This study evaluated the prognostic value of the cumulative cisplatin dose (CCD) for long-term survival outcomes after concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma (NPC). A CCD of ≥240 mg/m2 was not an independent prognostic factor in patients with locoregionally advanced NPC at high risk of distant metastasis, and 200 mg/m2 cisplatin may be adequate to achieve a survival benefit.
Keywords: Nasopharyngeal carcinoma, Concurrent chemotherapy, Cumulative cisplatin dose, Locoregionally advanced, Prognosis
Abstract
Background.
The objective of this study was to evaluate the prognostic value of the cumulative cisplatin dose (CCD) for long-term survival outcomes after concurrent chemoradiotherapy (CCRT) in locoregionally advanced nasopharyngeal carcinoma (NPC).
Methods.
Patients were included in an open-label phase III multicenter randomized controlled trial performed at seven institutions in China, and the 298 patients receiving CCRT only were assessed. Patient survival between different CCD groups were compared.
Results.
Median CCD for the 298 patients was 240 mg/m2 (range, 40–320 mg/m2); 113 (37.9%) patients received a CCD of <240 (≤200) mg/m2, and 185 (62.1%) received a CCD of ≥240 mg/m2. For CCD of ≥240 mg/m2 vs. <240 mg/m2, the estimated 5-year overall survival, disease-free survival, locoregional relapse-free survival, and distant metastasis-free survival rates were 83.2% vs. 76.2% (p = .403), 73.5% vs. 67.8% (p = .461), 90.4% vs. 86.8% (p = .551), and 82.6% vs. 79.7% (p = .632), respectively. Multivariate analysis demonstrated that CCD (240 mg/m2) was not an independent prognostic factor in either the entire cohort or stage III/IV subgroup.
Conclusion.
A CCD of ≥240 mg/m2 was not an independent prognostic factor in patients with locoregionally advanced NPC at high risk of distant metastasis, and 200 mg/m2 cisplatin may be adequate to achieve a survival benefit.
Implications for Practice:
The current standard treatment for locoregionally advanced nasopharyngeal carcinoma (NPC) is cisplatin-based concurrent chemoradiotherapy (CCRT), and the cisplatin is delivered every 3 weeks (100 mg/m2) for three cycles. However, the prognostic value of cumulative cisplatin dose (CCD) delivered during CCRT is controversial. The present study investigated the prognostic value of CCD and demonstrated that a CCD of 200 mg/m2 during CCRT is adequate to achieve satisfactory survival outcomes for patients with locoregionally advanced NPC. This finding is of great importance to clinicians because it could allow patients to avoid excessive treatment and toxicities.
Abstract
摘要
背景. 本研究旨在评价顺铂累积剂量(CCD)对局部晚期鼻咽癌(NPC)患者同步放化疗(CCRT)后长期生存转归的预后价值。
方法. 在中国的7家机构开展了一项开放标签的III期多中心、随机对照临床试验, 本研究仅评估其中298例仅接受CCRT治疗的患者。比较不同CCD组的患者的生存。
结果. 298例患者的中位CCD为240 mg/m2(范围40∼320 mg/m2), 113例(37.9%)患者接受的CCD < 240(≤200)mg/m2, 而185例(62.1%)接受的CCD≥240 mg/m2。CCD≥240 mg/m2和<240 mg/m2患者的估算5年总生存率、无疾病生存率、局部无复发生存率和无远处转移生存率分别为83.2% vs. 76.2%(P=0.403)、73.5% vs. 67.8%(P=0.461)、90.4% vs. 86.8%(P=0.551)和82.6% vs. 79.7%(P=0.632)。多变量分析证实CCD(240 mg/m2)不是总队列或III/IV期亚组的独立预后因素。
结论. CCD≥240 mg/m2不是具有远处转移高风险的局部晚期NPC患者的独立预后因素, 顺铂200 mg/m2可能足以达到生存获益。The Oncologist 2016;21:1369–1376
对临床实践的提示: 目前局部晚期鼻咽癌(NPC)的标准治疗是含顺铂的同步放化疗(CCRT), 顺铂每3周给药一次(100 mg/m2), 共3周期。但CCRT治疗过程中顺铂累积剂量(CCD)的预后价值仍有争议。本研究调查了CCD的预后价值, 并证实局部晚期NPC患者的CCRT中给予CCD 200 mg/m2足以达到满意的生存转归。本研究结果对临床医生意义重大, 因为这些结果使得患者得以避免过度治疗及毒性。
Introduction
Nasopharyngeal carcinoma (NPC) arises from the nasopharyngeal epithelium and has an extremely unbalanced geographical distribution. The age-standardized incidence in 2008 was 20–50 per 100,000 for males in southern China, compared with only 0.5 per 100,000 males in predominantly white populations of European origin [1]. As a result of anatomic constraints and its high radiosensitivity, radiotherapy is the primary and only curative treatment for NPC. Patients with early stage disease usually have an excellent prognosis after radiotherapy alone; however, locoregionally advanced NPC has a poor prognosis. The 5-year overall survival rates are 87%–96% for stage I–II NPC and 67%–77% for stage III–IV [2]. Based on the 6th edition of International Union Against Cancer (UICC)/American Joint Commission on Cancer (AJCC) staging system [3], 60%–70% of newly diagnosed cases are locoregionally advanced stage III or IV NPC [4]. Therefore, improved treatment strategies are required for advanced-stage NPC.
