Treatment-related hyperglycemia is associated with several anticancer agents. This review summarizes available information on hyperglycemia associated with agents that inhibit key molecules within the larger context of adverse event profiles, namely, the tyrosine kinase receptors insulin growth factor receptor 1 and epidermal growth factor receptor, and the intracellular signaling molecules phosphatidylinositol 3-kinase, AKT, and mammalian target of rapamycin.
Keywords: Anticancer agents, Molecular targeted therapy, Receptors, Growth factor, Tyrosine kinase, mTOR protein, Proto-oncogene protein Akt
Abstract
Molecularly targeted cancer therapy has rapidly changed the landscape of oncologic care, often improving patients’ prognosis without causing as substantial a quality-of-life decrement as cytotoxic chemotherapy does. Nevertheless, targeted agents can cause side effects that may be less familiar to medical oncologists and that require the attention and expertise of subspecialists. In this review, we focus on hyperglycemia, which can occur with use of new anticancer agents that interact with cell proliferation pathways. Key mediators of these pathways include the tyrosine kinase receptors insulin growth factor receptor 1 (IGF-1R) and epidermal growth factor receptor (EGFR), as well as intracellular signaling molecules phosphatidylinositol 3-kinase (PI3K), AKT, and mammalian target of rapamycin (mTOR). We summarize available information on hyperglycemia associated with agents that inhibit these molecules within the larger context of adverse event profiles. The highest incidence of hyperglycemia is observed with inhibition of IGF-1R or mTOR, and although the incidence is lower with PI3K, AKT, and EGFR inhibitors, hyperglycemia is still a common adverse event. Given the interrelationships between the IGF-1R and cell proliferation pathways, it is important for oncologists to understand the etiology of hyperglycemia caused by anticancer agents that target those pathways. We also discuss monitoring and management approaches for treatment-related hyperglycemia for some of these agents, with a focus on our experience during the clinical development of the EGFR inhibitor rociletinib.
Implications for Practice:
Treatment-related hyperglycemia is associated with several anticancer agents. Many cancer patients may also have preexisting or undiagnosed diabetes or glucose intolerance. Screening can identify patients at risk for hyperglycemia before treatment with these agents. Proper monitoring and management of symptoms, including lifestyle changes and pharmacologic intervention, may allow patients to continue benefiting from use of anticancer agents.
Introduction
Cancer is the second most common cause of death in the U.S. and Europe after heart disease [1, 2]. In recent years, targeted therapies have delivered important and substantial benefits to patients. These agents inhibit cancer-promoting cellular pathways and can improve overall survival [3]. Compared with traditional cytotoxic chemotherapy, the incidences of low blood counts, severe fatigue, nausea, and vomiting tend to be lower with novel agents; many of these agents have also been associated with improved quality of life [4–9]. Nevertheless, many targeted agents have a side-effect profile that differs from that of traditional chemotherapy. In particular, many newer targeted agents have been found to induce treatment-related hyperglycemia. In this article, we review the agents that are known to cause treatment-related hyperglycemia and provide an overview of monitoring and management for this toxicity (Table 1).
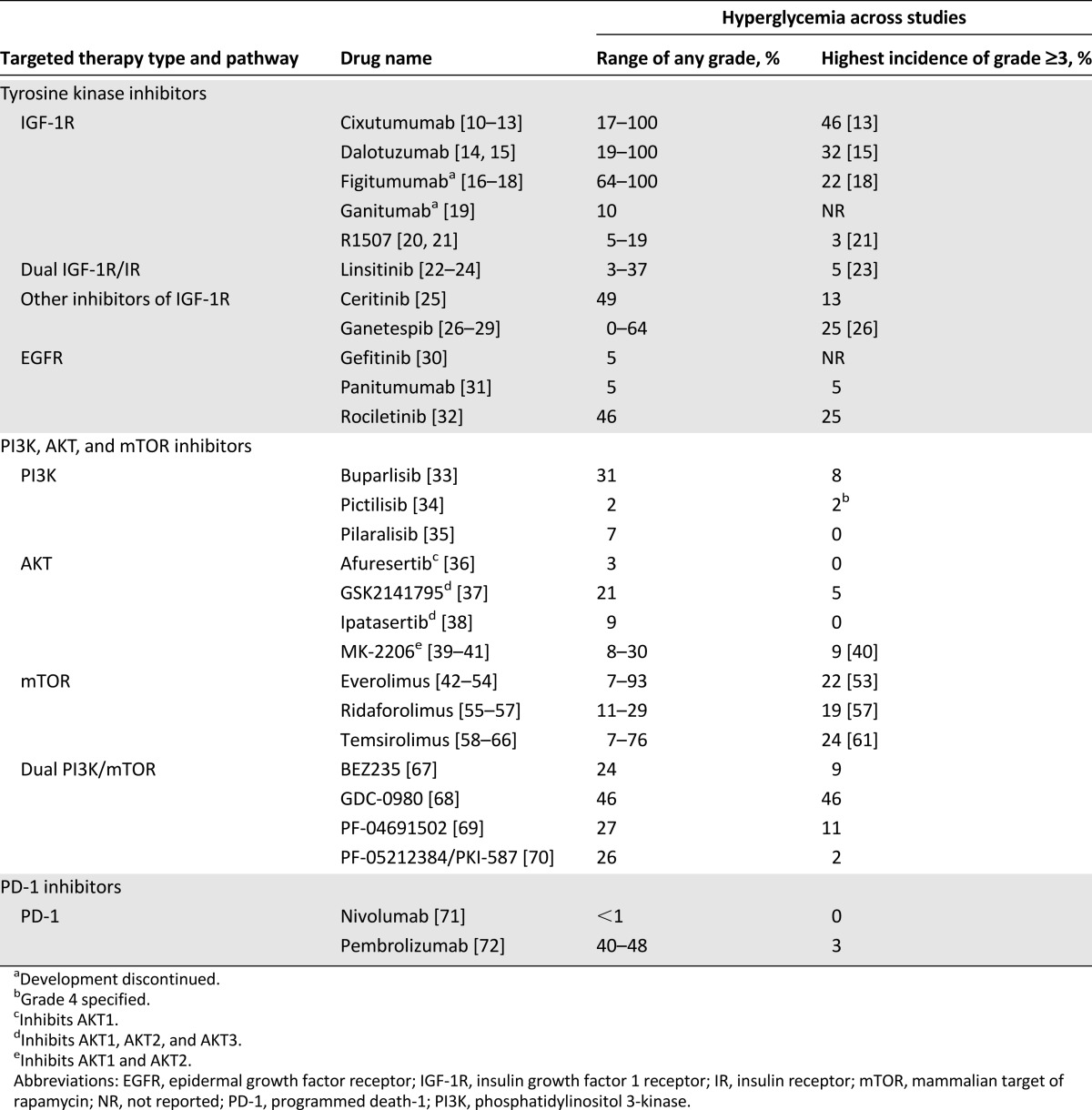
Table 1.
