Abstract
Lessons Learned
Patients with metastatic castration-resistant prostate cancer did not tolerate the combination of alisertib with abiraterone and prednisone.
There was no clear signal indicating that adding alisertib might be beneficial for those patients progressing on abiraterone.
Background.
We hypothesized that Aurora A kinase (AK) contributes to castrate resistance in prostate cancer (PCa) and that inhibiting AK with alisertib can resensitize PCa cells to androgen receptor (AR) inhibitor abiraterone.
Methods.
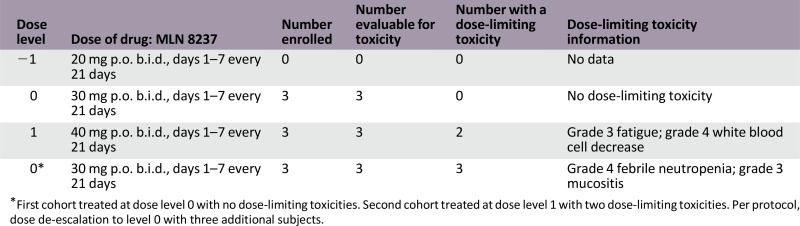
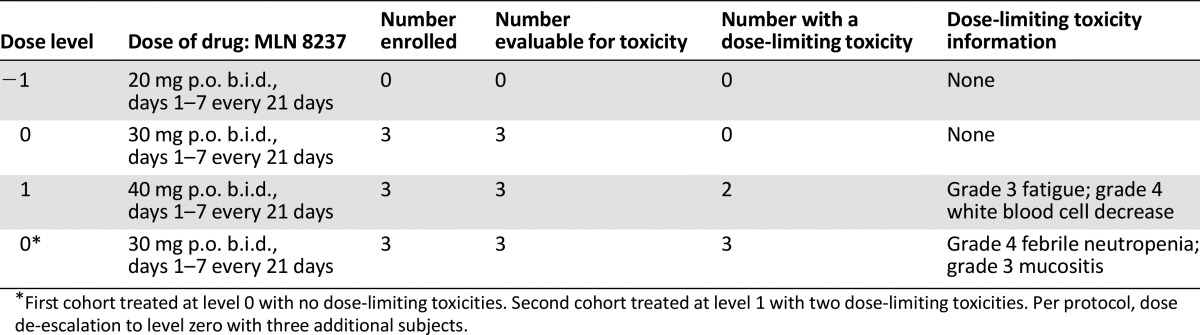
This was a phase I/II trial to determine the safety and efficacy of alisertib when given in combination with abiraterone plus prednisone (AP). Metastatic castration-resistant prostate cancer (mCRPC) patients were treated with dose escalation (alisertib at 30, 40, and 50 mg orally b.i.d., days 1–7 every 21 days) per standard 3+3 design.
Results.
