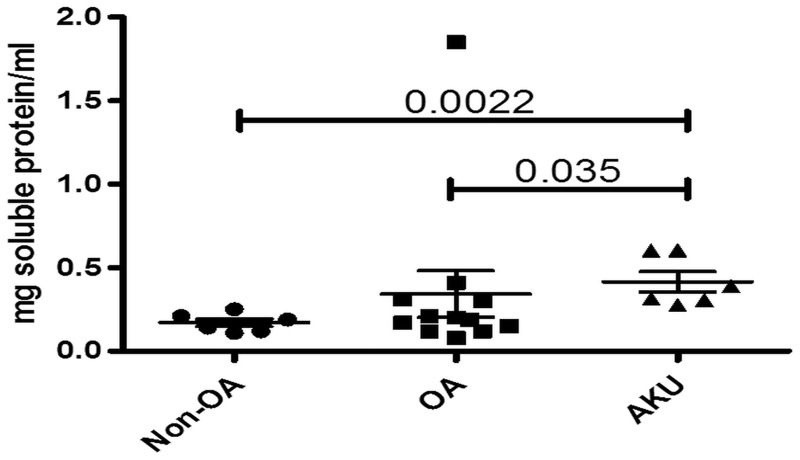
Our results show that significantly more total protein was extractable from AKU cartilage than from either non-OA or OA samples, P = 0.0022 and 0.035, respectively (Fig. 1). There was no significant difference between the milligrams of soluble protein/millilitre between non-OA and OA samples (P = 0.4245).
Fig. 1.
Higher total protein in alkaptonuria and OA cartilage compared with non-OA samples
Graph showing extractable protein (milligram soluble protein per millilitre) from AKU (n = 6), OA (n = 12) and non-OA (n = 6) independent cartilage specimens. Error bars represent the mean of the n in each group ±95% CI. AKU: alkaptonuria.
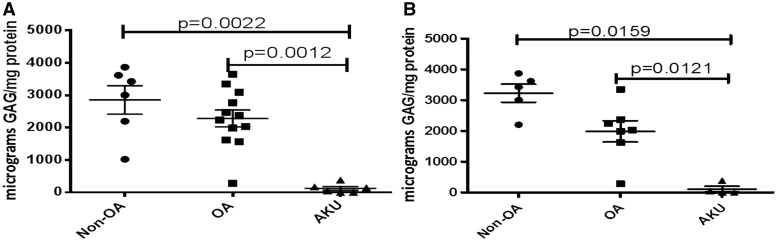
However, the mean amount of extractable GAG (as a proportion of the total protein) was significantly less in AKU cartilage samples compared with either non-OA or OA samples, P = 0.0022 and P = 0.0012, respectively (Fig. 2A). Similar significant disease-related differences were also reflected in the smaller hip-only set; AKU vs non-OA and OA, P = 0.0159 and P = 0.0121, respectively (Fig. 2B).
Fig. 2.
Lower GAG in alkaptonuria cartilage compared with OA and non-OA samples
Graph showing extractable GAG (microgram GAG per milligram protein) from AKU (n = 6), OA (n = 12) and non-OA (n = 6) independent cartilage specimens. (A) All samples, both hips and knees. (B) Hip-only samples. Error bars represent the mean of the n in each group ±95% CI. AKU: alkaptonuria.
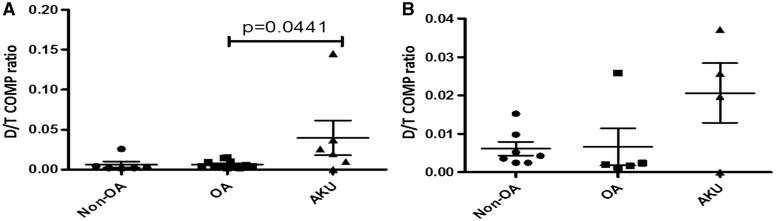
A greater amount of aged COMP protein, expressed as a proportion of deamidated to total COMP (D/T ratio), was extractable from AKU cartilage compared with from OA samples (P = 0.0441) (Fig. 3A). There was no significant difference between non-OA and AKU cartilage (P = 0.1797). Analysis of the hip-only set showed no significant differences between AKU and OA (P = 0.5556) or non-OA cartilages (P = 0.2303) (Fig. 3B).
