Abstract
This review discusses the evolution of platinum and taxane first-line chemotherapy for ovarian cancer and the most recent trials and their impact on the current standard of care.
The optimal chemotherapy management of advanced ovarian cancer has been the subject of numerous randomized clinical trials. These trials have helped define our standard of care. However, the lack of randomized data in every clinical setting forces the practicing oncologist to make inferences from previous trials. For the last 24 years, a combination of a platinum compound and a taxane has been the standard regimen for ovarian cancer. This review highlights some of the principal past trials and recent trials and their effect on our standard of care.
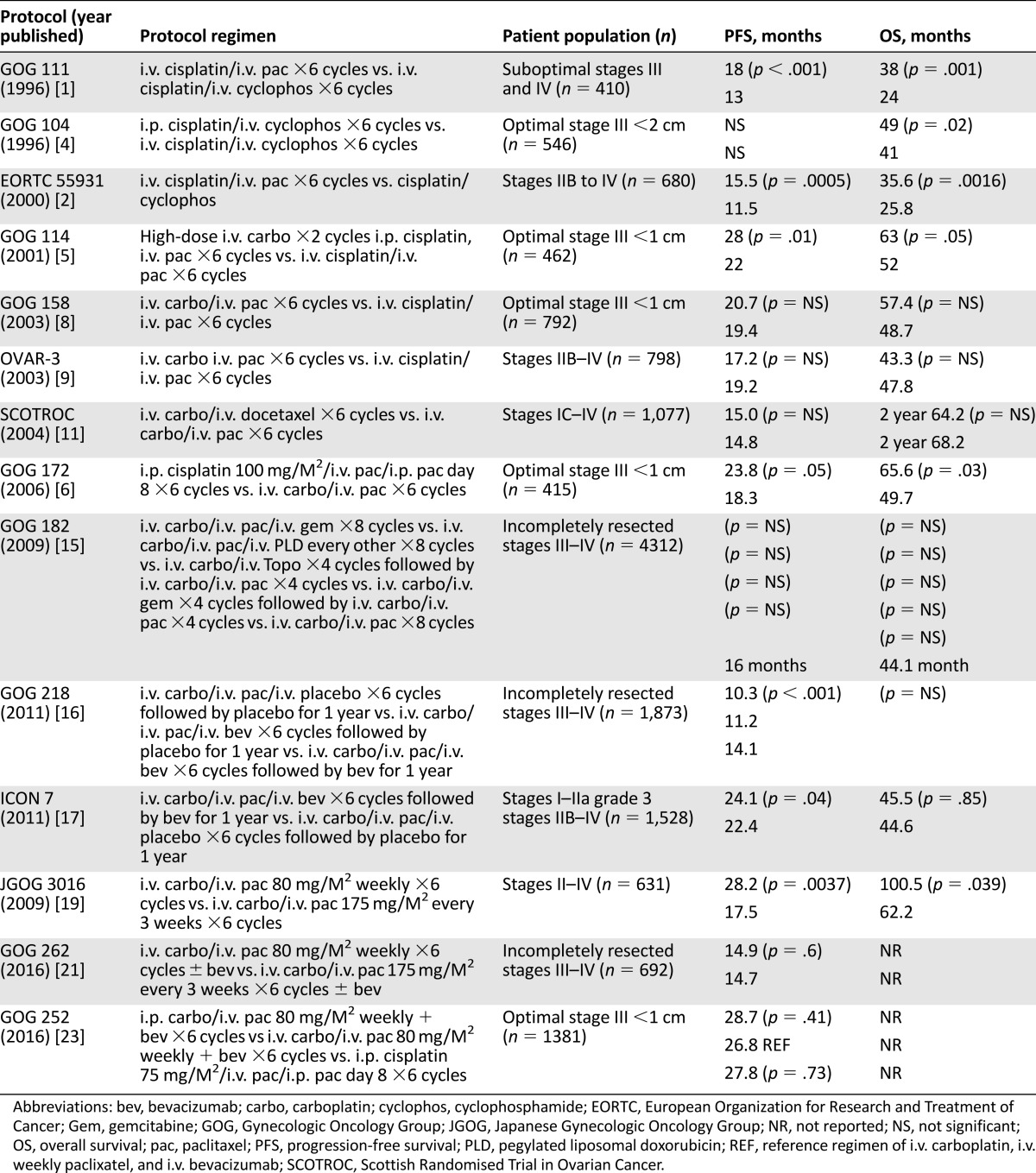
The combination of paclitaxel and a platinum compound chemotherapy for advanced ovarian cancer was established by a prospective randomized trial published 20 years ago. Since that original trial, numerous studies have confirmed these results and addressed the role of different platinum compounds, different taxanes, different routes, and different schedules of administration. In the last few months, the mature results of several prospective chemotherapy trials in ovarian cancer have further defined what the author believes should be considered the standard of care. The purpose of this current review is to discuss the evolution of platinum and taxane first-line chemotherapy for ovarian cancer and the most recent trials and their impact on the current standard of care (Table 1).
