Abstract
Objective
To evaluate the use of adjuvant therapy after primary surgery for stage I–III uterine carcinosarcoma (CS).
Methods
A multi-institutional retrospective study of women with stage I–III CS was conducted. Analyses were stratified by stage (I/II and III). Patients were categorized according to adjuvant therapy: observation (OBS), radiation (RT), chemotherapy (CT) or multimodal therapy (CT + RT). Overall survival (OS) and progression-free survival (PFS) were analyzed using log-rank tests and Cox proportional hazards models.
Results
303 patients were identified across four institutions: 195 with stage I/II and 108 with stage III disease. In stage I/II disease, 75 (39.9%) received OBS, 33 (17.6%) CT, 37 (19.7%) RT, and 43 (22.9%) CT + RT. OBS was associated with a fourfold increased risk of death compared to CT (adjusted hazard ratio (aHR) = 4.48, p = 0.003). Patients receiving CT + RT had significantly improved PFS compared to those receiving CT alone (aHR = 0.43, p = 0.04), but no difference in OS. In the stage III cohort, 16 (15.0%) received OBS, 34 (31.8%) CT, 20 (18.7%) RT, and 37 (34.6%) CT + RT. OBS was associated with worse OS and PFS compared to CT (OS: aHR = 2.46, p = 0.04; PFS: aHR = 2.39, p = 0.03, respectively). A potential improvement in PFS was seen for those treated with CT + RT compared to CT alone, however it was not statistically significant (aHR = 0.53, p = 0.09).
Conclusions
Observation after surgery was associated with poor outcomes in uterine CS compared to CT and RT alone. Multimodality therapy for women with stage I/II disease was associated with improved PFS compared to chemotherapy alone. Novel treatment options are needed to improve outcomes in this aggressive disease.
Keywords: Uterine carcinosarcoma, Multimodal therapy, Survival, MMMT, Chemotherapy, Radiation
1. Introduction
In 2015, 54,870 women will be diagnosed with endometrial cancer and 10,170 will die of the disease [1]. Uterine carcinosarcoma (CS), previously known as malignant mixed mullerian tumor, is an exceedingly rare neoplasm, comprising 2–5% of all uterine tumors, with an incidence of fewer than 3 per 100,000 women each year. [2]. The five year survival is estimated to be no more than 40%, which confers the worst prognosis of all uterine cancers [3]. The most important prognostic factor to be identified to date is tumor stage [4]. Due to the aggressive nature of CS, there is a continuing need to identify the best adjuvant therapy regimen to improve outcomes.
Uterine CS was initially classified as a uterine sarcoma and termed a malignant mixed mullerian tumor. Recently, these tumors have been reclassified as metaplastic carcinomas based on in vitro, immunohistochemistry and molecular studies [5–8]. In 2009, the FIGO committee introduced a new staging system for uterine sarcomas however; CS remained classified for staging purposes with carcinomas of the endometrium due to their biologically similar disease pattern [9]. Cell lines established from CS can differentiate into either epithelial, mesenchymal components, or both [10]. Clonality studies show derivation of these tumors from a monoclonal cancer cell with carcinomatous and sarcomatous components of the tumor sharing common genetic alteration [11]. The original classification was the rationale for using sarcoma-based chemotherapy.
Several studies have attempted to identify the optimal adjuvant therapy for patients with CS. The National Comprehensive Cancer Network (NCCN) recommends adjuvant chemotherapy even for patients with early stage disease [12]. The Gynecologic Oncology Group (GOG) 108 trial compared ifosfamide/cisplatin with ifosfamide alone, while GOG 161 examined the combination of ifosfamide/paclitaxel compared to ifosfamide alone [13,14]. The combination arms yielded higher response rates and an improved progression free survival (PFS); however a survival advantage was only observed with ifosfamide/paclitaxel [14]. GOG 150 compared 3 cycles of chemotherapy (cisplatin-ifosfamide-mesna) to whole abdominal radiation in stage I–IV CS, and showed no statistical significant difference in outcomes. However, the recurrence rate at 5 years was 21% lower (RH = 0.789, 95% CI: 0.530–1.176), p = 0.245) and the death rate was 29% lower (RH = 0.712,95% CI: 0.484–1.048, p = 0.085) for those who received chemotherapy [15]. This was beneath the threshold considered clinically important (as determined a priori); however, this study concluded that the differences observed favored adjuvant chemotherapy in this population. Many centers today are using platinum–taxane combinations in CS as these tumors are now regarded as de-differentiated carcinomas of the endometrium rather than sarcomas. The results of GOG 261 comparing paclitaxel plus carboplatin to ifosfamide plus paclitaxel in patients with stage I–IV disease are eagerly awaited [16].
There have been several retrospective reviews examining the effects of post-operative pelvic radiation in this population. These studies have shown a consistent decrease in pelvic recurrences but no impact on overall survival [17–23]. A 2008 European Organization for Research and Treatment of Cancer [EORTC protocol 55,874] trial of sarcoma patients included 91 women with early stage CS and showed that pelvic radiation decreased local recurrence but did not improve OS [24]. This finding is most likely attributed to the high incidence of distant metastasis found when CS recurs, indicating the need for systemic therapy.
There have been nonrandomized trials which have suggested a benefit for multimodal therapy (chemotherapy combined with radiation) in this population. Einstein et al. (2012) (N = 27), found that radiotherapy “sandwiched” between chemotherapy was feasible and had favorable outcomes when compared to historic outcomes for women with uterine CS [25]. A study of stage III–IV patients with uterine CS reported improved disease free survival with multimodal therapy compared to all other groups (observation, radiation alone, chemotherapy alone) [26]. To date, no large randomized studies comparing multimodal therapy to chemotherapy, radiation, or observation in patients with uterine carcinosarcoma has been conducted and further data are needed.
Recommendations from the NCCN for adjuvant therapy include chemotherapy with or without radiation and whole abdominal radiation with or without vaginal brachytherapy [12]. In this retrospective, multi-institutional observational study, our goal was to assess the association between type of adjuvant therapy received and outcomes in women with stage I–III uterine CS, and specifically to assess outcomes of women treated with multimodality therapy vs. either modality alone.
