A microengineered model was used to provide in vitro quantitative evidence of the ability of mesoangioblasts to restore dystrophin, in terms of protein accumulation and distribution, within myotubes derived from patients with Duchenne muscular dystrophy (DMD). Results showed healthy myoblasts and mesoangioblasts restored dystrophin expression in DMD myotubes. However, mesoangioblasts showed unexpected efficiency with respect to myoblasts in dystrophin production and length of the dystrophin membrane domain.
Keywords: Cellular therapy, Muscular dystrophy, Pericytes, Tissue regeneration, Technology
Abstract
Restoration of the protein dystrophin on muscle membrane is the goal of many research lines aimed at curing Duchenne muscular dystrophy (DMD). Results of ongoing preclinical and clinical trials suggest that partial restoration of dystrophin might be sufficient to significantly reduce muscle damage. Different myogenic progenitors are candidates for cell therapy of muscular dystrophies, but only satellite cells and pericytes have already entered clinical experimentation. This study aimed to provide in vitro quantitative evidence of the ability of mesoangioblasts to restore dystrophin, in terms of protein accumulation and distribution, within myotubes derived from DMD patients, using a microengineered model. We designed an ad hoc experimental strategy to miniaturize on a chip the standard process of muscle regeneration independent of variables such as inflammation and fibrosis. It is based on the coculture, at different ratios, of human dystrophin-positive myogenic progenitors and dystrophin-negative myoblasts in a substrate with muscle-like physiological stiffness and cell micropatterns. Results showed that both healthy myoblasts and mesoangioblasts restored dystrophin expression in DMD myotubes. However, mesoangioblasts showed unexpected efficiency with respect to myoblasts in dystrophin production in terms of the amount of protein produced (40% vs. 15%) and length of the dystrophin membrane domain (210–240 µm vs. 40–70 µm). These results show that our microscaled in vitro model of human DMD skeletal muscle validated previous in vivo preclinical work and may be used to predict efficacy of new methods aimed at enhancing dystrophin accumulation and distribution before they are tested in vivo, reducing time, costs, and variability of clinical experimentation.
Significance
This study aimed to provide in vitro quantitative evidence of the ability of human mesoangioblasts to restore dystrophin, in terms of protein accumulation and distribution, within myotubes derived from patients with Duchenne muscular dystrophy (DMD), using a microengineered model. An ad hoc experimental strategy was designed to miniaturize on a chip the standard process of muscle regeneration independent of variables such as inflammation and fibrosis. This microscaled in vitro model, which validated previous in vivo preclinical work, revealed that mesoangioblasts showed unexpected efficiency as compared with myoblasts in dystrophin production. Consequently, this model may be used to predict efficacy of new drugs or therapies aimed at enhancing dystrophin accumulation and distribution before they are tested in vivo.
Introduction
Duchenne muscular dystrophy (DMD) is a genetic disease caused by mutations in the gene encoding the protein dystrophin [1]. Dystrophin is a critical component of the dystrophin-glycoprotein complex (DGC) in muscle that links the actin cytoskeleton to the extracellular matrix of myofibers. The lack of a functional dystrophin protein causes loss of proper localization of many of the DGC components at the sarcolemma of muscle fibers, leading to membrane instability and myofiber degeneration [2]. DMD primarily affects skeletal muscles and results in progressive paralysis and premature death. At the moment, no successful treatments are available, but new drug, gene, and cell therapy strategies are under clinical investigation [3, 4].
Many of the therapeutic strategies and research lines for DMD are focused on the restoration of the protein dystrophin. In 2 recently completed clinical trials, 15% [5] and 18% [6] of normal levels of dystrophin resulted in a moderate but significant clinical benefit during a 12-week study. Indeed, reports indicate that dystrophin production as low as 30% of that found in healthy animals or individuals prevents muscular X-linked dilated cardiomyopathy (XLDC) in humans [7, 8].
In this scenario, cell therapy has good potential. In particular, strategies able to restore the compartment of muscle stem cells are among the most promising because they would not require continuous injections to sustain the muscle regeneration. However, cell therapy is a complex regenerative process that includes intra-arterial (or intramuscular) cell delivery, crossing the blood vessel wall, survival, migration, and contribution to skeletal muscle regeneration by fusion with regenerating muscle fibers, and by entering the satellite cell compartment [8, 9]. From this perspective, pericytes are a promising cell source because of their peculiar characteristics: These cells surround the endothelium of small vessels and can differentiate into different mesoderm cell types, including skeletal muscle [8, 10]. When delivered into the arterial circulation, mouse mesoangioblasts cross the blood vessel wall and participate in skeletal muscle regeneration, ameliorating signs of muscular dystrophy in animal models such as the α-sarcoglycan-null [11] and mdx [8] mice and the golden retriever muscular dystrophy dog [12]. When cells similar to mouse mesoangioblasts were isolated from human adult skeletal muscle, they were shown to correspond to a subset of pericytes expressing alkaline phosphatase [13]. Cells derived from in vitro expansion of human skeletal muscle vasculature pericytes, which we deemed mesoangioblasts, have recently been transplanted in DMD patients in a phase I/IIa clinical trial (EudraCT no. 2011-000176-33) whose results showed safety and limited efficacy [14].