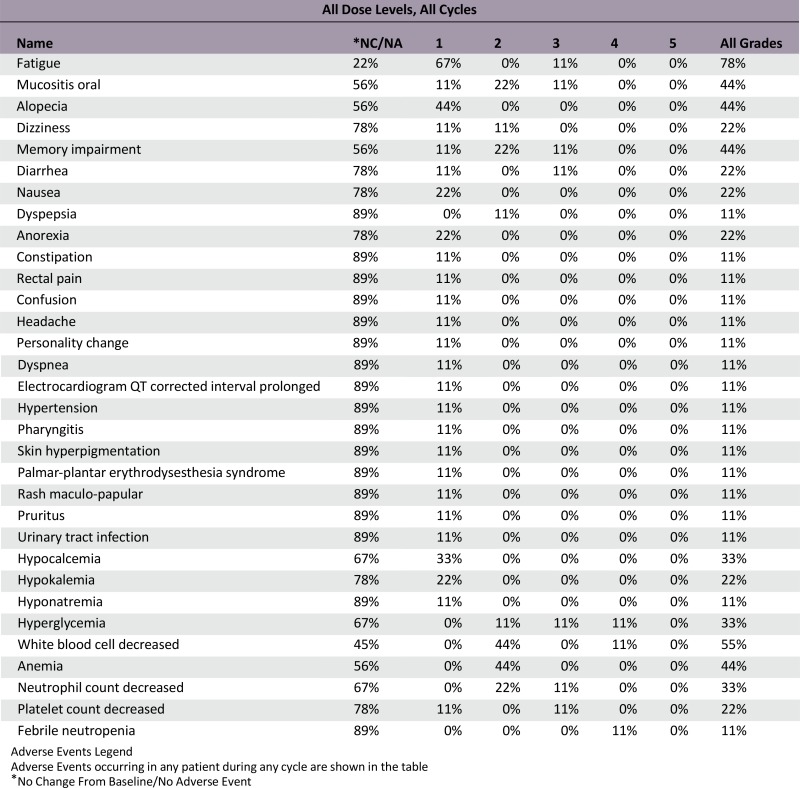
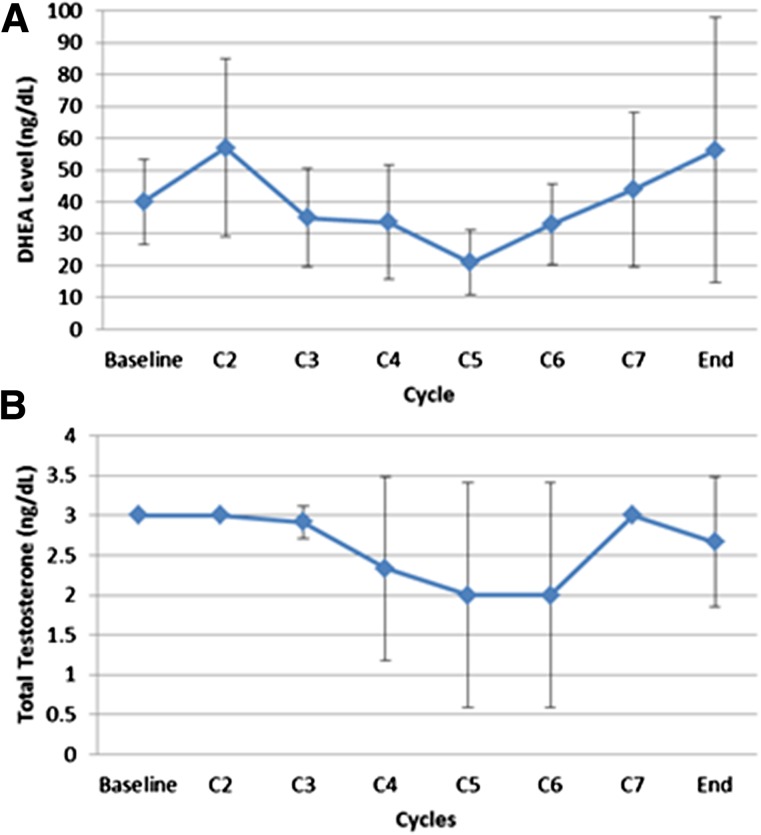
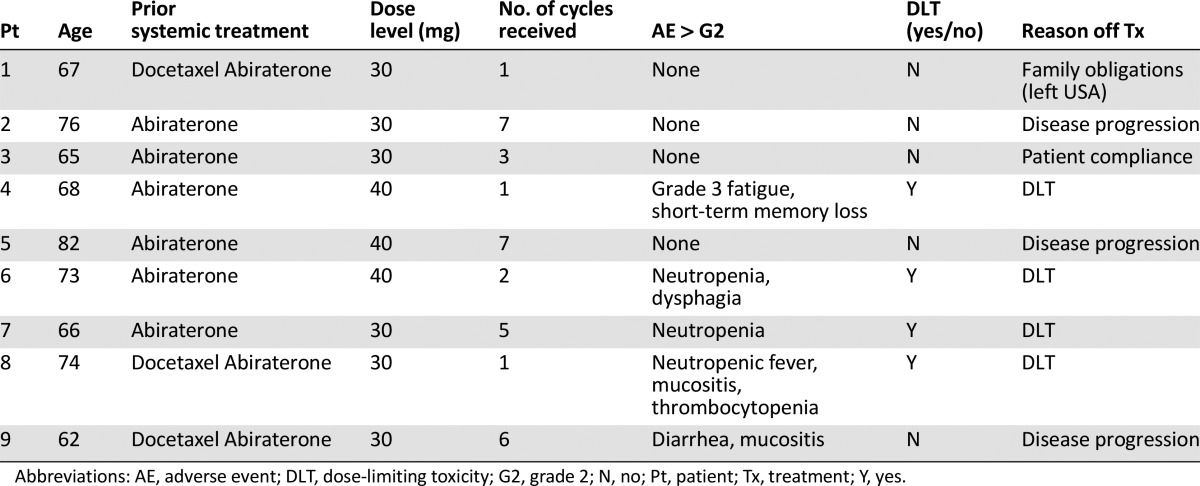
Nine of 43 planned subjects were enrolled. The maximum tolerated dose (MTD) was not reached, and the dose-limiting toxicities (DLTs) included neutropenic fever (1 of 9), neutropenia (1 of 9), fatigue with memory impairment (1 of 9), and diarrhea/mucositis (1 of 9). No prostate-specific antigen (PSA) decrease or circulating tumor cell (CTC) changes were observed during the study. Pharmacodynamically, adding alisertib did not affect total testosterone or dehydroepiandrosterone (DHEA) levels. There was some change in neuroendocrine markers after therapy. Mean duration on study was 2.5 months. The trial was terminated early.
Conclusion.
A tolerable dose of alisertib in combination with AP in mCRPC was not established in this study. There was no clear signal indicating that alisertib might be beneficial for patients with mCRPC progressing on abiraterone.
Abstract
作者总结
经验
• 转移性去势抵抗性前列腺癌患者不能耐受alisertib与阿比特龙和泼尼松的联合方案。
• 在接受阿比特龙治疗后发生进展的患者中, 尚无明确迹象表明联合alisertib治疗可带来临床获益。
摘要