Fig. 3.
Higher delaminated to total COMP ratio in alkaptonuria cartilage compared with OA cartilage
Graph showing extractable D-COMP as a proportion of total COMP (D/T) from AKU (n = 6), OA (n = 12) and non-OA (n = 6) independent cartilage specimens. (A) All samples, both hips and knees. (B) Hip-only samples. Error bars represent the mean of the n in each group ±95% CI. D-COMP: deamidated COMP; AKU: alkaptonuria; D/T: D-COMP to T-COMP ratio.
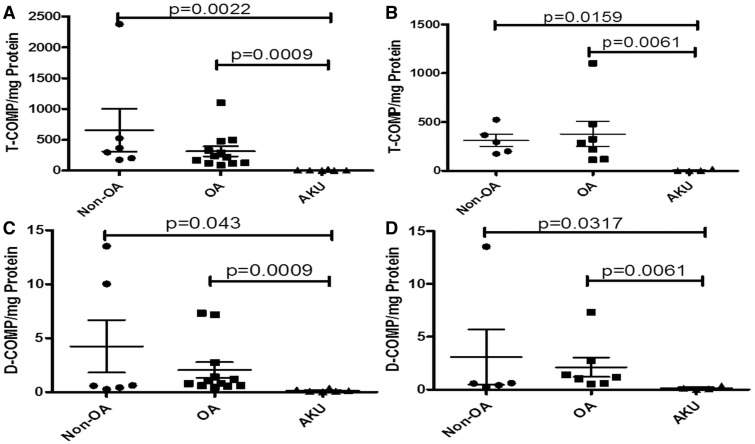
Significantly less total COMP (as a proportion of total protein) was extractable from AKU cartilage than from either non-OA or OA samples, P = 0.0022 and P = 0.0009, respectively (Fig. 4A). Similar significant disease-related differences were reflected in the smaller hip-only set; AKU vs non-OA and OA, P = 0.0159 and P = 0.0061, respectively (Fig. 4B).
Fig. 4.
Lower total COMP and lower deamidated COMP in alkaptonuria cartilage compared with OA and non-OA cartilage
Graph showing extractable T-COMP (T-COMP per milligram protein) from AKU (n = 6), OA (n = 12) and non-OA (n = 6) cartilage specimens. (A) All samples, both hips and knees. (B) Hip-only samples. Graph showing extractable D-COMP (D-COMP/mg protein) from AKU (n = 6), OA (n = 12) and non-OA (n = 6) cartilage specimens. (C) All samples, both hips and knees. (D) Hip-only samples. Error bars represent the mean of the n in each group ±95% CI. D-COMP: deamidated COMP; T-COMP: total COMP; AKU: alkaptonuria.
Significantly less D-COMP (as a proportion of total protein) was extractable from AKU cartilage than from either non-OA or OA samples, P = 0.043 and P = 0.0009, respectively (Fig. 4C). Similar significant disease-related differences were also reflected in the smaller hip-only set; AKU vs non-OA and OA, P = 0.0317 and P = 0.0061, respectively (Fig. 4D).
