Analysis of a multicenter Italian series of patients with male breast cancer treated with eribulin is reported. Patients were retrospectively identified from 19 reference centers. Eribulin was well-tolerated, with few adverse events. The results of the present study suggest the use of eribulin as therapy for male breast cancer.
Keywords: Clinical practice, Eribulin, Male breast cancer, Treatment
Abstract
Background.
Evidence on the management and treatment of male breast cancer is scant. We report the analysis of a multicenter Italian series of patients with male breast cancer treated with eribulin. To our knowledge, this is the first report on the use or eribulin in this setting.
Patients and Methods.
Patients were retrospectively identified in 19 reference centers. All patients received eribulin treatment, according to the standard practice of each center. Data on the identified patients were collected using a standardized form and were then centrally reviewed by two experienced oncologists.
Results.
A total of 23 patients (median age, 64 years; range, 42–80) were considered. The median age at the time of diagnosis of breast cancer was 57 years (range, 42–74). HER2 status was negative in 14 patients (61%), and 2 patients (9%) had triple-negative disease. The most common metastatic sites were the lung (n = 14; 61%) and bone (n = 13; 56%). Eribulin was administered for a median of 6 cycles (range, 3–15). All patients reported at least stable disease; two complete responses (9%) were documented. Eribulin was well-tolerated, with only four patients (17%) reporting grade 3 adverse events and two (9%) with treatment interruptions because of toxicity. Eight subjects (35%) did not report any adverse event during treatment. For patients with a reported fatal event, the median overall survival from the diagnosis of metastatic disease was 65 months (range, 22–228).
Conclusion.
Although hampered by all the limitations of any retrospective case series, the results of the present study suggest, for the first time, the use of eribulin as therapy for male breast cancer.
Implications for Practice:
Evidence on the management and treatment of male breast cancer is eagerly awaited. Although hampered by all the limitations of any retrospective case series, the results of the present study suggest, for the first time, the use of eribulin as therapy for male breast cancer.
Introduction
Male breast cancer is a rare solid neoplasm, accounting for ∼1% of all cancers in men. It has been associated with a 70%–80% survival rate at 5 years [1, 2]. The incidence of this neoplasm has been increasing [1, 3]. A number of biological differences between male and female breast cancer exist. Furthermore, male breast cancer is usually diagnosed at a more advanced age and stage than its female counterpart and presents with more aggressive features [2, 4, 5].
Despite these differences, the current management of male breast cancer has been determined from what is known for female breast cancer [2, 4, 6]. Antiestrogen therapy, with tamoxifen, aromatase inhibitors, and/or fulvestrant, remains the mainstay of treatment for male breast cancer patients [6, 7]. However, owing to the rarity of the disease, the use of these molecules has been investigated mostly in retrospective, small-size, and often monocentric populations [4, 6]. Therefore, new evidence from multicenter cohorts on the management and therapy of male breast cancer is eagerly awaited [1, 2].
Eribulin mesylate is a structurally simplified analog of halichondrin B, a natural product isolated from the marine sponge Halichondria okadai. This molecule induces mitotic blockade at the G2-M phase and apoptosis, thus inhibiting microtubule polymerization without affecting depolymerization [8]. The efficacy and safety of eribulin in the treatment of female breast cancer have been demonstrated in the pivotal, phase III EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician's Choice Versus E7389) trial, which compared eribulin with the treatment of physician’s choice in locally advanced or metastatic breast cancer patients who had received at least two previous chemotherapy regimens, including both anthracyclines and taxanes [9]. Given the favorable outcomes and the advantage in overall survival shown with eribulin, the European Commission issued in 2011 a marketing authorization valid throughout the European Union for eribulin for patients with metastatic breast cancer, after anthracycline and taxane treatment. Moreover, other evidence, collected in a field-practice setting, lend further support to the use of eribulin for the treatment of female breast cancer [10–15].