背景. 假设Aurora A激酶(AK)可促成前列腺癌(PCa)发生去势抵抗, 而使用alisertib抑制AK可使PCa细胞对雄激素受体(AR)抑制剂阿比特龙治疗恢复敏感。
方法. 本项I/II期临床试验旨在确定alisertib联合阿比特龙和泼尼松(AP)治疗的安全性和有效性。研究为标准3+3设计, 转移性去势抵抗性前列腺癌(mCRPC)患者接受剂量递增治疗: alisertib 30、40和50 mg口服, 每日2次, 第1∼7天, 21天为一周期。
结果. 计划招募43例患者, 研究共纳入9例。本研究未达到最大耐受剂量(MTD), 剂量限制毒性(DLT)包括中性粒细胞减少性发热(1/9例)、中性粒细胞减少(1/9例)、疲乏伴记忆受损(1/9例), 以及腹泻/黏膜炎(1/9例)。研究期间未观察到前列腺特异性抗原(PSA)水平下降及循环肿瘤细胞(CTC)数量改变。从药效学上来说, 联合alisertib没有影响总睾酮或脱氢表雄酮(DHEA)水平。治疗后神经内分泌标记物有所变化。治疗平均时间为2.5个月。研究提早终止。
结论. 本研究未确立alisertib联合AP在mCRPC患者中的耐受剂量。没有明确的征象提示alisertib治疗可为阿比特龙治疗后发生进展的mCRPC患者带来临床获益。The Oncologist 2016;21:1296–1297e
Discussion
Abiraterone acetate is active and approved for use in patients with metastatic castration-resistant prostate cancer [1, 2], but resistance does develop, and the mechanism of drug resistance is under active investigation [3]. Preclinical studies have shown AK as a potential target for advanced PCa, especially for PCa with neuroendocrine differentiation. We investigated whether the addition of alisertib, an AK inhibitor, to an AP regimen was tolerable and effective to reverse resistance to abiraterone.