Because the NPC-0099 trial initially reported that a combination of chemotherapy and radiotherapy was superior to radiotherapy alone for advanced-stage disease [5], numerous subsequent clinical trials had confirmed the benefit of chemotherapy in advanced-stage NPC [6–9]. In addition, our previous study indicated that adjuvant chemotherapy had no prognostic value in advanced-stage NPC [10]. Therefore, cisplatin-based concurrent chemoradiotherapy (CCRT) is deemed the main standard treatment for stage II–IVB NPC that is recommended by National Comprehensive Cancer Network guidelines.
Many previous studies have reported the prognostic value of cumulative cisplatin dose (CCD) delivered during CCRT, and a CCD of 200 mg/m2 was found to be associated with significantly improved prognosis in NPC [11–13] and other head and neck cancers [12]. However, most recently, Ou et al. [14] revealed that a total cisplatin dose of 300 mg/m2 was an independent prognostic factor for distant metastasis and overall survival. These controversial results need further investigation. Moreover, the prognostic value of CCD in locoregionally advanced NPC has never been investigated. Therefore, the aim of study was to figure out this problem based on a prospective phase III clinical trial [10].
Materials and Methods
Patient Selection
Patients selected for this study were derived from an open-label phase III multicenter randomized controlled trial (NCT00677118) at seven institutions in China in which patients received CCRT alone or CCRT with adjuvant chemotherapy (AC). The trial inclusion criteria have previously been described in detail [10]. Briefly, eligible patients were 18–70 years old with nonmetastatic histologically proven nonkeratinizing stage III–IV NPC, with the exception of T3-4N0 NPC (6th edition of UICC/AJCC). All participants had Karnofsky scores ≥ 70 and adequate bone marrow, liver, and renal function. Patients who had received previous chemotherapy or radiotherapy or who had other malignant tumors were excluded. Between June 1, 2006, and March 5, 2010, a total of 508 patients were recruited; of those, 257 (50.6%) patients were assigned to receive CCRT, and 251 (49.4%) were assigned to receive CCRT plus AC.
The protocol of this study was approved by the ethics committee or institutional review board at each center. Therefore, the patients receiving only CCRT were included in this study. Informed consent was obtained from all patients.
Clinical Staging
Routine staging included a complete medical history, clinical examination of head and neck, direct fiber-optic nasopharyngoscopy, magnetic resonance imaging (MRI) of skull base and entire neck, chest radiography, whole-body bone scan, abdominal sonography, and positron emission tomography-computed tomography (CT). The tumor-associated markers immunoglobulin A antibodies to Epstein-Barr virus (EBV) viral capsid antigen and to EBV early antigen were quantified. All patients had a dental evaluation before radiotherapy and were restaged according to the 6th edition of the UICC/AJCC system [3].
Chemotherapy
The chemotherapy component of CCRT consisted of 40 mg/m2 cisplatin given as a 2-hour intravenous infusion every week for a maximum of seven cycles, beginning on the first day of radiotherapy [15, 16]. Dose modifications were not permitted. Complete blood and biochemistry analyses were performed every week. Chemotherapy was delayed until the absolute neutrophil count was ≥1.5 × 109/L or the white cell count was ≥3 × 109/L, and was stopped completely if patients refused to receive further chemotherapy, creatinine clearance was <40 mL/minute, or a ≥grade 3 toxicity developed.