Thus, eribulin might also be worth investigating in the treatment of metastatic male breast cancer. We report the analysis of a multicenter, Italian series of patients with male breast cancer. To our knowledge, this is the first report on the use or eribulin in this setting.
Patients and Methods
The patients for inclusion in the present case series were retrospectively identified from 19 reference centers for the treatment of breast cancer located throughout the Italian territory. All patients were required to be male, to be affected by metastatic breast cancer, and to have received eribulin treatment, according to the standard practice of each center and the clinical judgment of the treating oncologists. No other limitations in patient selection were applied. All patients provided written informed consent before inclusion in the present analysis. Data from the selected patients were collected at each center using a standardized form and were then centrally reviewed by two experienced oncologists.
Results
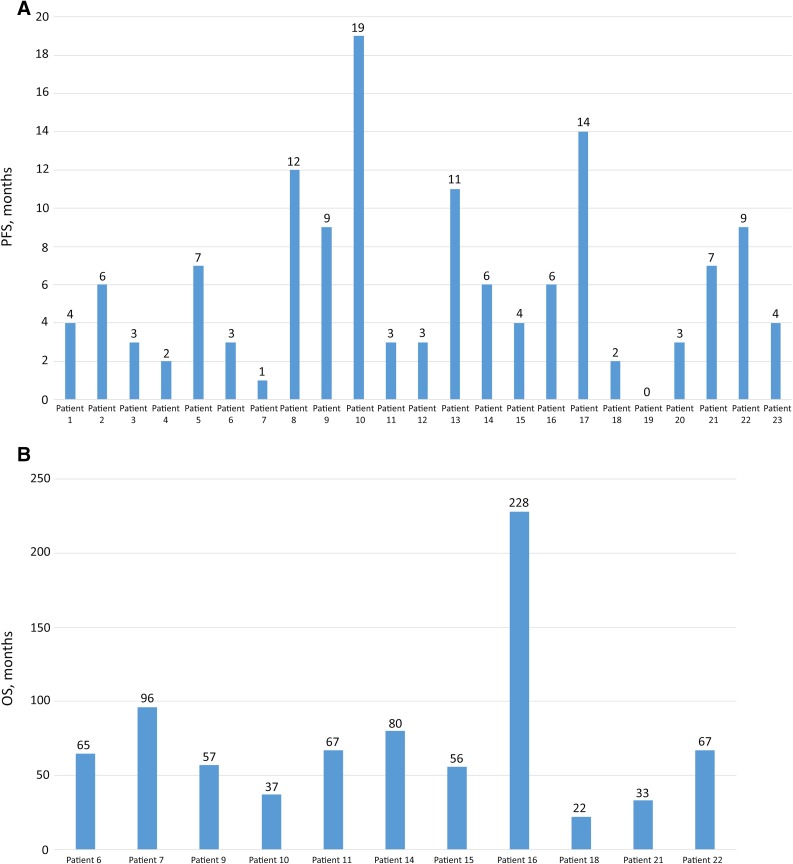
A total of 23 patients (median age, 64 years; range, 42–80) were identified and considered for the present case series. Their characteristics and information on the treatment received and clinical outcomes are listed in Table 1. Figure 1 shows the individual data on progression-free survival and overall survival.
Table 1.
Summary of patient and disease characteristics and treatment outcomes
Figure 1.
Individual data on survival for all patients. (A): PFS shown from the initiation of eribulin treatment (not evaluated in patient 19 owing to the lack of progression at the time of analysis). (B): OS shown from the diagnosis of metastatic disease (for patients with a fatal event only).
Abbreviations: PFS, progression-free survival; OS, overall survival.