Clinical trials are expensive and time-consuming processes required before drugs and therapies reach the market and the clinics; many drugs are withdrawn and many therapies fail during clinical trials. Moreover, it may be extremely useful to test a large number of variables that may synergize to increase efficacy (e.g., combination of different drugs with cell/gene therapy), but it is, in fact, almost impossible because of logistic and economic reasons. To overcome this problem, the concept of “clinical trial in a dish” or “clinical trial on a chip” has been recently proposed [15]. Such complementary approaches could provide therapy efficiency information at an early stage of protocol development. For instance, Liang et al. [16] validated the capacity of a library of human induced pluripotent stem cell-derived cardiomyocytes to be used as a clinical trial-in-a-dish model for accurate detection of patient-specific drug responses and drug-induced cardiotoxicity profiles. The concept of a clinical trial-on-a-chip is based on the advances in microtechnology and microfluidics, and development of physiologically relevant three-dimensional organs or tissues on the microscale level. The aim is to provide a less expensive, faster, and more accurate way to screen drugs or therapies for efficacy and toxicity. Several reviews of the potential of this approach have been published [15, 17–19].
So far, no study has reported the use of skeletal muscle for clinical evaluation of therapy in a dish. However, the derivation of physiologically relevant skeletal muscle tissues in vitro is not straightforward. Physiological stimuli and interactions must be reproduced and finely controlled. In particular, the development of a skeletal muscle model and a DMD assay requires the formation of a mature tissue with a high degree of differentiation and a proper expression of dystrophin, which is a late marker not commonly detected in standard in vitro cultures. For this reason, primary myoblasts freshly isolated from patient should be used because they can be expanded and successfully differentiated into mature myotubes, the functional unit of skeletal muscle. The major drawback of this cell source, the relatively low number of derived cells, could be overcome with the design of microscaled assay able to maintain physiological relevance.
We developed a human-based skeletal muscle tissue-on-a-chip derived from healthy and DMD donors [20, 21]. We engineered the culture substrate, in terms of mechanical and topological properties, for optimizing human myoblast differentiation and obtaining the expression of dystrophin in vitro [20].
In this work, we aimed at exploiting the developed human-based skeletal muscle tissue-on-a-chip for testing a number of experimental variables that may subsequently benefit the design of a protocol for future clinical trials, leading to more efficacious stem cell therapies. We used a microengineered DMD model of skeletal muscle to test the ability of human mesoangioblasts, in comparison with human myoblasts, to restore levels of dystrophin expression and distribution along the myotube when cocultured with myoblasts from DMD patients. Wild-type cells (i.e., dystrophin-positive [Dys+] myoblasts or mesoangioblasts derived from skeletal muscle vasculature from healthy subjects) were cocultured with DMD cells (dystrophin negative [Dys−]) at different ratios. The coculture of Dys+ with Dys− cells induced the formation of myotubes containing nuclei encoding for dystrophin and DMD nuclei not encoding for this protein.
Materials and Methods
Cell Culture
Myoblasts (Mbs) are referred to here as the in vitro counterpart of satellite cell-derived myoblasts. Mesoangioblasts (Mabs) are referred to as an in vitro-expanded perivascular cell population sharing markers of skeletal muscle pericytes and likely corresponding to a subpopulation thereof. Mesoangioblasts isolated from postnatal mammalian muscle express markers (>95%) common to mesenchymal stem cells such as CD44, CD90, and CD13 but not endothelial or hematopoietic markers such as CD31, CD34, and CD45. They do not express the satellite cell marker CD56. They express, to variable extents in different preparations, the “pericyte” markers platelet-derived growth factor receptor β, smooth α actin, neural-glial-2 chondroitin sulphate proteglycan, alkaline phosphatase, and CD146 [22].
Human primary myoblasts were provided by the Telethon BioBank (Telethon Research Service, Istituto Nazionale Neurologico “Carlo Besta,” Milan, Italy, http://www.istituto-besta.com). They are derived from healthy donors (Dys+ cells) and from DMD affected donors (Dys− cells). The mutation in DMD donors is the deletion of exon 45. Myoblasts were expanded on standard 100-mm tissue-culture Petri dishes previously coated with 0.5% gelatin solution (gelatin from porcine skin; Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) with the following proliferation medium: 60% high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM Glutamax; Thermo Fisher), 20% Medium M199 (Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com), 20% fetal bovine serum (Thermo Fisher), 10 ng/ml epidermal growth factor (PeproTech, Rocky Hill, NJ, https://www.peprotech.com), 2 ng/ml β-fibroblast growth factor (β-FGF; PeproTech), 10 μg/ml insulin (insulin from bovine pancreas; Sigma-Aldrich), and 1% penicillin-streptomycin-glutamine mix solution (Thermo Fisher).
Human mesoangioblasts were derived from skeletal muscle biopsy specimens from healthy subjects, as previously described [22]. Biopsy specimens were obtained from the Orthopedic Surgery Department of the San Raffaele Hospital, Milan, Italy, after authorization from the Institute Ethics Committee and signature of informed consent by the patient or his or her parents or caregivers.