2. Materials and methods
2.1. Study parameters
Institutional Review Board (IRB) approval was obtained by all institutions prior to study initiation. A retrospective chart review was conducted of patients diagnosed with stage I–III uterine CS between January 1, 1997 and December 31, 2012 at the University of Minnesota, University of North Carolina, Duke University and Johns Hopkins University. All patients with surgically confirmed stage I–III disease based on the 1988 International Federation of Gynecology and Obstetrics (FIGO) staging system for carcinoma of the corpus uteri [27] and documented therapy information were included. Patients with stage IV disease were excluded from analysis as the majority of these patients received only chemotherapy. Clinical data were extracted from inpatient and outpatient charts. Demographic and clinical data including age, race, body mass index (BMI), smoking status, parity, cancer and treatment history, date of diagnosis, date of surgery, final pathology results, grade, lymphovascular space involvement (LVSI), node positivity, anatomic site positivity (presence or absence of residual disease, disease present in cervix, ovaries, uterine serosa or vagina), and treatment type were recorded. Patient outcomes included presence and location of disease recurrence and death.
2.2. Statistical analysis
Patients were categorized into four groups based on treatment received: observation only (OBS), chemotherapy alone (CT), radiation alone (RT), or multimodal therapy (combination of chemotherapy and radiation; CT + RT). Temporality of multimodal therapy (chemotherapy followed by radiation, radiation followed by chemotherapy, or sandwich protocol) could not be examined due to small numbers in each group. Chemotherapy (CT) was utilized as the comparison group due to historic data and the use of chemotherapy primarily as adjuvant therapy for CS in the past studies. Due to differences in both treatment practices and disease outcomes, analyses was stratified by stage (early — I/II and advanced — III).
Patient demographic and clinical data were summarized; means ± standard deviations (SD) and percentages are presented unless otherwise noted. Comparisons of baseline characteristics by treatment received were conducted using Chi-Square tests, Fisher’s Exact tests, and linear regression models as appropriate. The presence of and site of recurrence during follow-up were compared by treatment using Chi-Square and Fisher’s Exact tests.
PFS was calculated from date of initial treatment to the date of first known progression, recurrence or death, or censored at date of last follow-up if disease-free and alive. OS was calculated from the date of initial treatment to date of death or censored at the date of last follow-up if still alive. OS and PFS were summarized using Kaplan–Meier methods; the median time to event for each group is presented when estimable and treatment groups were compared using log-rank tests. Additionally, Cox proportional hazards models were conducted to include known predictors of OS and PFS and to address possible confounding. The effect of treatment type on OS and PFS was calculated after adjusting for variables determined prior to the analysis: clinic site, race, cancer history, residual disease, LVSI, stage, age at diagnosis, parity, and year at diagnosis. Hazard ratios and 95% confidence intervals (CIs) are presented. Data were analyzed using SAS 9.3 (Cary, NC) and p-values less than 0.05 were considered statistically significant.
3. Results
A total of 303 patients were identified as meeting the inclusion criteria at the four sites for this study; 8 were excluded due to missing treatment information.
3.1. Early stage (I/II) uterine carcinosarcoma
Of them, 195 patients with stage I/II uterine CS were identified; 188 had treatment information and were included in the analysis (Table 1). The majority had stage I disease (82.5%), the mean age at diagnosis was 67.8 ± 11.1 years, and the mean BMI at surgery of 32.2 ± 8.4 kg/m2.
Table 1.
Demographic and clinical characteristics of patients with stage I/II uterine carcinosarcoma by treatment received.
| Variable | OBS (N = 75)
|
CT (N = 33)
|
RT (N = 37)
|
CT + RT (N = 43)
|
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | ||
| Age | 75 | 70.3 (11.4) | 33 | 66.5 (11.5) | 37 | 67.2 (12.0) | 43 | 64.9 (8.6) | 0.07 |
| Year of diagnosis | 75 | 2005.3 (4.3) | 33 | 2007.9 (4.4) | 37 | 2004.4 (3.7) | 43 | 2007.9 (2.8) | <0.0001 |
| Body mass index (BMI) | 66 | 31.4 (7.3) | 33 | 32.6 (9.4) | 30 | 33.1 (9.3) | 42 | 32.2 (8.7) | 0.82 |
| Parity | 73 | 2.8 (1.9) | 31 | 2.3 (2.6) | 36 | 3.9 (3.0) | 42 | 2.5 (1.8) | 0.02 |
| N | % | N | % | N | % | N | % | p-value | |
|
| |||||||||
| Race | 0.05 | ||||||||
| White | 27 | 37.0 | 21 | 65.6 | 17 | 47.2 | 22 | 51.2 | |
| Black | 44 | 60.3 | 9 | 28.1 | 17 | 47.2 | 17 | 39.5 | |
| Hispanic | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 1 | 2.3 | |
| Other | 1 | 1.4 | 2 | 6.3 | 2 | 5.6 | 3 | 7.0 | |
| Missing | 2 | 1 | 1 | 0 | |||||
| Smoking | 0.20 | ||||||||
| Yes | 13 | 18.1 | 9 | 29.0 | 9 | 27.3 | 5 | 11.6 | |
| No | 59 | 81.9 | 22 | 71.0 | 24 | 72.7 | 38 | 88.4 | |
| Missing | 3 | 2 | 4 | 0 | |||||
| Clinic site | 0.02 | ||||||||
| 1 | 21 | 28.0 | 13 | 39.4 | 11 | 29.7 | 23 | 53.5 | |
| 2 | 10 | 13.3 | 9 | 27.3 | 9 | 24.3 | 8 | 18.6 | |
| 3 | 29 | 38.7 | 10 | 30.3 | 14 | 37.8 | 9 | 20.9 | |