The trial was terminated early because of toxicity and lack of clinical benefit. The first three patients in cohort 1 (30 mg b.i.d., days 1–7 every 21 days) did not experience a DLT. Two patients experienced DLTs in cohort 2 (40 mg level) (fatigue with memory impairment or neutropenic fever), resulting in dose de-escalation Three additional patients were treated at 30 mg b.i.d., and two developed DLTs (neutropenia and diarrhea/mucositis). Evaluation of side-effect profile among the nine patients demonstrated poor tolerability of alisertib and abiraterone/prednisone combination. Bone marrow suppression is a known side effect from alisertib [4, 5], but the rate of grade 3/4 toxicities was higher in our study compared with others. It is important to note that previous studies used alisertib as monotherapy in solid tumors. To improve patient tolerance, it might be reasonable to use a different dose and schedule for patients with relatively slow-growing tumors such as prostate cancer.
The efficacy is difficult to assess in this phase I trial. Three patients were taken off the study because of disease progression. Seven (of 9) patients had an increase in PSA during the study. Four (of 9) patients in the trials had ≥5 CTCs at baseline, but no conversion was observed at the end of therapy. Mean duration on the study was 2.5 months. These results suggest an unfavorable efficacy-to-toxicity ratio for this combination. The trial was prematurely terminated, and the phase II portion was not performed.
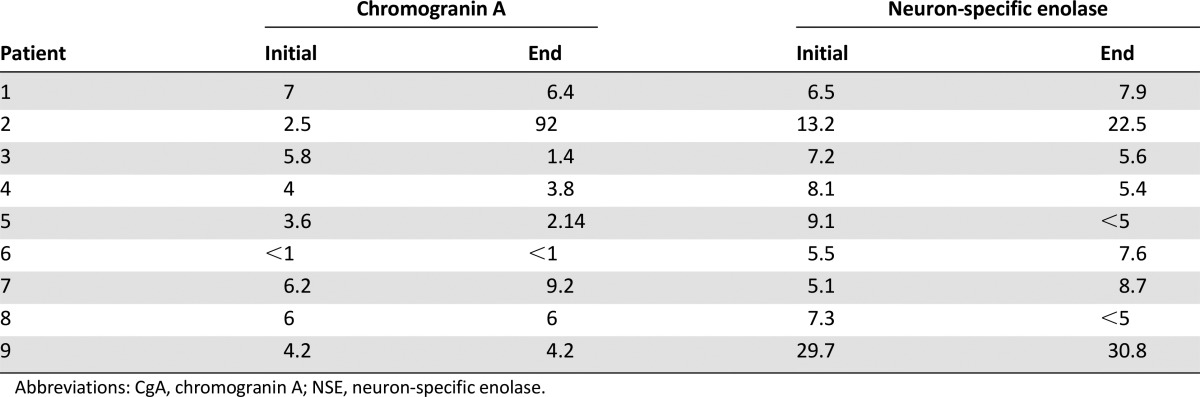
From measuring the total testosterone and DHEA levels during the study, we believe alisertib does not interfere with the ability of abiraterone to inhibit biosynthesis of androgens. For neuroendocrine biomarkers, we observed three (of nine) patients who had a sustained decreased in chromogranin A levels and four (of nine) patients who had a decrease in neuron-specific enolase levels. The significance of these changes is not clear, given the small sample size of the study. Fluorescence in situ hybridization analysis of collected CTCs did not demonstrate AK amplification. Further study is certainly needed to make any conclusions.
