Abstract
Objective
To determine the clinical and electroencephalographic findings associated with prognosis in non-neonate children following cardiac arrest.
Design
Retrospective observational study.
Setting
Pediatric Intensive Care Unit and Cardiac Intensive Care Unit.
Patients
Non-neonate children with a history of cardiac arrest > 2 minutes and EEG monitoring within 72 hours of return of spontaneous circulation.
Measurements and Main Results
Clinical and features, neurophysiological data and Pediatric Cerebral Performance Category (PCPC) scores were collected. EEG traces were reviewed in a blinded manner, all seizures and EEG findings noted, and the EEG was scored at 1 hour, 24 hours and CEEG end. Discrete data regarding specific characteristics of the EEG background and seizures were studied. Univariate and multivariate analyses were performed to identify associations between clinical variables, EEG findings and PCPC score at hospital discharge. Multivariate analysis of 73 children revealed duration of cardiac arrest < 20 minutes or continuous EEG background activity within 12 hours post-ROSC were associated with good short term neurological outcome. Change in EEG background score over time and EEG data collected after the initial hour were not associated with outcome.
Conclusions
Following pediatric cardiac arrest, an initially normal EEG or generalized slowing of the EEG background was associated with good neurologic outcome at hospital discharge.
Keywords: seizures, cardiac arrest, EEG, coma, prognosis, pediatric
Introduction
Clinicians are often asked to prognosticate regarding the potential for severe neurological impairment in children following cardiac arrest, in whom significant long-term neurological disability or death often occurs (1). Numerous investigations into the capabilities of clinical features, laboratory markers, imaging characteristics and electrophysiological testing have yielded mixed results in children (2, 3). Adult data is often more robust and has led to consensus statements regarding prognostication in survivors of cardiac arrest (4, 5), whereas no current analogue exists for children.
Electroencephalograms (EEG) have been extensively utilized to aid prognostication and monitoring for nonconvulsive seizures in adults and children following cardiac arrest (6, 7) and in neonates with hypoxic-ischemic encephalopathy (8), yet few studies have reported non-neonate pediatric EEG findings associated with neurological outcome (9, 10). The potential presence or absence of seizures further complicates prognostication following cardiac arrest, as electroclinical and electrographic-only seizures occur have been reported in 36–44% of children following cardiac or respiratory arrest (11–13). Unfortunately, previous reports have not evaluated the effect of seizure burden on prognosis in children, apart from discrete epochs during continuous EEG (CEEG) or with intermittent EEG (9).
Prognostic guidance from EEG data in adults following cardiac arrest has been clearer, yet remains imperfect (4). Features of EEG occurring at varying time points highly associated with poor outcome include absence of reactivity, low voltage, persistent burst suppression, periodic epileptiform discharges and status epilepticus, although significant exceptions have also been reported (4, 5). Post-anoxic myoclonus and myoclonic status has been highly associated with poor neurological outcome, most notably when associated with epileptiform activity (5, 14). Other studies have found a lack of correlation between “malignant EEG patterns” and neurological outcome in adults treated with hypothermia after cardiac arrest (15). EEG obtained within the first 24 hours in adults with hypoxic coma is more predictive than later EEG findings (16). More recent recommendations include using a combination of absence of EEG reactivity, presence of burst-suppression or status epilepticus ≥72 hours after return of spontaneous circulation (ROSC) to predict poor outcome (4), whereas older AAN guidelines (5) concluded the presence of either generalized suppression, burst-suppression with generalized epileptiform activity or generalized periodic complexes on a flat background were suggestive of poor outcome.
We aimed to study the relationship between EEG findings and neurological outcome at discharge in children following cardiac arrest. We hypothesized that individuals with poor short term neurological outcomes would be more likely to have worsening EEG findings or EEGs containing poor reactivity, voltage suppression, a high seizure burden or status epilepticus compared to those individuals with improving EEGs or EEGs with reactivity, normal voltage or lower seizure burden.
Materials and Methods
A retrospective cohort review of children with a history of cardiac arrest admitted to the pediatric intensive care unit (PICU) or cardiac intensive care unit (CICU) at St. Louis Children’s Hospital was performed under an institutional review board-approved human studies protocol at the Washington University School of Medicine. To identify patients with cardiac arrest, we queried our institution’s Virtual PICU Systems (VPS, LLC) database from 2009–2014, searching for a diagnosis of cardiac arrest at the time of admission or at any point during the ICU stay. The VPS database is a multicenter database of PICUs, and is used primarily for benchmarking and quality assessments (17). VPS data were provided by VPS, LLC. No endorsement or editorial restriction of the interpretation of these data has been implied or stated. Inclusion criteria were: age at hospital admission >44 weeks post-conceptual age and <18 years, diagnosis of cardiac arrest with chest compressions for >2 minutes and EEG within 72 hours of return of spontaneous circulation (ROSC). Exclusion criteria were primary diagnosis of head trauma, neurosurgical intervention, or post-ROSC pharmacologic coma.
Baseline characteristics were obtained by chart review and included: age; gender; ICU location; arrest location, etiology and duration; initial cardiac rhythm; treatment with therapeutic hypothermia or extracorporeal membrane oxygenation; preexisting comorbidities such as seizure disorder, developmental delay, previous stroke or previous neurosurgical procedure; interval between cardiac arrest and EEG; antiseizure medications, neuromuscular blockade and sedation medications at time of EEG; sodium, calcium, pH, aspartate transaminase (AST) and alanine transaminase (ALT) at time of EEG.