| 4 | 15 | 20.0 | 1 | 3.0 | 3 | 8.1 | 3 | 7.0 | |
| Cancer history | 0.04 | ||||||||
| Yes | 14 | 18.7 | 13 | 39.4 | 5 | 13.5 | 8 | 18.6 | |
| No | 61 | 81.3 | 20 | 60.6 | 32 | 86.5 | 35 | 81.4 | |
| Radiation history | 0.45 | ||||||||
| Yes | 4 | 5.3 | 4 | 12.1 | 1 | 2.7 | 2 | 4.7 | |
| No | 71 | 94.7 | 29 | 87.9 | 36 | 97.3 | 41 | 95.4 | |
| LSVI | 0.18 | ||||||||
| Yes | 20 | 27.0 | 11 | 66.7 | 17 | 47.2 | 17 | 39.5 | |
| No | 54 | 73.0 | 22 | 33.3 | 19 | 52.9 | 26 | 60.5 | |
| Missing | 1 | 0 | 1 | 0 | |||||
| Stage | 0.001 | ||||||||
| I | 70 | 93.3 | 29 | 87.9 | 26 | 70.3 | 30 | 69.8 | |
| II | 5 | 6.7 | 4 | 12.1 | 11 | 29.7 | 13 | 30.2 | |
| Residual disease | 0.30 | ||||||||
| Yes | 0 | 0.0 | 1 | 3.0 | 1 | 2.7 | 1 | 2.4 | |
| No | 75 | 100.0 | 32 | 96.7 | 36 | 97.3 | 41 | 97.6 | |
| Missing | 0 | 0 | 0 | 1 | |||||
| Cervix + disease | 0.003 | ||||||||
| No | 69 | 92.0 | 30 | 90.9 | 26 | 70.3 | 30 | 71.4 | |
| Yes | 6 | 8.0 | 3 | 9.1 | 11 | 29.7 | 12 | 28.6 | |
| Missing | 0 | 0 | 1 | ||||||
| Ovaries + disease | 0.18 | ||||||||
| No | 75 | 100.0 | 32 | 97.0 | 37 | 100.0 | 43 | 100.0 | |
| Yes | 0 | 0.0 | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | |
| Uterine serosa + disease | 1.00 | ||||||||
| No | 74 | 98.7 | 33 | 100.0 | 37 | 100.0 | 42 | 97.7 | |
| Yes | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 1 | 2.3 | |
| Vagina + disease | – | ||||||||
| No | 75 | 100.0 | 33 | 100.0 | 37 | 100.0 | 41 | 100.0 | |
| Yes | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Missing | 0 | 0 | 0 | 2 | |||||
| Recurrence outcomes | |||||||||
| Recurrence | 0.42 | ||||||||
| Yes | 30 | 42.9 | 15 | 45.5 | 11 | 30.6 | 14 | 32.6 | |
| No | 40 | 57.1 | 18 | 54.6 | 25 | 69.4 | 29 | 67.4 | |
| Unknown | 5 | 0 | 1 | 0 | |||||
| Site of first recurrence | 0.07 | ||||||||
| Vaginal only | 10 | 34.5 | 7 | 46.7 | 2 | 18.2 | 1 | 7.1 | |
| Other pelvic | 10 | 34.5 | 6 | 40.0 | 2 | 18.2 | 6 | 42.9 | |
| Extra pelvic/multiple | 9 | 31.0 | 2 | 13.3 | 7 | 63.6 | 7 | 50.0 | |
Treatment received included: OBS: 75 (39.9%), CT: 33 (17.6%), RT: 37 (19.7%), and CT + RT: 43 (22.9%). There was a statistically significant difference between treatment groups with respect to several key characteristics. The mean age of the OBS group was 70.3, and was the oldest of the 4 groups (p = 0.07). With respect to race, there was a higher proportion of African Americans (60.3%) in the OBS group compared to the CT (28.1%), RT (47.2%), and CT + RT groups (39.5%) (p = 0.05). Those with a history of a prior cancer received CT (39.4%) more commonly than OBS (18.7%), RT (13.5%), and CT + RT (18.6%) (p = 0.04). The majority of patients had stage I disease, though the proportion differed by treatment group (93.3% in OBS, 87.9% in CT, 70.3% in RT, and 69.8% in CT + RT; p = 0.001). Cervical involvement was different between groups, with those receiving RT (29.7%) and CT + RT (28.6%) having the greatest proportion (p = 0.003). The year of diagnosis was significantly different between groups, with those receiving OBS and RT being diagnosed significantly earlier (p < 0.0001). Parity was also statistically different between groups, with the RT group having the highest mean parity (p = 0.02). Of the 37 patients who received radiation alone in early stage disease, 62% (22) received WPRT, 25% (9) WPRT/brachytherapy and 11% (4) brachytherapy alone and 2 patients were missing data. Of the 43 patients who received CT + RT, 30% (13) had WPRT, 37% (16) had WPRT/brachytherapy and 32% (14) received brachytherapy alone. In early stage disease the majority of women receiving chemotherapy alone received paclitaxel/carboplatin (54%), the next most common regimen was paclitaxel/ifosfamide (27%), followed by ifosfamide/cisplatin (18%). The majority (62%) of patients with early stage disease receiving CT + RT had paclitaxel/carboplatin with cisplatin/ifosfamide being the second most common agents used.
The median follow-up was 31 months (range 1–160). There was no statistical difference in the rate of recurrence between treatment groups (Table 1). The majority of recurrences in the RT and CT + RT groups occurring outside of the pelvis (63.6% and 50.0%, respectively) compared to 31.0% and 13.3% in the OBS and CT alone groups, however this was not statistically significantly (p = 0.07).
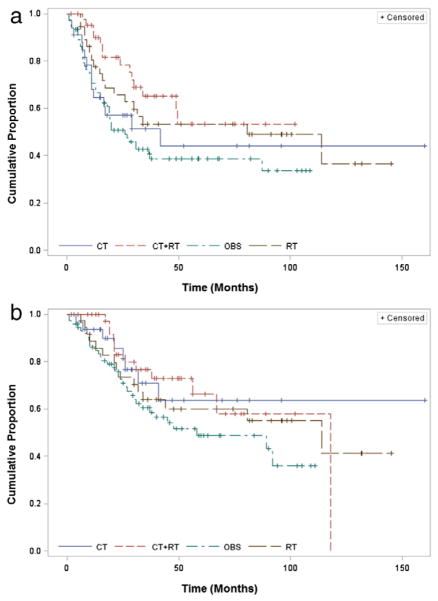
Graphs of the OS and PFS by treatment group are presented in Fig. 1a and b. In the multivariate analysis, there were statistically significant differences in survival outcomes by clinical variables, including clinic site, age, disease stage, and residual disease (Table 2). Increased disease stage and age were associated with worse PFS and disease stage and presence of residual disease was associated with worse OS. Both also showed differences by site. After adjustment for potential confounders, OBS was associated with a more than four-fold increased risk of death compared to CT alone (adjusted hazard ratio (aHR) = 4.48 [95% CI: 1.67–12.02], p = 0.003). OBS was associated with a two-fold increased risk of death compared to RT alone (aHR = 2.07 [95% CI: 1.01–4.22], p = 0.05). Multimodality therapy was associated with improved PFS compared to CT alone (aHR = 0.43 [95% CI: 0.19–0.95], p = 0.04), but there was no difference in OS (aHR = 0.94 [95% CI: 0.34–2.65], p = 0.91). There was not a statistically significant difference in PFS or OS between RT and CT alone.