Table 1.
First-line chemotherapy regimens for ovarian cancer
First-Line Therapy With Cisplatin and Paclitaxel
Gynecologic Oncology Group (GOG) protocol 111 compared paclitaxel and cisplatin to cyclophosphamide and cisplatin in patients with suboptimal (suboptimal, >1 cm residual disease following primary debulking) stages III and IV disease following primary cytoreductive surgery [1]. Improvements were seen with the paclitaxel-based regimen in progression-free survival (PFS) and overall survival (OS) by 5 months and 14 months, respectively. A subsequent trial by the European Organization for Research and Treatment of Cancer (EORTC), the Nordic Gynecological Cancer Study Group, the National Cancer Institute of Canada Clinical Trials Group, and the Scottish Randomised Trial in Ovarian Cancer (SCOTROC) confirmed these results in a slightly broader patient population that included patients with stages IIB, IIC, III, and IV cancer with optimal and suboptimal residual disease [2]. This trial also demonstrated improvement in progression-free and overall survival consistent with a GOG trial. However, as noted in their manuscript, “the trial did not have the power to compare the chemotherapy regimens in the subsets of patients having optimal or suboptimal residual disease.” Previous trials by the GOG had evaluated three groups of patients on the basis of their risk of disease progression. Suboptimal patients were defined as stage III patients with greater than 1 cm diameter of residual disease or stage IV disease. Optimal stage III patients had less than 1 cm diameter of residual disease. High-risk early-stage disease included patients with stage IA and stage IB tumors with grade 3 histology, stage IC, IIA, IIB, and IIC. Although, subgroup analyses did not specifically evaluate optimal stage III patients and stage II patients, the combination chemotherapy regimen was rapidly adopted in these more favorable patient subgroups. Additionally, although no specific study evaluated the use of paclitaxel and cisplatin in high-risk early-stage ovarian cancer, the benefit of this combination in this patient population was inferred. Inference as defined by the Merriam-Webster dictionary is the act or process of reaching a conclusion about something from known facts or evidence [3].
Intraperitoneal Chemotherapy
At the same time as the GOG 111 publication, the first important trial of intraperitoneal chemotherapy SWOG 8501, GOG 104 was published [4]. This trial compared i.v. cisplatin 100 mg/m2 and i.v. cyclophosphamide 1,000 mg/m2 with i.p. cisplatin 100 mg/m2 and i.v. cyclophosphamide 1,000 mg/m2. The use of intraperitoneal chemotherapy decreased the likelihood of residual disease at second-look laparotomy and improved progression-free and overall survival. However, the concern regarding this regimen was the lack of paclitaxel, which had become a new standard. A subsequent study GOG 114 utilized paclitaxel, comparing i.v. cisplatin 75 mg/m2 and i.v. paclitaxel 135 mg/m2 for 24 hours for six cycles to an experimental arm that included high-dose i.v. carboplatin for two cycles: i.p. cisplatin 100 mg/m2 and i.v. paclitaxel 135 mg/m2 for 24 hours for six additional cycles [5]. Although the experimental combination was marginally better, severe hematologic toxicity from high-dose carboplatin limited drug delivery in the experimental arm. Additionally, the trial was very complex, with four variables changed, including the following: the route of administration (i.p. therapy), the number of courses of chemotherapy (six versus eight), the differential dose of cisplatin (75 versus 100 mg/m2), and the use of a new agent, carboplatin. This made interpretation of the trial difficult.