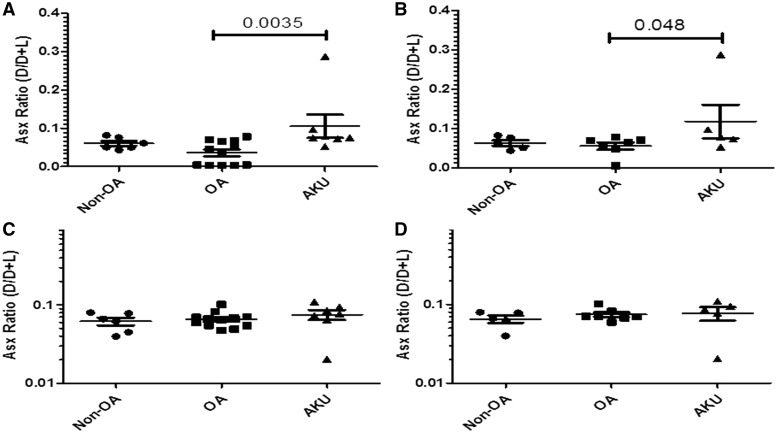
A general measure of aged protein, (Asx D/D + L), representing a ratio of racemized Asx (D form) to non-racemized Asx (L form), was used to further assess the biological age of two cartilage fractions: the Gu-HCl extractable protein from cartilage (Fig. 5 A and B), primarily representing proteoglycan; and the Gu-HCl insoluble protein from cartilage (Fig. 5 C and D), representing collagen and collagen-associated proteins. Compared with OA, the soluble protein fractions from AKU cartilage were significantly enriched in aged (racemized) protein represented by the mean Asx D/D + L ratio, P = 0.0076. Compared with non-OA, the mean Asx ratio from AKU cartilage was higher, but this was not statistically significant (P = 0.1797) (Fig. 5A). Similar disease-related differences were not reflected in the smaller OA vs AKU hip-only set (P = 0.1091) or between non-OA and AKU (P = 0.0931) (Fig. 5B). The mean Asx ratio in the insoluble cartilage fraction was significantly higher for AKU vs OA for all samples (P = 0.0277), and also trended higher for AKU vs non-OA for all samples (P = 0.0931) (Fig. 5C). The hip-only set also showed similar trends, with higher Asx ratios in AKU vs non-OA and OA (P = 0.0635 and 0.0727, respectively) (Fig. 5D).
Fig. 5.
Racemized Asx content in PBS and insoluble extract alkaptonuric cartilage vs OA and non-OA
Graphs showing the amount of racemized Asx (D form) as a proportion of non-racemized Asp (L form) represented by the D/D+L ratio in AKU, OA and non-OA cartilage specimens. (A) Asx ratio (D/D+L) in PBS extract of all samples: both hips and knees from AKU (n = 6), OA (n = 12) and non-OA (n = 6) independent cartilage specimens. (B) Asx ratio (D/D+L) in PBS extract from hip-only samples from AKU (n = 4), OA (n = 7) and non-OA (n = 5) independent cartilage specimens. (C) Asx ratio (D/D+L) in insoluble extract of all samples: both hips and knees from AKU (n = 6), OA (n = 12) and non-OA (n = 6) independent cartilage specimens, (D) Asx ratio (D/D+L) in insoluble extract from hip-only samples from AKU (n = 4), OA (n = 7) and non-OA (n = 5) for independent cartilage specimens. Error bars represent the mean of the n in each group ± 95% CI. AKU: alkaptonuria; racemized Asx: aspartate and asparagine; Asp: aspartate; D: dextrorotary (d-isomer); L: levorotary (l-isomer).
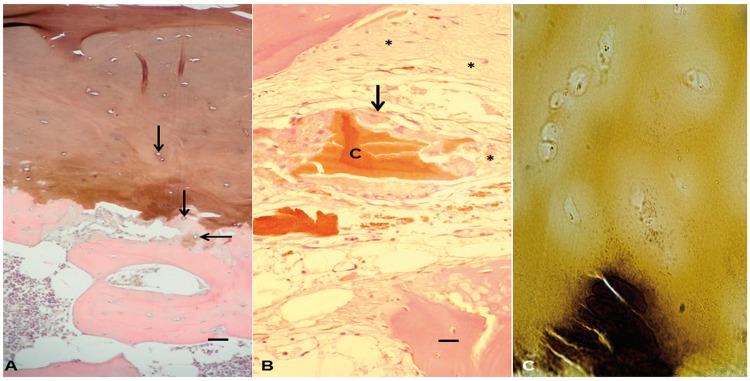
Histological examination of alkaptonuric cartilages revealed full-depth pigmentation of numerous areas across all samples, with many empty chondrocyte lacunae; regions with retained chondrocytes displayed intracellular pigmentation (Fig. 6A). Some areas of calcified cartilage matrix displayed a granular-like pigment, often in proximity to empty chondrocyte lacunae. The bone domain showed mature bone with characteristic lamellar structure and multiple osteocytes without pigmentation. In a number of areas, there were regions where pigmented cartilage was observed without articular calcified cartilage or subchondral bone beneath it. In areas where articular cartilage was absent from the bone surface, pieces of impacted cartilage were observed within the marrow space, surrounded by a variety of mono- and multinucleated cells, many of which displayed intracellular pigmentation (Fig. 6B). Histological examination by von Kossa staining and alizarin red staining (data not shown) revealed that there was no deposition of calcium salts such as hydroxyapatite associated with pigment. This contrasted with the mineralized bone seen beneath the cartilage (Fig. 6C).