Electroencephalography was performed as clinically indicated using either a Stellate Harmonie (Natus; Pleasanton, CA, USA) or Nihon Kohden (Tokyo, Japan) digital EEG system using an extended international 10–20 electrode placement with modifications when necessary. One ACGME fellowship-trained neurophysiologist (A.P.O.) performed the blinded retrospective interpretation of CEEG. One reviewer was deemed sufficient, as previous studies have demonstrated significant or moderate inter-observer reproducibility for the major scored EEG components in this study, such as overall interpretation, continuity, burst suppression and seizures (18). Standardized American Clinical Neurophysiology Society (ACNS) CCEEG terminology (19) was utilized and the reviewer had previously passed the ACNS ICU EEG terminology certification test. The recordings were analyzed qualitatively and scored similar to previous studies (9, 10, 20) as follows: 0, normal; 1, slow and organized (i.e. contained sleep architecture or posterior dominant rhythm); 2, slow and disorganized; 3, discontinuous; 4, burst suppression; 5, suppression. Normal age-specific ranges for posterior dominant EEG background rhythms were: 3–4 Hz at 6 months; 4–6 Hz from 12 months to three years; 6–8 Hz from three to six years; greater than 8.5 Hz after six years (10, 21). Additional EEG characteristics recorded included: frequency and duration of seizures and status epilepticus; reactivity; variability; and EEG duration. Seizure frequency was determined using the maximum seizure burden per hour and categorized as low seizure burden (seizures occurring less than 12 minutes per hour), moderate seizure burden (seizures occurring between 12 and 29 minutes per hour) and status epilepticus. Normal voltage was defined as > 20 microvolts. Recordings were reviewed in their entirety.
We utilized two approaches for analyzing EEG results with respect to neurological outcome. In order to assess the relationship between timing of EEG findings following ROSC, we analyzed EEG findings in the first hour of monitoring relative to ROSC. However, this approach did not adequately assess the association of neurological outcomes and timing of EEG findings within an EEG monitoring session. Therefore, in an attempt to address the question of impact of duration of EEG, we analyzed EEG findings with respect to the start of EEG monitoring. This approach also preserved the blinded re-interpretation of EEG. Specific time points were scored at one hour following EEG initiation, 24 hours after EEG initiation and EEG end. If a new EEG finding (i.e.: seizure, epileptiform discharge, etc.) occurred, it was documented at the next point in order to capture all seizures or other transient changes that may have impacted outcome measures. For instance, if a seizure occurred during hour 12, it was recorded at the 24-hour or EEG end time point for statistical comparisons. Study data were collected and managed using REDCap electronic data capture tools hosted at Washington University in St. Louis (22).
The primary outcome measured was neurological outcome as determined by PCPC score (23). The categories are as follows: 1, normal; 2, mild disability; 3, moderate disability; 4, severe disability; 5, coma and vegetative state; 6, death. Admission PCPC score, PICU discharge PCPC score and hospital discharge PCPC score were determined through retrospective chart review. Poor outcome was categorized as decrease in PCPC score from admission to discharge by > 1 or discharge PCPC scores of 4–6 if not preexisting. Other outcome variables included PICU length of stay and hospital length of stay. Differences between means were determined using Student’s t-test for normally distributed data or the Mann-Whitney U test for non-normally distributed data, as appropriate. Differences in proportions were determined using the Fisher’s exact test. To identify the factors associated with outcome, we performed logistic regression with outcome as the dependent variable and demographic, clinical care, and EEG variables as the independent variables. A Bonferroni correction was applied to univariate analysis with statistical significance set a p < 0.002.
All variables with p ≤ 0.2 on univariate testing were eligible for inclusion in the multivariable model. To determine the association between results of EEG monitoring and neurological outcome, we created the model in stepwise fashion. First, we entered all patient and non-EEG clinical variables found to be significant on univariate testing into the model. This model was then adjusted, using stepwise evaluation of all variables for significance and model fit. Next, we added the additional EEG variables from the first hour of monitoring found to be significant on univariate testing. We then re-tested the model for significance and goodness of fit. We assigned statistical significance at a p-value ≤0.05. All analyses were conducted using STATA (STATA Corp, College Station, TX).
Results
Data were collected from 73 individuals ages 1.6 to 242 months (mean and median ages of 70 months and 31 months, respectively). Clinical characteristics of children with good neurological outcome and poor neurological outcome are summarized in Table 1. Longer duration of cardiac arrest was associated with poor neurological outcome after multivariable analysis. Several clinical characteristics significant on univariate analysis were likely related to duration of arrest, including: initial cardiac rhythm, initial AST level, treatment with therapeutic hypothermia, and treatment with ECMO. Children receiving dexmedetomidine at time of EEG initiation were more likely to have good neurological outcome, and children receiving lorazepam at the time of EEG initiation were more likely to have a poor outcome but neither reached statistical significance after Bonferroni correction. All other antiseizure and sedative medications usage was similar between the two groups. Children with poor outcome had hospital LOS roughly a third the duration of children who were discharged with a good outcome (p=0.001).