Cancer drugs with known side effect of hyperglycemia
Novel anticancer agents have been developed to target several important cancer characteristics, including sustained proliferative signaling, evasion of growth suppressors, induction of angiogenesis, and avoidance of immune destruction. Sustained proliferation is largely controlled by specific growth and antiapoptotic pathways, notably the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathways. Many of the agents targeting these pathways are small-molecule tyrosine kinase inhibitors, which block ligand-mediated dimerization and activation of downstream effectors. Cell surface receptors can also be inhibited by monoclonal antibodies that interfere with ligand-receptor docking.
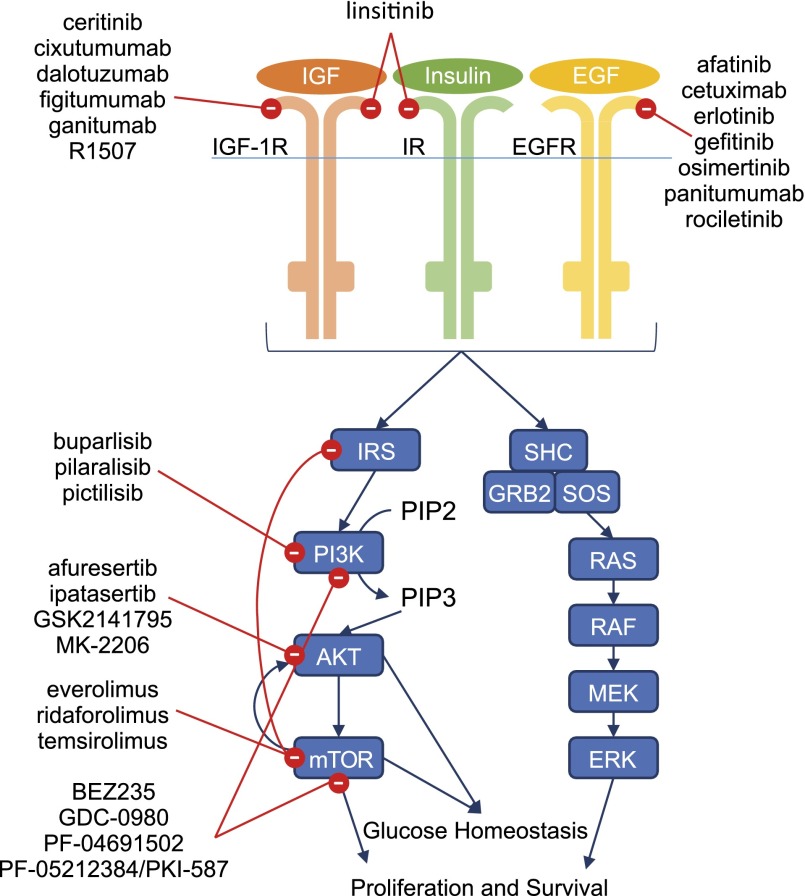
The body regulates blood glucose levels in several ways. Excess serum glucose increases the secretion of insulin from the pancreatic β cells. The action of insulin begins when the hormone binds to the insulin receptor (IR) in the cell membrane. In addition to promoting cellular uptake of glucose, IR activates intracellular pathways, including PI3K/AKT/mTOR, affecting glucose homeostasis by increasing glycogen synthesis and decreasing glycolysis [73–75]. Insulin growth factor receptor 1 (IGF-1R) is partially homologous to IR and is an important mediator of growth and anabolic effects [76–78]. Activation of IGF-1R via its ligand insulin growth factor 1 (IGF-1) inhibits growth hormone release from the pituitary; high levels of growth hormone promote insulin resistance and increased gluconeogenesis [78]. Increased levels of growth hormone also stimulate hepatic production of IGF-1 as part of a negative feedback loop. Figure 1 illustrates the current understanding of these proteins and their pathway interactions, as well as the targeted cancer therapies that inhibit them.
Figure 1.
Cellular control of hyperglycemia. Glucose homeostasis is maintained at a cellular level through activation of the intracellular PI3K/AKT/mTOR pathway downstream of IGF-1R and IR. These receptors, along with EGFR, can also activate the Ras/MAPK/ERK pathway, which plays a role in cellular proliferation and survival. Targeted therapies designed to inhibit cancer cell proliferation and promote apoptosis act on these pathways at multiple points (red circles).
Abbreviations: EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; IGF, insulin growth factor; IGF-1R, insulin growth factor receptor 1; IR, insulin receptor.
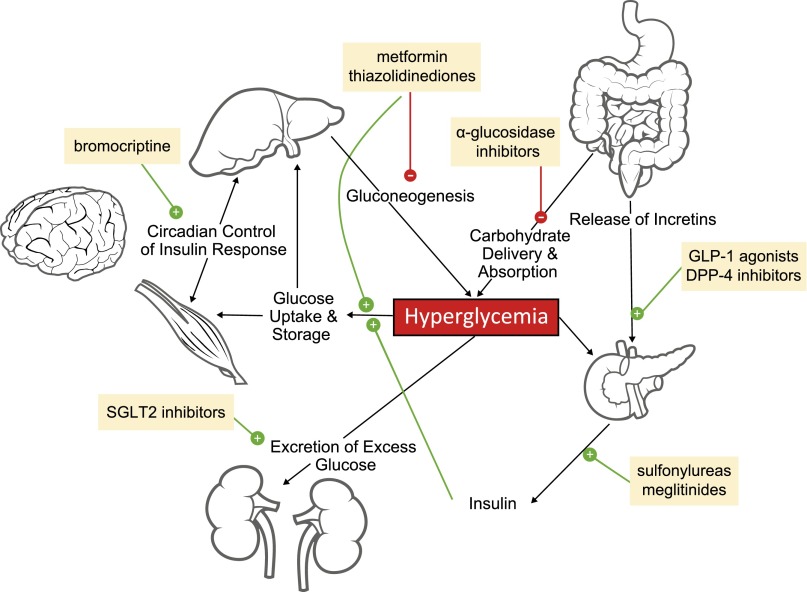
Hyperglycemia has systemic effects that may result in constitutional symptoms (e.g., fatigue, anorexia, weight loss, polyuria, polydipsia, blurred vision, nausea, diarrhea, dehydration, and renal insufficiency) [73, 74]. If left untreated, these conditions may cause a progressive decline in quality of life and functional status. Even if a patient is deriving antitumor benefit from a targeted agent, onset of constitutional symptoms or other adverse events may lead to dose reductions or treatment discontinuation, potentially resulting in reduced efficacy. By having a good understanding of the etiology of treatment-related hyperglycemia and the pathways that are associated with this adverse event, clinicians may be in a better position to manage and mitigate treatment-related hyperglycemia (Fig. 2).
Figure 2.
Systemic control of hyperglycemia. Hyperglycemia is controlled systemically (solid black lines) through the release of insulin, which promotes glucose uptake and storage in organs such as the liver and muscles and excretion of excess glucose by the kidneys. Several drugs (yellow boxes) can be used to counteract hyperglycemia, for example, by inhibiting gluconeogenesis or promoting the production of insulin. Stimulatory signals are indicated by green circles containing white “+” symbols, and inhibitory signals are displayed as red circles containing white “–” symbols.
