This was the largest published study assessing cognitive function in older adults with early-stage breast cancer that included a group of patients treated with modern chemotherapy regimens. Approximately one half of the patients had objective cognitive decline after adjuvant treatment. The oldest patients were more likely to have cognitive decline with chemotherapy, particularly with docetaxel.
Keywords: Cognition deficits, Breast cancer, Elderly, Chemotherapy, Neuropsychology
Abstract
Background.
The impact of chemotherapy on cognition among elderly patients has received little attention, although such patients are more prone to presenting with age-related cognitive deficits and/or cognitive decline during chemotherapy. The present study assessed the cognitive function in older adults treated for early-stage breast cancer (EBC).
Patients and Methods.
The participants were newly diagnosed EBC patients aged ≥65 years without previous systemic treatment or neurological or psychiatric disease and matched healthy controls. They underwent two assessments: before starting adjuvant therapy and after the end of chemotherapy (including doxorubicin ± docetaxel [CT+ group], n = 58) or radiotherapy for patients who did not receive chemotherapy (CT− group, n = 61), and at the same interval for the healthy controls (n = 62). Neuropsychological and geriatric assessments were performed. Neuropsychological data were analyzed using the Reliable Change Index.
Results.
Forty-nine percent of the patients (mean age, 70 ± 4 years) had objective cognitive decline after adjuvant treatment that mainly concerned working memory. Among these patients, 64% developed a cognitive impairment after adjuvant treatment. Comorbidity was not associated with cognitive decline. No significant difference in objective cognitive decline was found between the two groups of patients; however, the CT+ group had more subjective cognitive complaints after treatment (p = .008). The oldest patients (aged 70–81 years) tended to have more objective decline with docetaxel (p = .05).
Conclusion.
This is the largest published study assessing cognitive function in older adults with EBC that included a group of patients treated with modern chemotherapy regimens. Approximately half the patients had objective cognitive decline after adjuvant treatment. The oldest patients were more likely to have cognitive decline with chemotherapy, particularly with docetaxel.
Implications for Practice:
This is the largest published study assessing cognitive function in older adults with early-stage breast cancer that included a group of patients treated with modern chemotherapy regimens. Approximately half the patients had objective cognitive decline after adjuvant treatment. The oldest patients were more likely to have cognitive decline with chemotherapy, particularly with docetaxel. Cognitive deficits could affect patients’ quality of life and their compliance to treatment. Assessing cognitive dysfunctions in the elderly cancer population is a challenge in clinical practice, but it could influence the choice of the most appropriate therapy, including the use of oral drugs.
Introduction
The impact of adjuvant chemotherapy on cognition has mainly been studied in middle-age women (age, <60 years) treated with chemotherapy for breast cancer. The findings from longitudinal studies suggest that 15%–25% of patients experience post-treatment cognitive decline [1]. Furthermore, 20%–30% of breast cancer patients will have cognitive impairment before starting adjuvant treatment [2], suggesting a global effect of cancer on cognition.
Although the cancer incidence is greater in older adults, clinical trials usually concern younger participants. Several studies have demonstrated that elderly patients are at greater risk of treatment toxicity [3], although cognitive functioning was poorly assessed.
Age is a risk factor for cognitive decline, and older adults are thought to be more vulnerable to the adverse cognitive effects of cancer and its treatments [4–7]. Indeed, 11%–41% of elderly patients had cognitive dysfunctions before adjuvant breast cancer treatment [8, 9]. Chemotherapy could be a risk factor for inducing cognitive decline or increasing dysfunction. Furthermore, treatment-induced accelerated cognitive aging can be expected in vulnerable populations [2]. To date, only one prospective study with a small sample has assessed cognition after adjuvant chemotherapy, particularly in elderly breast cancer patients [8].
The effect of modern chemotherapy regimens on cognition, including anthracyclines and taxanes, has not been specifically assessed in elderly patients. Nevertheless, these drugs induce cognitive impairment in young breast cancer patients [10–12], and preclinical studies have shown that docetaxel can induce cognitive impairment [13, 14]. The present multicenter prospective study assessed the impact of adjuvant treatment on cognition among older adults with early-stage breast cancer (EBC) compared with healthy controls.
Patients and Methods
Participants
Newly diagnosed older women with EBC were recruited from three French comprehensive cancer centers. The recruitment details have been published previously [9].
A sample of healthy controls who met the same inclusion (except for the cancer diagnosis) and exclusion criteria were recruited by community advertisements. The healthy controls were age-, sex-, and education-matched to patients.
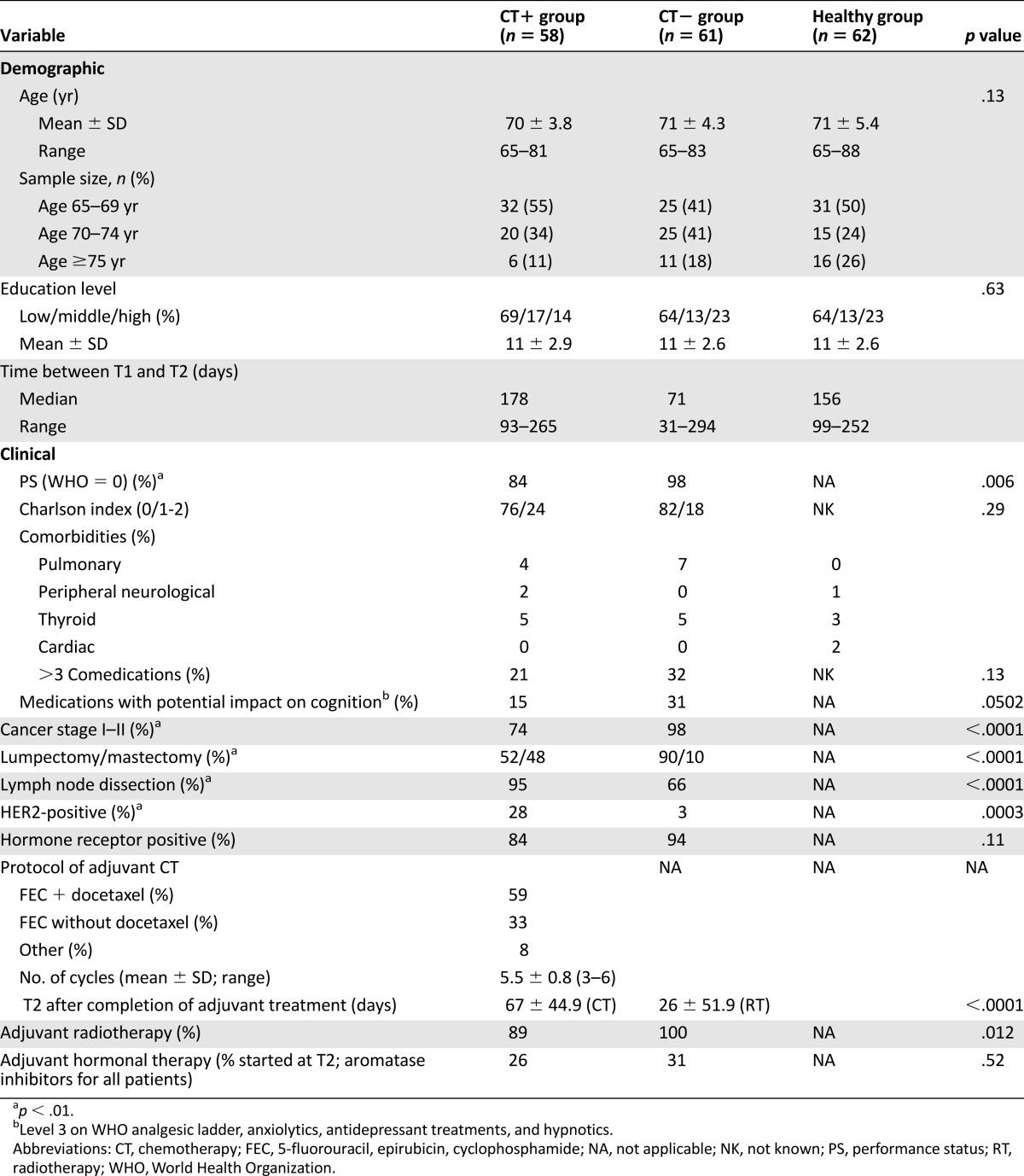
The pretreatment assessment (T1) occurred after surgery but before the start of adjuvant therapy. The follow-up assessment (T2) was conducted in patients after the end of the first adjuvant treatment: adjuvant chemotherapy (CT+ group; within a mean of 67 ± 44.9 days after chemotherapy), and after radiotherapy in patients who did not receive chemotherapy (CT− group; within a mean of 26 ± 51.9 days after radiotherapy). The median time between T1 and T2 was 178 days (range, 93–265) for the CT+ group and 71 days (range, 31–294) for the CT− group. The interval for healthy controls was approximately the mean of that of the two patient groups (Table 1). All participants provided written informed consent for the study, which was approved by the local ethics committee and is registered at ClinicalTrials.gov (ClinicalTrials.gov identifier, NCT01333735).