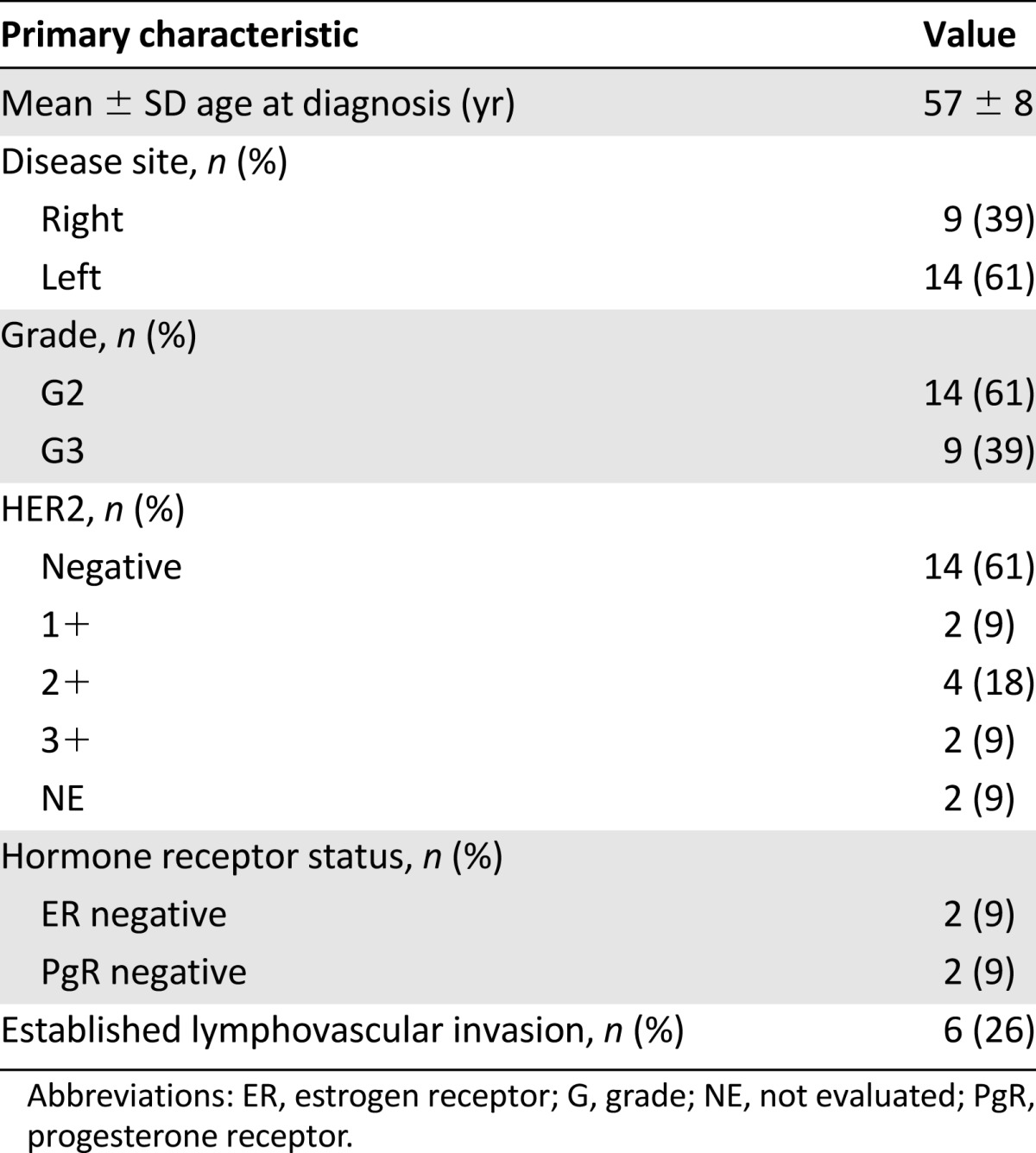
Overall, the median age at the diagnosis of breast cancer was 57 years (range, 42–74), and the median age at the detection of metastasis was 60 years (range, 45–77). Breast cancer was graded as grade 3 in 9 patients (39%) and grade 2 in 14 (61%). No cases of grade 4 disease were documented. HER2 status was negative in 14 patients (61%; i.e., with a score of 0 by immunohistochemistry), and 3 patients (13%) had triple-negative disease. The median Ki-67 value was 23% (range, 10%–50%). The most common metastatic sites were the lung (n = 14; 61%) and bone (n = 13; 56%). Only two patients (9%) did not undergo surgery. The median number of treatment lines before the initiation of eribulin was 3 (range, 2–11; Table 2).