Attempting to resolve controversies with intraperitoneal chemotherapy, the GOG initiated their third large intraperitoneal trial, GOG 172 [6]. The trial design compared i.v. paclitaxel 135 mg/m2 for 24 hours, followed by i.v. cisplatin 75 mg/m2 versus i.v. paclitaxel 135 mg/m2 for 24 hours, followed by i.p. cisplatin 100 mg/m2 and i.p. paclitaxel 60 mg/m2 on day 8 for six cycles. This trial resulted in the most significant improvements in progression-free and overall survival seen with i.p. therapy to date, with a 5.5-month improvement in PFS and 15.9-month improvement in OS. However, only 42% of patients enrolled completed all six courses because of toxicity. Specifically, nonhematologic toxicity was significantly increased, including neurotoxicity and renal and gastrointestinal toxicity. Quality of life with the i.p. regimen was statistically worse and did not resolve until after the first year of treatment. On the basis of the significant improvements seen with this trial and the prior trials collectively, the National Cancer Institute (NCI) issued a clinical alert recommending the use of i.p. therapy for optimal stage III ovarian cancer [7]. Presumably because of the concerns for toxicity, no specific i.p. regimen was recommended in the NCI clinical alert.
Replacing Cisplatin With the Less Toxic Carboplatin
The next generation of studies—GOG 158 and Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) OVAR-3—established the equivalence of the less toxic carboplatin and paclitaxel in comparison with cisplatin and paclitaxel [8, 9]. The GOG trial compared i.v. cisplatin 75 mg/m2 and i.v. paclitaxel 135 mg/m2 administered for 24 hours to i.v. carboplatin at an area under the curve (AUC) of 7.5 and i.v. paclitaxel 175 mg/m2 administered for 3 hours, whereas the AGO trial compared cisplatin 75 mg/m2 and paclitaxel 185 mg/m2 administered for 3 hours to carboplatin AUC of 6 and paclitaxel 185 mg/m2 administered for 3 hours. Both trials were large, approximately 800 patients each, to ensure equivalence and established the less toxic carboplatin combination as the new standard. However, the dose that the consensus conference accepted—i.v. carboplatin at an AUC of 5–6 and i.v. paclitaxel 175 mg/m2 administered for 3 hours—was not the dose utilized in either of the two trials [10]. This is another example of how inference is used to determine the optimal treatment strategy.
Subsequently, the SCOTROC trial evaluated an alternative taxane, docetaxel, comparing i.v. docetaxel 75 mg/m2 and i.v. carboplatin (AUC of 5) to i.v. paclitaxel 175 mg/m2 for 3 hours and i.v. carboplatin AUC of 5 [11]. This demonstrated nearly identical PFS and OS with different toxicities. Docetaxel and carboplatin caused less peripheral neurotoxicity but more hematologic toxicity than did paclitaxel and carboplatin. Therefore, substitution of docetaxel for paclitaxel is one strategy for patients with worsening peripheral neuropathy.
Platinum/Taxane Triplets
Numerous second-line chemotherapy agents—including topotecan, pegylated liposomal doxorubicin, and gemcitabine—were approved for or studied in ovarian cancer in the 1990s [12–14]. The next important study, GOG 182, compared four experimental arms incorporating topotecan, gemcitabine, or liposomal doxorubicin in combination with carboplatin and paclitaxel versus carboplatin and paclitaxel alone [15]. This trial failed to demonstrate improvements in progression-free or overall survival with any of the experimental regimens in comparison with carboplatin and paclitaxel. The use of carboplatin and paclitaxel remained the standard first-line regimen.