Radiotherapy
All patients were treated with 2.0–2.27 Gy per fraction with five daily fractions per week for 6–7 weeks, administered as megavoltage photons using either two-dimensional radiotherapy, intensity-modulated radiotherapy (IMRT), or three-dimensional conformal radiotherapy, in accordance with the treatment policy adopted by each center. Cumulative radiation doses were ≥66 Gy to the primary tumor and 60–66 Gy to the involved neck area. All potential sites of local infiltration and bilateral cervical lymphatics were irradiated to ≥50 Gy.
Follow-Up
Patient follow-up was measured from first day of therapy to last examination or death. Participants were assessed every 3 months during first 3 years and every 6 months thereafter until death. The primary endpoint (time to first defining event) was overall survival (OS); disease-free survival (DFS), locoregional relapse-free survival (LRRFS), and distant metastasis-free survival (DMFS) were secondary endpoints. All local recurrences were diagnosed via fiber-optic endoscopy and biopsy or MRI scan (or both) of nasopharynx and skull base showing progressive bone erosion and soft tissue swelling. Regional recurrences were diagnosed by clinical examination of neck and, in doubtful cases, by fine-needle aspiration or MRI of neck. Distant metastases were diagnosed by clinical symptoms, physical examinations, and imaging methods, including chest radiography, bone scan, MRI, CT, and abdominal sonography. When possible, salvage treatments, including reirradiation, chemotherapy, and surgery, were given after documented relapse or in persistent disease, in accordance with the standard practice of each center.
Statistical Analysis
The chi-square test was used to compare clinical characteristics. Time-to-event data were described using Kaplan-Meier curves and time-to-event intervals with the log-rank test. The multivariate Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Covariates included host factors (i.e., sex, performance status, and age), tumor factors (i.e., T and N