Table 2.
Summary of primary disease characteristics
Eribulin was administered for a median of 6 cycles (range, 3–15). All the patients reported at least stable disease. In addition, two complete responses (9%) and nine partial responses (39%) were documented.
Eribulin was overall well tolerated, with only four patients (17%) reporting grade 3 adverse events and two patients (9%) interrupting treatment because of toxicity. Eight subjects (35%) did not report any adverse event during eribulin therapy.
At the last follow-up point, 12 patients (52%) were still alive. For patients with a reported fatal event, the median overall survival from the diagnosis of metastatic disease was 65 months (range, 22–228). A full description of some cases of particular interest is reported in the following paragraphs (the patient numbers correspond to the patient numbers in Table 1).
Patient 5
A 54-year-old man was referred to the treating institution in March 2009 for histologically confirmed breast carcinoma. Staging of the disease was negative for distant metastases, and patient had undergone total right mastectomy with surgical axillary lymph nodes. The histologic examination revealed poorly differentiated ductal carcinoma, pT3pN2 (8 of 20), grade 3; estrogen receptor, 90%; progesterone receptor, 60%; HER2+, 3. In April 2009, he started adjuvant chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide for six cycles, followed by trastuzumab for 1 year and aromatase inhibitors for 5 years. In March 2013, because of the appearance of intense pelvic pain symptoms, a total body computed tomography (CT) scan was performed, which showed an osteolytic lesion at the left ischiopubic branch, multiple vertebral lesions, multiple bilateral pulmonary lesions and liver lesion. In April 2013, he started chemotherapy with paclitaxel plus trastuzumab with zoledronic acid until October 2013, when chemotherapy was interrupted because of disease progression with the appearance of multiple symptomatic brain lesions. The patient started palliative whole brain radiotherapy and second-line therapy with vinorelbine plus trastuzumab. In March 2014, a total body CT scan was performed to restage the disease. The CT scan showed disease progression of known lung and liver lesions. The patient then started third-line therapy with lapatinib plus capecitabine until July 2014. The total body CT scan performed in July 2014 showed disease progression of the lung and liver lesions, with the appearance of new liver and bone lesions. At that time, the patient had an Eastern Cooperative Oncology Group performance status of 2 for tumor-related pain. After a cardiology examination (electrocardiogram and echocardiogram), which showed good heart function, in July 2014 the patient started fourth-line therapy with eribulin. A radiological partial response, with radiological reduction of more than 50% in the sum of the diameters of the target lesions and stability of the other nontarget lesions, was obtained after only 2 months of treatment. This clinical and radiological response was maintained at subsequent disease restaging, performed every 2 months, with clinical improvement (Eastern Cooperative Oncology Group performance status of 0), pain relief, and the absence of relevant side effects. Treatment was continued until February 2015 without dose modifications. Eribulin was then interrupted because of disease progression and a decline in the patient’s clinical condition.
Patient 7
The patient came to the treating institution for metastatic breast cancer at the age of 69 years. Given the presence of severe dilated cardiomyopathy, adjuvant therapy was not performed. At the first relapse, the patient started hormonal therapy, achieving good control of the disease. Eribulin was used after three lines of therapy after the detection of four brain metastases. The patient showed a complete response to the brain injuries but died of a heart attack 1 month after the initiation of eribulin.