Fig. 6.
H&E-stained section of alkaptonuric femoral head
(A) Histological section showing full depth articular cartilage pigmentation with numerous empty chondrocyte lacunae (arrows). There is pericellular pigmentation around chondrocyte lacunae in the calcified cartilage. There are also areas where the calcified cartilage is not continuous with either the subchondral bone below it or the hyaline articular cartilage above it. Bar = 50 µm. (B) Shards of pigmented cartilage can be seen impacted within the marrow space of the bone marrow cavity (‘C’). The cartilage is surrounded by inflammatory cells, some of which show intracellular pigmentation (arrow). The fibrous tissue response of the marrow cavity can be seen in numerous strands of matrix peripherally around the cartilage (asterisk). Bar = 10 µm. (C) A 10-µm non-decalcified cryostat section of AKU tissue stained with von Kossa. Densely mineralized bone (black) can be seen beneath isogenous zones of chondrocytes located in the hyaline articular cartilage, which shows ochronontic colouration but absence of the similar black staining that would indicate calcium deposition.
Discussion
Although few in absolute numbers, the AKU samples in this study represent 0.6% of the currently known global population with AKU and 9.375% of the currently known UK AKU population, highlighting one of the difficulties in studying rare diseases [2, 4]. Here we present the first data analysing biomarkers of ageing and turnover comparing alkaptonuric to OA and non-OA cartilage samples. AKU samples demonstrated an older profile of biomarkers of ageing across a number of analysed parameters, both by ratio of aged (deamidated) to total COMP and by ratio of aged (racemized) aspartate to total aspartate in protein. These results were consistent with lower anabolism of AKU cartilage, that is, low turnover resulting in enrichment of age-related post-translation modification of cartilage protein, including the higher ratios of deamidated to total COMP and higher ratios of racemized aspartate to total aspartate. This was of interest, considering that the mean age and range of age was higher for OA than for AKU, which would be expected to yield more age-related changes in OA.
Previously it has been suggested that turnover in articular cartilage is greatest at the superficial layer and that the turnover rate decreases as depth from the articular surface increases [28]. This may initially be true in AKU, but once pigmentation commences, the highest rate of turnover, in the form of catabolism, appears in the deepest cartilage, with resorption of hyaline articular calcified cartilage, following resorption of the subchondral bone [8]. With the biochemical tools of deamidated COMP and racemized aspartate, it would be possible in future to evaluate turnover by depth of cartilage to determine whether protein turnover reflects apparent turnover based on calcified cartilage and subchondral bone pathology.
Interestingly, despite the presence of pigment and potential for matrix cross-linking via pigment adducts, the amount of protein extractable from AKU cartilage was higher than that from OA and non-OA. Higher levels of molecular degeneration could lead to more readily extractable protein. In the case of AKU, a large proportion of both non-collagen– and collagen-bound proteins might be affected, resulting in a higher amount of extractable protein relative to OA and non-OA. This explanation is plausible given the trend for OA cartilage to yield more extractable protein than non-OA and for AKU to yield still higher amounts of extractable protein.