categories), radiotherapy (i.e., radiotherapy technique), and cumulative cisplatin dose (i.e., <240 mg/m2 vs. ≥240 mg/m2). All statistical tests were two-sided; p < .05 was considered statistically significant. STATA statistical package (STATA 12; StataCorp LP, College Station, TX, http://www.stata.com) was used for all analyses.
Results
Baseline Characteristics
In the prospective trial, 17.5% (44 of 251) of patients in the CCRT plus AC arm refused adjuvant chemotherapy and were included in this study, and 3 of 257 (1.2%) patients in the CCRT arm did not receive chemotherapy and were excluded from this study. Therefore, 298 patients were included in this analysis. The male (n = 218) to female (n = 80) ratio was 2.7:1, median age was 45 years old (range, 18–68 years old). Median CCD for all 298 patients was 240 mg/m2 (range, 40–320 mg/m2). Only 30 (10.1%) patients received a CCD of <200 mg/m2. In total, 113 (37.9%) patients received a CCD of <240 (≤200) mg/m2, and 185 (62.1%) patients received a CCD of ≥240 mg/m2; the basic characteristics of these groups are summarized in Table 1. Although patients with a CCD of ≥240 mg/m2 had a higher percentage of N3 disease than patients with a CCD of <240 mg/m2 (p = .039), the overall stages were well balanced between the two groups (p = .077).
Table 1.
Basic characteristics of the 298 patients with locoregionally advanced NPC receiving concurrent chemoradiotherapy

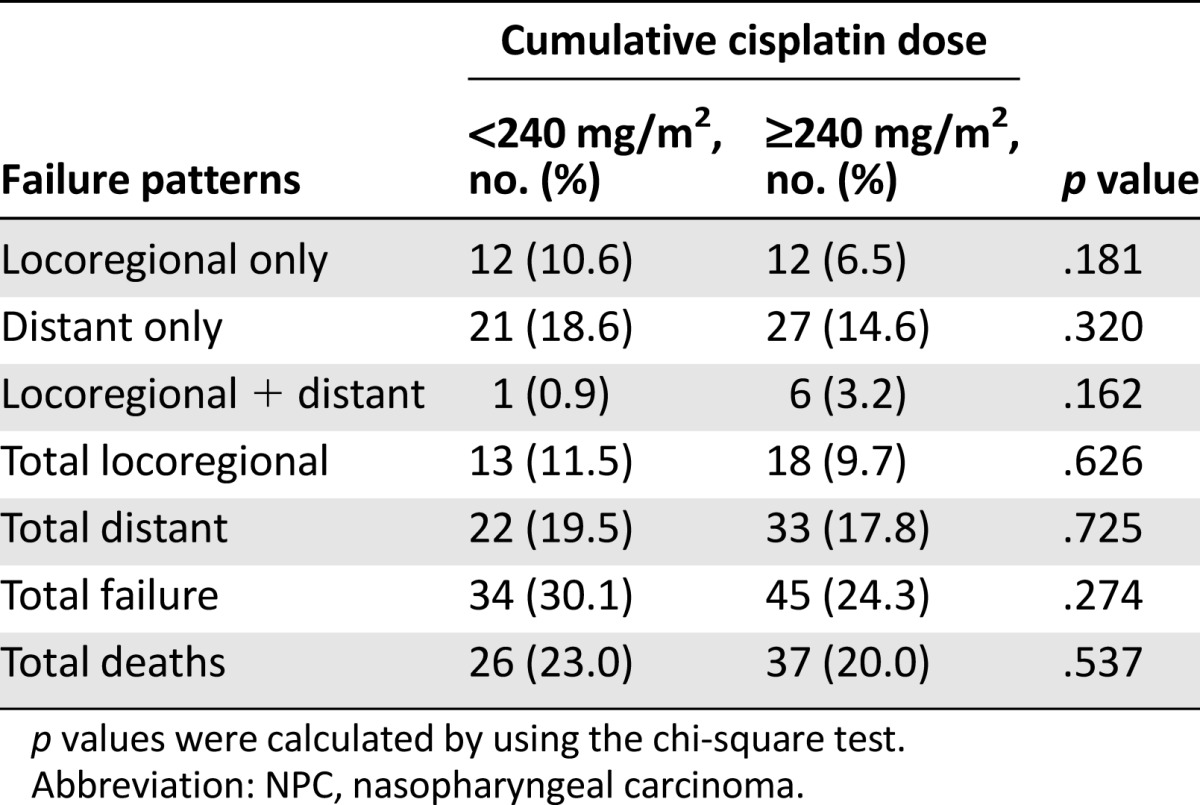
Patterns of Treatment Failure
By last follow-up (April 30, 2015), 14 of 298 (5.4%) patients had been lost to follow-up. Median follow-up duration for the entire cohort was 66.3 months (range, 2.2–103.9 months). The patterns of treatment failure are summarized in Table 2. Results showed that 13 of 113 (11.5%) patients in the CCD of <240 mg/m2 group and 18 of 185 (9.7%) in the ≥240 mg/m2 group experienced locoregional failure, and 22 of 113 (19.5%) patients in the <240 mg/m2 group and 33 of 185 (17.8%) patients in the ≥240 mg/m2 group developed distant metastases. Moreover, 26 of 113 (23.0%) patients in the <240 mg/m2 group and 37 of 185 (20%) patients in the ≥240 mg/m2 group died.