Briefly, the biopsy specimen was cleaned of fat and connective tissue, minced to fragments that were plated on collagen-coated dishes (6 or 9 cm, depending on the size of the dish) and cultured with 1 or 2 ml (in the 6- or 9-cm dishes, respectively) of Megacell medium (described next). More medium was gently added the next day. After a week, when cells had outgrown from the explant, loosely attached or floating cells were collected by gentle pipetting and subcultured as P1.
Human mesoangioblasts derived from healthy donors were expanded in a gelatin-coated dish with proliferation medium composed of MegaCell DMEM (Sigma-Aldrich) supplemented with 5% heat-inactivated fetal bovine serum (Thermo Fisher), 2 mM l-glutamine (Thermo Fisher), 1% nonessential amino acids (Thermo Fisher), 0.1 mM β-mercaptoethanol (Thermo Fisher), 5 ng/ml β-FGF (PeproTech), and 1% penicillin-streptomycin (Thermo Fisher). Mesoangioblasts were induced to differentiate into myotubes by changing the proliferation medium with the same differentiating medium used for human primary myoblasts. Human primary myoblasts and mesoangioblasts were induced to form myotubes in the following differentiating medium: 98% DMEM Glutamax (Thermo Fisher), 2% horse serum (Thermo Fisher), 30 µg/ml insulin, and 1% penicillin-streptomycin-glutamine mix solution (Thermo Fisher). We used three batches of mesoangioblasts, three batches of myoblasts from healthy individuals, and one batch of myoblasts from DMD patients.
Dys+ and Dys− Coculture on Micropatterned Substrate
The engineered culture system was prepared as previously described [20, 21]. Briefly, hydrogels with muscle-like stiffness were fabricated with acrylamide/bisacrylamide (29:1) 40% solution (Sigma-Aldrich) over a 25-mm glass coverslip. The prepolymer was diluted in phosphate-buffered saline (Sigma-Aldrich) to the final concentrations of 10%. The photoinitiator (Irgacure 2959; BASF, Basel, Switzerland, https://www.basf.com) was added to the acrylamide/bis-acrylamide solution to reach a final concentration of 20 mg/ml, and mixed thoroughly. Hydrogel polymerization occurred by exposing the prepolymer solution to UV light for 3 minutes (high-pressure mercury vapor lamp [Philips HPR 125 W; Philips Lighting Holding, Eindhoven, The Netherlands, http://www.lighting.philips.com] emitting at 365 nm with an incident light intensity of 20 mW/cm2). Nonpolymerized acrylamide was removed using distilled water. Hydrogel films were immersed in ultrapure distilled water for 48 hours to ensure complete removal of the unreacted monomeric units or photoinitiator, and final sterilization occurred after 20 minutes of exposure to UV light under a sterile hood. The hydrogel has a diameter of 18 mm.
Matrigel (2.5% volume per volume in DMEM) was used for the micropattern. Micropatterning geometry (parallel lanes, 1 cm2) was optimized for myoblasts and pericytes, according to our previous work [21]. Dys+ and Dys− cells were cocultured in ratios of 1:1, 1:2, and 1:9. A 300-µl aliquot of the cell suspension (1 × 105 cells/ml) was dropped on the micropatterned hydrogel and the cultures were kept at 37°C, 5% CO2. Cocultures were maintained in a 1:1 mix of each specific proliferation medium for 1 or 2 days, followed by 8 days of culture in differentiating medium.
Western Blotting
The cultures were treated with 50 µl of ice-cold lysis buffer: 50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.5 mM dithiothreitol (DTT); 10% glycerol; 1 mM EDTA; 10 mM MgCl2; 2% SDS; 1% Triton X-100; 1 mM phenylmethylsulfonyl fluoride; 1 mM NaV; 5 mM NaF; 3 mM β-glycerol (all Sigma-Aldrich); and complete EDTA-free protease inhibitor cocktail (Roche, Basel, Switzerland, http://www.roche.com). Lysis buffer was dropped directly onto the hydrogel surfaces and incubated at 4°C for 1 hour. After 1 hour of treatment, lysis buffer was resuspended on the hydrogel to collect all the cellular contents. Cell fractions were sedimented by centrifugation at 13,000g for 20 minutes at 4°C, and supernatant was collected. Protein extract (10 µg per lane) was solubilized in loading buffer (Thermo Fisher) and 10% DTT (Thermo Fisher), and heated for 10 minutes at 70°C. Proteins were resolved in 3%–8% precast gels (NuPAGE Tris-Acetate gel; Thermo Fisher) and then transferred on polyvinylidene difluoride membranes (Thermo Fisher) under a potential difference of 45 V and 400 mA for 6 hours. Membranes were blocked with 5% nonfat dry milk (Bio-Rad, Hercules, CA, http://www.bio-rad.com) in TBST (TBS, 0.05% Tween 20) and then probed with primary antibodies for dystrophin (Abcam, Cambridge, UK, http://www.abcam.com), myosin heavy chain II (Sigma-Aldrich), and β-actin (Sigma-Aldrich), and then with the proper horseradish peroxidase-conjugated secondary antibodies: goat anti-rabbit antibody (Thermo Fisher) and goat anti-mouse antibody (Bio-Rad). Proteins were visualized by enhanced chemiluminescence (Thermo Fisher), and dystrophin content was quantified by densitometry using ImageJ software (U.S. National Institutes of Health). For each culture condition, we quantified the intensity of dystrophin and myosin heavy chain bands, and normalized them with the housekeeping protein β-actin.