Table 1.
Clinical characteristics and neurological outcomes.
| Good Neurological Outcome (n=23) |
Poor Neurological Outcome (n=50) |
p value | |
|---|---|---|---|
| Age in months, mean (SD) | 83.4 (83.4) | 65.0 (76.5) | 0.17 |
| Male gender, n (%) | 12 (52.2) | 27 (54.0) | 1.00 |
| Arrest location: | 0.62 | ||
| IHCA, n (%) | 10 (43.5) | 26 (52.0) | |
| OHCA n(%) | 13 (56.5) | 24 (48.0) | |
| ICU location: | 0.61 | ||
| PICU, n (%) | 15 (65.2) | 28 (56.0) | |
| CICU, n (%) | 8 (34.8) | 22 (44.0) | |
| Arrest etiology, n (%) | 0.63 | ||
| Asphyxia/respiratory | 13 (56.5) | 21 (42.0) | |
| Cardiac | 7 (30.4) | 23 (46.0) | |
| Near drowning | 1 (4.4) | 3 (6.0) | |
| Other | 1 (4.4) | 1 (2.0) | |
| Unknown | 1 (4.4) | 2 (4.0) | |
| Arrest duration in minutes, mean (SD) | 12.4 (9.4) | 36.2 (24.0) | 0.001 |
| ROSC to EEG onset interval (hours), median (IQR) [range] |
18 (6,21) [3.8 – 66.7] |
10 (6,15) [4.1 – 60.0] |
0.09 |
| ROSC to EEG onset interval n (%) | 0.17 | ||
| Less than or equal to 12 hours | 10 (43.5) | 33 (66.0) | |
| Between 12 and less than or equal to 24 Hours |
8 (34.8) | 11 (22.0) | |
| Longer than 24 hours | 5 (21.7) | 6 (12.0) | |
| Initial Cardiac Rhythm | 0.03 | ||
| Pulseless electrical activity | 3 (13.0) | 12 (24.0) | |
| Ventricular fibrillation/tachycardia | 3 (13.0) | 5 (10.0) | |
| Bradycardia | 11 (47.8) | 13 (26.0) | |
| Asystole | 0 | 12 (24.0) | |
| Unknown | 6 (26.1) | 8 (16.0) | |
| Therapeutic hypothermia: yes, n (%) | 3 (13.0) | 20 (40.0) | 0.03 |
| Admission PCPC, n (%) | |||
| ≤ 4 | 23 (100) | 50 (100) | N/A |
| ≥ 5 | 0 | 0 | N/A |
| ECMO: yes, n (%) | 3 (13.1) | 19 (38.0) | 0.04 |
| Pre-existing conditions, n (%) | 0.36 | ||
| None | 15 (65.2) | 40 (80.0) | |
| Epilepsy | 4 (17.4) | 5 (10.0) | |
| Neurodevelopmental delay | 3 (13.1) | 4 (8.0) | |
| Previous stroke | 0 | 1 (2.0) | |
| Previous intracranial procedure | 1 (4.4) | 0 | |
| Medications at EEG onset, n (%) | |||
| Midazolam | 10 (43.5) | 25 (50.0) | 0.63 |
| Lorazepam | 0 | 9 (18.0) | 0.05 |
| Levetiracetam | 5 (21.7) | 9 (18.0) | 0.75 |
| Fosphenytoin | 2 (8.7) | 6 (12.0) | 1.00 |
| Phenobarbital | 3 (13.1) | 8 (16.0) | 0.74 |
| Valproic acid | 2 (8.7) | 1 (2.0) | 0.23 |
| Pentobarbital | 0 | 0 | N/A |
| Neuromuscular blockade | 9 (39.1) | 19 (38.0) | 1.00 |
| Narcotic infusion | 17 (73.9) | 35 (70.0) | 0.79 |
| Dexmedetomidine | 6 (26.1) | 4 (8.0) | 0.06 |
| Laboratory findings at EEG onset | |||
| Serum sodium (mmol/L), mean (SD) | 144 (10) | 144 (6.1) | 0.83 |
| Ionized calcium (mg/dL), mean (SD) | 4.9 (0.8) | 4.8 (0.6) | 0.58 |
| Serum calcium (mg/dL), mean (SD) | 8.9 (1.4) | 8.9 (1.4) | 0.86 |
| Serum pH, mean (SD) | 7.35 (0.06) | 7.33 (0.12) | 0.88 |
| AST (units/L), mean (SD) | 207 (322) | 444 (558) | 0.02 |
| ALT (units/L), mean (SD) | 95 (123) | 199 (273) | 0.28 |
| Length of Stay (LOS) | |||
| PICU LOS (days), median (IQR) | 12 (4,23) | 7 (3,17) | 0.32 |
| Hospital LOS (days), median (IQR) | 21 (15,64) | 7 (3,19) | 0.001 |
SD = standard deviation; IHCA = in-hospital cardiac arrest; OHCA = out-of-hospital cardiac arrest; ICU = intensive care unit; PICU = pediatric intensive care unit; CICU = cardiac intensive care unit; PCPC = Pediatric Cerebral Performance Category score; ECMO = extracorporeal membrane oxygenation; AST = aspartate transaminase; ALT = alanine transaminase; LOS = length of stay; IQR = interquartile range
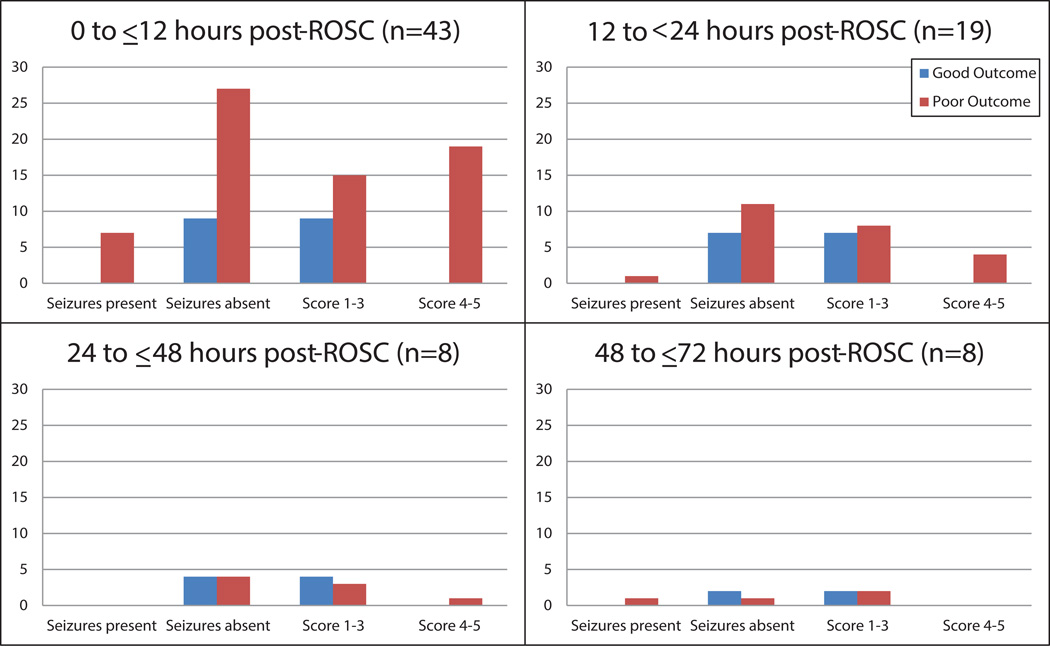