Table 2.
Failure patterns for the 298 patients with locoregionally advanced NPC receiving concurrent chemoradiotherapy
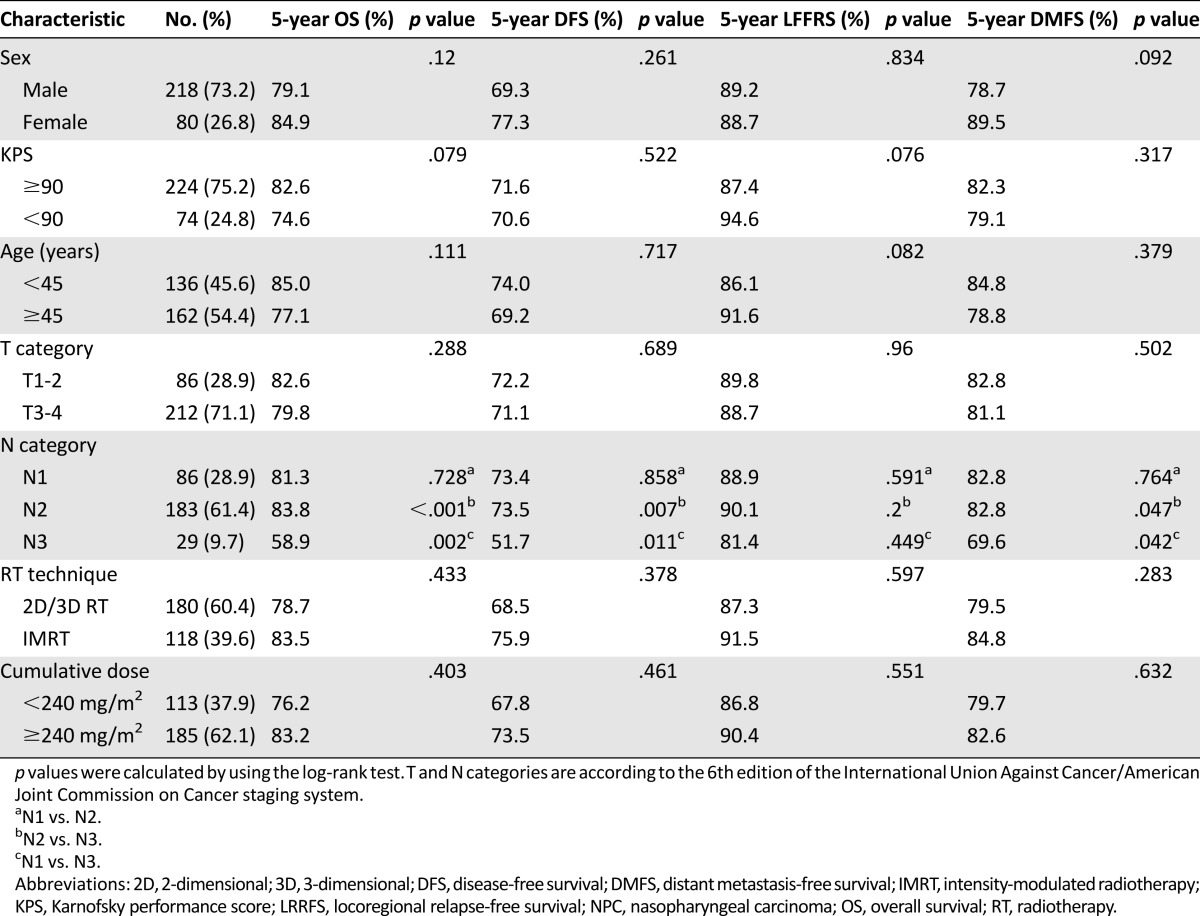
Univariate and Multivariate Analysis
The results of univariate analysis are presented in Table 3. Univariate analysis revealed that the N category was significantly associated with 5-year OS, DFS, and DMFS. Notably, these trends were significant in patients with N3 disease, but not in patients with N1 and N2 disease. No significant differences in 5-year OS, DFS, LRRFS, and DMFS were observed between patients with a CCD of <240 mg/m2 and of ≥240 mg/m2 (Fig. 1). Moreover, to further assess the effect of low and high CCD on prognosis, patient survival between the highest CCD quartile (280 mg/m2) and lowest CCD quartile (200 mg/m2) were compared (supplemental online Appendix).
Table 3.
Univariate analysis of 5-year OS, DFS, LRRFS, and DMFS in the 298 patients with locoregionally advanced NPC
Figure 1.
Kaplan-Meier OS (A), DFS (B), LRRFS (C), and DMFS (D) curves for patients with NPC stratified as the cumulative cisplatin dose of <240 mg/m2 and ≥240 mg/m2 groups.
Abbreviations: DFS, disease-free survival; DMFS, distant metastasis-free survival; LRRFS, local-regional relapse-free survival; OS, overall survival.
Multivariate analysis was performed to adjust for potential prognostic factors, including sex, Karnofsky performance score, age, T category, N category, radiotherapy technique, and CCD. In accordance with the results of univariate analysis, the CCD was not identified as an independent prognostic factor in patients with locoregionally advanced NPC receiving CCRT (p = .117 for OS, p = .198 for DFS, p = .41 for LRRFS, and p = .314 for DMFS). Only the N category was an independent prognostic factor for 5-year OS (HR, 3.435; 95% CI, 1.655–7.130; p = .001), DFS (HR, 2.324; 95% CI, 1.195–4.520; p = .013), and DMFS (HR, 2.378; 95% CI, 1.028–5.501; p = .043; Table 4).
Table 4.