Adding Antiangiogenic Therapy
Frustrated with the lack of improvement in GOG 182, the GOG turned to biologic agents in their next study. Built on a backbone of carboplatin and paclitaxel, GOG 218 evaluated bevacizumab in combination with chemotherapy and in combination with chemotherapy and as maintenance for 12 months versus placebo in a three-arm trial [16]. A similar trial in Europe (ICON 7) compared carboplatin and paclitaxel with bevacizumab in combination with chemotherapy and as maintenance for 12 months versus carboplatin and paclitaxel alone [17]. Both trials demonstrated a benefit in PFS, with bevacizumab in combination with chemotherapy and as maintenance for 12 months. In an ICON7 subgroup analysis the greatest benefit of bevacizumab was seen in suboptimal stage III and stage IV patients [18].
Dose-Dense Paclitaxel
On the basis of the improvement with dose-dense paclitaxel in breast cancer, this approach was studied in a randomized Japanese ovarian cancer trial (Japanese Gynecologic Oncology Group [JGOG] 3016) [19, 20]. Dose-dense paclitaxel (80 mg/m2 weekly) plus carboplatin at an AUC of 6 was compared with every-3-week paclitaxel (175 mg/m2) and carboplatin at an AUC of 6. This trial included patients with stage II–IV disease and included patients receiving neoadjuvant chemotherapy. A significant improvement in PFS (28.2 vs. 17.5 months) and OS (100.5 vs. 62.2 months) was seen in the patients treated with a dose-dense paclitaxel schedule. The biggest benefit was evident in patients with suboptimal stage III and stage IV disease. Subgroup analysis failed to demonstrate a significant improvement in patients with optimal residual disease. Additionally, patients with clear cell and mucinous histology did not benefit from the dose-dense paclitaxel schedule.
Recent Trials
The next two trials discussed were recently reported trials that have better defined the standard of care. In the first trial, GOG 262, the GOG chose to confirm the JGOG 3016 study in a group of patients with stage III suboptimal residual and stage IV disease. The trial design included carboplatin at an AUC of 6 in both arms, with a randomization between dose-dense paclitaxel (80 mg/m2) weekly versus every-3-week paclitaxel at the standard dose of 175 mg/m2 [21]. Because of the favorable findings with bevacizumab in GOG 218 and ICON 7, the trial allowed and provided bevacizumab to patients who elected to receive it. The confounding factor in this trial is that 84% of patients elected to receive bevacizumab. This trial demonstrated inferiority of 3-weekly paclitaxel and carboplatin without bevacizumab. The patients with every- 3-week paclitaxel and bevacizumab, weekly paclitaxel and bevacizumab, and weekly paclitaxel without bevacizumab had similar progression-free survivals. Despite the fact that only 16% of patients did not have bevacizumab, those patients treated with weekly paclitaxel versus every-3- week paclitaxel had a statistically superior PFS with a hazard ratio of 0.62 (95% confidence interval [CI], 0.40 to 0.95; p = .03). However, the authors concluded that weekly paclitaxel is not superior to every-3-week paclitaxel. This, of course, was true, because weekly versus 3-weekly paclitaxel was the basis of the randomization, but the fact that patients received bevacizumab confounded the interpretation of the results. Weekly paclitaxel has been reported to be antiangiogenic, and this may account for its superiority to 3-weekly treatment [22].