Patient 21
A 62-year-old man came to the treating institution after he had undergone left radical mastectomy with ipsilateral axillary lymph node dissection 5 years earlier, when he was first diagnosed at the age of 57. The histological examination revealed an infiltrating ductal breast carcinoma, grade 3, pT1cN0M0; estrogen receptor, 0%; progesterone receptor, 0%; and negative HER2 status. The patient underwent adjuvant chemotherapy with six cycles of 5-fluorouracil, epirubicin, and cyclophosphamide. In February 2010, after a suspected locoregional recurrence appeared on the mastectomy scar, he underwent excision of the nodule. Examination of the surgical specimen confirmed the previous histological and biological features (i.e., triple-negative breast cancer). The patient underwent adjuvant radiotherapy at the recurrence site and chemotherapy with carboplatin area under the curve (AUC) 5, every 21 days for six cycles. In February 2012, following the onset of pain in the thoracic spine, restaging was undertaken with a total body CT scan and bone scan, which showed bone metastases. Therefore, the patient started treatment with paclitaxel plus bevacizumab for six courses, followed by bevacizumab maintenance until July 2013. He also received zoledronic acid until August 2014.
In September 2013, after clinical and instrumental bone progression, the patient began treatment with vinorelbine, 5-fluorouracil, and folic acid day 1–3 every 3 weeks for eight cycles until January 2014. Because further clinical and instrumental disease progression at the bone was documented in February 2014, eribulin 1.23 mg/m2 on days 1 and 8 every 3 weeks was started and was continued for seven cycles, without any relevant toxicity, until September 2014 when the disease progressed. In November 2014, the patient died of disease progression.
Patient 22
Patient 22 was a 68-year-old man, diagnosed in October 2009 with stage IV, luminal B-like left breast cancer, with synchronous bone and mediastinal lymph node metastases. He received first-line chemotherapy with epirubicin and docetaxel, with disease progression after 3 months. In order to better understand the breast cancer subtype, he underwent mastectomy with lymphadenectomy. The histopathological analysis confirmed the diagnosis of invasive ductal carcinoma, histological grade 3, positive for hormone receptors. The breast surgical margins were free. After surgery, the patient received first-line hormonal therapy consisting of letrozole. He also underwent radiation therapy to the symptomatic bone metastases.
Four months later, the disease had progressed. The patient was treated with two more lines of chemotherapy (anthracyclines and taxanes; carboplatin and gemcitabine). Treatment with eribulin (1.23 mg/m2) was then instituted and continued for nine cycles. The main toxicities were asthenia, nausea, and stypsis (all grade 1). The patient’s disease was stable for 9 months. He later died 67 months after the diagnosis of metastatic disease because of a car accident.
Discussion
Although it usually presents with aggressive features, male breast cancer remains a poorly characterized disease. The current management of this condition has been determined from the results of clinical trials of female breast cancer patients. However, the existence of marked biological differences between male and female breast cancer has been shown repeatedly [1, 2, 4].
At present, dedicated studies are needed to investigate the biological and clinical characteristics of male breast cancer and to determine the specific management for this disease [1]. However, owing to the rarity of male breast cancer, the conduction of prospective studies has been difficult [4]. Therefore, most pieces of evidence for male breast cancer must be collected from retrospective analyses, a type of study that can, however, be informative in the oncological setting [16].
In the present retrospective, multicenter case series of 23 subjects, we have analyzed the clinical outcomes of eribulin for patients with metastatic male breast cancer. To our knowledge, this is the first report of eribulin use in this population. Although the great majority of patients had been heavily pretreated, eribulin was continued for a median of six cycles and was associated with a clinical response in almost one half of the patients. No unexpected safety concerns were reported, and approximately one third of evaluated subjects did not experience any relevant adverse event during eribulin treatment.
Conclusion
Although hampered by all the limitations of any retrospective case series and the lack of specific analyses on survival owing to the heterogeneity of patients, we believe that the present analysis adds to the current knowledge of the management of male breast cancer. Our results suggest for the first time the potential use of eribulin in the therapy of this disease. While waiting for other studies to either confirm or discard these preliminary findings, eribulin might be worth consideration in the treatment of pretreated patients with male breast cancer.