Neurophysiologic findings at 12 hours post-ROSC including a background score of 0–1, normal voltage, reactivity, and variability were significantly associated with good outcome on univariate analysis (Table 2). During the first hour of EEG, lower overall EEG background score or the presence of normal voltage, reactivity and variability correlated with good outcome on univariate analysis. All patients with seizures within the first hour of EEG monitoring or within 24-hours following ROSC (Figure 1) had poor outcomes in this cohort but did not reach statistical significance (Table 3). All individuals with an overall score of 4 or 5, indicating a burst suppressed or suppressed EEG within 12 hours of ROSC, had poor outcome (Figure 1). At greater than 24 hours, all eight individuals with overall EEG background scores ≥4 had a poor neurological outcome. All six patients with myoclonic status epilepticus at any point during EEG monitoring were discharged with the poor neurological outcomes of coma or vegetative state (one individual) or death, a result that did not reach statistical significance (p = 0.17).
Table 2.
Electroencephalogram (EEG) findings during first hour of EEG monitoring and neurological outcomes
| Good Outcome (n=23) |
Poor Outcome (n=50) |
p value | |
|---|---|---|---|
| Interval between ROSC and EEG initiation ≤ 12 hr | n=10 EEGs | n=33 EEGs | |
| EEG background score, n (%) | 0.008 | ||
| Score 0–1 (normal or slow and organized) | 8 (80.0) | 7 (21.2) | |
| 2 (slow and disorganized) | 2 (20.0) | 6 (18.2) | |
| 3 (discontinuous) | 0 | 2 (6.1) | |
| 4 (burst suppression) | 0 | 8 (24.2) | |
| 5 (suppression) | 0 | 10 (30.3) | |
| Normal voltage | 10 (100) | 12 (36.4) | 0.001 |
| Reactive | 6 (60.0) | 6 (18.2) | 0.017 |
| Variable | 6 (60.0) | 6 (18.2) | 0.017 |
| Seizures, n (%) | n=0 seizures | n=7 seizures | 0.13 |
| Low seizure burden | 0 | 1 (14.3) | |
| Moderate seizure burden | 0 | 1 (14.3) | |
| Status epilepticus | 0 | 5 (71.4) | |
| Interval between ROSC and EEG initiation > 12 ≤ 24 hr | n=8 EEGs | n=11 EEGs | |
| EEG background score, n (%) | 0.13 | ||
| Score 0–1 (normal or slow and organized) | 4 (50.0) | 3 (27.3) | |
| 2 (slow and disorganized) | 4 (50.0) | 4 (36.4) | |
| 3 (discontinuous) | 0 | 0 | |
| 4 (burst suppression) | 0 | 1 (9.1) | |
| 5 (suppression) | 0 | 3 (27.3) | |
| Normal voltage | 6 (75.0) | 7 (63.6) | 1.00 |
| Reactive | 5 (62.5) | 3 (27.3) | 0.18 |
| Variable | 5 (62.5) | 3 (27.3) | 0.18 |
| Seizures, n (%) | n=0 seizures | n=1 seizure | 0.58 |
| Low seizure burden | 0 | 0 | |
| Moderate seizure burden | 0 | 0 | |
| Status epilepticus | 0 | 1 (9.1) | |
| Interval between ROSC and EEG initiation > 24 hr | n=5 EEGs | n=6 EEGs | |
| EEG background, n (%) | 0.26 | ||
| Score 0–1 (normal or slow and organized) | 5 (100) | 3 (50.0) | |
| 2 (slow and disorganized) | 0 | 2 (33.3) | |
| 3 (discontinuous) | 0 | 0 | |
| 4 (burst suppression) | 0 | 0 | |
| 5 (suppression) | 0 | 1 (16.7) | |
| Normal voltage | 5 (100) | 4 (66.7) | 0.46 |
| Reactive | 4 (80.0) | 3 (50.0) | 0.55 |
| Variable | 4 (80.0) | 3 (50.0) | 0.55 |
| Seizures, n (%) | n=0 seizures | n=1 seizure | 0.55 |
| Low seizure burden | 0 | 0 | |
| Moderate seizure burden | 0 | 1 (16.7) | |
| Status epilepticus | 0 | 0 |
Low seizure burden = seizures occurring less than 12 minutes per hour; moderate seizure burden = seizures occurring between 12 and 29 minutes per hour; status epilepticus = greater than 30 minutes of seizure per hour
Figure 1.