The second important trial presented at the Society of Gynecologic Oncology meeting in San Diego, California, on March 21, 2016, was GOG 252 [23]. This trial was very carefully developed with the intent to evaluate the role of intraperitoneal therapy by changing only one variable: drug delivery by either the intraperitoneal or the intravenous route. This study compared i.v. carboplatin, i.v. paclitaxel administered weekly, and bevacizumab to i.p. carboplatin, i.v. paclitaxel administered weekly, and bevacizumab. A third arm in this study was a modified version of the i.p. arm of GOG 172. This arm administered paclitaxel 135 mg/m2 i.v. for 3 hours, cisplatin 75 mg/m2 i.p. day 2 and paclitaxel 60 mg/m2 i.p. day 8 with bevacizumab. This study accrued 1,381 evaluable patients and demonstrated statistically similar PFS in all three arms. The only significant finding was the increased toxicity of the modified 172 i.p. arm. An ongoing Japanese trial (Intraperitoneal Therapy for Ovarian Cancer With Carboplatin Trial [iPocc]) is also comparing i.v. carboplatin, i.v. paclitaxel administered weekly to i.p. carboplatin, i.v. paclitaxel, administered weekly [24]. The primary endpoint of this trial is progression- free survival, and it is not expected to be completed until 2020. But because accrual goals are significantly smaller (654), it seems unlikely that they will see a statistical difference, because this was not seen in the larger GOG trial.
From the results of these trials we need to infer the optimal therapy for first-line therapy. Clearly, in the well-designed GOG 252 protocol, the i.p. administration of carboplatin was unable to demonstrate a benefit over i.v. carboplatin therapy. Therefore, what would be the purpose of delivering chemotherapy via the more complicated i.p. route? One possibility is that the benefit with i.p. therapy is limited to cisplatin at a dose of 100 mg/m2. But in view of the toxicity of the modified 172 i.p. arm at 75 mg/m2, it does not seem reasonable to increase the dose of cisplatin to 100 mg/m2 to incur even greater toxicity. Furthermore, an ancillary study of GOG 172 evaluating the efficacy of i.p. therapy in patients with deficiency in homologous rercombinant DNA repair demonstrated a statistical benefit to i.p. therapy only in the homologous recombination (HR)-deficient patients (HR = 0.67 [0.47–0.97], p = .032) [25]. In patients with an intact homologous recombination, DNA repair had no benefit with i.p. therapy (HR = 0.98 [0.69–1.38], p = .895). In view of the newer potential therapies for patients with homologous recombination deficiency such as poly-ADP ribose polymerase (PARP) inhibitors with a HR of 0.18 as maintenance therapy after a platinum-sensitive recurrence, is it reasonable to use such a toxic primary therapy [26]? Although currently, because PARP inhibitors are approved by the U.S. Food and Drug Administration (FDA) only in the recurrent disease setting, the use as first-line therapy or as first-line maintenance or both is currently being evaluated in phase 3 studies.
Does that leave us with arm 1 of GOG 252 (dose-dense paclitaxel, carboplatin, and bevacizumab) as the new standard of care? I do not think so. GOG 262 demonstrates that in the suboptimal stage III and stage IV populations, dose-dense paclitaxel and carboplatin are not inferior to dose-dense paclitaxel, carboplatin, and bevacizumab. Numerous studies have addressed different settings for the use of bevacizumab. In first-line therapy, GOG 218 and ICON 7 demonstrated improvements in PFS but not in OS. Similarly, in platinum-sensitive recurrent ovarian cancer, The Oceans trial and GOG 213 demonstrated improvements in PFS but not in survival [27, 28]. To date, on the basis of the Aurelia trial, the FDA has approved the use of bevacizumab only in platinum-resistant ovarian cancer [29].
On the basis of GOG 262, the new standard for suboptimal stage III and stage IV should be dose-dense paclitaxel and carboplatin, corroborating the Japanese trial. By inference from arm 1 of GOG 252 and the equivalence in GOG 262, the new standard for optimal stage III disease should also be dose-dense paclitaxel and carboplatin. In early-stage disease with high-grade serous histology, a histologic subset in which additional chemotherapy with six versus three cycles of therapy results in a hazard ration of HR = 0.33 (p = .04), dose-dense paclitaxel and carboplatin could be considered as an alternative to every-3-week paclitaxel administration [30]. Again, one has to remember that the use of platinum and paclitaxel for early-stage disease is recommended by the National Comprehensive Cancer Network guidelines in the absence of level 1 evidence.