Immunofluorescence
Primary antibodies used in this study were against myosin heavy chain II (Sigma-Aldrich) and dystrophin (Abcam). A standard immunohistochemistry protocol was used [20]. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich); samples were mounted with a polyvinyl alcohol product, and viewed under a fluorescence confocal microscope (Leica, Wetzlar, Germany, http://www.leica-microsystems.com).
Results
Assay Validation
The microengineered DMD model used in this study has been developed in our laboratory [20, 21] and it can be placed in a well of a standard six-multiwell plate. It allows the amount of reagents and the number of cells per sample to be reduced: The culture surface is 0.5 cm2 and as few as 3 × 104 cells per sample can be used. To analyze the contribution of mesangioblasts derived from skeletal muscle vasculature and of myoblasts in the restoration of dystrophin, we designed an experimental strategy based on coculture at different ratios of Dys+ and Dys− human cells in a microengineered in vitro model of human DMD skeletal muscle (Fig. 1). We defined Dys+ cells as either myoblasts or mesoangioblasts derived from skeletal muscle vasculature from healthy subjects; “Dys− cells” refers to myoblasts from DMD patients.
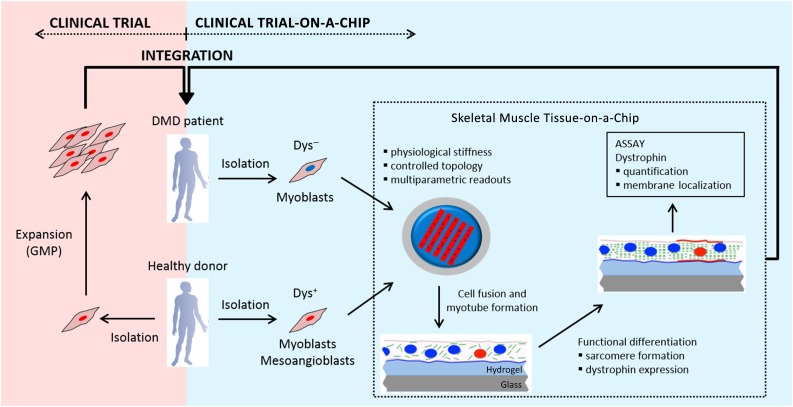
Figure 1.
Description of the experimental strategy used in this study. Clinical trials (light red box) on stem cell therapies are based on the isolation and expansion of stem cells from a healthy donor following GMP guidelines. These cells are then injected into the DMD patient. Clinical trial-on-a-chip (light blue box) is based on the exploitation of the skeletal muscle tissue-on-a-chip, which allows multiparametric and high-throughput experiments to be performed in vitro. Dys+ and Dys− cells are cocultured at different ratios within the skeletal muscle tissue-on-a-chip. The proportion used for the core set of experiments was one Dys+ to nine Dys− cells. Dys+ and Dys− cells fuse and result in myotubes composed of Dys+ and Dys− nuclei. The microengineered model induces the functional maturation of the myotubes, promoting MyHC sarcomeric organization and dystrophin expression within 8 days. The cocultures are analyzed in terms of dystrophin accumulation and localization within the membrane. The knowledge acquired is then integrated within the clinical trial process. Abbreviations: DMD, Duchenne muscular dystrophy; Dys+, myoblasts or mesoangioblasts derived from skeletal muscle vasculature from healthy subjects; Dys−, myoblasts from DMD patients; GMP, Good Manufacturing Practice.
We recently demonstrated that human myoblasts (both Dys+ and Dys−) differentiated optimally in our system and gave rise to fully differentiated myotubes [20, 21]. Sarcomeric striations of myosin heavy chain (MyHC) were visible (Fig. 2A) and dystrophin was expressed and located at the membrane (Fig. 2C). Mesoangioblasts also differentiate in our system: We obtained myotubes striated for MyHC (Fig. 2B) with membrane-localized dystrophin (Fig. 2D). As expected, myotubes derived from myoblasts of DMD patients showed sarcomeric organization of MyHC (Fig. 2E) but no dystrophin expression (Fig. 2F).