Methods
Published, English-language articles were identified by searching PubMed for the following: (“hyperglycemia” OR “hyperglycaemia”) AND (“inhibitor”) AND (“tyrosine kinase” OR “PI3K” OR “AKT” OR “mTOR” OR “IR” OR “IGF-1R” OR “EGFR” OR “PD-1”). Results were screened to identify clinical trials of anticancer agents used as monotherapy to avoid confounding factors present in combination studies. Additional information was obtained by reviewing oncology-focused congress abstracts published within the past 10 years and prescribing information for anticancer agents known to cause treatment-related hyperglycemia.
Treatment-Induced Hyperglycemias
IGF-1R Inhibitors
IGF-1R activates the Ras/MAPK/extracellular regulated kinase (ERK) and PI3K/AKT/mTOR pathways, which regulate cell proliferation, inhibit apoptosis, and are associated with other cancer-related processes (Fig. 1) [79]. IGF-1R is a cell surface receptor and, as such, can be targeted by monoclonal antibodies or small molecules. Many IGF-1R inhibitors also block IR as a result of receptor homology [76, 80].
IGF-1R-Specific Monoclonal Antibodies
Monoclonal antibodies that target IGF-1R that are currently in clinical development include dalotuzumab and cixutumumab. Figitumumab, ganitumab, and R1507 were previously under evaluation but their clinical development has been discontinued. Hyperglycemia was listed as a common adverse event (AE) in all but 2 of 12 studies reported in the literature for these agents [10–21]. Other common AEs that have been observed with these agents include fatigue, nausea, and anorexia, which may have been associated with hyperglycemia. The incidence of hyperglycemia for these 5 agents ranged from 10% to 100% (any grade) and from 0% to 46% (grade 3), and appeared to be dependent on dose and the frequency of administration.
In a phase I study of dalotuzumab (10–30 mg/kg weekly) in patients with mixed solid tumors, the overall incidence of hyperglycemia was 19% (1 patient had grade 3 hyperglycemia) [14]. In a small phase II study of patients with neuroendocrine tumors treated with dalotuzumab (10 mg/kg weekly), all patients experienced hyperglycemia; the incidence of grade 3 or greater hyperglycemia was 32% [15].
In several phase I and II studies of cixutumumab for the treatment of mixed solid tumors, hyperglycemia incidence was dose dependent. The incidence of all-grade hyperglycemia ranged from 17% to 100% (5%–46% for grade 3 hyperglycemia) [10–13]. The lowest incidence of hyperglycemia occurred with biweekly administration of cixutumumab (10 mg/kg) [10, 11]. Weekly administration of cixutumumab, even at a lower dose (6 mg/kg), resulted in a notably higher incidence of hyperglycemia [13].
In a study of figitumumab for mixed solid tumors, the rate of hyperglycemia (all grade) was 64% over a range of doses (3–20 mg/kg every 3 weeks) [16]. During the dose-expansion phase of the study, hyperglycemia (all grade) was observed in 100% of patients who received 20 mg/kg of figitumumab; 21% of patients experienced grade 3 hyperglycemia [17]. In both studies, glucose, insulin, and human growth hormone (hGH) were monitored when feasible in patients receiving the 20 mg/kg dose. Elevations in glucose and hGH levels were not clinically significant by the end of each study, but most patients had increased levels of insulin [16, 17]. In a larger study of patients with metastatic colorectal cancer, treatment with 20 mg/kg or 30 mg/kg figitumumab every 3 weeks resulted in rates of hyperglycemia (all grade) of 26% and 33%, respectively; the majority were grade 3 events [18].
Ganitumab was tested in a small phase I study in patients with mixed solid tumors or non-Hodgkin lymphoma [19]. In that study, 10% of 50 nondiabetic patients experienced hyperglycemia.
R1507 was examined in phase I and phase II studies before development was suspended. In the phase I dose-escalation study (dosing range, 1–9 mg/kg weekly) in patients with mixed solid tumors, clinically significant hyperglycemia was only observed in 2 of 37 patients, both of whom had abnormal glucose tolerance at baseline [20]. In a larger phase II study of R1507 in patients with Ewing’s sarcoma (9 mg/kg weekly or 27 mg/kg every 3 weeks), hyperglycemia was a common AE, occurring in 19% (any grade) and 3% (grade 3) of patients [21].
As demonstrated by these studies, the incidence of hyperglycemia is variable with monoclonal antibodies that have activity against IGF-1R. This variability may be partially attributed to the small study sizes and patient heterogeneity. Regardless, hyperglycemia was common across these studies, highlighting the need to actively monitor patients for hyperglycemia following initiation of these therapies.
Small-Molecule Inhibitors of IGF-1R and IR
Given the sequence homology between IR and IGF-1R, small molecules designed to target the kinase domain of IGF-1R can also inhibit signaling through IR. For example, the small molecule linsitinib has demonstrated dual IGF-1R and IR inhibition [76]. In two phase I dose-escalation studies in mixed solid tumors and a placebo-controlled phase III study in patients with locally advanced or metastatic adrenocortical carcinoma, hyperglycemia was a common AE [22–24]. Other common AEs in those studies included nausea, vomiting, and fatigue. Rates of hyperglycemia (all grade), regardless of dose, were 17% and 37% in the 2 phase I studies and 3% in the phase III study. Hyperglycemia generally occurred at the highest doses tested (≥300 mg daily) when administered at more frequent dosing intervals. The phase III study used 150 mg daily as the clinical dose, which may explain the lower incidence of hyperglycemia. Patients with documented diabetes were excluded from the majority of these studies. In a very small cohort of nine diabetic patients in one of the phase I studies, five patients reported grade 1 hyperglycemia; three patients reported transient grade 2 or 3 hyperglycemia. These patients did not require alterations in diabetes medications [22].
Other Inhibitors of IGF-1R
The small molecule ganetespib was selected to inhibit the molecular chaperone Hsp90, leading to degradation of key oncogenic proteins, including IGF-1R, EGFR, vascular endothelial growth factor, c-MET, and human epidermal growth factor receptor 2 (HER2) [26]. In a small, phase I, dose-escalation study in patients with hepatocellular carcinoma, hyperglycemia was listed as a common AE along with diarrhea, fatigue, aspartate aminotransferase elevation, and anemia [26]. Any-grade hyperglycemia was experienced by 64% of patients; grade 3 or 4 hyperglycemia was experienced by 25% of patients. Notably, hyperglycemia was not listed as a common AE in an earlier phase I study of patients with solid malignancies nor in larger phase II studies in non-small cell lung cancer (NSCLC) and breast cancer [27–29].
Ceritinib is a small-molecule inhibitor of anaplastic lymphoma kinase, which is frequently mutated in lung cancer and has also been shown to inhibit IGF-1R [25]. In a phase I trial, in which the majority of patients had NSCLC, gastrointestinal AEs including diarrhea, nausea, vomiting, and abdominal pain were the most common side effects; the incidence of hyperglycemia was 49% (all grade) and 13% (grade 3 or 4) [25].