Acknowledgments
Editorial assistance for the preparation of our manuscript was provided by Luca Giacomelli, on behalf of Content Ed Net; this assistance was funded by Eisai. The sponsoring company was not offered the opportunity to access the raw data or revise the paper and had no role in the decision to submit it for publication.
Footnotes
For Further Reading: Giovanna Masci, Michele Caruso, Francesco Caruso et al. Clinicopathological and Immunohistochemical Characteristics in Male Breast Cancer: A Retrospective Case Series. The Oncologist 2015;20:586–592.
Implications for Practice: There is little evidence that prognostic features established in female breast cancer, such as grading and Ki-67 labeling index, could be applied to male breast cancer as well. This study found that grade 3 was associated with shorter overall survival and a trend for Ki-67 >20%; this could help in choosing the best treatment option in the adjuvant setting. Many questions remain regarding the impact of HER-2 positivity on survival and treatment with adjuvant anti-HER-2 therapy. Regarding metastatic male breast cancer, the results suggest that common regimens of chemo-, endocrine and immunotherapy used in female breast cancer are safe and effective for men. Male breast cancer patients show a higher incidence of second primary tumors, especially prostate and colon cancers and should therefore be carefully monitored.
Author Contributions
Conception/Design: Francesco Giotta, Luigi Acito, Giampiero Candeloro, Gennaro Gadaleta-Caldarola, Guido Giordano, Rossana Gueli, Antonio Lugini, Valentina Magri, Marta Mandarà, Giovanna Masci, Salvatore Pisconti, Mirco Pistelli, Anna Rizzi, Nello Salesi, Alessio Schirone, Giovanni Scognamiglio, Maria Tedeschi, Patrizia Zucchinelli
Provision of study material or patients: Francesco Giotta, Luigi Acito, Giampiero Candeloro, Gennaro Gadaleta-Caldarola, Guido Giordano, Rossana Gueli, Antonio Lugini, Valentina Magri, Marta Mandarà, Giovanna Masci, Salvatore Pisconti, Mirco Pistelli, Anna Rizzi, Nello Salesi, Alessio Schirone, Giovanni Scognamiglio, Maria Tedeschi, Patrizia Zucchinelli
Collection and/or assembly of data: Francesco Giotta, Luigi Acito, Giampiero Candeloro, Gennaro Gadaleta-Caldarola, Guido Giordano, Rossana Gueli, Antonio Lugini, Valentina Magri, Marta Mandarà, Giovanna Masci, Salvatore Pisconti, Mirco Pistelli, Anna Rizzi, Nello Salesi, Alessio Schirone, Giovanni Scognamiglio, Maria Tedeschi, Patrizia Zucchinelli
Data analysis and interpretation: Francesco Giotta, Luigi Acito, Giampiero Candeloro, Pietro Del Medico, Gennaro Gadaleta-Caldarola, Guido Giordano, Rossana Gueli, Antonio Lugini, Valentina Magri, Marta Mandarà, Giovanna Masci, Salvatore Pisconti, Mirco Pistelli, Anna Rizzi, Nello Salesi, Alessio Schirone, Giovanni Scognamiglio, Maria Tedeschi, Patrizia Zucchinelli
Manuscript writing: Francesco Giotta, Luigi Acito, Giampiero Candeloro, Pietro Del Medico, Gennaro Gadaleta-Caldarola, Guido Giordano, Rossana Gueli, Antonio Lugini, Valentina Magri, Marta Mandarà, Giovanna Masci, Salvatore Pisconti, Mirco Pistelli, Anna Rizzi, Nello Salesi, Alessio Schirone, Giovanni Scognamiglio, Maria Tedeschi, Patrizia Zucchinelli
Final approval of manuscript: Francesco Giotta, Luigi Acito, Giampiero Candeloro, Gennaro Gadaleta-Caldarola, Guido Giordano, Rossana Gueli, Antonio Lugini, Valentina Magri, Marta Mandarà, Giovanna Masci, Salvatore Pisconti, Mirco Pistelli, Anna Rizzi, Nello Salesi, Alessio Schirone, Giovanni Scognamiglio, Maria Tedeschi, Patrizia Zucchinelli
Disclosures
The authors indicated no financial relationships.