In summary, adding alisertib to abiraterone regimen seems intolerable in mCRPC. The optimal dose and schedule of alisertib could not be determined. There was no clear signal indicating that alisertib might be beneficial for patients with mCRPC progressing on abiraterone, and further development of this treatment combination is not warranted.
Trial Information
- Disease
Prostate cancer
- Stage of disease / treatment
Metastatic/advanced
- Prior Therapy
1 prior regimen
- Type of study - 1
Phase I
- Type of study - 2
3+3 dose escalation
- Primary Endpoint
Safety
- Primary Endpoint
Maximum tolerated dose
- Secondary Endpoint
PSA kinetics after alisertib is added to abiraterone and prednisone regimen
- Secondary Endpoint
Baseline and post-therapy CTCs enumeration
- Secondary Endpoint
Baseline and post-therapy chromogranin A and neuron-specific enolase levels
- Additional Details of Endpoints or Study Design
-
1. DHEA and testosterone kinetics after alisertib
2. Aurora kinase A gene amplification status
- Investigator's Analysis
Poorly tolerated/not feasible
Drug Information
- Drug 1
- Generic/working name
MLN 8237
- Trade name
Alisertib
- Company name
Millennium: The Takeda Oncology Company
- Drug type
Small molecule
- Drug class
Mitotic - Aurora kinase
- Dose
milligrams (mg) per flat dose
- Route
oral (p.o.)
- Schedule of administration
Dose Level −1: Alisertib, 20 mg p.o. b.i.d., days 1–7 every 21 days
Dose Level 0 (starting): Alisertib, 30 mg p.o. b.i.d., days 1–7 every 21 days
Dose Level 1: Alisertib, 40 mg p.o. b.i.d., days 1–7 every 21 days
Dose Level 2: Alisertib, 50 mg p.o. b.i.d., days 1–7 every 21 days
The first cohort was treated at dose level 0 with no DLTs. The second cohort was treated at dose level 1 with two DLTs. Per protocol, dose de-escalation was to level 0 with three additional subjects.
Patient Characteristics
- Number of patients, male
9
- Number of patients, female
0
- Stage
Stage IV, Metastatic
- Age
Median (range): 68 (62–82)
- Number of prior systemic therapies
Median (range): 1 (1–2)
- Performance Status: ECOG
0 — 7
1 — 2
2 — 0
3 — 0
Unknown — 0
- Other
Not collected
- Cancer types or histologic subtypes
Adenocarcinoma of prostate: 9
Primary Assessment Method
- Number of patients screened
9
- Number of patients enrolled
9
- Number of patients evaluable for toxicity
9
- Number of patients evaluated for efficacy
9
- Evaluation method
Clinic visit at 12 weeks or progression: CT\MRI Abd and Pelvis; CT of chest or CXR; Bone Scan; PSA; Androgen Panel
- Response assessment CR
0
- Response assessment PR
0
- Response assessment SD
0
- Response assessment PD
n = 3
- Response assessment OTHER
n = 6
Adverse Events
Serious Adverse Events
Dose-Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated reason
Toxicity
- Investigator’s assessment
Poorly tolerated/not feasible
Abiraterone acetate is active and approved for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) [1, 2]. Unfortunately, disease progression inevitably occurs, and patients require additional therapy, which remains an unmet medical need. The mechanism of resistance to abiraterone is not clear and is still under active investigation [3]. It is widely accepted that prostate cancer (PCa) is composed of heterogeneously distinct cell subtypes, including neuroendocrine prostate cancer (NEPC). NEPC is generally considered an aggressive and lethal variant of PCa that most commonly arises from existing PCa [6]. Clinically, NEPC often has high serum levels of chromogranin A (CgA) and neuron-specific enolase (NSE) [7]. The presence of the neuroendocrine (NE) tumor subpopulation can be gauged noninvasively by measuring circulating levels of secretory products, primarily CgA [8]. Increased plasma CgA was observed in 64.3% of patients with CRPC [9]. Increased NSE was seen in 35% of patients [10]. These NEPC can be considered to be castration-resistant and androgen receptor (AR)-independent because AR signaling is not a key player for survival of these cells [11, 12]. The development of the NE features (transformation) in androgen-deprived conditions may contribute to castrate resistance and/or abiraterone resistance. Molecularly, Aurora Kinase (AK) (and concurrent MYCN oncogene) amplification was identified by fluorescence in situ hybridization (FISH) in 75% of primary PCa of patients with NEPC (>95% cells) [11]. By using tissue biopsies from metastatic sites, up to 53% metastatic PCa were found to harbor AK amplification [11].