Table 1.
Demographic and clinical characteristics of patients and healthy controls
Measures
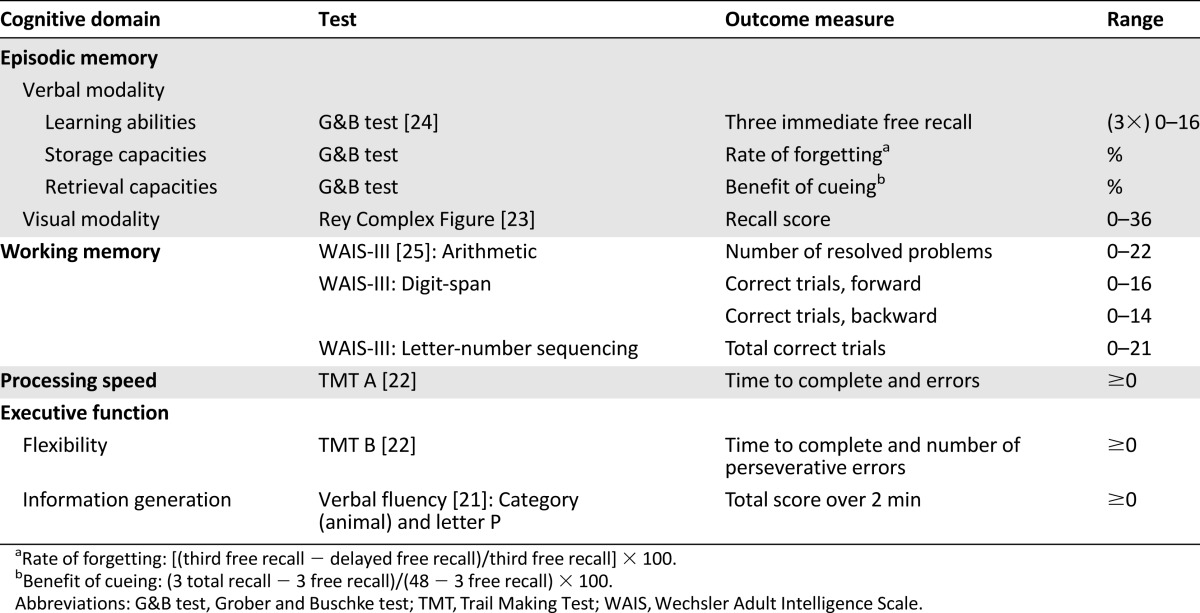
Neuropsychological testing included the domains most likely to be affected by cancer treatment [15–20], including episodic memory (verbal and visual modalities), working memory, processing speed, and executive functions (Table 2) [21–25]. We used a comprehensive battery of assessments with standard and recommended instruments.
Table 2.
Neuropsychological tests grouped by main cognitive domains
The subjective assessment consisted of self-report measures of cognitive complaints (Functional Assessment of Cancer Therapy, Cognitive Scale [FACT-Cog], version 3 [26, 27], with four subscales [Perceived Cognitive Impairments [PCI], Impact on Quality of Life [QoL], Comments from Others, and Perceived Cognitive Abilities [PCA]), depression (Beck Depression Inventory [BDI] [28]), anxiety (Spielberger State-Trait Anxiety Inventory [STAI] [29]), and fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-Fatigue], version 4 [30]). The geriatric assessment included the Geriatric Depression Scale (GDS) [31], the Instrumental Activities of Daily Living (IADL) [32], the Activities of Daily Living (ADL) [33], the Charlson comorbidity index [34], the number of medications, and the main medical history.
The sociodemographic measures included age and education level. The clinical variables were the performance status (PS), medications with a potential impact on cognition (level 3 on the World Health Organization analgesic ladder, anxiolytics, antidepressant treatments, and hypnotics), cancer stage, type of surgery, HER2-positive, hormone receptor status, adjuvant treatments, and weight.
Blood samples were obtained for measurement of hematologic parameters, thyroid function (thyroid-stimulating hormone [TSH]), C-reactive protein (CRP), folates, and albumin to assess for anemia, inflammation, and level of nutrition. The relationships between these variables and cognition were studied. The healthy controls completed the battery of neuropsychological tests, FACT-Cog, BDI, STAI, and FACIT-Fatigue.
Procedure
Details about the study procedure have been published previously [9].
Statistical Analysis
Descriptive statistics were generated for the sociodemographic and clinical variables. Data from the two neuropsychological assessments were analyzed using a practice effect-adjusted Reliable Change Index (RCI) [35, 36]. The RCI was used to determine the standardized change scores for each patient on every neuropsychological score to compare the T2 and T1 scores. The calculation included the practice effect (i.e., the change from T1 to T2 on the same measure in the healthy group). The Iverson formula includes an adapted standard error of the difference that incorporates T2’s variability. A significant change was found at ±1.645 [35] (−1.645 indicated decline; and +1.645 indicated improvement). The RCI scores were grouped together in the cognitive domains. A change in a domain was considered significant when at least one of the domain scores changed significantly (decline or improvement). For the self-report questionnaires, a difference of more than 10% between T1 and T2 was considered clinically significant [37]. Pairwise comparisons by group between the two assessments were performed for the other measures.
We used the t test, Wilcoxon test, analysis of variance, or Fisher’s exact test to compare the means and proportions, as appropriate, and the paired t test to compare changes over time within each group. Subgroups were compared according to the treatment received and age (≥75 or ≥70 years vs. younger) regarding the cognitive and biological scores. Differences in the CT+ group in the cognitive domains were examined within subgroups according to age, docetaxel administration, and 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) dose intensity.