One-hour EEG findings by elapsed time post-ROSC. The vast majority of EEGs were obtained within 24 hours following ROSC. All patients with seizures, myoclonic status epilepticus or EEG background scores of 4–5 had poor outcome, regardless of timing of EEG.
Table 3.
Electroencephalogram (EEG) findings by duration of EEG and neurological outcomes
| Good Outcome (n=23) |
Poor Outcome (n=50) |
p value | |
|---|---|---|---|
| EEG duration (hours), median (IQR) | 25 (11,83) | 29 (1,66) | 0.71 |
| EEG background score: 1 hour, n (%) | n=23 EEGs | n=50 EEGs | < 0.001 |
| Score 0–1 (normal or slow/organized) | 17 (73.9) | 13 (26.0) | |
| 2 (slow and disorganized) | 6 (26.1) | 12 (24.0) | |
| 3 (discontinuous) | 0 | 2 (4.0) | |
| 4 (burst suppression) | 0 | 9 (18.0) | |
| 5 (suppression) | 0 | 14 (28.0) | |
| Normal voltage | 21 (91.3) | 23 (46.0) | < 0.001 |
| Reactive | 15 (65.2) | 12 (24.0) | 0.001 |
| Variable | 15 (65.2) | 12 (24.0) | 0.001 |
| Total number of patients with seizures in first hour, n (%) |
0 | 9 (18.0) | 0.05 |
| EEG background score: 24 hour | n=12 EEGs | n=28 EEGs | 0.36 |
| Score 0–1 | 7 (30.4) | 12 (42.8) | |
| 2 | 5 (21.7) | 8 (28.6) | |
| 3 | 0 | 0 | |
| 4 | 0 | 5 (17.9) | |
| 5 | 0 | 3 (10.7) | |
| Normal voltage | 10 (83.3) | 17 (60.7) | 0.27 |
| Reactive | 8 (66.7) | 13 (46.4) | 0.31 |
| Variable | 8 (66.7) | 13 (46.4) | 0.31 |
| Total number of patients with seizures in first 24 hours, n (%) |
1 (4.3) | 10 (20.0) | 0.16 |
| EEG backgroundscore: >24 hours | n=12 EEGs | n=28 EEGs | 0.63 |
| Score 0–1 | 8 (66.7) | 15 (53.6) | |
| 2 | 4 (33.3) | 9 (32.1) | |
| 3 | 0 | 2 (7.1) | |
| 4 | 0 | 7 (25.0) | |
| 5 | 0 | 5 (17.9) | |
| Normal voltage | 10 (83.3) | 22 (78.6) | 1.00 |
| Reactive | 10 (83.3) | 14 (50.0) | 0.08 |
| Variable | 10 (83.3) | 14 (50.0) | 0.08 |
| Total number of patients with seizures recorded after first day, n (%) |
2 (16.7) | 2 (7.1) | 0.57 |
| Total number of patients with seizures recorded at any time point |
2 (8.7) | 11 (22.0) | 0.21 |
| Myoclonic status epilepticus | 0 | 6 (12.0) | 0.17 |
Following univariate regression analysis, nine patient or clinical care variables and eight EEG variables met our significance threshold for inclusion in multivariable testing. In the first model using patient and clinical care variables, use of lorazepam was removed due to its perfect correlation with poor outcome. The remaining clinical variables were entered into the model and removed in a stepwise fashion until we identified a model with best fit (Table 4, Model 1). This final model included only two variables, each associated with increased odds of good outcome (six-fold increase in the odds of good outcome with arrest duration < 20 minutes and ten-fold increase in the odds of a good outcome with use of a dexmedetomidine infusion for clinical sedation). Although the model was statistically significant (chi square statistic with 2 degrees of freedom = 13.1, p=0.001), it had only fair performance (r2=0.25, ROC=0.78). Pearson’s chi square goodness of fit testing had a p=0.02, indicating poor overall fit. We then added the EEG variables, and identified a second model with improved fit (Table 4, Model 2). In this model, two variables were removed for their perfect correlation with poor outcome: use of lorazepam and seizures in the first hour of EEG monitoring. This second model included the initially identified clinical variables as well as normal or near-normal EEG background in the first hour, which was associated with a four-fold increase in the odds of good outcome (Table 4). This second model was also significant (chi square statistic with 3 degrees of freedom = 16.5, p=0.0009), and had better performance than the first model (r2=0.36, ROC=0.81). On goodness of fit testing, this model had a Pearson’s chi square goodness of fit p=0.21, indicating good overall fit.