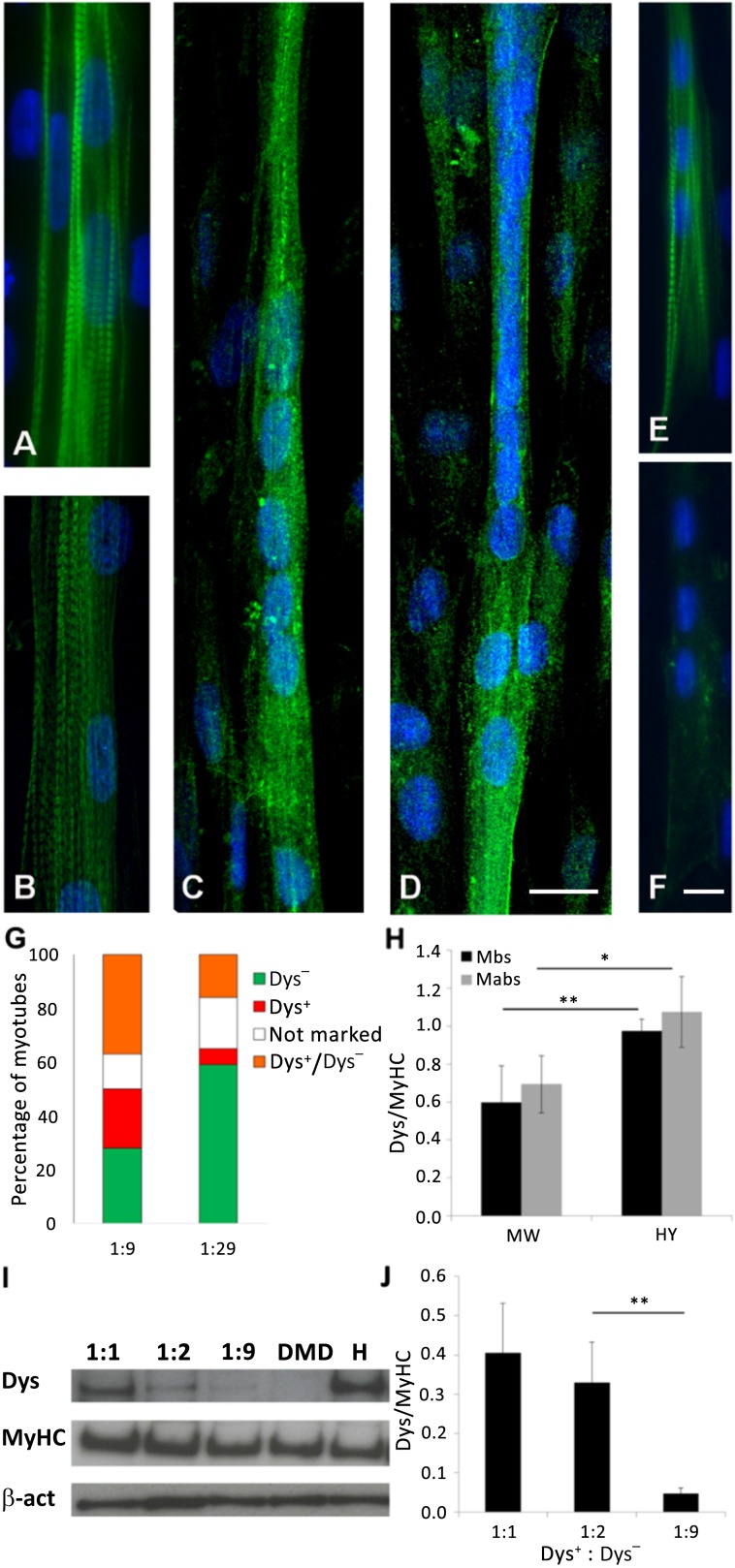
Figure 2.
Validation of the assay. We verified the differentiation of primary myoblasts and mesoangioblasts on our device and characterized the coculture assay. (A–F): Functional maturation of human myoblasts (A, C), human mesoangioblasts (B, D), and human DMD primary myoblasts (E, F) was confirmed through immunofluorescence against MyHC (A, B, E) and dystrophin (C, D, F); n = 3 independent biological replicates (E). (G): Graph representing the percentage of myotubes derived from Dys− cells (green), Dys+ cells (red), not marked cells (white), and Dys− cells fused with Dys+ cells (orange); n = 4 independent biological replicates. (H): Graph representing the ratio between the band intensity of dystrophin and MyHC in Dys+ Mbs and Dys+ Mabs. The cultures were grown in standard 48-multiwell plates and on the microengineered skeletal muscle chip. (I): A representative Western blot of the coculture of myoblasts. The 1:1, 1:2, and 1:9 samples are the ratio of Dys+ to Dys− myoblasts. (J): Quantification of the bands’ intensity. Error bars indicate SD (H, J). ∗, p < .05; ∗∗, p < .01 (two-sided t test). Abbreviations: β-act, β-actin; DMD, Duchenne muscular dystrophy; Dys, dystrophin; Dys+, myoblasts or mesoangioblasts derived from skeletal muscle vasculature from healthy subjects; Dys−, primary myoblasts affected by Duchenne muscular dystrophy; H, Dys+ myoblasts from healthy donor; HY, microengineered skeletal muscle chip; Mabs, mesoangioblasts; Mbs, myoblasts; MyHC, myosin heavy chain; MW, multiwell.
Because this study is based on the coculture of human Dys− and Dys+ myogenic cells, we developed an assay to distinguish the two cell populations. We tested a number of standard methodologies (supplemental online Fig. 1), but most failed, for different reasons. The only method that allowed us to identify hybrid myotubes derived from fusion of Dys+ with Dys− cells was the use of lipophilic tracers Dil and DiO. We marked Dys+ cells with Dil (red) and Dys− cells with DiO (green) and performed the coculture experiments with 2 different ratios of cells from the 2 populations: 1 Dys+ cell to every 9 Dys− cells and 1 Dys+ cell to every 29 Dys− cells (n = 3 independent experiments) (Fig. 2G; supplemental online Fig. 1D–1F).