We estimated the mean scores and their differences between groups (CT+ group, CT− group, healthy controls) over time for all neuropsychological data, cognitive complaints, and anxiety and depression scores by a repeated measures linear mixed model with an unrestricted covariance structure, including age at baseline, delay between T1 and T2, groups, assessment time, and groups × assessment time interaction. Given the large number of analyses performed with this model, p < .01 was applied to minimize type I error.
Correlations between the cognitive complaints and other measures were assessed with Spearman's rank or Pearson’s correlation coefficient, as appropriate. Cognitive domains were considered as categorical variables (decline: yes vs. no) and associated with other measures. Given the large number of correlations, we considered p < .01 to minimize type I error. Analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, NC, http://www.sas.com).
Results
Sample Characteristics
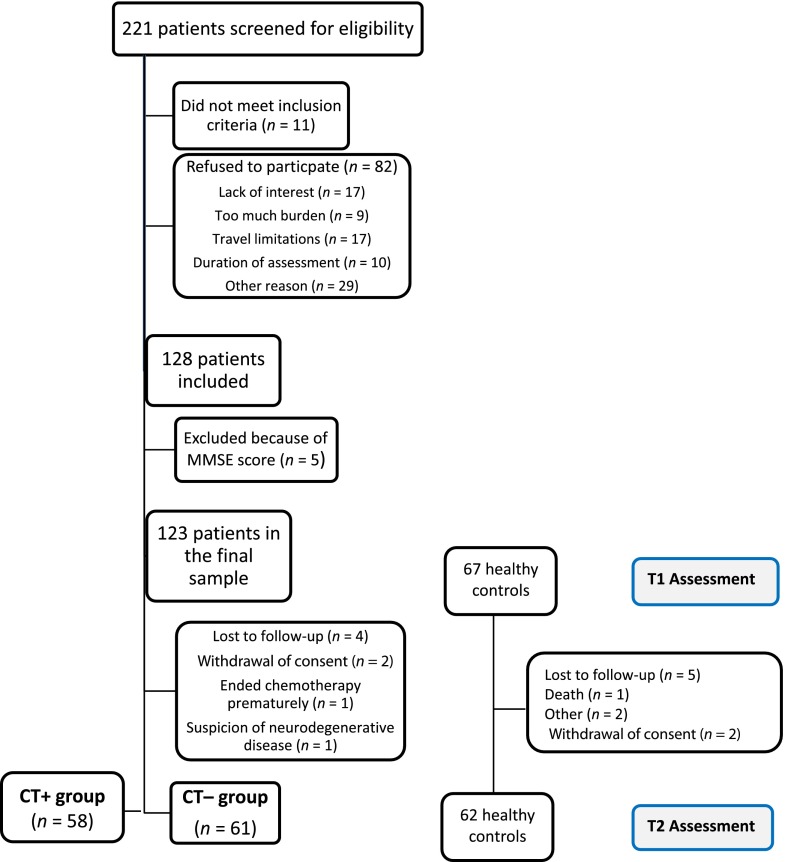
At the baseline assessment, of 221 older women with EBC screened, 11 were ineligible, and 82 were not enrolled in the trial for the following reasons: lack of interest (n = 17), too much burden (n = 9), travel limitations (n = 17), duration of the assessment (n = 10), or other reasons (n = 29). This yielded a 61% participation rate. Moreover, five patients were excluded from the analysis because of a score below the threshold of dementia [38]. Hence, the final sample consisted of 123 patients (stage I, 60%; stage II, 27%; stage III, 13%). However, 4 patients completed only the T1 (Fig. 1). Therefore, the final sample for the T1-T2 analysis was 58 CT+ patients, 61 CT− patients, and 62 healthy controls. Among the patients, 57 were <70 years (48%), 45 were 70–74 years (38%), and 17 were ≥75 years (14%). The participants’ demographic and medical information is summarized in Table 1. The breast cancer stage, surgery type, lymph node dissection, and HER2 status were significantly different between the patient groups (p < .01). No significant difference was found for the Charlson index, main medical history, comedications, medications with a potential impact on cognition, hormone receptor status, or initiation of hormonal therapy. A significant difference was found in the performance status between the patient groups (CT− group had better PS; p < .01).
Figure 1.
Flow diagram of participant follow-up.
Abbreviation: MMSE, mini-mental state examination.
In the CT+ group, 91% of patients (n = 53) received an anthracycline-based regimen. For 59% (n = 34) of these patients, the regimen included docetaxel. Also, 21% of patients received FEC 75 (n = 12) and 67% FEC 100 (n = 39). No significant difference in age and education was observed among the three groups at baseline.
Baseline (T1)
According to the baseline results, 41% of patients had objective cognitive impairment (46% in the CT+ group [26 of 57]; 38% in the CT− group [23 of 61]). No significant difference between the patient groups was observed at baseline regarding the objective cognitive scores, subjective cognitive complaints, anxiety, depression, fatigue, or geriatric and biological measures. Only digit span forward was better in the CT− group (p < .01), and the PCA (FACT-Cog) was higher in the CT+ group (p = .018).
Compared with the EBC patients, the healthy group was less anxious than the CT+ group (p < .001). The healthy group also had more subjective cognitive complaints than the CT+ group (PCI and PCA subscales; p = .01), but this had less impact on quality of life (FACT-Cog, QoL subscale; p = .02).
Neuropsychological Outcomes
RCI Analysis (Objective Cognitive Results)
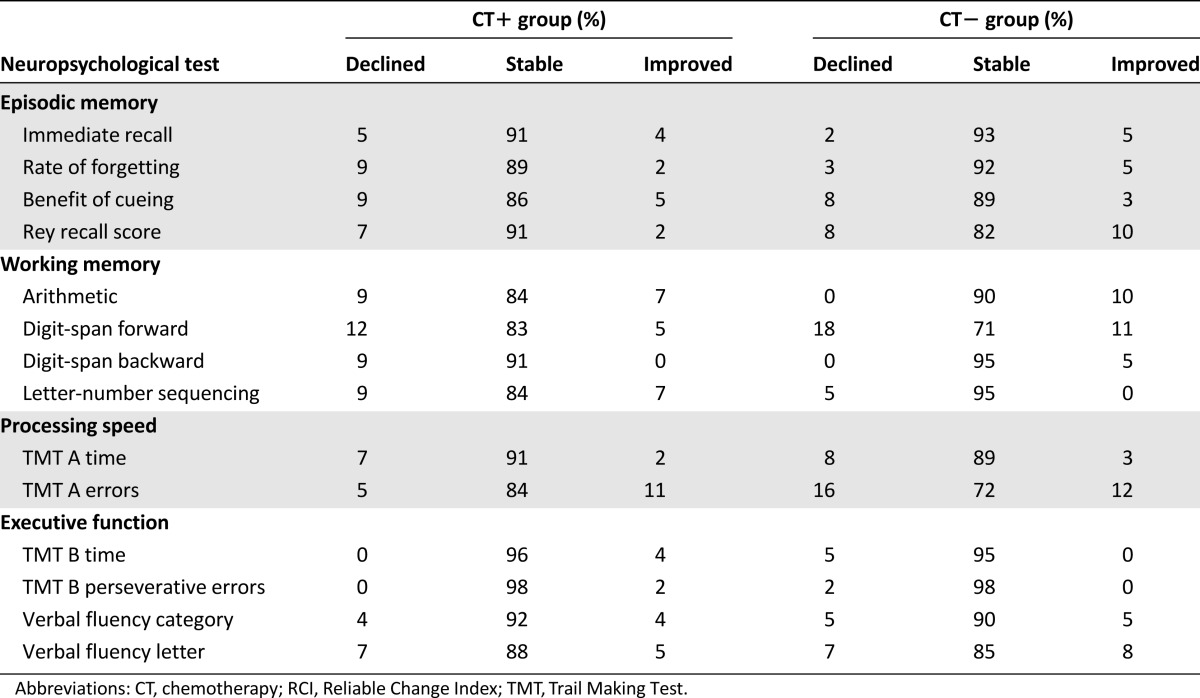
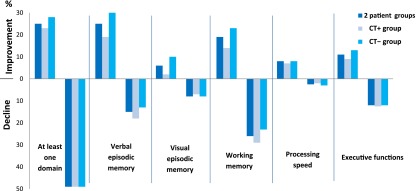
Tables 3 and 4 and Figure 2 show the change in neuropsychological scores and objective cognitive domains between T1 and T2 compared with healthy women. Forty-nine percent of patients had an objective decline and 25%, objective improvement in at least one domain. The domain with the greatest objective decline was working memory (25% of patients).