Table 4.
Multivariable logistic regression exploring the relationship of clinical variables with patient outcome.
| Model 1: Clinical Variables Only | |||
|---|---|---|---|
| Variable | Odds Ratio of good outcome |
95% Confidence Interval |
p-value |
| Arrest duration < 20 minutes | 6.1 | 1.7–22.2 | 0.006 |
| Dexmedetomidine infusion | 10.1 | 1.5–68.2 | 0.02 |
| Model 2: Clinical and EEG Variables | |||
|---|---|---|---|
| Variable | Odds Ratio of good outcome |
95% Confidence Interval |
p-value |
| Arrest duration < 20 minutes | 3.8 | 0.96–15.1 | 0.06 |
| Dexmedetomidine infusion at time of EEG |
8.2 | 1.2–57.1 | 0.03 |
| Normal/slow EEG background in first hour of EEG monitoring |
4.1 | 1.1–15.9 | 0.04 |
Model 1 notes:
1. Lorazepam administration was removed from the model due to perfect prediction of poor outcome.
2. Model characteristics: chi square with 2 degrees of freedom = 13.1, p=0.001; r2 = 0.25; ROC = 0.78; Pearson’s chi square goodness of fit p=0.02.
3. Classification table results: model correctly classified 75.9% of subjects.
Model 2 notes:
1. The following variables were removed from the model due to perfect prediction of poor outcome: (1) lorazepam infusion and (2) seizures within the first hour of EEG monitoring
2. Model characteristics: chi square with 3 degrees of freedom = 16.5, p=0.0009; r2 = 0.36; ROC = 0.81; Pearson’s chi square goodness of fit p=0.21.
3. Classification table results: model correctly classified 75.9% of subjects.
Change over time in an individual’s EEG characteristics was also examined for association with neurological outcome. EEG data at one hour and 24 hours were available for 40/73 individuals (55%). Improving or worsening of overall EEG background score, voltage, or reactivity/variability did not correlate with neurological outcome (p=0.857, p=1.00 and p=0.255, respectively). The development or abatement of seizures between the two time points was rare and not analyzed.
Discussion
Limitations in the pediatric data regarding prognostication following cardiac arrest necessitate further study, and our report aims to address several. First, the prognostic utility and timing or duration of EEG following cardiac arrest is not well established in children. Previous studies utilized epochs from EEG traces started “as soon as the patient was stabilized” (9) or within seven days following cardiac arrest (10). A heterogeneous study of children with hypoxic ischemic encephalopathy utilized routine EEG within 24 hours of admission (20). Our data reveal an association between good prognosis and the presence of a normal EEG or generalized slowing of the EEG background during the first hour of EEG when obtained within the first 12 hours of ROSC (Table 2), thus more clearly defining the timeframe during which EEG findings may be of more prognostic significance.
Previous reports have utilized intermittent EEG (10, 20) or limited epochs from CEEG (9). This analytic technique minimizes a significant strength of CEEG, the dynamic and continuous nature of data collection and exposes these data to omission of transient events, such as seizures, rhythmic or periodic patterns or transitory changes in EEG background. We present a large cohort of which 73% percent received EEG monitoring greater than one hour. We evaluated change in EEG variables in a continuous fashion that may have been omitted in previous studies, although standard epochs at one hour and 24 hours were used for some statistical comparisons.
Increasingly, data are revealing the association between poor neurological outcome in children and the presence of moderate seizure burden (>12 minutes/hour) or status epilepticus (>30 minutes/hour) (13, 24). The majority of patients in our cohort with seizures detected on EEG had poor neurologic outcome. However, the small number of patients with seizures did not reveal statistically significant associations between seizures and poor neurologic outcome. All studied individuals with myoclonic status epilepticus had poor neurological outcome, although this was not statistically significant due to small numbers. This finding is supportive of some previous adult guidelines (5), but has not been reported in children following cardiac arrest. While several clinical variables available without EEG monitoring were associated with outcome on univariate analysis, our study results emphasize the added utility of EEG monitoring in prognostication after cardiac arrest. Our multivariable model had better performance and goodness of fit when EEG variables were included.
A controversial aspect of neurophysiological monitoring of children in the intensive care unit remains the EEG duration. Studies focused on adults and children have primarily focused on the duration of EEG required to identify seizures and have supported both shorter duration EEG (25, 26) and more prolonged EEG monitoring (6, 12). Our data regarding the prognostic value of EEG emphasizes the initial hour of EEG monitoring, while no EEG characteristic examined at 24 hours was found to have significant association with neurological outcome (Table 3). Furthermore, changes in EEG characteristics over time were not associated with neurological outcome. Thus, a one-hour EEG may be adequate for prognostic purposes.