We observed 4 types of myotubes: myotubes formed only by Dys− cells, with a green fluorescence (Dys− myotubes); myotubes formed only by Dys+ cells, with a red fluorescence (Dys+ myotubes); myotubes without fluorescence (not-marked myotubes), because these tracers marked approximately 80% of the cells; and myotubes formed by Dys+ and Dys− cells, with an orange fluorescence (Dys−/Dys+ myotubes). Dys−/Dys+ myotubes represented 51% and 42% of myotubes (in 1:9 and 1:29 cocultures, respectively) expressing dystrophin (because Dys− myotubes do not express dystrophin). We thus concluded that the fusion of Dys− and Dys+ cells contributed to 50% of the dystrophin signal in the coculture experiments.
Unfortunately, tracking Dys− and Dys+ cell nuclei, which would help provide better characterization of the systems, is not straightforward for long-term cell fusion experiments. Staining with Hoechst 33258 [22] (supplemental online Fig. 1A) resulted in reduced cell viability after 5 days of culture. Human myoblasts infected with an adenovirus expressing the green fluorescent protein (supplemental online Fig. 1B) showed low infection efficiency (approximately 50%). The use of a lentivirus encoding for a nuclear LacZ showed high efficiency (90%); however, the β-galactosidase was translocated to all the nuclei inside the myotube. After 10–12 days, we observed myotubes with all positive nuclei, myotubes with all negative nuclei, and, in few cases, gradient of staining (supplemental online Fig. 1C). Thus, it was not possible to track clearly nuclei origin inside Dys− and Dys+ myotubes.
The expression and accumulation of dystrophin were then analyzed using Western blotting. First, we verified that the culture of Dys+ cells in our microengineered model maintained the same ratio of dystrophin to MyHC as that of cultures in standard multiwell plates (48 wells) for both myoblasts and mesoangioblasts (Fig. 2H). The results showed that our microengineered model induced a differentiation consistent with standard culture substrate and increased the expression of dystrophin (n = 3 independent biological replicates; Fig. 2H). In addition, we determined the Dys-to-MyHC ratio in vivo (Western blot of a biopsy specimen of human muscle). The in vivo Dys-to-MyHC ratio was 0.08, roughly one-tenth of the ratio found in myotubes formed in vitro (1.07 for mesoangioblasts and 0.97 for myoblasts, on hydrogel) (data not shown). We expected this result because the average diameter of a myotube is 10 µm, whereas the diameter of a muscle fiber is 100 µm. Dystrophin covers only the periphery of the myotube or muscle fiber, whereas MyHC occupies the whole fiber. Therefore, the Dys-to-MyHC ratio between in vivo and in vitro conditions should be around 1:10.
To determine the lower ratio of Dys+ and Dys− cells with a detectable expression of dystrophin, we performed cocultures of Dys+ and Dys− myoblasts at different ratios: 1:1, 1:2, and 1:9. We quantified the production of dystrophin and MyHC as the intensity of Western blot bands. Figure 2I shows a representative Western blot. The quantification of Western blot bands are reported in Figure 2J (n = 4 independent biological replicates). As expected, the production of dystrophin was directly proportional to the number of Dys+ cells in culture. Based on these results, we decided to use the 1:9 ratio because the dystrophin band was still detectable.
Dystrophin Accumulation
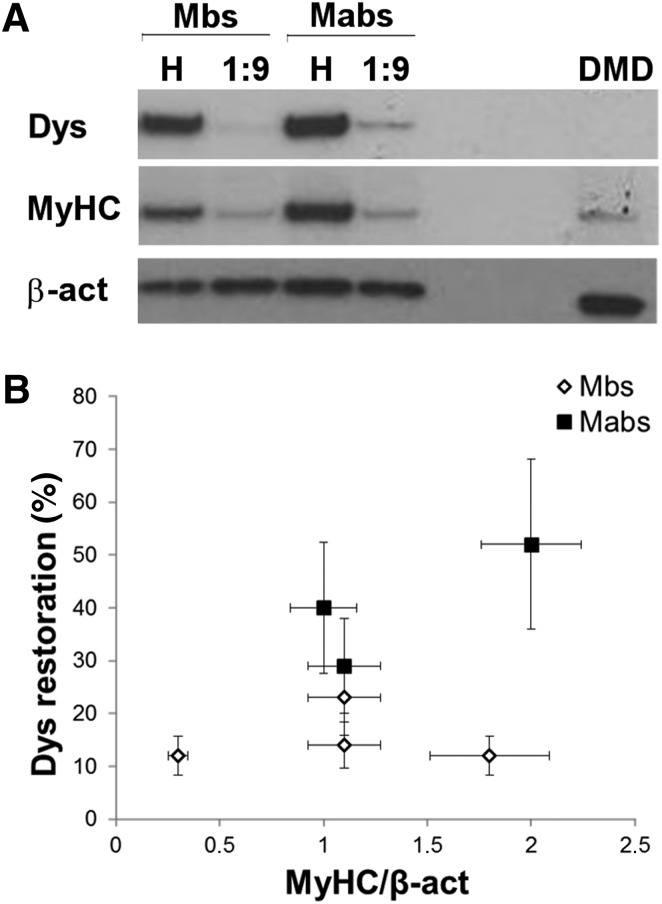
We analyzed dystrophin expression by Dys+ myoblasts and mesoangioblasts, when cocultured with Dys− myoblasts in a 1:9 ratio, by Western blotting (Fig. 3A). The graph in Figure 3B reports the percentage of dystrophin restoration versus MyHC intensity. The percentage of dystrophin restoration was defined as the intensity of dystrophin from the coculture normalized by the intensity of dystrophin from samples of Dys+ cells (myoblasts or mesoangioblasts, accordingly). Dystrophin restoration was represented as a function of MyHC expression because the latter represents the differentiation degree of the culture. Because dystrophin expression strictly correlated with the differentiation degree of the culture, we observed that, although the differentiation timing was kept constant (8 days), cultures reached different levels of differentiation, in particular for Dys− myoblasts. Therefore, to compare different samples, dystrophin restoration was reported as a function of MyHC expression. The results showed that dystrophin restoration was always higher in mesoangioblasts cocultures than in myoblasts cocultures (n = 3 independent biological replicates for each cell type; Fig. 3B).