Fig. 1.
Progression-free and overall survival for patients with stage I/II uterine carcinosarcoma. Kaplan–Meier curves for a) progression-free survival and b) overall survival are presented by treatment received. OBS = observation only; CT = chemotherapy alone; RT = radiation alone; CT + RT = multimodal therapy.
Table 2.
Multivariate cox proportional hazards model results for progression-free and overall survival for patients with stage I/II uterine carcinosarcoma.
| Variable | Progression-free survival
|
Overall survival
|
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CT + RT vs. CT | 0.43 (0.19–0.95) | 0.04 | 0.94 (0.34–2.65) | 0.91 |
| RT vs. CT | 0.81 (0.37–1.76) | 0.59 | 2.13 (0.79–5.71) | 0.13 |
| OBS vs. CT | 1.73 (0.84–3.57) | 0.14 | 4.48 (1.67–12.02) | 0.003 |
| Site 4 vs. Site 1 | 0.41 (0.18–0.91) | 0.03 | 0.28 (0.10–0.77) | 0.01 |
| Site 3 vs. Site 1 | 0.62 (0.36–1.06) | 0.08 | 0.49 (0.26–0.94) | 0.03 |
| Site 2 vs. Site 1 | 0.43 (0.21–0.89) | 0.02 | 0.33 (0.14–0.77) | 0.01 |
| Race (White vs. Black/Other) | 1.18 (0.71–1.97) | 0.53 | 1.52 (0.82–2.82) | 0.19 |
| Cancer history (Yes vs. No) | 0.91 (0.51–1.64) | 0.76 | 1.23 (0.62–2.44) | 0.55 |
| Residual disease (Yes vs. No) | 2.26 (0.49–10.34) | 0.30 | 4.88 (0.97–24.51) | 0.05 |
| LVSI (Yes vs. No) | 0.80 (0.48–1.35) | 0.40 | 1.29 (0.70–2.36) | 0.41 |
| Stage (II vs. I) | 3.28 (1.82–5.94) | <0.0001 | 2.86 (1.47–5.59) | 0.002 |
| Age (continuous) | 1.03 (1.00–1.05) | 0.03 | 1.01 (0.98–1.04) | 0.45 |
| Year of diagnosis (continuous) | 1.05 (0.99–1.11) | 0.13 | 1.06 (0.99–1.14) | 0.11 |
| Parity (continuous) | 0.97 (0.87–1.09) | 0.59 | 1.06 (0.99–1.14) | 0.40 |
3.2. Advanced stage (III) uterine carcinosarcoma
A total of 108 patients with Stage III uterine CS were identified; 107 had treatment information and were included in the analysis (Table 3). The majority had stage IIIC disease (63.6%), the mean age at diagnosis was 67.1 ± 10.1 years, most were Caucasian (52.8%), mean BMI was 31.5 ± 6.9 kg/m2. Only 16 (15.0%) were in the OBS group; the majority received CT: 34 (31.8%), RT: 20 (18.7%), or CT + RT: 37 (34.6%). There were statistically significant differences between treatment received and race (p = 0.01), with the majority of OBS and RT groups being African American (75.0% and 65.0%) compared to the CT (41.2%) and CT + RT (30.6%) groups. The presence of LVSI was statistically different between treatment groups, with those in the RT group having the fewest patients with LVSI compared to the other groups (p = 0.02). Evidence of residual disease and ovarian involvement was statistically significantly different between groups, with the OBS group having the highest percentage of patients with residual disease and ovarian disease (p = 0.04 and 0.02, respectively).
Table 3.
Demographic and clinical characteristics of patients with stage III uterine carcinosarcoma by treatment received.
| Observation (N = 16) | CT only (N = 34) | RT only (N = 20) | CT + RT (N = 37) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | p-value |
| Age | 16 | 70.9 (11.1) | 34 | 67.3 (9.6) | 20 | 68.0 (9.1) | 37 | 65.0 (10.5) | 0.25 |
| Year of diagnosis | 16 | 2005.7 (3.7) | 34 | 2006.1 (5.0) | 20 | 2004.5 (4.6) | 37 | 2006.7 (3.9) | 0.33 |
| Body mass index (BMI) | 13 | 32.0 (6.5) | 30 | 32.1 (7.5) | 18 | 32.2 (8.1) | 33 | 30.4 (6.0) | 0.73 |
| Parity | 15 | 3.5 (3.2) | 33 | 2.6 (1.8) | 18 | 2.6 (1.6) | 36 | 2.8 (1.9) | 0.57 |
| N | % | N | % | N | % | N | % | p-value | |
|
| |||||||||
| Race | 0.01 | ||||||||
| White | 4 | 25.0 | 20 | 58.8 | 7 | 35.0 | 25 | 69.4 | |
| Black | 12 | 75.0 | 14 | 41.2 | 13 | 65.0 | 11 | 30.6 | |
| Missing | 0 | 0 | 0 | 1 | |||||
| Smoking | 0.19 | ||||||||
| Yes | 6 | 40.0 | 4 | 12.1 | 4 | 22.2 | 7 | 20.0 | |
| No | 9 | 60.0 | 29 | 87.9 | 14 | 77.8 | 28 | 80.0 | |
| Missing | 1 | 1 | 2 | 2 | |||||
| Clinic site | 0.13 | ||||||||
| 1 | 7 | 43.8 | 12 | 35.3 | 3 | 15.0 | 18 | 48.7 | |
| 2 | 2 | 12.5 | 6 | 17.7 | 2 | 10.0 | 7 | 18.9 | |
| 3 | 6 | 37.5 | 11 | 32.4 | 14 | 70.0 | 9 | 24.3 | |
| 4 | 1 | 6.3 | 5 | 14.7 | 1 | 5.0 | 3 | 8.1 | |
| Cancer history | 0.78 | ||||||||
| Yes | 3 | 18.8 | 5 | 14.7 | 5 | 25.0 | 6 | 16.2 | |