In vitro studies support the fact that there is a functional relationship between AK and PCa: (a) AK is overexpressed in PCa, especially in anti-androgen-resistant PCa [13, 14]; (b) AK phosphorylates and interacts with AR, enhancing AR DNA binding [15]; (c) AK induces cell growth in the presence and absence of androgen; (d) AK induces/enhances AR activity and potentiates androgen action in AR; and (e) targeting AK reverses the androgen-independent phenotype in vitro [15]. Features of mCRPC likely include AK (and concurrent MYCN) amplification/overexpression with NE phenotype. AK is potentially an important drugable target for CRPC with or without neuroendocrine differentiation. Therefore, we investigated whether mCRPC is sensitive to AK suppression by alisertib, especially in the subgroup of patients with neuroendocrine phenotype. We hypothesized that adding alisertib to the existing androgen-deprivation therapy regimen may reverse the resistance to abiraterone.
We designed a phase I/II, open-label, single-institution trial to determine the safety and efficacy of the AK inhibitor alisertib when given in combination with abiraterone plus prednisone (AP). In the phase I portion, we evaluated the maximum tolerated dose of alisertib. In the phase II study, we planned to evaluate the proportion of patients who had no disease progression after alisertib is added to abiraterone and prednisone.
The trial was terminated early because of toxicity and lack of clinical benefit. The first three patients in cohort 1 (30 mg b.i.d., days 1–7 every 21 days) did not experience a dose-limiting toxicity (DLT). Two patients experienced DLTs in cohort 2 (fatigue with memory impairment and neutropenic fever), resulting in dose de-escalation. Three additional patients were treated at 30 mg b.i.d., and two developed DLTs (neutropenia and diarrhea/mucositis). Evaluation of the side-effect profile among the nine patients demonstrated poor tolerability of the alisertib and abiraterone/prednisone combination. Five (of 9) patients required a treatment delay. Bone marrow suppression is a known side effect from alisertib [4, 5], and although the rate of grade 3/4 toxicities was higher in our study compared with others, it is important to note that previous studies used alisertib as monotherapy. To improve patient tolerance, it might be reasonable to use a different dose and schedule for patients with relatively slow-growing tumors such as prostate cancer.
The efficacy of alisertib and abiraterone is difficult to assess in this phase I trial. Three patients were taken off the study because of disease progression. Seven (of 9) patients had an increase in prostate-specific antigen (PSA) during the study. The effect of alisertib on circulating tumor cell (CTC) enumeration before and after treatment was also measured. Four (of 9) patients in the trials had ≥5 CTCs at baseline, but no conversion was observed at the end of therapy. These results suggest an unfavorable efficacy to toxicity ratio of alisertib with abiraterone and prednisone. Therefore, the trial was terminated earlier, and the phase II portion was not performed.
We looked at the possible pharmacodynamic interactions between alisertib and abiraterone. The addition of alisertib did not significantly affect the total testosterone and dehydroepiandrosterone (DHEA) levels. This suggests that alisertib does not interfere with the ability of abiraterone to inhibit the biosynthesis of androgens.
For neuroendocrine biomarkers, we observed 3 (of 9) patients who had a sustained decreased in chromogranin A levels and 4 (of 9) patients who had a decrease in neuron-specific enolase levels. The significance of these changes is not clear, given the small sample size of the study. FISH analysis of collected CTCs did not demonstrate AK amplification. Further study is certainly needed to make any conclusions.