Figure 3.
Analysis of the accumulation of dystrophin within coculture experiments. (A): A representative Western blot of the coculture experiments of Mbs or Mabs derived from skeletal muscle vasculature from healthy subjects (Dys+) and Mbs from patients with Duchenne muscular dystrophy (Dys−), and Dys+ Mabs and Dys−. (B): Quantification of the percentage of dystrophin restoration as function of MyHC expression; n = 3 independent biological replicates. Error bars represent SD. Abbreviations: β-act, β-actin; DMD, Duchenne muscular dystrophy; Dys−, primary myoblasts affected by Duchenne muscular dystrophy; Dys, dystrophin; H, sample with only Dys+ cells (Mbs or Mabs); Mabs, mesoangioblasts; Mbs, myoblasts; MyHC, myosin heavy chain.
Dystrophin Expression Domain on the Myotube Surface
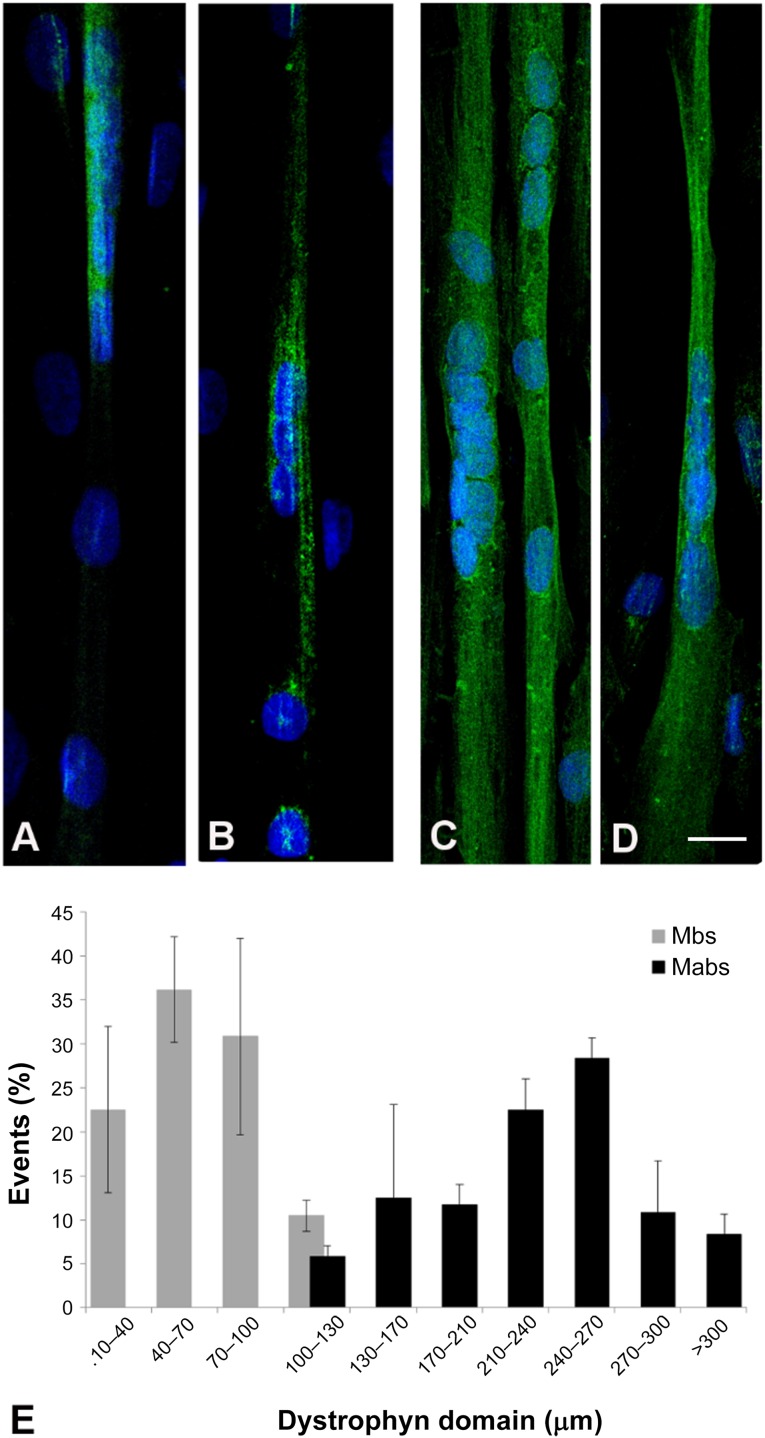
In terms of dystrophin localization, immunofluorescence analysis indicated that Dys+ myoblasts cocultured with Dys− myoblasts (at the 1:9 ratio) gave rise to myotubes where dystrophin was expressed in a defined portion of the myotube (Fig. 4A, 4B). Dys+ mesoangioblasts in the same conditions, on the other hand, gave rise to myotubes expressing dystrophin along the majority of their length; that is, the protein was expressed almost along the entire myotube (Fig. 4C, 4D). We analyzed the length of the myotube in which dystrophin was expressed (the “dystrophin domain”). The distribution of the dystrophin domain length is depicted in Figure 4E (independent biological replicates: Mbs, n = 3; Mabs, n = 2). The dystrophin restoration domain sustained by myoblasts had an average length of 40–100 µm, whereas mesoangioblast nuclei contributed to restore dystrophin for 210–240 µm.