Table 3.
Change in neuropsychological test scores (RCI scores) between baseline (T1) and after adjuvant treatment (T2) compared with healthy women
Table 4.
Change in neuropsychological test scores (RCI scores) between baseline (T1) and after adjuvant treatment (T2) compared with healthy women by patient subgroup
Figure 2.
Frequency of patients with improvement or decline in cognitive domains (Reliable Change Index based on healthy control scores). Forty-nine percent of patients had a decline in at least one domain. The domain with the greatest decline was working memory (25% of patients). No significant difference was observed between the two patient groups.
Overall, no significant difference was observed between the two patient groups in objective change in at least one domain or any cognitive domain. Among the patients who had objective cognitive decline (49%; n = 58 of 118), 12% (n = 7 of 58) already had objective cognitive impairment before adjuvant treatment and 64% (n = 37 of 58) developed objective cognitive impairment after adjuvant treatment (according to the T1 data based on normative data [9]). The other 24% of patients (n = 14 of 58) had cognitive decline without impairment (scores greater than the threshold of impairment based on normative data [9]). No difference was observed between the groups for episodic memory processes (learning, retrieval, storage; Table 3).
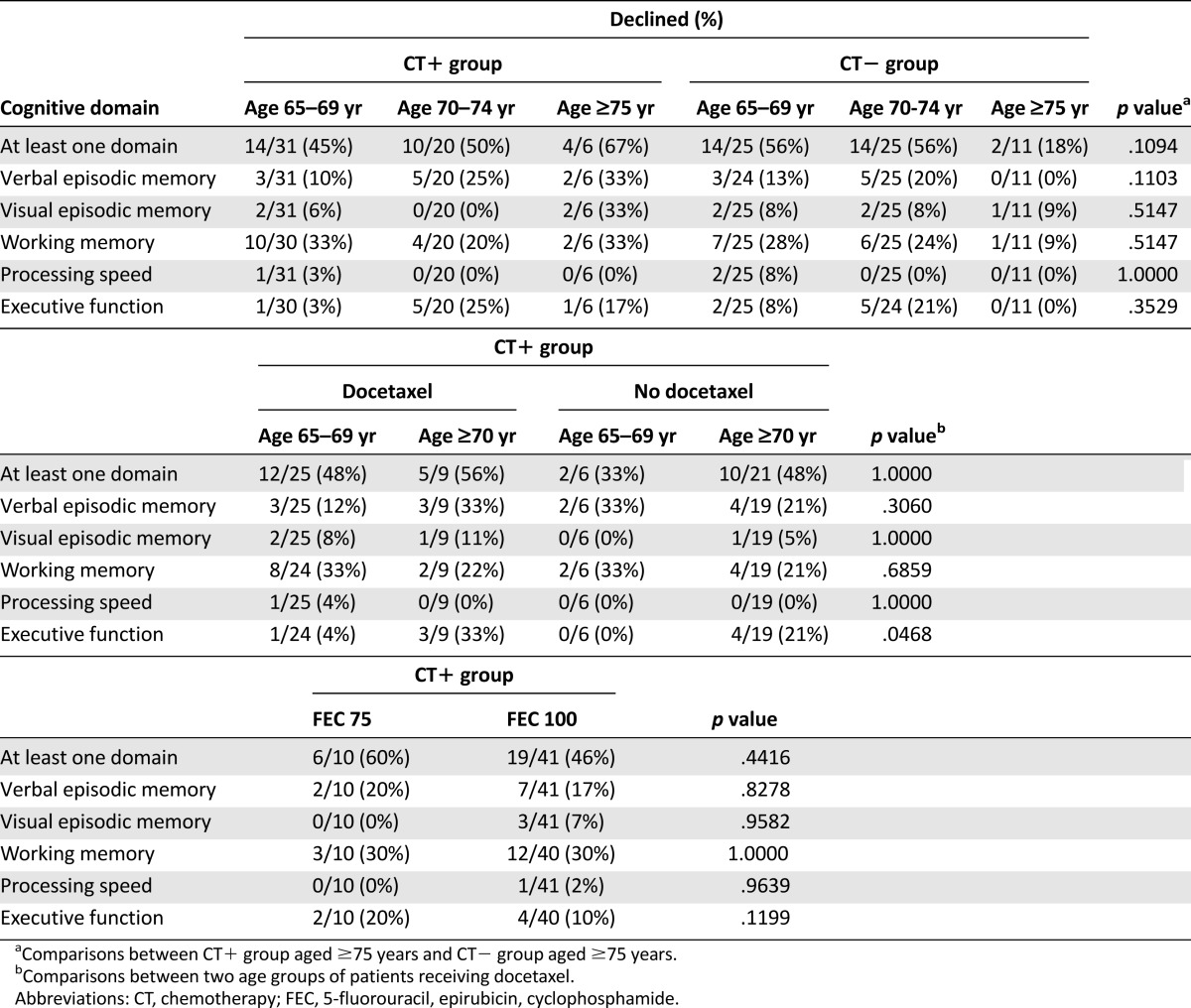
However, the oldest patients were more likely to have objective cognitive decline, especially those in the CT+ group (Table 4). The oldest patients (≥75 years) treated with CT tended to have more objective decline in at least one domain compared with those in the CT− group (67% in the CT+ group [n = 4 of 6] vs. 18% in the CT− group [n = 2 of 11]; p = .10).
Among the patients receiving docetaxel, those aged ≥70 years were more likely than those aged <70 years to experience objective decline in executive functions (p = .047, Table 4). No chemotherapy dose-dependent effect (FEC 75 vs. FEC 100) was observed for objective cognitive decline. No relationship was found between the duration of chemotherapy and objective cognitive decline.