Multivariate analysis of variables correlated with clinical outcomes in the 298 patients with locoregionally advanced NPC
Subgroup Analysis for Stage III and IV NPC
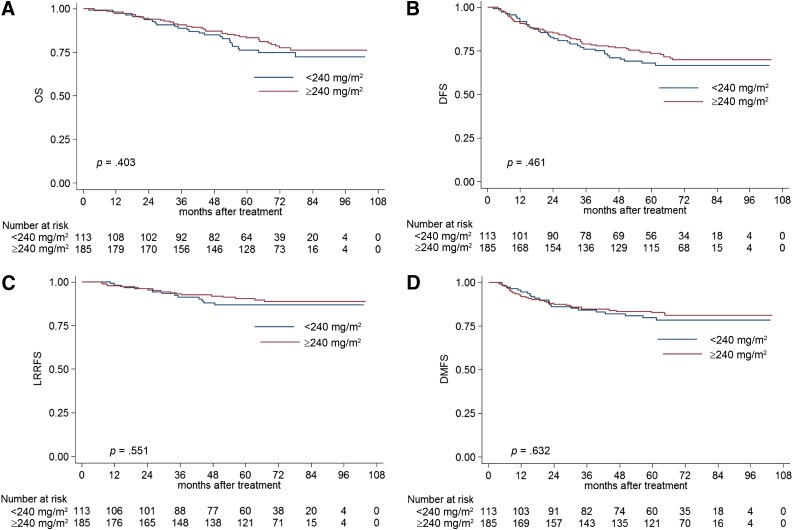
Subgroup analysis was conducted in patients with different clinical stages of NPC to further investigate the prognostic value of the CCD. In stage III NPC, the estimated 5-year OS, DFS, LRRFS, and DMFS rates for patients with a CCD of <240 mg/m2 vs. ≥240 mg/m2 were 80.3% vs. 86.5% (p = .434; Fig. 2A), 71.5% vs. 79.8% (p = .354; Fig. 2B), 89.6% vs. 91.7% (p = .944; Fig. 2C), and 82.3% vs. 87.9% (p = .344; Fig. 2D), respectively. The CCD was not identified as an independent prognostic factor in stage III NPC by multivariate analysis.
Figure 2.
Kaplan-Meier OS (A), DFS (B), LRRFS (C), and DMFS (D) curves for NPC patients with stage III disease stratified as the cumulative cisplatin dose of <240 mg/m2 and ≥240 mg/m2 groups.
Abbreviations: DFS, disease-free survival; DMFS, distant metastasis-free survival; LRRFS, local-regional relapse-free survival; OS, overall survival.
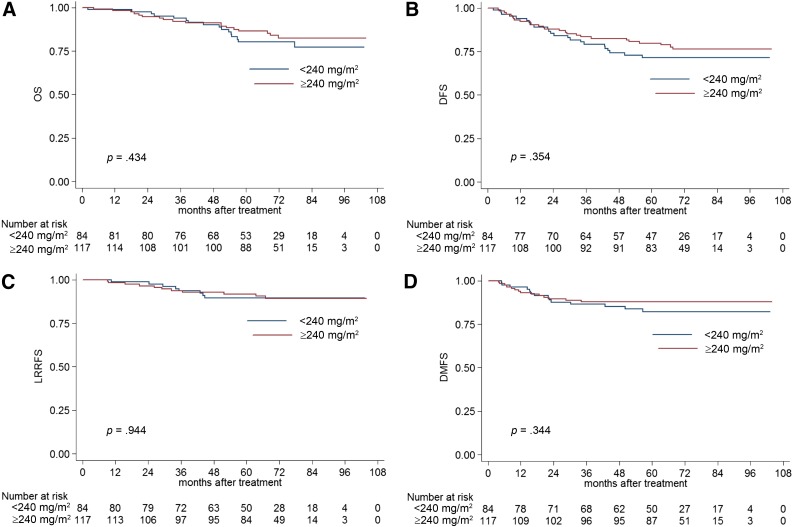
In stage IV, the estimated 5-year OS, DFS, LRRFS, and DMFS rates for patients with a CCD of <240 mg/m2 vs. ≥240 mg/m2 were 63.0% vs. 77.3% (p = .257; Fig. 3A), 56.0% vs. 62.2% (p = .47; Fig. 3B), 78.0% vs. 87.6% (p = .24; Fig. 3C), and 70.9% vs. 72.8% (p = .733; Fig. 3D), respectively. Multivariate analysis also revealed the CCD was not an independent prognostic factor in stage IV NPC.
Figure 3.
Kaplan-Meier OS (A), DFS (B), LRRFS (C), and DMFS (D) curves for NPC patients with stage IV disease stratified as the cumulative cisplatin dose of <240 mg/m2 and ≥240 mg/m2 groups.
Abbreviations: DFS, disease-free survival; DMFS, distant metastasis-free survival; LRRFS, local-regional relapse-free survival; OS, overall survival.
Discussion
Based on this analysis of a prospective phase III clinical trial, patients with locoregionally advanced (T3-4N1-3 or T1-2N2-3) NPC undergoing only CCRT who received a CCD of ≥240 mg/m2 had similar outcomes to patients who received a CCD of <240 mg/m2. Additionally, CCD had no prognostic value in subgroup analysis of stage III/IV NPC. Consistent with previous results [10], the N category was the only prognostic factor in the entire cohort.