| No | 13 | 81.3 | 29 | 85.3 | 15 | 75.0 | 31 | 83.8 | |
| Radiation history | 0.84 | ||||||||
| Yes | 1 | 6.3 | 3 | 8.8 | 3 | 15.8 | 4 | 10.8 | |
| No | 15 | 93.8 | 31 | 91.2 | 16 | 84.2 | 33 | 89.2 | |
| Missing | 0 | 0 | 1 | 0 | |||||
| LSVI | 0.02 | ||||||||
| Yes | 14 | 87.5 | 27 | 87.1 | 8 | 47.1 | 26 | 74.3 | |
| No | 2 | 12.5 | 4 | 12.9 | 9 | 52.9 | 9 | 25.7 | |
| Missing | 0 | 3 | 3 | 2 | |||||
| Stage | 0.64 | ||||||||
| IIIA | 6 | 37.5 | 11 | 32.4 | 5 | 25.0 | 8 | 21.6 | |
| IIIB | 1 | 6.3 | 1 | 2.9 | 3 | 15.0 | 4 | 10.8 | |
| IIIC | 9 | 56.3 | 22 | 64.7 | 12 | 60.0 | 25 | 67.6 | |
| Positive nodes | 0.56 | ||||||||
| Yes | 8 | 53.3 | 14 | 45.2 | 8 | 42.1 | 14 | 37.8 | |
| No | 7 | 46.7 | 17 | 54.8 | 11 | 57.9 | 23 | 62.2 | |
| Missing | 1 | 3 | 1 | 0 | |||||
| Residual disease | 0.04 | ||||||||
| Yes | 4 | 25.0 | 6 | 18.8 | 0 | 0.0 | 2 | 5.7 | |
| No | 12 | 75.0 | 26 | 81.3 | 17 | 100.0 | 33 | 94.3 | |
| Missing | 0 | 2 | 3 | 2 | |||||
| Cervix + disease | 0.12 | ||||||||
| No | 4 | 25.0 | 21 | 61.8 | 9 | 50.0 | 19 | 51.4 | |
| Yes | 12 | 75.0 | 13 | 38.2 | 9 | 50.0 | 18 | 48.7 | |
| Missing | 0 | 0 | 2 | 0 | |||||
| Ovaries + disease | 0.02 | ||||||||
| No | 8 | 50.0 | 18 | 52.9 | 15 | 88.2 | 27 | 75.0 | |
| Yes | 8 | 50.0 | 16 | 47.1 | 2 | 11.8 | 9 | 25.0 | |
| Missing | 0 | 0 | 3 | 1 | |||||
| Uterine serosa + disease | 0.11 | ||||||||
| No | 7 | 43.8 | 26 | 76.5 | 14 | 77.8 | 26 | 72.2 | |
| Yes | 9 | 56.3 | 8 | 23.5 | 4 | 22.2 | 10 | 27.8 | |
| Missing | 0 | 0 | 2 | 1 | |||||
| Vagina + disease | 0.10 | ||||||||
| No | 13 | 81.3 | 32 | 94.1 | 13 | 76.5 | 34 | 94.4 | |
| Yes | 3 | 18.8 | 2 | 5.9 | 4 | 23.5 | 2 | 5.6 | |
| Missing | 0 | 0 | 3 | 1 | |||||
| Recurrence outcomes | |||||||||
| Recurrence | 0.36 | ||||||||
| Yes | 9 | 64.3 | 21 | 67.7 | 11 | 55.0 | 17 | 47.2 | |
| No | 5 | 35.7 | 10 | 32.3 | 9 | 45.0 | 19 | 52.8 | |
| Unknown | 2 | 3 | 1 | 1 | |||||
| Site of initial documented recurrence | 0.001 | ||||||||
| Vaginal only | 6 | 66.7 | 1 | 4.8 | 2 | 18.2 | 2 | 12.5 | |
| Other pelvic | 1 | 11.1 | 2 | 9.5 | 0 | 0.0 | 5 | 31.3 | |
| Extra pelvic/multiple | 2 | 22.2 | 18 | 85.7 | 9 | 81.8 | 9 | 56.3 | |
The median follow-up was 19.0 months (range 1–119). There was not a statistical difference in recurrence rates between the treatment groups (p = 0.36), however, site of initial documented recurrence was significantly different (Table 3; p = 0.001), with the majority of those in the CT and RT groups recurring outside of the pelvis (85.7% and 81.8%, respectively) while those in the CT + RT group recurred both in the pelvis (31.3%) and outside the pelvis (56.3%).
In advanced stage disease, 20 received RT alone and of those 50% (9) received WPRT, 27% (5) WPRT and brachytherapy, 11% (2) WAR, 5% (1) brachytherapy alone, and one had extended field RT. For stage III patients receiving CT + RT, 43% (16) received WPRT, 43% 16) WPRT/brachytherapy, 8% (3) brachytherapy alone, and 5% (2) received extended field RT. The majority of this group receiving CT or CT + RT received paclitaxel and carboplatin; 39% and 48%, respectively. This was followed by ifosfamide/paclitaxel (24%) in CT alone arm and cisplatin/ifosfamide (21%) in the CT + RT arm.
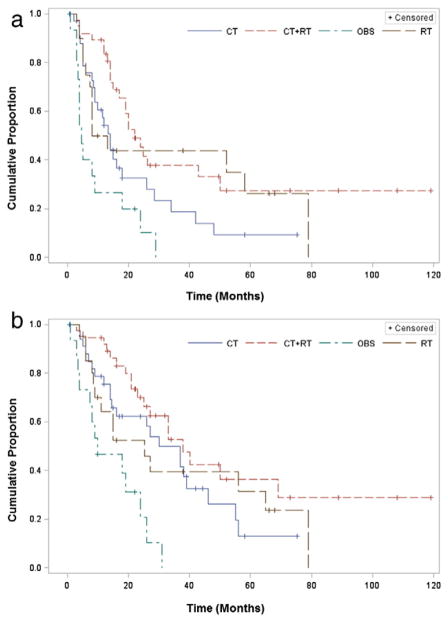
Graphs of the OS and PFS by treatment group are presented in Fig. 2a and b. There were statistically significant differences in PFS and OS by clinic site and disease stage among patients with stage III disease (Table 4). The presence of LVSI was also associated with worse PFS and OS. After adjusting for potential confounders, OBS was associated with lower PFS and OS compared to CT alone (aHR = 2.39 [95% CI: 1.11–5.17], p = 0.03 and aHR = 2.46 [95% CI: 1.05–5.79], p = 0.04, respectively). The data suggest a possible improvement in PFS for those treated with CT + RT compared to CT alone (aHR = 0.53 [95% CI: 0.26–1.11], p = 0.09) though this association was not statistically significant. The association was similar but also not significant for OS (aHR = 0.58 [95% CI: 0.25–1.33], p = 0.20). There was not a significant difference in PFS or OS between RT and CT alone (PFS: aHR = 0.94 [95% CI: 0.35–2.50], p = 0.90 and OS: aHR = 1.26 [95% CI: 0.45–3.50], p = 0.66).