The use of paclitaxel and carboplatin as first-line therapy in ovarian cancer has undergone refinements during the last 20 years. These refinements have optimized efficacy and tolerability of the regimen. Our most recent studies continue to support the use of dose-dense paclitaxel and carboplatin in ovarian cancer.
Disclosures
The author indicated no financial relationships.
References
- 1.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 2.Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 3.Inference. 2016. In: Merriam-Webster.com. Available at http://www.merriam-webster.com/dictionary/inference. Accessed March 25, 2016.
- 4.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 5.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 7.NCI clinical announcement on intraperitoneal chemotherapy in ovarian cancer (January 5, 2006). Available at http://ctep.cancer.gov/highlights/20060105_ovarian.htm. Accessed March 22, 2016.
- 8.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 9.du Bois A, Lück HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 10.du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: Final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol. 2005;16(suppl 8):viii7–viii12. doi: 10.1093/annonc/mdi961. [DOI] [PubMed] [Google Scholar]
- 11.Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–1691. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 12.ten Bokkel Huinink W, Gore M, Carmichael J, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997;15:2183–2193. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]
- 13.Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander M, Millward MJ, Bell D, et al. A phase II study of gemcitabine in platinum pre-treated patients with advanced epithelial ovarian cancer. Ann Oncol. 1998;9:1343–1345. doi: 10.1023/a:1008469212268. [DOI] [PubMed] [Google Scholar]
- 15.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 17.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 18.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 20.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 21.Chan JK, Brady MF, Penson RT, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016;374:738–748. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird RD, Tan DS, Kaye SB. Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev Clin Oncol. 2010;7:575–582. doi: 10.1038/nrclinonc.2010.120. [DOI] [PubMed] [Google Scholar]
- Walker JL, Brady MF, DiSilvestro PA et al. A phase III clinical trial of bevacizumab with IV versus IP chemotherapy in ovarian, fallopian tube and primary peritoneal carcinoma NCI-supplied agent(s): Bevacizumab (NSC #704865, IND #7921) NCT01167712 a GOG/NRG trial (GOG 252). Paper presented at: American Society of Clinical Oncology Annual Meeting; June 3–7, 2016; Chicago, IL. [Google Scholar]
- 24.Intraperitoneal therapy for ovarian cancer with carboplatin trial (iPocc). Clinicaltrials.gov identifier: NCT01506856. Available at: https://clinicaltrials.gov/ct2/show/NCT01506856. Accessed March 22, 2016.
- 25.Lesnock JL, Darcy KM, Tian C, et al. BRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: A Gynecologic Oncology Group Study. Br J Cancer. 2013;108:1231–1237. doi: 10.1038/bjc.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 27.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RL, Brady MF, Herzog TJ et al. A phase III randomized controlled clinical trial of carboplatin and paclitaxel alone or in combination with bevacizumab followed by bevacizumab and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian, peritoneal primary and fallopian tube cancer (Gynecologic Oncology Group 0213). 2015;137(suppl 1):3–4.
- 29.FDA approves Genentech's Avastin (bevacizumab) plus chemotherapy to treat women with platinum-resistant recurrent ovarian cancer. Available at: http://www.gene.com/media/press-releases/14578/2014-11-14/fda-approves-genentechs-avastin-bevacizu. Accessed March 22, 2016.
- 30.Chan JK, Tian C, Fleming GF, et al. The potential benefit of 6 vs. 3 cycles of chemotherapy in subsets of women with early-stage high-risk epithelial ovarian cancer: An exploratory analysis of a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:301–306. doi: 10.1016/j.ygyno.2009.10.073. [DOI] [PubMed] [Google Scholar]