In summary, adding alisertib to an abiraterone regimen seems intolerable in mCRPC. The optimal dose and schedule of alisertib could not be determined. There was no clear signal indicating that alisertib might be beneficial for patients with mCRPC progressing on abiraterone, and further development of this treatment combination is not warranted.
Supplementary Material
Figure 1.
Effects of alisertib on androgen synthesis. (A): DHEA levels during the study treatment. (B): Total testosterone levels during the study treatment.
Abbreviations: C2, cycle 2; DHEA, dehydroepiandrosterone.
Dose-limiting toxicities
Table 1.
Summary of the phase I safety data
Table 2.
Changes of CgA and NSE levels after treatment with alisertib
Footnotes
ClinicalTrials.gov Identifier: NCT01848067
Sponsor: Thomas Jefferson University
Principal Investigator: Jianqing Lin
IRB Approved: Yes
Click here to access other published clinical trials.
Disclosures
Nancy Lewis: Novartis (E); Massimo Cristofanilli: Dompe, Vorlex, Agendia (C/A), Pfizer (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dees EC, Cohen RB, von Mehren M, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: Safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–4784. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 5.Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: A five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. doi: 10.1016/S1470-2045(15)70051-3. [DOI] [PubMed] [Google Scholar]
- 6.Stein ME, Bernstein Z, Abacioglu U, et al. Small cell (neuroendocrine) carcinoma of the prostate: etiology, diagnosis, prognosis, and therapeutic implications—a retrospective study of 30 patients from the rare cancer network. Am J Med Sci. 2008;336:478–488. doi: 10.1097/MAJ.0b013e3181731e58. [DOI] [PubMed] [Google Scholar]
- 7.Flechon A, Pouessel D, Ferlay C, et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: Results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann Oncol. 2011;22:2476–2481. doi: 10.1093/annonc/mdr004. [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Dogliotti L, Mosca A, et al. Potential clinical value of circulating chromogranin A in patients with prostate carcinoma. Ann Oncol. 2001;12(suppl 2):S153–S157. doi: 10.1093/annonc/12.suppl_2.s153. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar D, Singh SK, Mandal AK, et al. Plasma chromogranin A: Clinical implications in patients with castrate resistant prostate cancer receiving docetaxel chemotherapy. Cancer Biomark. 2010;8:81–87. doi: 10.3233/CBM-2011-0198. [DOI] [PubMed] [Google Scholar]
- 10.Loriot Y, Massard C, Gross-Goupil M, et al. Combining carboplatin and etoposide in docetaxel-pretreated patients with castration-resistant prostate cancer: A prospective study evaluating also neuroendocrine features. Ann Oncol. 2009;20:703–708. doi: 10.1093/annonc/mdn694. [DOI] [PubMed] [Google Scholar]
- 11.Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aparicio A, Tzelepi V, Araujo JC, et al. Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient’s tumor: Morphological, immunohistochemical, and gene expression profiles. Prostate. 2011;71:846–856. doi: 10.1002/pros.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buschhorn HM, Klein RR, Chambers SM, et al. Aurora-A over-expression in high-grade PIN lesions and prostate cancer. Prostate. 2005;64:341–346. doi: 10.1002/pros.20247. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa J, Miyake H, Takenaka A, et al. Persistent expression of Aurora-A after neoadjuvant hormonal therapy as a predictor of a poor clinical outcome in patients undergoing radical prostatectomy for prostate cancer. BJU Int. 2007;100:310–314. doi: 10.1111/j.1464-410X.2007.06982.x. [DOI] [PubMed] [Google Scholar]
- 15.Shu SK, Liu Q, Coppola D, et al. Phosphorylation and activation of androgen receptor by Aurora-A. J Biol Chem. 2010;285:33045–33053. doi: 10.1074/jbc.M110.121129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.