Changes in Subjective and Biological Scores
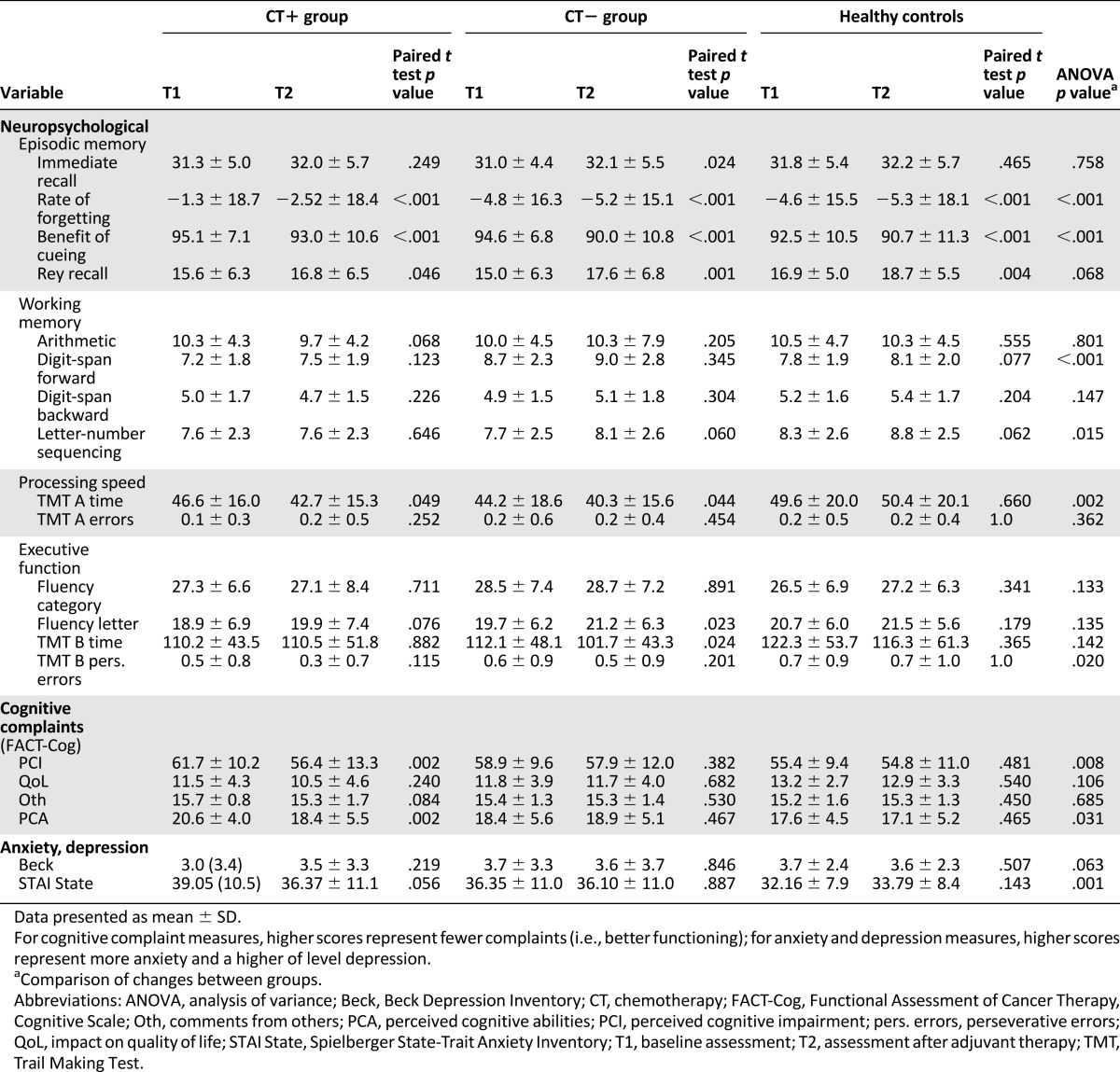
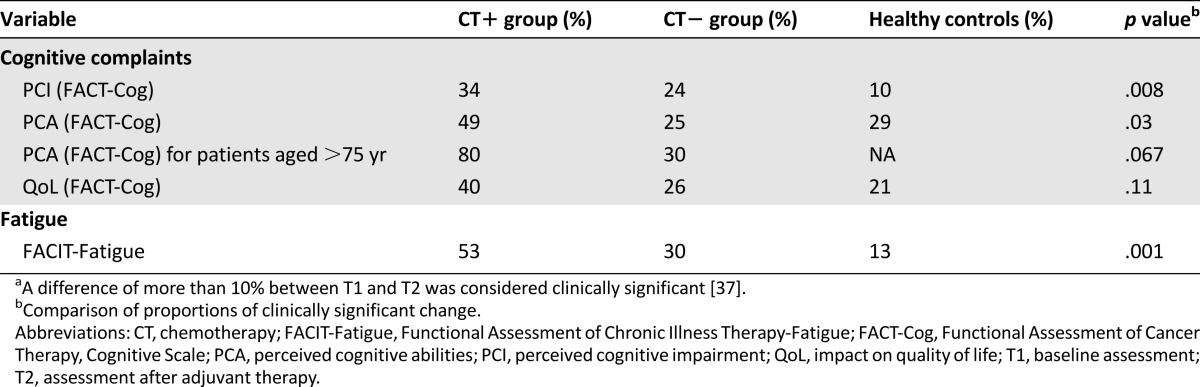
Table 5 shows the subjective assessment scores and Table 6 the clinically significant change in subjective scores. The CT+ group had a significantly greater increase in subjective cognitive complaints after treatment than the CT− and healthy groups (PCI subscale; p = .008; Table 6). A clinically significant subjective decline in the PCA subscale score was observed mainly in the CT+ group (p = .03) and mainly concerned CT+ patients older than 75 years compared with CT− patients (p = .067).
Table 5.
Neuropsychological, cognitive complaints, quality of life, anxiety, and depression scores
Table 6.
Clinically significanta change in subjective scores between T1 and T2
The CT+ group also expressed significantly more fatigue after treatment (p < .001). However, the CT+ group experienced significantly less anxiety (p = .001) after treatment compared with the other groups. The intragroup depression score did not change significantly between the assessments.
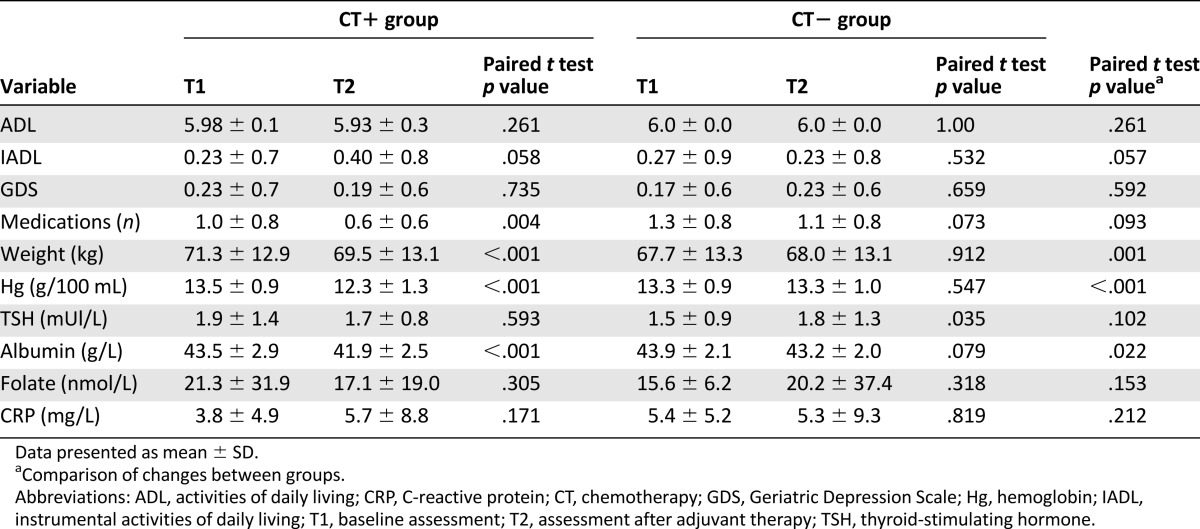
Treatment did not significantly affect the geriatric scores (ADL, IADL, GDS, number of medications), or folate, CRP, or TSH levels. However, the CT+ group decreased more in weight, hemoglobin, and albumin level after treatment than the CT− group (p = .001, p < .001, and p = .022, respectively; Table 7). No effect of the CT regimen or dose intensity was found on subjective cognitive complaints or fatigue.