References
- 1.White J, Kearins O, Dodwell D, et al. Male breast carcinoma: Increased awareness needed. Breast Cancer Res. 2011;13:219. doi: 10.1186/bcr2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masci G, Caruso M, Caruso F, et al. Clinicopathological and immunohistochemical characteristics in male breast cancer: A retrospective case series. The Oncologist. 2015;20:586–592. doi: 10.1634/theoncologist.2014-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115:429–430. doi: 10.1007/s10549-008-0053-y. [DOI] [PubMed] [Google Scholar]
- 4.Ottini L, Palli D, Rizzo S, et al. Male breast cancer. Crit Rev Oncol Hematol. 2010;73:141–155. doi: 10.1016/j.critrevonc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Yu XF, Yang HJ, Yu Y, et al. A prognostic analysis of male breast cancer (MBC) compared with post-menopausal female breast cancer (FBC) PLoS One. 2015;10:e0136670. doi: 10.1371/journal.pone.0136670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Lauro L, Pizzuti L, Barba M, et al. Role of gonadotropin-releasing hormone analogues in metastatic male breast cancer: Results from a pooled analysis. J Hematol Oncol. 2015;8:53. doi: 10.1186/s13045-015-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan MH, Allerton R, Pettit L. Hormone therapy for breast cancer in men. Clin Breast Cancer. 2015;15:245–250. doi: 10.1016/j.clbc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Towle MJ, Salvato KA, Wels BF, et al. Eribulin induces irreversible mitotic blockade: Implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res. 2011;71:496–505. doi: 10.1158/0008-5472.CAN-10-1874. [DOI] [PubMed] [Google Scholar]
- 9.Cortés J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 10.Tesch H, Schneeweiss A. Practical experiences with eribulin in patients with metastatic breast cancer. Anticancer Drugs. 2016;27:112–117. doi: 10.1097/CAD.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 11.Smith JW, II, Vukelja S, Hoffman AD, et al. Phase II, multicenter, single-arm, feasibility study of eribulin combined with capecitabine for adjuvant treatment in estrogen receptor-positive, early-stage breast cancer. Clin Breast Cancer. 2016;16:31–37. doi: 10.1016/j.clbc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Martella F, Bacci C, Giordano C, et al. Eribulin mesylate in advanced breast cancer: Retrospective review of a single institute experience. Future Oncol. 2015;11(suppl):31–36. doi: 10.2217/fon.15.151. [DOI] [PubMed] [Google Scholar]
- 13.Leo L, Caputo F, Sarno AD, et al. Response to eribulin in a difficult-to-treat, heavily pretreated breast cancer patient: A case report. Future Oncol. 2015;11(suppl):27–30. doi: 10.2217/fon.15.150. [DOI] [PubMed] [Google Scholar]
- 14.Iorfida M, Mazza M. Long-lasting control with eribulin in a taxane pretreated metastatic breast cancer patient. Future Oncol. 2015;11(suppl):23–26. doi: 10.2217/fon.15.149. [DOI] [PubMed] [Google Scholar]
- 15.Chang AY, Ying XX. Brain metastases from breast cancer and response to treatment with eribulin: A case series. Breast Cancer (Auckl) 2015;9:19–24. doi: 10.4137/BCBCR.S21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzo M, Cartenì G, Pappagallo G. We need both randomized trials and real-world data: The example of everolimus as second-line therapy for mRCC. Future Oncol. 2014;10:1893–1896. doi: 10.2217/fon.14.182. [DOI] [PubMed] [Google Scholar]