EGFR Inhibitors
The tyrosine kinase receptor EGFR is not directly involved in glucose metabolism. Hyperglycemia following use of EGFR inhibitors, including gefitinib, panitumumab, erlotinib, afatinib, cetuximab, and osimertinib, is uncommon [30, 31, 81, 82].
Rociletinib is a third-generation EGFR tyrosine kinase inhibitor that targets the most common EGFR-activating mutations (L858R and del19) and the acquired primary resistance mutation T790M [83]. In a phase I/II dose-escalation study, treatment-related hyperglycemia (all grade) was reported in 46% of patients who received rociletinib [32]. Nausea, fatigue, and diarrhea were the other most common AEs. The incidence of hyperglycemia was dose dependent; it was reported in 35%, 45%, 59%, and 67% of patients who received 500, 625, 750, or 1,000 mg b.i.d., respectively [32]. Hyperglycemia was the most common grade 3 event irrespective of dose.
In preclinical NSCLC models, IGF-1R and IR signaling are believed to be among the mediators of resistance to EGFR inhibitors. In the rociletinib TIGER-X study, hyperglycemia was not expected before the onset of the study because rociletinib had no effect on glucose levels in preclinical toxicology studies or an oral glucose tolerance test in the rat. In humans, rociletinib has three major metabolites: M460, M502, and M544. Interestingly, rociletinib has a differential metabolic profile in humans compared with rodents. As such, low levels of M460 and M502 are observed in rodents, whereas higher levels are observed in humans. These metabolites were found to have activity against IGF-1R and IR; thus, the rociletinib-induced hyperglycemia observed in patients likely results from inhibition of these pathways by M460 and M502, and not from the parent molecule itself.
PI3K, AKT, and mTOR Inhibitors
Downstream effectors of IGF-1R and EGFR include PI3K, AKT, and mTOR (Fig. 1). These intracellular mediators can only be inhibited through use of small molecules. Agents that target the PI3K/AKT/mTOR pathway are intended to interfere with cancer cell growth and survival; however, inhibition of this pathway may also lead to hyperglycemia by interrupting the intracellular response to insulin, causing decreased glucose transport, decreased glycogen synthesis, and increased glycolysis (Fig. 1) [73–75]. Activation of AKT via PI3K inhibits nuclear localization of the transcription factor FoxO1, preventing transcription of genes involved in gluconeogenesis. AKT is also involved in activation of glucose transport into the cells and glycogen synthesis. AKT is required for mTOR activation, which plays a key role in nutrient sensing of the cell. Glucose metabolism is mediated by mTOR through activation of hypoxia-inducible factor 1α, a transcription factor that upregulates expression of glucose transporters and glycolytic genes. Chronic inhibition of mTOR has been linked to decreased proliferation and destruction of insulin-producing pancreatic β cells, as well as the development of insulin resistance [84].
PI3K Inhibitors
The PI3K inhibitors currently in early clinical development include pilaralisib, pictilisib, and buparlisib, which inhibit the kinase activity of all PI3K isoforms by preventing binding with adenosine 5′-triphosphate. The most common AEs observed with PI3K inhibitors include rash, nausea, and diarrhea; the incidence of hyperglycemia reported in the literature has generally been low [33–35]. In phase I dose-escalation studies of pilaralisib and pictilisib, less than 8% of patients were reported to have hyperglycemia [34, 35]. In a phase I dose-escalation study of buparlisib in patients with advanced solid tumors, the incidence of hyperglycemia (all grade) was higher (31%), and 8% of patients experienced grade 3 or 4 hyperglycemia [33]. Three of four patients receiving the highest dose (150 mg) experienced hyperglycemia (all grade). The buparlisib study excluded diabetic patients, and managed symptoms with standard antidiabetic therapies.
AKT Inhibitors
The AKT1, AKT2, and AKT3 isoforms share partial sequence homology, and inhibitors in development target some or all of the isoforms. The incidence of hyperglycemia in phase I studies was generally lower with agents specific for one or two isoforms than with agents that inhibit all three isoforms. Other common AEs associated with AKT inhibitors include rash, fatigue, nausea, vomiting, and diarrhea. In a phase I study of the AKT1-specific inhibitor afuresertib for the treatment of multiple myeloma, any-grade hyperglycemia was reported in <3% of patients treated across the range of doses tested [36]. MK-2206, an agent that targets AKT1 and AKT2, has been investigated in phase I and phase II studies. In the phase I study, patients with advanced solid tumors treated with 60 mg every other day experienced infrequent (<8%) grade 1 or 2 hyperglycemia [39]. In phase II studies with MK-2206, the incidence of hyperglycemia was 10% (2 of 21 subjects; both events grade 3) in patients with nasopharyngeal carcinoma (200 mg/week) [40] and was more frequent (30%) in patients with advanced gastric cancer (60 mg every other day) [41]. Hyperglycemia (all grade) was observed in 9% and 21% of patients, respectively, in phase I dose-escalation studies of ipatasertib and GSK2141795, both of which target the 3 AKT isoforms [37, 38]. Notably, most of the aforementioned studies excluded patients with high fasting blood-glucose (FBG) levels.
mTOR-Specific Inhibitors
With mTOR inhibitors, the incidence of hyperglycemia (all grade) ranges from as low as 7% to as high as 93%, and the incidence of grade 3 or 4 hyperglycemia is generally higher with mTOR inhibitors than with AKT or PI3K inhibitors. An important caveat is that exclusion of patients with diabetes or those with uncontrolled glucose levels was not consistent across studies or agents. Other common AEs observed with mTOR inhibitors include rash, diarrhea, fatigue, stomatitis, anemia, asthenia, and anorexia. In this review, we discuss the mTOR inhibitors temsirolimus, everolimus, and ridaforolimus, which are analogs of rapamycin, the first mTOR inhibitor discovered. These agents bind to both mTOR and a key coactivator, FKBP12, inducing a conformational change that prevents binding of raptor, which is required for activation of downstream signaling molecules (including 4EBP1 and S6K1) [85].
With mTOR inhibitors, the incidence of hyperglycemia (all grade) ranges from as low as 7% to as high as 93%, and the incidence of grade 3 or 4 hyperglycemia is generally higher with mTOR inhibitors than with AKT or PI3K inhibitors. An important caveat is that exclusion of patients with diabetes or those with uncontrolled glucose levels was not consistent across studies or agents.