Fig. 2.
Progression-Free and Overall Survival for Patients with Stage III Uterine Carcinosarcoma. Kaplan–Meier curves for a) progression-free survival and b) overall survival are presented by treatment received. OBS = observation only; CT = chemotherapy alone; RT = radiation alone; CT + RT = multimodal therapy.
Table 4.
Multivariate Cox proportional hazards model results for progression-free and overall survival for patients with stage III uterine carcinosarcoma.
| Variable | Progression-free survival
|
Overall survival
|
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Multimodal therapy vs. chemotherapy alone | 0.58 (0.27–1.25) | 0.17 | 0.51 (0.22–1.16) | 0.10 |
| Radiation therapy vs. chemotherapy alone | 0.95 (0.36–2.50) | 0.91 | 1.19 (0.42–3.38) | 0.74 |
| Observation alone vs. Chemotherapy alone | 2.41 (1.16–5.21) | 0.03 | 2.40 (1.03–5.56) | 0.04 |
| Site 4 vs. Site 1 | 6.15 (1.70–22.32) | 0.01 | 4.61 (1.03–20.63) | 0.05 |
| Site 3 vs. Site 1 | 1.61 (0.72–3.59) | 0.25 | 1.52 (0.61–3.79) | 0.37 |
| Site 2 vs. Site 1 | 1.82 (0.67–4.96) | 0.24 | 1.83 (0.91–5.49) | 0.28 |
| Race (White vs. Black) | 0.66 (0.34–1.28) | 0.22 | 0.56 (0.27–1.19) | 0.13 |
| Cancer history (Yes vs. No) | 1.31 (0.63–2.70) | 0.47 | 1.75 (0.76–4.07) | 0.19 |
| Residual disease (Yes vs. No) | 1.72 (0.78–3.80) | 0.18 | 1.69 (0.70–4.08) | 0.25 |
| LVSI (Yes vs. No) | 2.56 (1.11–5.89) | 0.03 | 3.82 (1.41–10.38) | 0.01 |
| Stage IIIC vs. IIIA | 2.78 (1.38–5.61) | 0.004 | 2.89 (1.30–6.43) | 0.01 |
| Stage IIIB vs. IIIA | 0.52 (0.06–4.14) | 0.53 | 0.44 (0.04–4.37) | 0.48 |
| Age (continuous) | 1.00 (0.97–1.03) | 0.98 | 1.00 (0.96–1.03) | 0.95 |
| Year of diagnosis (continuous) | 1.02 (0.95–1.10) | 0.60 | 1.04 (0.95–1.13) | 0.43 |
| Parity (continuous) | 1.02 (0.86–1.21) | 0.82 | 1.00 (0.83–1.21) | 0.98 |
4. Discussion
In this retrospective multi institutional study, we found that in patients with early stage CS, there was over a four-fold increase in the risk of death in patients who were observed compared to those who received adjuvant chemotherapy. There was significantly higher PFS in patients with early stage disease in the CT + RT group compared to the CT group. However, this did not translate into a difference in overall survival raising the question if toxicity of multi-modal therapy is worth the price if no OS benefit is observed. The improvement in PFS could be due to delay in recurrence in those receiving CT + RT. While there was a trend toward improved PFS in advanced staged patients receiving multimodal therapy, this did not reach statistical significance.
The findings of our study are similar to those of by Cantrell et al. (2012) showing that patients with early stage disease who received adjuvant therapy (chemotherapy +/− radiation therapy) had improved PFS but not OS when compared to observation alone [28]. However, our study did find a 4 fold increased risk of death for those patients who did not receive adjuvant therapy compared to those receiving CT, suggesting that there may be benefit to adjuvant therapy in those with early stage disease. Of note, in Stage III disease, the OBS group did have the highest percentage of women with residual disease and ovarian involvement. Logically this would seem the population that most likely would benefit from adjuvant therapy. The rationale for observation rather than adjuvant therapy was not consistently provided and represents the innate bias that is present in retrospective studies. We hypothesize that if these patients had extensive residual disease remaining following surgery perhaps adjuvant therapy was not offered as it may have been thought to be futile in such aggressive disease. This could have contributed to the poorer outcome seen in the OBS group. It is unclear why improvement in PFS was not observed as well in the CT vs OBS group; there could be selection bias in the treatment groups, with definite differences, such as age and race, present between the groups. Although it is possible that there is a subgroup of patients that may not require adjuvant therapy, identifying those patients based on risk factors in this study was not feasible. Due to small sample sizes, we were unable to identify an early stage subgroup in which observation was associated with equal or better outcomes when compared to the adjuvant treatment cohorts. Genomic data may assist in differentiating those patients who are less likely to recur and therefore may ultimately not require adjuvant therapy. Until such markers are confirmed, however, our study suggests the use of adjuvant therapy for early staged uterine CS.
In the multivariate analyses, several factors were associated with poorer outcomes in both the early and advanced stage disease groups. In the early stage group, residual disease and stage II disease were associated with poorer outcomes, which is consistent with previous studies identifying stage as a predictor of outcome in CS [4,28]. In the advanced group, LVSI and higher stage were associated with worse outcome. Interestingly, in both groups, we observed statistical differences in PFS and OS according to treatment site. This finding is supported by a recent SEER database study of early stage elderly CS patients which showed a difference between outcomes based on region of the country where patients were treated [29]. Other studies have supported different outcomes according to region treated, particularly in elderly patients [30]. Our findings may represent additional regional differences that exist among the patients treated at differing sites that are associated with OS and PFS and cannot be captured using medical records, such as socioeconomic status, insurance status, geographic location, and education. There were racial differences according to treatment site. We found that 60% of the early staged patients observed following surgery were black. It is well known that black women with endometrial cancer have a poorer prognosis compared to white women. The factors contributing to this are multifactorial and include later diagnosis, disparities in access and treatment, comorbidities, and tumor genetic differences [31,32]. Therefore, we adjusted for race in these analyses, however other potential confounders such as socioeconomic status and education of the population treated at the different institutions may have contributed to the differences observed in survival according to site treated. There may have also been differences between sites in follow up practices, physician bias, and data collection.