Until recently, little was known about the molecular events occurring in AKU cartilage [8]. Most information available is representative of the end stage of the disease, when the molecular mechanisms are almost/already complete [8]. Our observation of lower levels of extractable GAG in AKU is consistent with an OA trajectory [29, 30]; however, the extractable amounts we observed in our AKU patient samples were significantly less than for OA. Similarly, total COMP was significantly less extractable from AKU cartilage. The significantly reduced amounts of extractable GAG and COMP in AKU cartilage could be hypothesized as arising through two scenarios. First, they are lost very early or steadily (due to accelerated molecular degeneration, ageing, trauma, biochemical alteration of the extracellular milieu or some combination of these factors) and not replaced (due to chondrocyte pathology induced by the pigment). One of the symptoms of AKU is an early onset, rapidly progressing osteoarthropathy. If proteoglycan in AKU is lost very early and much more rapidly than in general OA, this may be a factor in the accelerated disease progression observed in AKU.
Second, they are bound to the cartilage extracellular matrix by the presence of ochronotic polymer and cannot be extracted. If proteoglycans are bound within the ochronotic matrix, this is likely a result of the polymerization process occurring in water spaces of the cartilage. HGA, which will polymerize to form ochronotic pigment, is a small, very highly water-soluble molecule and likely binds matrix proteins that are anchored in and around collagen fibres [10]. However, this latter possibility seems unlikely given the ability to extract more total protein from AKU cartilage than from OA or non-OA cartilage.
Regardless of the scenario, both possibilities lead to a cartilage matrix in AKU that is far from normal. It has already been shown that pigmentation commences pericellularly [8]. Given the intricate relationship that exists between chondrocytes and their pericellular matrices [31, 32], this likely exacerbates processes that drive alteration in tissue composition. Alterations in the pericellular matrix have been linked to OA [33]. Second, pigmented cartilage in AKU is significantly stiffer than that seen in OA and normal cartilage [8]. It is likely that the observed changes in cartilage matrix composition described here precede microscopic changes in the articular cartilage and subchondral bone plate and may contribute to their progression by allowing HGA to polymerize in spaces once occupied by GAG, or utilize GAG as its nucleation site. There is a possibility that the greater loss of GAG in AKU allows for greater extracellular protein penetration, and this, at least in part, might account for higher total protein content in AKU samples. This is potentially combined with an increased crosslinking in the matrix, binding larger amounts of protein in. One could also posit that there is increased protein production due to a cellular attempt to mount a repair response, producing more proteins that are incorporated into the matrix. The latter explanation seems unlikely given the apparent low turnover of AKU cartilage specimens, based on the COMP D/T and racemized Asp results.
The histological images presented demonstrate typical changes observed previously in alkaptonuric joints [8]. Cartilage is full of ochronotic pigment, existing in association with collagen [10] or with other matrix proteins. The bone changes indicate altered mechanical loading, with resorption of subchondral bone and calcified cartilage [9, 10]. We did not see levels of calcium deposition consistent with mineralized tissue, compared with bone within our sections. Lack of calcium associated with pigmentation is consistent with our previous work showing that calcium is not present in association with ochronotic pigment by backscattered electrons scanning electron microscope (BSE-SEM) or IKI-enhanced BSE-SEM [8, 33]. These morphological changes are likely contributed to by the matrix changes described here.
While proteoglycan loss in these patients has physiological significance, there are clinical implications. Radiographic presentation of AKU patients is almost always representative of some degenerative changes that mimic OA; joint space narrowing is often described, particularly in the intervertebral disc spaces [8]. Cartilage thinning is not well documented; the majority of specimens obtained at surgery are mostly normal, apart from their ochronosis [8]. Those that do possess what would be described as lesions appear ochronotic and are typified by mechanical damage from contact between brittle cartilage on opposing surfaces, rather than by the enzymatic degradation and lesions typically observed with OA. Because the presence of pigment initially protects cartilage from overt breakdown, early radiographs appear normal, while cartilage is pigmenting/pigmented [34]. Only when mechanical trauma dislodges cartilage may radiological changes be observed; therefore, other diagnostic measures for assessing the state of joints of individuals with AKU, particularly during early joint pathological stages, may be more appropriate. Along with HGA [8], the damaged cartilage fragments are often seen embedded in the synovium and synovial fluid aspirate [35, 36].