Several pediatric studies have reported specific EEG findings associated with prognosis in non-neonate children following cardiac arrest (9, 10) or more heterogeneous cohorts, including hypoxic ischemic coma, head trauma and near drowning events (27–32). Kessler and colleagues prospectively obtained CEEG in 35 children receiving therapeutic hypothermia following cardiac arrest and found poor outcomes were more likely in individuals with continuous but unreactive tracings or with discontinuous or isoelectric tracings during both hypothermia and after rewarming to normothermia (9). A retrospective review of EEG from the seven days following cardiac arrest in 34 children noted discontinuous or isoelectric EEGs were correlated with poor neurologic outcome (10). Children with hypoxic ischemic encephalopathy having discontinuous EEG backgrounds or generalized epileptiform discharges on routine EEG were more likely to have unfavorable neurological outcome (20). Older studies have also reported poor prognosis in children associated with high voltage slowing (28, 29, 33), absence of reactivity (30, 33), poor variability (29, 33), sharp waves (29), burst suppression (28, 29) and marked voltage attenuation (27). Positive outcomes in these children have been associated with rapid EEG improvement (31), reactivity (29), and normal sleep patterns (27, 29).
Our study reinforces the validity of some previous correlations between EEG findings and outcomes. Univariate analysis identified lower overall EEG background score and the presence of normal voltage, reactivity and/or variability correlated with good outcome. Multivariable analysis reinforced that a normal or slow EEG background during the first one-hour of EEG monitoring, especially within the first 12 hours post-ROSC, correlated with good neurologic outcome.
Previous reports focused on the prognosis of EEG findings in similar cohorts did not provide extensive clinical information. We utilized univariate (Table 1) and multivariable analysis (Table 4) of more comprehensive clinical data. Lorazepam administration at the time of EEG monitoring was more common in children with poor outcome. Good neurological outcome was associated with dexmedetomidine infusion at the time of EEG monitoring on univariate analysis and cardiac arrest duration less than 20 minutes on multivariate analysis. The relationship between sedation administration and outcomes likely reflects the link between seizures and outcomes more than treatment effect, as lorazepam is commonly used in patients having suspected or definite seizures or myoclonus. The correlation between shorter duration of cardiac arrest and better neurological outcomes in our cohort upholds previous data (34), but includes the further context of neurophysiological data.
We included children treated with hypothermia and normothermia, as temperature likely did not impact EEG findings. Although children treated with hypothermia in our cohort were statistically more likely to have a poor outcome, this likely reflects the severity of illness and not EEG related findings. The use of therapeutic hypothermia following cardiac arrest has been shown not to improve outcomes in children (35) and adults (36) and EEG findings modulated by moderate hypothermia are likely minimal (37). Thus, the heterogeneity of temperature in this cohort is unlikely to have significantly influenced the data collection or outcomes.
The current study is limited in several respects but provides clinical guidance and possible directions for future study. While our data set is large compared to similar studies, these data should be interpreted with some degree of caution. For example, all patients in our cohort with burst suppression or voltage attenuation during the first hour of EEG had poor outcome, yet the number of patients is not large enough to predict a similar outcome for future patients. Our data may be clinically helpful as a biomarker for illness severity and used to guide to future studies, but should not be used in isolation to determine goals of care.
We analyzed EEG data at variable time points following ROSC and reported the findings based on EEG duration rather than elapsed time after ROSC, mainly due to the blinded nature of the retrospective EEG review and in an attempt to analyze the added yield of more prolonged EEG. We attempted to overcome this limitation by then examining the significant EEG characteristics in the first hour of EEG in discrete post-ROSC periods (Table 2 and Figure 1) and demonstrated these remained substantially different in the first 12 hours post-ROSC. Another limitation is EEG data after 24 hours were available for only 40/73 (55%) of children, diminishing the power of more prolonged EEG in this study and potentially underestimating the utility in more prolonged EEG. However, the proportion of individuals with good outcome and prolonged EEG (52%) and those with poor outcome and prolonged EEG (56%) was nearly exactly matched. As has been noted in previous studies using EEG (10) or other prognostication methods following cardiac arrest (38), the EEG variables or other clinical features may have led to a self-fulfilling prophecy and severely abnormal results may have influenced the family and clinical team’s decision to redirect care. Furthermore, a single or limited group of outcome measures over short duration may have less prognostic power than longer-term follow-up utilizing multiple neurocognitive measures or biomarkers of neurologic injury (39).
Conclusions
Our findings further delineate the role of EEG in children following cardiac arrest. Good prognostic indicators include a shorter duration of cardiac arrest and continuous EEG background activity within the first 12 hours post-ROSC. Less additional prognostic value may be derived from prolonged EEG monitoring or EEG monitoring greater than 24 hours post-ROSC. Although these data should not be solely relied upon for the guidance of goals of care, they provide insight into the neurological prognosis of these critically ill children and may assist in optimizing the timing and duration of EEG.
Acknowledgments
Source of Funding: This study was performed without direct financial support. Institutional REDCap support is provided through Clinical and Translational Science Award (CTSA) Grant No. UL1 TR000448 and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant No. P30 CA091842.