A particular interest when considering adjuvant therapy for patients with CS is the location of recurrence. In the EORTC 2008 trial of sarcoma patients, while pelvic recurrences were decreased by pelvic radiation, OS was not affected, which the authors suggested was because the recurrences were distant [24]. Secord et al. found that patients with stage IIIC endometrial cancer treated with chemotherapy alone were more likely to develop vaginal or pelvic recurrences compared to those treated with radiation or combination therapy [33]. Several other retrospective studies have also shown an improvement in local recurrence with radiotherapy, but these have not translated into improved OS [21,34]. We found that in the early stage group, although there were no differences in recurrence rates comparing the treatment groups, there was evidence of a difference in site of recurrence. The RT and CT + RT groups had a greater number of extra pelvic recurrences, whereas those receiving CT alone tended to recur in the pelvis. The results among patients with advanced stage disease were similar and statistically significant by site. Though the majority of recurrences in the CT and RT alone groups were extra pelvic, those in the CT + RT group recurred equally in the pelvis and distantly. We believe this speaks to the aggressiveness of the disease when it presents at more advanced stages. Despite a multi-modal approach to the disease, it recurs both in field and distantly, again highlighting the need for new therapies in advanced stage CS.
There are limitations to this study. As this was a retrospective study, treatment modalities were not randomized. Due to the low incidence of disease, data were pooled from four large institutions which had different patient populations and treatment practices. Despite pooling 15 years of data from four institutions, the sample sizes were only moderate for survival analyses. In addition, the sample size did not allow for exploration of our secondary objective, which was to explore the role of temporality of the adjuvant therapies among those who received multimodal therapy. Finally, there is an innate selection bias with retrospective studies. In our study population among those with early stage disease who were observed, a large percentage was older and African American, which may be an explanation for our findings. It is unknown whether biological differences access to care or other confounding socioeconomic factors contributed to differences in the treatment planning offered to patients.
In this large, multi-center analysis of women with uterine CS, observation was associated with worse outcomes. Our data shows that multimodality therapy was associated with improved PFS compared to chemotherapy alone in early stage disease. However, given the lack of difference in OS in stage I/II disease when comparing CT + RT to CT alone, and with no significant survival differences observed in advanced disease comparing CT + RT to CT, it appears that systemic relapse is the norm. Although beneficial in preventing local relapse, the lack of radiation effect with regard to overall survival is notable. The optimal chemotherapy has yet to be determined. With further understanding that CS is actually an endometrial cancer with a de-differentiated component, new classification within clinical trials as well as novel therapeutics and treatment approaches should be considered to improve outcomes in women with this disease.
HIGHLIGHTS.
Multimodal therapy for stage I/II uterine CS shows improved PFS over chemotherapy.
In early stage CS, there is a 4 times greater risk of death without adjuvant therapy.
Novel therapeutics are necessary to improve overall survival in patients with CS.
Acknowledgments
Funding statement
This research was supported by the NIH T-32 training grant 5T32-CA132715 and NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This research was presented as an oral plenary session at the Society of Gynecologic Oncology annual meeting on March 25th, 2013.
Conflict of interest statement
Dr. Dickson, Dr. Geller and Ms. Isaksson Vogel report funding from the National Institutes of Health during the study period. The other authors declare that there are no conflicts of interest.
References
- 1.American Cancer Society. Endometrial Cancer Statistics. 2014. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;(63):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Amant F, Cadron I, Fuso L, Berteloot P, de Jonge E, Jacomen G, Van Robaeys J, Neven P, Moerman P, Vergote I. Endometrial carcinosarcomas have a different prognosis and pattern of spread compared to high-risk epithelial endometrial cancer. Gynecol Oncol. 2005;98:274–280. doi: 10.1016/j.ygyno.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, Yordan E, Brady MF. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71:1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 5.Mayall F, Rutty K, Campbell F, Goddard H. p53 immunostaining suggests that uterine carcinosarcomas are monoclonal. Histopathology. 1994;24:211–214. doi: 10.1111/j.1365-2559.1994.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 6.Abeln EC, Smit VT, Wessels JW, de Leeuw WJ, Cornelisse CJ, Fleuren GJ. Molecular genetic evidence for the conversion hypothesis of the origin of malignant mixed mullerian tumours. J Pathol. 1997;183:424–431. doi: 10.1002/(SICI)1096-9896(199712)183:4<424::AID-PATH949>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Kounelis S, Jones MW, Papadaki H, Bakker A, Swalsky P, Finkelstein SD. Carcinosarcomas (malignant mixed mullerian tumors) of the female genital tract: comparative molecular analysis of epithelial and mesenchymal components. Hum Pathol. 1998;29:82–87. doi: 10.1016/s0046-8177(98)90394-x. [DOI] [PubMed] [Google Scholar]
- 8.Abargel A, Avinoach I, Kravtsov V, Boaz M, Glezerman M, Menczer J. Expression of p27 and p53: comparative analysis of uterine carcinosarcoma and endometrial carcinoma. Int J Gynecol Cancer. 2004;14:354–359. doi: 10.1111/j.1048-891x.2004.014221.x. [DOI] [PubMed] [Google Scholar]