Our data demonstrate the alterations that occur in the cartilage of alkaptonuric patients. The loss of numerous matrix proteins raises potential issues for treating AKU. Nitisinone has been suggested as a potential therapy [16, 17, 19, 37]; however, it should be borne in mind that this would most benefit individuals without established pigmentation. These data represent an advance in the understanding of AKU cartilage turnover that could stimulate new therapeutic options. For instance, our results suggest that individuals may also benefit from growth factor and other anabolic treatments, as there appears to be a lack of an anabolic response to the pigmentation process, particularly in those who have some established pigmentation.
Acknowledgements
We are extremely grateful to all patients who donated their tissues as part of this study. The authors would like to thank Dr Hazel Sutherland for her technical assistance with histology. Author contributions: A.T., J.A.G., L.R.R. and V.B.K. conceived the study. A.T., J.B.C., J.P.D., M.F.H. and J.L.H. undertook experimental procedures. All authors contributed to interpreting data, drafting, reading and approving the final version of the manuscript.
Funding: This study was supported by an Osteoarthritis Research Society International scholarship award, the AKU Society, Rosetrees Trust, the National Institute of Health/National Institute of Arthritis Musculoskeletal and Skin Diseases [AR050245] and the National Institute of Health/National Institute on Ageing [AG028716]. Funders had no input into the study design, collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Kraus VB. Rare osteoarthritis: ochronosis and Kashin-Beck disease In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology, 6th edn, Chapter 185. Philadelphia: Mosby Elsevier, 2014: 1536–40. [Google Scholar]
- 2.Mistry JB, Bukhari M, Taylor AM. Alkaptonuria. Rare Dis 2013;1:e27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrod AE. The incidence of alkaptonuria: a study in chemical individuality. Lancet 1902;II:1616–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Ranganath L, Taylor AM, Shenkin A. et al. Identification of alkaptonuria in the general population: a United Kingdom experience describing the challenges, possible solutions and persistent barriers. J Inherit Metab Dis 2011;34:723–30. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AM, Wilson PJ, Ingrams DR. et al. Calculi and intracellular ochronosis in the submandibular tissues from a patient with alkaptonuria. J Clin Pathol 2010;63:186–8. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AM, Batchelor TJ, Adams VL. et al. Ochronosis and calcification in the mediastinal mass of a patient with alkaptonuria. J Clin Pathol 2011;64:935–6. [DOI] [PubMed] [Google Scholar]
- 7.La Du BN, Zanonni VG, Laster L, Seegmiller JE. The nature of the defect in tyrosine metabolism in alcaptonuria. J Biol Chem 1958;230:251–60. [PubMed] [Google Scholar]
- 8.Taylor AM, Boyde A, Wilson PJ. et al. The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum 2011;63:3887–96. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AM, Boyde A, Davidson JS. et al. Identification of trabecular excrescences, novel microanatomical structures, present in bone in osteoarthropathies. Eur Cell Mater 2012; 23:300–8. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AM, Wlodarski B, Prior IA. et al. Ultrastructural examination of tissue in a patient with alkaptonuric arthropathy reveals a distinct pattern of binding of ochronotic pigment. Rheumatology 2010;49:1412–4. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AM. An investigation into the pathogenesis of ochronosis in the arthropathy in Alkaptonuria (AKU) University of Liverpool, Liverpool, UK, 2011. [Google Scholar]
- 12.Chow WY, Taylor AM, Reid DG, Gallagher JA, Duer MJ. Collagen atomic scale molecular disorder in ochronotic cartilage from an alkaptonuria patient, observed by solid state NMR. J Inherit Metab Dis 2011;34:1137–40. [DOI] [PubMed] [Google Scholar]