Table 7.
Geriatric and biological scores
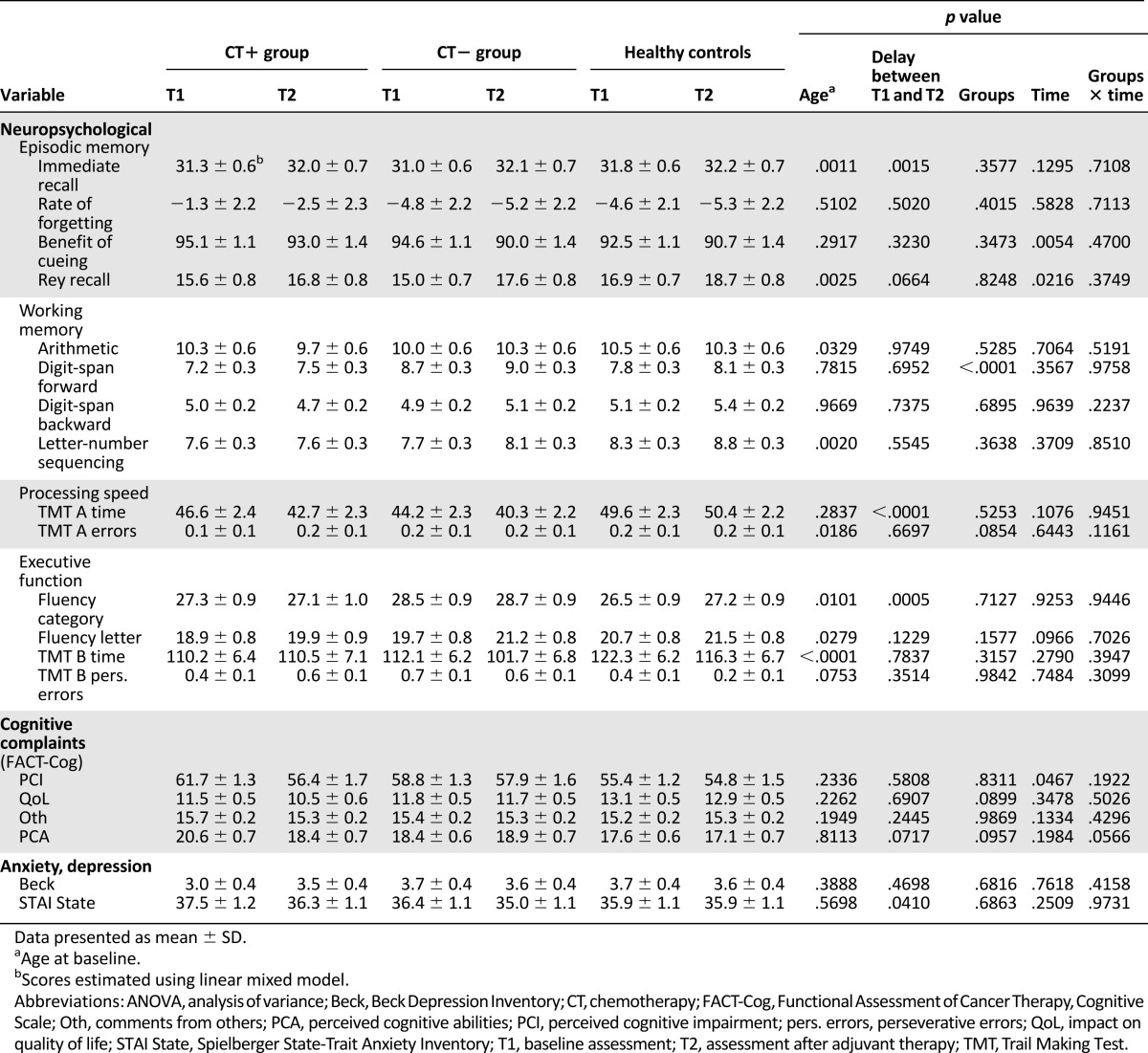
Mixed Model Analysis
Table 8 shows the results of the mixed model analysis. According to the groups × time analysis, no significant effect was found for any of the objective and subjective cognitive scores. Age had a significant effect on 4 of 14 objective cognitive scores and a delay between T1 and T2 had a significant effect on 3 of 14 objective cognitive scores (less delay resulted in a better score).
Table 8.
Estimated neuropsychological, cognitive complaints, quality of life, anxiety, and depression scores by group (linear mixed model)
Relationships Between Objective Cognitive Decline and Subjective and Biological Scores
Objective Cognitive Decline and Other Measures
Overall, no relationship was found between objective decline in any cognitive domain and subjective cognitive complaints. However, an objective decline in at least one domain was related to the QoL subscale (FACT-Cog; p = .006). Objective cognitive decline was not associated with education level, fatigue, anxiety, depression, medications with a potential effect on cognition, type of surgery, Charlson index, comedications, performance status, cancer stage, weight, initiation of aromatase inhibitors, or geriatric or biological scores. Medications potentially affecting cognition were related only to an objective decline in visual episodic memory (p = .0314). Among the objective cognitive domains, age was related to executive function decline (p = .0493), with patients aged >70 years having greater decline.
Subjective Cognitive Complaints and Other Cognitive Measures
Patients with more subjective cognitive complaints at T1 (PCI subscale) were those with more objective cognitive decline in verbal episodic memory (odds ratio, 0.956; 95% confidence interval, 0.920–0.994; p = .029). Also, patients with more subjective cognitive complaints at T1 were those who increased more in subjective cognitive complaints at T2 (PCI subscale; p = .013). Nevertheless, this increase in cognitive complaints was not clinically significant using the definition of less than 10%.
Discussion
The present study is the largest published prospective study assessing cognitive function in older adults with EBC that included a group treated with modern chemotherapy regimens (including anthracycline ± docetaxel) and a group of healthy controls. Regardless of the adjuvant treatment, approximately half the patients had objective cognitive decline after adjuvant treatment. However, the oldest patients treated with chemotherapy were more likely to experience objective cognitive decline than those treated without chemotherapy, especially when the regimen included docetaxel.
All Patients
Before adjuvant treatment, 41% of patients had objective cognitive impairment. The percentage of patients exhibiting pretreatment impairment was higher than that reported in studies of younger breast cancer patients (20%–30%) [2]. This finding supports the hypothesis that elderly patients might be more sensitive to the impact of cancer on cognition [1].