- 9.Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104:177–178. doi: 10.1016/j.ijgo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Gorai I, Yanagibashi T, Taki A, Udagawa K, Miyagi E, Nakazawa T, Hirahara F, Nagashima Y, Minaguchi H. Uterine carcinosarcoma is derived from a single stem cell: an in vitro study. Int J Cancer. 1997;72:821–827. doi: 10.1002/(sici)1097-0215(19970904)72:5<821::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Fujii H, Yoshida M, Gong ZX, Matsumoto T, Hamano Y, Fukunaga M, Hruban RH, Gabrielson E, Shirai T. Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer Res. 2000;60:114–120. [PubMed] [Google Scholar]
- 12.Greer BE, Koh WJ, Abu-Rustum N, Bookman MA, Bristow RE, Campos SM, Cho KR, Copeland L, Crispens MA, Eifel PJ, Huh WK, Jaggernauth W, Kapp DS, Kavanagh JJ, Lurain JR, III, Morgan M, Morgan RJ, Powell CB, Remmenga SW, Reynolds RK, Alvarez Secord A, Small W, Jr, Teng N. Uterine neoplasms. Clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2009;7:498–531. doi: 10.6004/jnccn.2009.0035. [DOI] [PubMed] [Google Scholar]
- 13.Sutton G, Brunetto VL, Kilgore L, Soper JT, McGehee R, Olt G, Lentz SS, Sorosky J, Hsiu JG. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: a gynecologic oncology group study. Gynecol Oncol. 2000;79:147–153. doi: 10.1006/gyno.2000.6001. [DOI] [PubMed] [Google Scholar]
- 14.Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC, Monk BJ, Ueland FR, Gynecologic OG. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a gynecologic oncology group study. J Clin Oncol. 2007;25:526–531. doi: 10.1200/JCO.2006.06.4907. [DOI] [PubMed] [Google Scholar]
- 15.Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, Cohn DE, Ioffe OB. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I–IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177–185. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GOG protocol 261, Paclitaxel and Carboplatin or Ifosfamide in treating patients with newly diagnosed persistent or recurrent uterine, ovarian, fallopian tube, or periotenum carcinosarcoma. www.cancer.gov (clinical trials website)
- 17.Dusenbery KE, Potish RA, Argenta PA, Judson PL. On the apparent failure of adjuvant pelvic radiotherapy to improve survival for women with uterine sarcomas confined to the uterus. Am J Clin Oncol. 2005;28:295–300. doi: 10.1097/01.coc.0000156919.04133.98. [DOI] [PubMed] [Google Scholar]
- 18.Dusenbery KE, Potish RA, Judson P. Limitations of adjuvant radiotherapy for uterine sarcomas spread beyond the uterus. Gynecol Oncol. 2004;94:191–196. doi: 10.1016/j.ygyno.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed mullerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys. 2004;58:786–796. doi: 10.1016/S0360-3016(03)01561-X. [DOI] [PubMed] [Google Scholar]
- 20.Gadducci A, Romanini A. Adjuvant chemotherapy in early stage uterine sarcomas: an open question. Eur J Gynaecol Oncol. 2001;22:352–357. [PubMed] [Google Scholar]
- 21.Le T. Adjuvant pelvic radiotherapy for uterine carcinosarcoma in a high risk population. Eur J Surg Oncol. 2001;27:282–285. doi: 10.1053/ejso.2000.1104. [DOI] [PubMed] [Google Scholar]
- 22.Knocke TH, Weitmann HD, Kucera H, Kolbl H, Pokrajac B, Potter R. Results of primary and adjuvant radiotherapy in the treatment of mixed mullerian tumors of the corpus uteri. Gynecol Oncol. 1999;73:389–395. doi: 10.1006/gyno.1999.5400. [DOI] [PubMed] [Google Scholar]
- 23.Chauveinc L, Deniaud E, Plancher C, Sastre X, Amsani F, de la Rochefordiere A, Rozemberg H, Clough KB. Uterine sarcomas: the Curie Institut experience. Prognosis factors and adjuvant treatments. Gynecol Oncol. 1999;72:232–237. doi: 10.1006/gyno.1998.5251. [DOI] [PubMed] [Google Scholar]
- 24.Reed NS, Mangioni C, Malmstrom H, Scarfone G, Poveda A, Pecorelli S, Tateo S, Franchi M, Jobsen JJ, Coens C, Teodorovic I, Vergote I, Vermorken JB European Organisation for R. Treatment of cancer gynaecological cancer G. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) Eur J Cancer. 2008;44:808–818. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Einstein MH, Klobocista M, Hou JY, Lee S, Mutyala S, Mehta K, Reimers LL, Kuo DY, Huang GS, Goldberg GL. Phase II trial of adjuvant pelvic radiation “sandwiched” between ifosfamide or ifosfamide plus cisplatin in women with uterine carcinosarcoma. Gynecol Oncol. 2012;124:26–30. doi: 10.1016/j.ygyno.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez Bosquet J, Terstriep SA, Cliby WA, Brown-Jones M, Kaur JS, Podratz KC, Keeney GL. The impact of multi-modal therapy on survival for uterine carcinosarcomas. Gynecol Oncol. 2010;116:419–423. doi: 10.1016/j.ygyno.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 27.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 28.Cantrell LA, Havrilesky L, Moore DT, O’Malley D, Liotta M, Secord AA, Nagel CI, Cohn DE, Fader AN, Wallace AH, Rose P, Gehrig PA. A multi-institutional cohort study of adjuvant therapy in stage I–II uterine carcinosarcoma. Gynecol Oncol. 2012;127:22–26. doi: 10.1016/j.ygyno.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Garg G, Yee C, Schwartz K, Mutch DG, Morris RT, Powell MA. Patterns of care, predictors, and outcomes of chemotherapy in elderly women with early-stage uterine carcinosarcoma: a population-based analysis. Gynecol Oncol. 2014;133:242–249. doi: 10.1016/j.ygyno.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodwin JS, Hunt WC, Samet JM. A population-based study of functional status and social support networks of elderly patients newly diagnosed with cancer. Arch Intern Med. 1991;151:366–370. [PubMed] [Google Scholar]
- 31.Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control. 2009;16:53–56. doi: 10.1177/107327480901600108. [DOI] [PubMed] [Google Scholar]
- 32.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among african americans in the United States. Gynecol Oncol. 2013;130:652–659. doi: 10.1016/j.ygyno.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secord AA, Geller MA, Broadwater G, Holloway R, Shuler K, Dao NY, Gehrig PA, O’Malley DM, Finkler N, Havrilesky LJ. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013;128:65–70. doi: 10.1016/j.ygyno.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Chi DS, Mychalczak B, Saigo PE, Rescigno J, Brown CL. The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcoma. Gynecol Oncol. 1997;65:493–498. doi: 10.1006/gyno.1997.4676. [DOI] [PubMed] [Google Scholar]