- 13.Pollak MR, Chou YH, Cerda JJ. et al. Homozygosity mapping of the gene for alkaptonuria to chromosome 3q2. Nat Genet 1993;5:201–4. [DOI] [PubMed] [Google Scholar]
- 14.Janocha S, Wolz W, Srsen S. et al. The human gene for alkaptonuria (AKU) maps to chromosome 3q. Genomics 1994;19:5–8. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Cañón JM, Granadino B, Beltrán-Valero de Bernabé D. et al. The molecular basis of alkaptonuria. Nat Genet 1996;14:19–24. [DOI] [PubMed] [Google Scholar]
- 16.Suwannarat P, O’Brien K, Perry MB. et al. Use of nitisinone in patients with alkaptonuria. Metabolism 2005;54:719–28. [DOI] [PubMed] [Google Scholar]
- 17.Introne WJ, Perry MB, Troendle J. et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab 2011;103:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor AM, Preston AJ, Paulk NK. et al. Ochronosis in a murine model of alkaptonuria is synonymous to that in the human condition. Osteoarthritis Cartilage 2012;20:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preston AJ, Keenan CM, Sutherland H. et al. Ochronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone. Ann Rheum Dis 2014;73:284–9. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AT, Milan AM, Christensen P. et al. Urine homogentisic acid and tyrosine: simultaneous analysis by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2014;963:106–12. [DOI] [PubMed] [Google Scholar]
- 21.Catterall JB, Hsueh MF, Stabler TV. et al. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem 2012;287:4640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catterall JB, Barr D, Bolognesi M, Zura RD, Kraus VB. Post-translational aging of proteins in osteoarthritic cartilage and synovial fluid as measured by isomerized aspartate. Arthritis Res Ther 2009;11:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCudden C, Kraus VB. Biochemistry of amino acid racemization and clinical application to musculoskeletal disease. Clin Biochem 2006;39:1112–30. [DOI] [PubMed] [Google Scholar]
- 24.Stabler TV, Byers SS, Zura RD, Kraus VB. Amino acid racemization reveals differential protein turnover in osteoarthritic articular and meniscal cartilages. Arthritis Res Ther 2009;11:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catterall JB, Zura RD, Bolognesi MP, Kraus VB. Aspartic acid racemization reveals a high turnover state in knee compared with hip osteoarthritic cartilage. Osteoarthritis Cartilage 2016;24:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrasekhar S, Esterman MA, Hoffman HA. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem 1987;161:103–8. [DOI] [PubMed] [Google Scholar]
- 27.Vilím V, Olejárová M, Machácek S. et al. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage 2002;10:707–13. [DOI] [PubMed] [Google Scholar]
- 28.Maroudas A. Glycosaminoglycan turn-over in articular cartilage. Philos Trans R Soc Lond B Biol Sci 1975;271:293–313. [DOI] [PubMed] [Google Scholar]
- 29.Malemud CJ. Changes in proteoglycans in osteoarthritis: biochemistry, ultrastructure and biosynthetic processing. J Rheumatol Suppl 1991;27:60–2. [PubMed] [Google Scholar]
- 30.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci U S A 2008;105:2266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage 2007;15:752–63. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng 2003;125:323–33. [DOI] [PubMed] [Google Scholar]
- 33.Guilak F, Alexopoulos LG, Upton ML. et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci 2006;1068:498–512. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher JA, Ranganath LR, Boyde A. Lessons from rare diseases of cartilage and bone. Curr Opin Pharmacol 2015;22:107–14. [DOI] [PubMed] [Google Scholar]
- 35.Maathuis PG, Driessen AP. Painted black. Ann Rheum Dis 2002;61:100–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damian LO, Felea I, Boloşiu C. et al. A case of alkaptonuria – ultrasonographic findings. Med Ultrason 2013;15:321–5. [DOI] [PubMed] [Google Scholar]
- 37.Mistry JB, Jackson DJ, Bukhari M, Taylor AM. A role for interleukins in ochronosis in a chondrocyte in vitro model of alkaptonuria. Clin Rheumatol 2016;35:1849–56. [DOI] [PubMed] [Google Scholar]