Forty-nine percent of patients had objective cognitive decline after adjuvant treatment that mainly concerned working memory. Our results are higher than those observed in a previous study using the same method of RCI analysis [39]. In that study, cognitive decline after adjuvant treatment was observed in 20% of the chemotherapy group and 26% of the group without chemotherapy. However, they did not include elderly patients (mean age, 51 and 59 years). Therefore, our results suggest that age might affect post-treatment cognitive functioning. Overall, regardless of the statistical method used, longitudinal studies of middle-age breast cancer women (age <60 years) have suggested that 15%–25% of patients will experience post-treatment cognitive decline [1]. Therefore, age seems to be a risk factor for cognitive decline, and older adults could be more vulnerable to the adverse cognitive effects of cancer and its treatments.
The domain mostly affected in our study was working memory, similar to the reports of younger breast cancer patients [12, 40, 41]. Daily, this alteration induces difficulties in the temporary maintenance of memory and manipulation of information during short periods and when performing activities.
In our study, we did not find an association between cognitive impairment and breast cancer stage and comorbidity, in contrast to the results of another study of elderly patients [42]. One explanation might be that our population had few comorbidities. Treatment-related objective cognitive decline was not associated with other measures (clinical, mood, or biological), and aromatase inhibitors do not seem to affect cognition [43]. Nevertheless, this lack of association between these factors/variables and objective cognitive decline might have resulted from the limited sample size. Only age had an effect on executive function.
Overall, although cognitive complaints were not related to the objective scores, just as was frequently the case in other studies [44], a decline in at least one cognitive domain was related to the cognitive complaint subscale: impact [of cognitive disorders] on quality of life. We also found that cognitive complaints could be predictive of cognitive decline. Cognitive complaints in subjects with normal neuropsychological scores could be a harbinger of further decline [45]. This phenomenon has been studied, in particular, in subjects who developed mild cognitive impairment or Alzheimer disease [46]. Therefore, it is important to assess cognitive complaints, including the impact on quality of life. This could make it possible to detect patients at risk of decline and to anticipate cognitive alterations by proposing adapted interventions such as cognitive training [47].
Comparison Between CT+ and CT− Groups
Overall, no significant difference was found in objective cognitive decline between our patient groups receiving or not receiving chemotherapy (RCI and mixed model analysis results). These results are consistent with others [48] of younger breast cancer patients, which showed that cognitive deficits were likely due to the general effects of the cancer diagnosis and treatments rather than to systemic treatment. Other studies have failed to confirm previous reports suggesting that adjuvant chemotherapy is associated with cognitive dysfunction among younger breast cancer patients [39, 49–52]. In our study, we probably overestimated the effect of radiotherapy on cognition by making the cognitive assessment of this group just after the end of radiotherapy; thus, the subjects probably had had insufficient time to recover from the fatigue induced by treatment. Although most young patients will recover within 1 year after chemotherapy [15, 53], this might not be the case for elderly patients, with chemotherapy inducing some direct damage to the brain [54]. Therefore, long-term follow-up of patients is important to demonstrate the real effect of the adjuvant treatment modalities administered and how they affect the recovery of cognitive functioning in elderly patients.
However, when we focused on the CT+ group, who were mainly treated with anthracycline ± docetaxel, 49% of the patients had an objective cognitive decline. In published cognitive studies of the elderly, very few patients received a CT regimen that included docetaxel. In a previous pilot study exploring postchemotherapy (mainly CMF [cyclophosphamide, methotrexate, and 5-fluorouracil]), cognitive functioning in elderly EBC patients (n = 28), Hurria et al. showed that 25% of patients experienced cognitive decline [8]. In other studies of elderly patients using the RCI, 52% of colon cancer patients and 33% of cancer patients (mainly breast and colon cancer, principally treated with FU-FA [5-fluorouracil, folinic acid] and CMF) experienced cognitive declines after chemotherapy [55, 56]. These rates are comparable to those for our patients, but those studies did not include a group of healthy controls to accurately assess the decline.
In terms of age group, the CT+ group aged ≥75 years tended to have a greater incidence of objective decline in at least one domain than did patients of the same age group treated without chemotherapy (67% vs. 18%). Nevertheless, this result needs to be confirmed owing to the low number of patients aged ≥75 years.
Furthermore, the oldest patients were more likely to experience objective cognitive decline when the regimen included docetaxel. The deleterious effect of docetaxel on cognition has been previously shown in preclinical studies [13, 14]; however, the effect of age was not assessed. No dose-dependent effect of FEC on cognition was found, but only our younger patients received the highest dose.
Cognitive complaints were more frequent after treatment for patients treated with chemotherapy than for those treated without it, particularly among the oldest patients. Although the CT+ group had fewer cognitive complaints before adjuvant treatment, they had a greater increase in complaints after it. Previous studies have not been conclusive on this issue. In one series of breast cancer patients aged ≥65 years, cognitive complaints after chemotherapy were present only in 10% of patients and no significant change occurred from baseline [57]. However, in another sample of breast cancer patients aged ≥65 years who had received adjuvant chemotherapy, 51% reported a decline in subjective cognitive function, which was most pronounced in patients who had reported pre-existing memory problems [58]. We also found that patients with more cognitive complaints before treatment were those with a greater increase in cognitive complaints after it.
Strengths and Limitations of the Study
The strengths of the present study are that it included the largest published sample of elderly EBC patients to date, including a group treated with modern chemotherapy regimens that included anthracyclines and docetaxel and a group of healthy controls. Our study used objective and subjective cognitive measures, a comprehensive battery of assessments using standard and recommended instruments, and geriatric and biological assessments.
Although they are not comparable, two complementary methods of statistical analysis were used: the RCI and a mixed model analysis. In addition, the RCI accounts for the results of each patient individually and for the test-retest effect. Using a subgroup analysis in which the treatment received and age were considered, the RCI showed different and complementary results to those of the mixed model.
Overall, the present study failed to confirm previous results that showed significant differences in objective cognitive functioning between patients who had received chemotherapy and those who had not. This result could have partly resulted from overestimation of the effect of radiotherapy on cognition by performing the cognitive assessment just after the end of radiotherapy in this group, such that the subjects probably had insufficient time to recover from the fatigue induced by the treatment. The post-treatment assessment differed between the patient groups. For practical reasons and so that the patients did not have to return to the hospital just for the study, the cognitive assessment was conducted with a follow-up consultation or medical examination. However, the delay between the two assessments (controlled in the mixed model) had no major effect on cognition (learning effect only for 3 of 14 objective cognitive scores).
The negative effect of CT on cognition among the group aged more than 75 years requires confirmation by a larger study that includes elderly patients.
Conclusion
The present study is the largest published prospective study assessing cognitive function in older adults with EBC that included a group of patients treated with modern chemotherapy regimens and a group of healthy controls. Regardless of the adjuvant treatment, approximately half the patients experienced objective cognitive decline after such treatment. The oldest patients were more likely to experience an objective cognitive decline with chemotherapy, in particular, when the regimen included docetaxel. Additional research is needed to understand, anticipate, and manage the short- and long-term effects of cancer therapy on cognitive function of elderly patients, especially those receiving chemotherapy.
Acknowledgments
We thank all the subjects for their participation, the clinicians in the breast cancer department, the Cancéropôle Nord-Ouest, the Centre de Traitement des Données du Cancéropôle Nord-Ouest, Lucie Laroche, Alexandra Leconte, Sophie Aumont, Marion Delarue, Maxime Feutry, and Ray Cook for copyediting. This work was supported by a national grant (Programme Hospitalier de Recherche Clinique, Grant APN 2008 n°06-08) and Sanofi.