Of note, although the triweekly regimen at 100 mg/m2 was the standard concurrent chemotherapy, the weekly regimen at 40 mg/m2 has been reported to be as effective as the triweekly regimen [15, 16]. In addition, the weekly regimen had fewer acute toxicities and therefore could improve the compliance of patients. The use of this regimen would not affect the results of this current study.
For the whole cohort, the percentages of patients receiving IMRT or conventional RT were well balanced between high- and low-CCD groups. However, in further analysis, the highest CCD quartile (280 mg/m2) group had a lower percentage of patients receiving IMRT compared with the lowest CCD quartile (200 mg/m2) group, and no survival difference was found between these two groups. Previous studies confirmed that IMRT is superior to conventional RT in terms of local control [17, 18], but has no significant influence on OS in NPC [19]. Additionally, concurrent cisplatin mainly increases the efficacy of radiotherapy. Therefore, it is difficult to confirm whether the local relapse-free survival benefit originated from a higher cisplatin dose, IMRT, or both. Thus, the use of nonuniform radiotherapy techniques complicates analysis of the effective cumulative cisplatin dose.
In the prospective NPC-9901 and NPC-9902 trials [20], at least two cycles of cisplatin (100 mg/m2) improved local-free survival and OS compared with one cycle. Moreover, 200 mg/m2 cisplatin was an appropriate cumulative dose in other retrospective studies of NPC [11, 12] and head and neck cancer [13, 21]. However, most recently, Ou et al. reported that a total cisplatin dose of >300 mg/m2 was an independent prognostic factor for OS, DFS, and DMFS in locally advanced NPC [14]. Of note, some of these patients also received induction or adjuvant chemotherapy. Several prospective clinical trials have shown that patients with locoregionally advanced NPC do not benefit from induction [22–26] or adjuvant chemotherapy [10]. Therefore, the lowest effectively cumulative cisplatin dose could have been inflated by cisplatin-based induction or adjuvant chemotherapy [14]. In addition, the results of this study were different from those of our previous study [27]. The main reasons may be that patients with stage I–IVB were recruited and were treated with IMRT in the study. Moreover, the data were retrospectively collected.
One of the main strengths of this study is that the patients were treated at seven institutions in China. Although the analysis was retrospective, the problems of poor data collection associated with retrospective studies do not apply, because the data were collected prospectively. However, no significant associations between the CCD (240 mg/m2) and long-term outcomes were observed. The lack of significant associations may be explained by the following factors. The aim of the phase III clinical trial [10] was to assess the efficacy of AC for reducing distant metastasis in locoregionally advanced NPC. Therefore, the patients in the present study had a high risk of distant metastasis. Concurrent cisplatin chemotherapy was administered to increase the effect of radiotherapy, as recommended by the National Comprehensive Cancer Network Guidelines; however, concurrent cisplatin did not reduce the risk of distant metastasis. Therefore, the survival benefits provided by concurrent cisplatin, even at a high dose, are limited.
A CCD of ≥240 mg/m2 had no prognostic significance, even in subgroup analysis of the stage III/IV subgroup. In the study by Loong et al. [11], subgroup analysis in stage II and III NPC revealed that a CCD of ≥200 mg/m2 led to better overall survival than a lower dose. Moreover, Lee et al. [20] also found that the number of cycles of concurrent chemotherapy was significantly associated with failure-free survival and OS in the stage III subgroup, but not the stage IV subgroup. These results indicate the CCD has prognostic value in patients with an intermediate or low risk of distant metastasis, but not in patients with a high risk of distant metastasis. All patients in this study had a high risk of distant metastasis, which may explain why the CCD did not have significant prognostic value.
The purpose of concurrent chemotherapy is to improve prognosis with minimal and acceptable toxicities. Therefore, it is important to establish the lowest concurrent cisplatin dose that achieves a survival benefit to avoid moderate or severe toxicities. Although we could not identify an optimal cutoff value, 200 mg/m2 cisplatin may be adequate to achieve satisfactory survival outcomes in locoregionally advanced NPC because no significant differences were observed between CCD of <240 mg/m2 (i.e., ≤200 mg/m2) and ≥240 mg/m2.
As with all retrospective studies, there are limitations to this study. The cohort was relatively small, and only a small number of potential prognostic factors were assessed. Future prospective studies of larger cohorts, including additional prognostic factors such as plasma Epstein-Barr virus DNA load [28–30], are warranted to confirm these results and define the most effective concurrent cisplatin chemotherapy regimen.