Everolimus is approved as a monotherapy in the U.S. for treatment of pancreatic neuroendocrine tumors (PNETs), advanced renal cell carcinoma (RCC), renal angiomyolipoma associated with tuberous sclerosis complex, and subependymal giant cell astrocytoma (SEGA). It is also approved in combination with the aromatase inhibitor exemestane for the treatment of hormone receptor-positive, HER2-negative breast cancer. As a combination therapy, this was not included in our review. In phase II and III studies of patients with PNET treated with everolimus (10 mg daily), the incidence of all-grade drug-related hyperglycemia ranged from 12% to 25% [42–44]. Hyperglycemia was among the major grade 3 or 4 drug-related AEs in these studies (range, 5%–18%). The phase III study excluded patients with uncontrolled blood glucose. Phase II and III studies with everolimus in patients with RCC reported a higher incidence of hyperglycemia at any grade (range, 50%–58%) than studies in patients with PNET, whereas grade 3 to 4 hyperglycemia was similar (range, 8%–12%) [45, 46]. In a phase II trial of patients with renal angiomyolipoma, fasting hyperglycemia (any grade) was reported in 14% of everolimus-treated patients; no grade 3 or 4 events were observed [86]. In phase II studies of patients with advanced urothelial cancer, advanced gastric cancer, metastatic pancreatic cancer, and bone or soft-tissue sarcomas, the incidence of hyperglycemia at any grade ranged from 66% to 93% [47–50]. In phase I studies of patients with advanced solid tumors, drug-related hyperglycemia was generally reported in <10% of patients [51, 52]. The incidence of hyperglycemia was 48% in patients with hepatocellular or hematologic malignancies [53, 54]. Hyperglycemia was not observed during the phase I/II or phase III trials of patients with SEGA [87, 88]; however, in a long-term follow-up of patients from the phase III trial, 14% reported hyperglycemia [89].
Activated T cells target cancerous cells but can also attack noncancerous normal tissues. This may lead to autoimmune destruction of pancreatic islet cells. Consequently, type 1 diabetes mellitus can occur, leading to decreased insulin levels and hyperglycemia.
Temsirolimus is approved in the U.S. for treatment of advanced RCC. The incidence of drug-related hyperglycemia in studies of patients with RCC treated with temsirolimus (25 mg weekly) ranged from 19% to 27% (all grade) and from 3% to 14% (grade 3 or 4) [58–60]. In phase II studies of patients with other cancers, including castration-resistant prostate cancer, metastatic breast cancer, advanced neuroendocrine cancer, glioblastoma, and NSCLC, rates of hyperglycemia (all grade) ranged from 7% to 76% [61–66]. The difference in rates of hyperglycemia for these studies was not dose dependent; surprisingly, the highest and lowest rates of hyperglycemia were observed with the lowest (25 mg/week) and highest (250 mg/week) doses, respectively [61, 66].
In studies of the mTOR inhibitor ridaforolimus, overall rates of all-grade hyperglycemia (11%–29%) in phase II and III studies were similar to those observed for everolimus and temsirolimus [55–57].
Dual PI3K/mTOR Inhibitors
PF-04691502, PF-05212384/PKI-587, and BEZ235 are molecules that target the catalytic domains of both PI3K and mTOR, which are structurally similar. Dual inhibition may be a valuable strategy because PI3K activity can be upregulated following mTOR inhibition [85]. In phase I studies of nondiabetic patients with solid tumors treated with these agents, the incidence of hyperglycemia (all grade) was in the range of 24%–27% (grade 3, 2%–11%) [67, 69, 70]. GDC-0980, another dual PI3K/mTOR inhibitor, was associated with grade 3 or 4 hyperglycemia in 46% of patients with endometrial cancer in a phase II trial [68]. Other common AEs with dual PI3K/mTOR inhibitors include fatigue, diarrhea, decreased appetite, nausea, rash, mucositis, vomiting, and constipation.
PD-1 Inhibitors
Pembrolizumab and nivolumab, antibodies that target the programmed death-1 (PD-1) receptor, are immune checkpoint inhibitors that promote T-cell activation and proliferation [90, 91]. Activated T cells target cancerous cells but can also attack noncancerous normal tissues [90, 91]. This may lead to autoimmune destruction of pancreatic islet cells. Consequently, type 1 diabetes mellitus can occur, leading to decreased insulin levels and hyperglycemia [90–92]. In phase I studies of pembrolizumab in patients with metastatic melanoma or NSCLC, the incidence of hyperglycemia (all grade) was 40% and 48%, respectively (grade 3, 2%; grade 4, 3%) [72]. Most likely, very few of the hyperglycemic events in this study represented an autoimmune diabetes; the others may have been from concomitant medications such as glucocorticoid medications. Diabetes mellitus was also reported in 1 of 206 patients in a phase III trial of nivolumab for the treatment of metastatic melanoma [71].
Screening, Monitoring, and Management
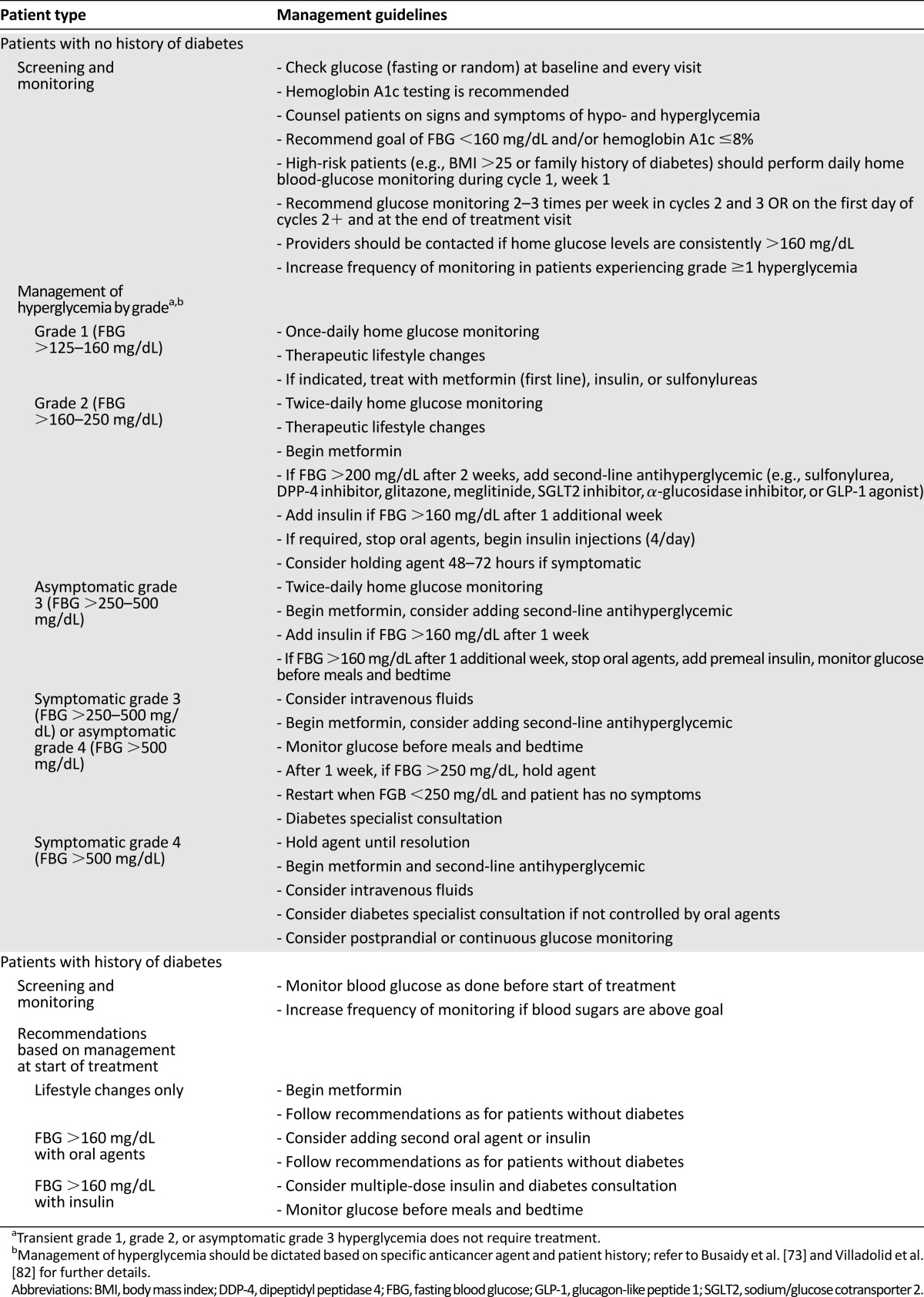
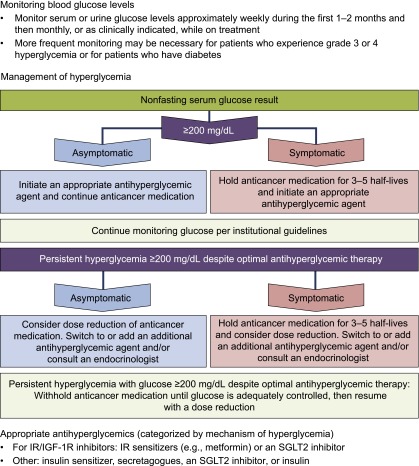