None
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
References
- 1.Young KD, Gausche-Hill M, McClung CD, et al. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114:157–164. doi: 10.1542/peds.114.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Kirschen MP, Topjian AA, Hammond R, et al. Neuroprognostication after pediatric cardiac arrest. Pediatr Neurol. 2014;51:663.e2–668.e2. doi: 10.1016/j.pediatrneurol.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–39. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- 4.Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: An advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014:1779–1789. doi: 10.1016/j.resuscitation.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Wijdicks EFM, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 6.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2015;32:96–108. doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Laerhoven H, de Haan TR, Offringa M, et al. Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review. Pediatrics. 2013;131:88–98. doi: 10.1542/peds.2012-1297. [DOI] [PubMed] [Google Scholar]
- 9.Kessler SK, Topjian AA, Gutierrez-Colina AM, et al. Short-term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocrit Care. 2011;14:37–43. doi: 10.1007/s12028-010-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishisaki A, Sullivan J, 3rd, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med. 2007;8:10–17. doi: 10.1097/01.pcc.0000256621.63135.4b. [DOI] [PubMed] [Google Scholar]
- 11.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jette N, Claassen J, Emerson RG, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 13.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seder DB, Sunde K, Rubertsson S, et al. Neurologic outcomes and postresuscitation care of patients with myoclonus following cardiac arrest. Crit Care Med. 2015;43:965–972. doi: 10.1097/CCM.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 15.Amorim E, Rittenberger JC, Baldwin ME, et al. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–132. doi: 10.1016/j.resuscitation.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmeijer J, Beernink TMJ, Bosch FH, et al. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology. 2015;85:137–143. doi: 10.1212/WNL.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetzel RC, Sachedeva R, Rice TB. Are all ICUs the same? Paediatr Anaesth. 2011;21:787–793. doi: 10.1111/j.1460-9592.2011.03595.x. [DOI] [PubMed] [Google Scholar]
- 18.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2011;28:15–19. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 20.Mandel R, Martinot A, Delepoulle F, et al. Prediction of outcome after hypoxic-ischemic encephalopathy: a prospective clinical and electrophysiologic study. J Pediatr. 2002;141:45–50. doi: 10.1067/mpd.2002.125005. [DOI] [PubMed] [Google Scholar]
- 21.Current Practice of Clinical Electroencephalography. Fourth. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 24.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crepeau AZ, Rabinstein AA, Fugate JE, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80:339–344. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 27.Tasker RC, Boyd S, Harden A, et al. Monitoring in non-traumatic coma. Part II: Electroencephalography. Arch Dis Child. 1988;63:895–899. doi: 10.1136/adc.63.8.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pampiglione G, Harden A. Resuscitation after cardiocirculatory arrest. Prognostic evaluation of early electroencephalographic findings. Lancet. 1968;1:1261–1265. doi: 10.1016/s0140-6736(68)92287-3. [DOI] [PubMed] [Google Scholar]
- 29.Cheliout-Heraut F, Sale-Franque F, Hubert P, et al. Cerebral anoxia in near-drowning of children. The prognostic value of EEG. Neurophysiol Clin Clin Neurophysiol. 1991;21:121–132. doi: 10.1016/s0987-7053(05)80066-8. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandrannair R, Sharma R, Weiss SK, et al. Reactive EEG patterns in pediatric coma. Pediatr Neurol. 2005;33:345–349. doi: 10.1016/j.pediatrneurol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Pampiglione G, Chaloner J, Harden A, et al. Transitory ischemia/anoxia in young children and the prediction of quality of survival. Ann N Y Acad Sci. 1978;315:281–292. doi: 10.1111/j.1749-6632.1978.tb50346.x. [DOI] [PubMed] [Google Scholar]
- 32.Evans BM, Bartlett JR. Prediction of outcome in severe head injury based on recognition of sleep related activity in the polygraphic electroencephalogram. J Neurol Neurosurg Psychiatry. 1995;59:17–25. doi: 10.1136/jnnp.59.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janati A, Erba G. Electroencephalographic correlates of near-drowning encephalopathy in children. Electroencephalogr Clin Neurophysiol. 1982;53:182–191. doi: 10.1016/0013-4694(82)90022-0. [DOI] [PubMed] [Google Scholar]
- 34.Matos RI, Watson RS, Nadkarni VM, et al. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127:442–451. doi: 10.1161/CIRCULATIONAHA.112.125625. [DOI] [PubMed] [Google Scholar]
- 35.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic Hypothermia after Out-of-Hospital Cardiac Arrest in Children. N Engl J Med. 2015 doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 37.Abend NS, Mani R, Tschuda TN, et al. EEG monitoring during therapeutic hypothermia in neonates, children, and adults. Am J Electroneurodiagnostic Technol. 2011;51:141–164. [PMC free article] [PubMed] [Google Scholar]
- 38.Greer DM, Rosenthal ES, Wu O. Neuroprognostication of hypoxic-ischaemic coma in the therapeutic hypothermia era. Nat Rev Neurol. 2014;10:190–203. doi: 10.1038/nrneurol.2014.36. [DOI] [PubMed] [Google Scholar]
- 39.Becker LB, Aufderheide TP, Geocadin RG, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124:2158–2177. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]