Conclusion
A cumulative cisplatin dose of 240 mg/m2 was not an independent prognostic factor in patients with locoregionally advanced NPC at high risk of distant metastasis, and 200 mg/m2 cisplatin may be adequate to achieve satisfactory survival outcomes in patients undergoing CCRT alone. Further prospective studies are warranted to confirm the results of the present study.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
This work was supported by Sun Yat-sen University Clinical Research 5010 Program Grant 2012011; Science and Technology Project of Guangzhou City, People’s Republic of China, Grant 14570006; and Planned Science and Technology Project of Guangdong Province, People’s Republic of China, Grant 2013B020400004.
Author Contributions
Conception/Design: Hao Peng, Jun Ma
Provision of study material or patients: Wen-Fei Li, Yan-Ping Mao, Jun Ma
Collection and/or assembly of data: Hao Peng, Lei Chen, Yuan Zhang, Wen-Fei Li, Fan Zhang, Li-Zhi Liu, Jun Ma
Data analysis and interpretation: Hao Peng, Lei Chen, Yuan Zhang, Fan Zhang, Rui Guo, Ai-Hua Lin, Jun Ma
Manuscript writing: Hao Peng, Wen-Fei Li, Rui Guo, Jun Ma
Final approval of manuscript: Ying Sun, Jun Ma
Disclosures
The authors indicated no financial relationships.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yi JL, Gao L, Huang XD, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65:161–168. doi: 10.1016/j.ijrobp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Greene FL. The American Joint Committee on Cancer: Updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 4.Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73:1326–1334. doi: 10.1016/j.ijrobp.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 6.Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536–539. doi: 10.1093/jnci/dji084. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966–6975. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 8.Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–637. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 9.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 11.Loong HH, Ma BB, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol. 2012;104:300–304. doi: 10.1016/j.radonc.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Wei W, Huang Z, Li S, et al. Pretreatment Epstein-Barr virus DNA load and cumulative cisplatin dose intensity affect long-term outcome of nasopharyngeal carcinoma treated with concurrent chemotherapy: Experience of an institute in an endemic area. Oncol Res Treat. 2014;37:88–95. doi: 10.1159/000360178. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK. Concurrent radiation chemotherapy for locally advanced head and neck carcinoma: Are we addressing burning subjects? J Clin Oncol. 2004;22:4657–4659. doi: 10.1200/JCO.2004.07.962. [DOI] [PubMed] [Google Scholar]
- 14.Ou X, Zhou X, Shi Q, et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: New insight into the value of total dose of cisplatin and radiation boost. Oncotarget. 2015;6:38381–38397. doi: 10.18632/oncotarget.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: Progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038–2044. doi: 10.1200/JCO.2002.08.149. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys. 2008;71:1356–1364. doi: 10.1016/j.ijrobp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684–3690. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: Combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer. 2011;47:656–666. doi: 10.1016/j.ejca.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Strojan P, Vermorken JB, Beitler JJ, et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: A systematic review. Head Neck. 2016;38(suppl 1):E2151–E2158. doi: 10.1002/hed.24026. [DOI] [PubMed] [Google Scholar]
- 22.Fountzilas G, Ciuleanu E, Bobos M, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: A randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. 2012;23:427–435. doi: 10.1093/annonc/mdr116. [DOI] [PubMed] [Google Scholar]
- 23.Hareyama M, Sakata K, Shirato H, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer. 2002;94:2217–2223. doi: 10.1002/cncr.10473. [DOI] [PubMed] [Google Scholar]
- 24.International Nasopharynx Cancer Study Group. VUMCA I Trial Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(> or = N2, M0) undifferentiated nasopharyngeal carcinoma: A positive effect on progression-free survival. Int J Radiat Oncol Biol Phys. 1996;35:463–469. doi: 10.1016/s0360-3016(96)80007-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee AW, Ngan RK, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 2015;121:1328–1338. doi: 10.1002/cncr.29208. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19:1350–1357. doi: 10.1200/JCO.2001.19.5.1350. [DOI] [PubMed] [Google Scholar]
- 27.Peng H, Chen L, Li WF, et al. The cumulative cisplatin dose affects the long-term survival outcomes of patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy. Sci Rep. 2016;6:24332. doi: 10.1038/srep24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AT, Lo YM, Zee B, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94:1614–1619. doi: 10.1093/jnci/94.21.1614. [DOI] [PubMed] [Google Scholar]
- 29.Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461–2470. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- 30.Lin JC, Wang WY, Liang WM, et al. Long-term prognostic effects of plasma Epstein-Barr virus DNA by minor groove binder-probe real-time quantitative PCR on nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1342–1348. doi: 10.1016/j.ijrobp.2007.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.