Hyperglycemia may negatively affect patient quality of life and interfere with treatment through dose reductions, delays, and discontinuations; however, the exact effect of hyperglycemia on treatment is often unclear because not all studies report detailed reasons for treatment interruptions. Because of these potential consequences, it is important for treating physicians to adequately screen patients, monitor glucose levels, and manage hyperglycemia as suggested in recently published guidelines (Table 2) [73, 82]. Although these guidelines represent the standard treatment for hyperglycemia, agents that cause severe insulin resistance or block IR may benefit from the treatment algorithm shown in Figure 3. Treating physicians can also work closely with an endocrinologist to ensure that hyperglycemia is being monitored and managed optimally.
Table 2.
Figure 3.
Algorithm for management of severe/persistent hyperglycemia. This algorithm, adapted from the protocol used in the TIGER-X study of rociletinib, may be applicable for use with other anticancer agents.
Abbreviations: IGF-1R, insulin growth factor receptor 1; IR, insulin receptor; SGLT2, sodium/glucose cotransporter 2.
Approximately half of the studies discussed thus far screened patients before treatment and excluded patients with preexisting diabetes mellitus or increased blood-glucose levels [10–15, 19–24, 33, 36, 39, 41, 42, 47, 48, 54, 62, 67–70]. In real-world clinical practice, patients who need anticancer treatment may present with preexisting or undiagnosed diabetes and glucose intolerance; screening patients for those conditions could help indicate which patients may require close monitoring.
During treatment, patients can be monitored for hyperglycemia (with fasting and/or postprandial blood-glucose levels and periodic hemoglobin A1c testing) and for insulin resistance (with insulin levels). Specific monitoring for hyperglycemia was common in many of the previously described studies, especially in studies of IGF-1R inhibitors [13, 16, 17, 19, 20, 33, 41, 62, 69]. Monitoring of all patients is of particular importance because patients considered low risk can still develop hyperglycemia. Additionally, although some agents have been associated with dose-dependent incidences of hyperglycemia, others (e.g., temsirolimus) have not.
Mild treatment-related hyperglycemia may be sufficiently managed through modifications in diet and exercise. Management of grade 3 and 4 hyperglycemia may involve dose reductions and/or the use of oral antihyperglycemic agents (Table 2; Fig. 2) [73, 93]. Insulin and insulin secretagogues are typically suitable options. These agents are used to increase cellular uptake of glucose. For patients who develop type 1 diabetes mellitus following treatment with PD-1 inhibitors, use of insulin is recommended [72]. Exceptions to the use of insulin should be made for patients who are receiving agents that inhibit IR (e.g., linsitinib or the M502 metabolite of rociletinib). In those instances, hyperglycemia should be managed with agents that decrease insulin resistance (e.g., metformin and thiazolidinediones) and increase glucose excretion (e.g., sodium-glucose linked transporter 2 inhibitors). Insulin and insulin secretagogues are unlikely to improve symptoms related to abnormal blood-glucose levels in this setting. Insulin sensitizers are not associated with hypoglycemia.
In many of the aforementioned studies, dose reductions and use of antihyperglycemic agents were sufficient to manage hyperglycemia; very few patients discontinued study drugs because of hyperglycemia [12, 13, 15, 19, 33, 45, 48, 53, 67, 70, 94]. In the rociletinib TIGER-X study, a specific protocol was implemented to manage M502-driven hyperglycemia. For FBG of >200 mg/dL, asymptomatic and symptomatic patients received an oral antihyperglycemic medication. Additionally, rociletinib was held for 48–72 hours in symptomatic patients. Once the drug was held, glucose levels tended to normalize within 24 hours and treatment could be reinitiated. This strategy may also be applicable with other anticancer agents associated with insulin resistance or those that block IR; this simplified management algorithm is provided in Figure 3.
Conclusion
In recent years, a better understanding of the cellular processes that drive cancer growth and survival has prompted the development of agents that target mediators of these processes, including IGF-1R, EGFR, PI3K, AKT, and mTOR. Many of these proteins are also involved in regulating glucose metabolism, and hyperglycemia is a recognized side effect of several targeted agents. Many clinical trials of these targeted agents exclude diabetic patients; however, in a real-world setting, a proportion of patients with cancer may also have preexisting conditions, including hyperglycemia and diabetes mellitus [95]. Screening in advance of treatment could help clinicians identify patients who will need closer observation. Proper hyperglycemia monitoring and management may ultimately lead to more successful outcomes with the use of these anticancer agents.
Acknowledgments
Writing and editorial support, including drafting and grammatical assistance, copyediting and preparation of the manuscript, and illustration production, was provided by Nathan Yardley, Stephanie Vadasz, Heather Sylvestro, and Shannon Davis of Infusion Communications (Haddam, CT), and was funded by Clovis Oncology, Inc. (Boulder, CO).