Author Contributions
Conception and design: Sabine Noal, Francis Eustache, Bénédicte Giffard, Florence Joly
Provision of study material or patients: Olivier Rigal, Sabine Noal, Jean-Emmanuel Kurtz, Christelle Lévy, Djelila Allouache, Corinne Veyret, Philippe Barthélémy
Collection and/or assembly of data: Marie Lange, Natacha Heutte, Chantal Rieux, Johan Lefel, Nadine Longato, Hélène Castel
Data analysis and interpretation: Marie Lange, Natacha Heutte, Francis Eustache, Bénédicte Giffard, Florence Joly
Manuscript writing: Marie Lange, Natacha Heutte, Olivier Rigal, Sabine Noal, Jean-Emmanuel Kurtz, Christelle Lévy, Djelila Allouache, Chantal Rieux, Johan Lefel, Bénédicte Clarisse, Corinne Veyret, Philippe Barthélémy, Nadine Longato, Hélène Castel, Francis Eustache, Bénédicte Giffard, Florence Joly
Final approval of manuscript: Marie Lange, Natacha Heutte, Olivier Rigal, Sabine Noal, Jean-Emmanuel Kurtz, Christelle Lévy, Djelila Allouache, Chantal Rieux, Johan Lefel, Bénédicte Clarisse, Corinne Veyret, Philippe Barthélémy, Nadine Longato, Hélène Castel, Francis Eustache, Bénédicte Giffard, Florence Joly
Disclosures
The authors indicated no financial relationships.
References
- 1.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012;21:1141–1148. doi: 10.1002/pon.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lange M, Rigal O, Clarisse B, et al. Cognitive dysfunctions in elderly cancer patients: A new challenge for oncologists. Cancer Treat Rev. 2014;40:810–817. doi: 10.1016/j.ctrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Adams-Price CE, Morse LW, Cross GW, et al. The effects of chemotherapy on useful field of view (UFOV) in younger and older breast cancer patients. Exp Aging Res. 2009;35:220–234. doi: 10.1080/03610730902720497. [DOI] [PubMed] [Google Scholar]
- 6.Eberhardt B, Dilger S, Musial F, et al. Short-term monitoring of cognitive functions before and during the first course of treatment. J Cancer Res Clin Oncol. 2006;132:234–240. doi: 10.1007/s00432-005-0070-8. [DOI] [PubMed] [Google Scholar]
- 7.Mandelblatt JS, Hurria A, McDonald BC, et al. Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Semin Oncol. 2013;40:709–725. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54:925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 9.Lange M, Giffard B, Noal S, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer. 2014;50:2181–2189. doi: 10.1016/j.ejca.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Jansen CE, Cooper BA, Dodd MJ, et al. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 11.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 12.Stewart A, Collins B, Mackenzie J, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: A prospective study. Psychooncology. 2008;17:122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 13.Seigers R, Loos M, van Tellingen O, et al. Cognitive impact of cytotoxic agents in mice. Psychopharmacology (Berl) 2015;232:17–37. doi: 10.1007/s00213-014-3636-9. [DOI] [PubMed] [Google Scholar]
- 14.Fardell JE, Zhang J, De Souza R, et al. The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology (Berl) 2014;231:841–852. doi: 10.1007/s00213-013-3301-8. [DOI] [PubMed] [Google Scholar]
- 15.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson KD, Hutchinson AD, Wilson CJ, et al. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39:297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30:3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koppelmans V, Breteler MM, Boogerd W, et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 21.Cardebat D, Doyon B, Puel M, et al. [Formal and semantic lexical evocation in normal subjects: Performance and dynamics of production as a function of sex, age and educational level] Acta Neurol Belg. 1990;90:207–217. [PubMed] [Google Scholar]
- 22.Reitan R. Validity of trail making tests as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 23.Rey A. [Manual of Copy and Memory Reproduction Test of Complex Geometric Figures] Paris: Éditions Centre de Psychologie Appliquée; 1959. [Google Scholar]
- 24.Van der Linden M, Adam S, Agniel A, et al. [Assessment of Memory Impairment] Marseille: Solal; 2004. [Google Scholar]
- 25.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 26.Joly F, Lange M, Rigal O, et al. French version of the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 3. Support Care Cancer. 2012;20:3297–3305. doi: 10.1007/s00520-012-1439-2. [DOI] [PubMed] [Google Scholar]
- 27.Wagner LI, Sweet J, Butt Z, et al. Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function Instrument. J Support Oncol. 2009;7:W32–W39. [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger SD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 30.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 31.Clément JP, Nassif RF, Léger JM, et al. [Development and contribution to the validation of a brief French version of the Yesavage Geriatric Depression Scale] Encephale. 1997;23:91–99. [PubMed] [Google Scholar]
- 32.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 33.Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: Relevant concepts and methods. Arch Clin Neuropsychol. 2012;27:248–261. doi: 10.1093/arclin/acr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iverson GL. Interpreting change on the WAIS-III/WMS-III in clinical samples. Arch Clin Neuropsychol. 2001;16:183–191. [PubMed] [Google Scholar]
- 37.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, Mchugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 41.Shilling V, Jenkins V, Morris R, et al. The effects of adjuvant chemotherapy on cognition in women with breast cancer—Preliminary results of an observational longitudinal study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32:1909–1918. doi: 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurria A, Patel SK, Mortimer J, et al. The effect of aromatase inhibition on the cognitive function of older patients with breast cancer. Clin Breast Cancer. 2014;14:132–140. doi: 10.1016/j.clbc.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchinson AD, Hosking JR, Kichenadasse G, et al. Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treat Rev. 2012;38:926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: A randomized controlled trial. Breast Cancer Res Treat. 2012;135:799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jim HS, Donovan KA, Small BJ, et al. Cognitive functioning in breast cancer survivors: A controlled comparison. Cancer. 2009;115:1776–1783. doi: 10.1002/cncr.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donovan KA, Small BJ, Andrykowski MA, et al. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 2005;104:2499–2507. doi: 10.1002/cncr.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehlsen M, Pedersen AD, Jensen AB, et al. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psychooncology. 2009;18:248–257. doi: 10.1002/pon.1398. [DOI] [PubMed] [Google Scholar]
- 51.Debess J, Riis JO, Engebjerg MC, et al. Cognitive function after adjuvant treatment for early breast cancer: A population-based longitudinal study. Breast Cancer Res Treat. 2010;121:91–100. doi: 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- 52.Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: A controlled longitudinal study. Breast Cancer Res Treat. 2010;123:25–34. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 53.Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- 54.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: A review of rodent research. Neurosci Biobehav Rev. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Cruzado JA, López-Santiago S, Martínez-Marín V, et al. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer. 2014;22:1815–1823. doi: 10.1007/s00520-014-2147-x. [DOI] [PubMed] [Google Scholar]
- 56.Minisini AM, De Faccio S, Ermacora P, et al. Cognitive functions and elderly cancer patients receiving anticancer treatment: A prospective study. Crit Rev Oncol Hematol. 2008;67:71–79. doi: 10.1016/j.critrevonc.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Freedman RA, Pitcher B, Keating NL, et al. Cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine on Cancer and Leukemia Group B 49907. Breast Cancer Res Treat. 2013;139:607–616. doi: 10.1007/s10549-013-2562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurria A, Goldfarb S, Rosen C, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Res Treat. 2006;98:343–348. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]