Author Contributions
Conception/Design: Jonathan W. Goldman
Provision of study material or patients: Jonathan W. Goldman
Collection and/or assembly of data: Jonathan W. Goldman
Data analysis and interpretation: Jonathan W. Goldman, Melody A. Mendenhall, Sarah R. Rettinger
Manuscript writing: Jonathan W. Goldman, Melody A. Mendenhall, Sarah R. Rettinger
Final approval of manuscript: Jonathan W. Goldman, Melody A. Mendenhall, Sarah R. Rettinger
Disclosures
Jonathan W. Goldman: Clovis Oncology (H, RF); Melody A. Mendenhall: Clovis Oncology (H). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Eurostat. Causes of death statistics - statistics explained. Available at http://ec.europa.eu/eurostat/statistics-explained/index.php/Causes_of_death_statistics#Main_statistical_findings. Accessed July 21, 2015.
- 2.Heron M. Deaths: Leading causes for 2010. Natl Vital Stat Rep. 2013;62:1–96. [PubMed] [Google Scholar]
- 3.National Cancer Institute. Targeted cancer therapies fact sheet - National Cancer Institute. Available at http://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Accessed July 21, 2015.
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: The TORCH randomized trial. J Clin Oncol. 2012;30:3002–3011. doi: 10.1200/JCO.2011.41.2056. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Feng J, Zhou C, et al. Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomised, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC) Ann Oncol. 2013;24:1615–1622. doi: 10.1093/annonc/mdt012. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: A phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342–3350. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 9.Yang JC-H, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 10.Higano CS, Berlin J, Gordon M, et al. Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors. Invest New Drugs. 2015;33:450–462. doi: 10.1007/s10637-015-0217-7. [DOI] [PubMed] [Google Scholar]
- 11.Schöffski P, Adkins D, Blay JY, et al. An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur J Cancer. 2013;49:3219–3228. doi: 10.1016/j.ejca.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Rajan A, Carter CA, Berman A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15:191–200. doi: 10.1016/S1470-2045(13)70596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abou-Alfa GK, Capanu M, O’Reilly EM, et al. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60:319–324. doi: 10.1016/j.jhep.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atzori F, Tabernero J, Cervantes A, et al. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 15.Reidy-Lagunes DL, Vakiani E, Segal MF, et al. A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors. Cancer. 2012;118:4795–4800. doi: 10.1002/cncr.27459. [DOI] [PubMed] [Google Scholar]
- 16.Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 17.Haluska P, Worden F, Olmos D, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65:765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becerra CR, Salazar R, Garcia-Carbonero R, et al. Figitumumab in patients with refractory metastatic colorectal cancer previously treated with standard therapies: A nonrandomized, open-label, phase II trial. Cancer Chemother Pharmacol. 2014;73:695–702. doi: 10.1007/s00280-014-2391-2. [DOI] [PubMed] [Google Scholar]
- 19.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 20.Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 21.Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: Results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puzanov I, Lindsay CR, Goff L, et al. A phase I study of continuous oral dosing of OSI-906, a dual inhibitor of insulin-like growth factor-1 and insulin receptors, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:701–711. doi: 10.1158/1078-0432.CCR-14-0303. [DOI] [PubMed] [Google Scholar]
- 23.Jones RL, Kim ES, Nava-Parada P, et al. Phase I study of intermittent oral dosing of the insulin-like growth factor-1 and insulin receptors inhibitor OSI-906 in patients with advanced solid tumors. Clin Cancer Res. 2015;21:693–700. doi: 10.1158/1078-0432.CCR-14-0265. [DOI] [PubMed] [Google Scholar]
- 24.Fassnacht M, Berruti A, Baudin E, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16:426–435. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- 25.Zykadia (ceritinib) capsules [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014. [Google Scholar]
- 26.Goyal L, Wadlow RC, Blaszkowsky LS, et al. A phase I and pharmacokinetic study of ganetespib (STA-9090) in advanced hepatocellular carcinoma. Invest New Drugs. 2015;33:128–137. doi: 10.1007/s10637-014-0164-8. [DOI] [PubMed] [Google Scholar]
- 27.Goldman JW, Raju RN, Gordon GA, et al. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer. 2013;13:152. doi: 10.1186/1471-2407-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Socinski MA, Goldman J, El-Hariry I, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res. 2013;19:3068–3077. doi: 10.1158/1078-0432.CCR-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jhaveri K, Chandarlapaty S, Lake D, et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer. 2014;14:154–160. doi: 10.1016/j.clbc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Moore AM, Einhorn LH, Estes D, et al. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: A Hoosier Oncology Group phase II trial. Lung Cancer. 2006;52:93–97. doi: 10.1016/j.lungcan.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Wadlow RC, Hezel AF, Abrams TA, et al. Panitumumab in patients with KRAS wild-type colorectal cancer after progression on cetuximab. The Oncologist. 2012;17:14. doi: 10.1634/theoncologist.2011-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sequist LV, Goldman JW, Wakelee HA, et al. Efficacy of rociletinib (CO-1686) in plasma-genotyped T790M-positive NSCLC patients. J Clin Oncol. 2015;33:8001. [Google Scholar]
- 33.Rodon J, Braña I, Siu LL, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32:670–681. doi: 10.1007/s10637-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 34.Sarker D, Ang JE, Baird R, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro GI, Rodon J, Bedell C, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:233–245. doi: 10.1158/1078-0432.CCR-13-1777. [DOI] [PubMed] [Google Scholar]
- 36.Spencer A, Yoon SS, Harrison SJ, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014;124:2190–2195. doi: 10.1182/blood-2014-03-559963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burris HA, Siu LL, Infante JR, et al. Safety, pharmacokinetics (PK), pharmacodynamics (PD), and clinical activity of the oral AKT inhibitor GSK2141795 (GSK795) in a phase I first-in-human study. J Clin Oncol. 2011;29:3003. [Google Scholar]
- 38.Tabernero J, Saura C, Roda Perez D, et al. First-in-human phase I study evaluating the safety, pharmacokinetics (PK), and intratumor pharmacodynamics (PD) of the novel, oral, ATP-competitive AKT inhibitor GDC-0068. J Clin Oncol. 2011;29:3022. [Google Scholar]
- 39.Yap TA, Yan L, Patnaik A, et al. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin Cancer Res. 2014;20:5672–5685. doi: 10.1158/1078-0432.CCR-14-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma BB, Goh BC, Lim WT, et al. Multicenter phase II study of the AKT inhibitor MK-2206 in recurrent or metastatic nasopharyngeal carcinoma from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group (MC1079) Invest New Drugs. 2015;33:985–991. doi: 10.1007/s10637-015-0264-0. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan RK, McDonough SL, Kennecke HF, et al. Phase 2 study of MK-2206, an allosteric inhibitor of AKT, as second-line therapy for advanced gastric and gastroesophageal junction cancer: A SWOG Cooperative Group Trial (S1005) Cancer. 2015;121:2193–2197. doi: 10.1002/cncr.29363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh DY, Kim TW, Park YS, et al. Phase 2 study of everolimus monotherapy in patients with nonfunctioning neuroendocrine tumors or pheochromocytomas/paragangliomas. Cancer. 2012;118:6162–6170. doi: 10.1002/cncr.27675. [DOI] [PubMed] [Google Scholar]
- 44.Kamp K, Gumz B, Feelders RA, et al. Safety and efficacy of everolimus in gastrointestinal and pancreatic neuroendocrine tumors after (177)Lu-octreotate. Endocr Relat Cancer. 2013;20:825–831. doi: 10.1530/ERC-13-0254. [DOI] [PubMed] [Google Scholar]
- 45.Amato RJ, Jac J, Giessinger S, et al. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer. 2009;115:2438–2446. doi: 10.1002/cncr.24280. [DOI] [PubMed] [Google Scholar]
- 46.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 47.Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–198. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milowsky MI, Iyer G, Regazzi AM, et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 2013;112:462–470. doi: 10.1111/j.1464-410X.2012.11720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon DH, Ryu MH, Park YS, et al. Phase II study of everolimus with biomarker exploration in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinum. Br J Cancer. 2012;106:1039–1044. doi: 10.1038/bjc.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo C, Lee J, Rha SY, et al. Multicenter phase II study of everolimus in patients with metastatic or recurrent bone and soft-tissue sarcomas after failure of anthracycline and ifosfamide. Invest New Drugs. 2013;31:1602–1608. doi: 10.1007/s10637-013-0028-7. [DOI] [PubMed] [Google Scholar]
- 51.O’Donnell A, Faivre S, Burris HA, 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 52.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: A phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 53.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 54.Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–5102. doi: 10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demetri GD, Chawla SP, Ray-Coquard I, et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31:2485–2492. doi: 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
- 56.Colombo N, McMeekin DS, Schwartz PE, et al. Ridaforolimus as a single agent in advanced endometrial cancer: Results of a single-arm, phase 2 trial. Br J Cancer. 2013;108:1021–1026. doi: 10.1038/bjc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oza AM, Pignata S, Poveda A, et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J Clin Oncol. 2015;33:3576–3582. doi: 10.1200/JCO.2014.58.8871. [DOI] [PubMed] [Google Scholar]
- 58.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 59.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 60.Lamm W, Vogl UM, Bojic M, et al. Safety and efficacy of temsirolimus in heavily pretreated patients with metastatic renal cell carcinoma. Acta Oncol. 2012;51:101–106. doi: 10.3109/0284186X.2011.589404. [DOI] [PubMed] [Google Scholar]
- 61.Kruczek K, Ratterman M, Tolzien K, et al. A phase II study evaluating the toxicity and efficacy of single-agent temsirolimus in chemotherapy-naïve castration-resistant prostate cancer. Br J Cancer. 2013;109:1711–1716. doi: 10.1038/bjc.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong AJ, Shen T, Halabi S, et al. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clin Genitourin Cancer. 2013;11:397–406. doi: 10.1016/j.clgc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 65.Reungwetwattana T, Molina JR, Mandrekar SJ, et al. Brief report: A phase II “window-of-opportunity” frontline study of the MTOR inhibitor, temsirolimus given as a single agent in patients with advanced NSCLC, an NCCTG study. J Thorac Oncol. 2012;7:919–922. doi: 10.1097/JTO.0b013e31824de0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 67.Bendell JC, Kurkjian C, Infante JR, et al. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Invest New Drugs. 2015;33:463–471. doi: 10.1007/s10637-015-0218-6. [DOI] [PubMed] [Google Scholar]
- 68.Makker V, Recio FO, Ma L, et al. Phase II trial of GDC-0980 (dual PI3K/mTOR inhibitor) in patients with advanced endometrial carcinoma: Final study results. J Clin Oncol. 2014;32:5513. [Google Scholar]
- 69.Britten CD, Adjei AA, Millham R, et al. Phase I study of PF-04691502, a small-molecule, oral, dual inhibitor of PI3K and mTOR, in patients with advanced cancer. Invest New Drugs. 2014;32:510–517. doi: 10.1007/s10637-013-0062-5. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro GI, Bell-McGuinn KM, Molina JR, et al. First-in-human study of PF-05212384 (PKI-587), a small-molecule, intravenous, dual inhibitor of PI3K and mTOR in patients with advanced cancer. Clin Cancer Res. 2015;21:1888–1895. doi: 10.1158/1078-0432.CCR-14-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 72.Keytruda (pembrolizumab) for injection [prescribing information] Whitehouse Station, NJ: Merck & Co., Inc.; 2015. [Google Scholar]
- 73.Busaidy NL, Farooki A, Dowlati A, et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 2012;30:2919–2928. doi: 10.1200/JCO.2011.39.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo S. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen HX, Sharon E. IGF-1R as an anti-cancer target--trials and tribulations. Chin J Cancer. 2013;32:242–252. doi: 10.5732/cjc.012.10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janssen JA, Varewijck AJ. IGF-IR targeted therapy: Past, present and future. Front Endocrinol (Lausanne) 2014;5:224. doi: 10.3389/fendo.2014.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma H, Zhang T, Shen H, et al. The adverse events profile of anti-IGF-1R monoclonal antibodies in cancer therapy. Br J Clin Pharmacol. 2014;77:917–928. doi: 10.1111/bcp.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Veeken J, Oliveira S, Schiffelers RM, et al. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: Implications for cancer therapy. Curr Cancer Drug Targets. 2009;9:748–760. doi: 10.2174/156800909789271495. [DOI] [PubMed] [Google Scholar]
- 80.King ER, Wong KK. Insulin-like growth factor: Current concepts and new developments in cancer therapy. Recent Patents Anticancer Drug Discov. 2012;7:14–30. doi: 10.2174/157489212798357930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gray J, Haura E. Update on third-generation EGFR tyrosine kinase inhibitors. Transl Lung Cancer Res. 2014;3:360–362. doi: 10.3978/j.issn.2218-6751.2014.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villadolid J, Ersek JL, Fong MK, et al. Management of hyperglycemia from epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) targeting T790M-mediated resistance. Transl Lung Cancer Res. 2015;4:576–583. doi: 10.3978/j.issn.2218-6751.2015.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–1709. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 84.Barlow AD, Nicholson ML, Herbert TP. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 2013;62:2674–2682. doi: 10.2337/db13-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vilar E, Perez-Garcia J, Tabernero J. Pushing the envelope in the mTOR pathway: The second generation of inhibitors. Mol Cancer Ther. 2011;10:395–403. doi: 10.1158/1535-7163.MCT-10-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–824. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 87.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 88.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 89.Afinitor (everolimus) tablets for oral administration afinitor disperz (everolimus tablets for oral suspension) [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. [Google Scholar]
- 90.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–e57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaudy C, Clévy C, Monestier S, et al. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38:e182–e183. doi: 10.2337/dc15-1331. [DOI] [PubMed] [Google Scholar]
- 92.Kochupurakkal NM, Kruger AJ, Tripathi S, et al. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One. 2014;9:e89561. doi: 10.1371/journal.pone.0089561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vergès B, Walter T, Cariou B. Endocrine side effects of anti-cancer drugs: Effects of anti-cancer targeted therapies on lipid and glucose metabolism. Eur J Endocrinol. 2014;170:R43–R55. doi: 10.1530/EJE-13-0586. [DOI] [PubMed] [Google Scholar]
- 94.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 95.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]