Figure 4.
Analysis of dystrophin domain length restored in the coculture experiments. (A–D): Immunofluorescence against dystrophin in the 1:9 coculture of Mbs or Mabs derived from skeletal muscle vasculature from healthy subjects (Dys+) and Mbs from patients with Duchenne muscular dystrophy (Dys−) (A, B) and 1:9 coculture of Dys+ Mabs and Dys− (C, D). (E): Quantification of dystrophin domain length; n = 3 (Mbs) and n = 2 (Mabs) independent biological replicates. Error bars represent SD. Abbreviations: Mabs, mesoangioblasts; Mbs, myoblasts.
We analyzed the number of nuclei per myotube in the dystrophin domain intervals to avoid the possibility that a longer dystrophin domain could be due to a higher number of nuclei per myotube (supplemental online Fig. 2). We observed that the average number of nuclei per myotube was comparable in myoblast and mesoangioblast cocultures.
Discussion
This study shows that human mesoangioblasts, derived from skeletal muscle vasculature, restored dystrophin expression and distribution in an in vitro model of human DMD skeletal muscle tissue-on-a-chip. Unexpectedly, they did so more efficiently than human skeletal myoblasts.
The human skeletal muscle tissue-on-a-chip used in this work is a versatile tool for studying human skeletal muscle differentiation in vitro. Recently, we demonstrated that human wild-type and DMD myoblasts can differentiate optimally in this model, thanks to the mechanical and topological stimuli exerted [20, 21]. In addition, dystrophin was expressed at significant levels and could be detected by immunofluorescence analysis [20]. These two main characteristics make this model suitable for studying human skeletal muscle differentiation in vitro. In this work, the developed human skeletal muscle tissue-on-a-chip was exploited for studying the ability of mesoangioblasts from skeletal muscle vasculature to restore dystrophin expression in hybrid myotubes formed with an excess of dystrophic myoblasts, and this was compared with satellite cell-derived myoblasts.
First, we verified that mesoangioblasts differentiated optimally in our microengineered model (Fig. 2). We observed that the substrate stiffness (15 kPa) and topology (parallel lanes) induced the differentiation of mesoangioblasts to functional myotubes (sarcomeric striation of MyHC) expressing dystrophin. Indeed, the culture in our tissue-on-a-chip induced a higher expression of dystrophin when compared with the standard culture in a dish: The ratio between dystrophin and MyHC was higher in the culture in our microengineered model than the one performed in a standard multiwell plate (Fig. 2H). Our model-on-a-chip also offers the additional advantage of an ordered topology of myotubes. In our model, myotubes grew only on the micropatterned area and were all oriented along the main direction of the patterning. Such spatial organization allows easy quantification of the dystrophin expression domain; this would be much more difficult in a standard culture of myotubes in which they are randomly oriented.
Human mesoangioblasts [9, 13] are a promising cell source for DMD therapy because they overcome some of the limitations associated with myoblast intramuscular injections [8]. In particular, they can be delivered through intra-arterial injections because they cross the endothelium and migrate extensively in the interstitial space, show long-term survival, partially restore muscle structure and function in dystrophic mice and dogs [11, 12], and contribute to the muscle satellite cell pool [8]. Here, we used a microengineered DMD model to test the ability of mesoangioblasts to restore dystrophin expression, in terms of protein accumulation and distribution along the surface of myotubes derived from DMD patients.
Interestingly, three different batches of mesoangioblasts showed the ability to restore a significant level of dystrophin, which was analyzed by immunofluorescence and Western blotting. In terms of dystrophin accumulation, restoration of dystrophin by mesoangioblasts was higher than the hypothetical 30% of the control (according to studies of animals or patients with XLDC) [7, 8], provided that a 1:9 ratio was achieved in vitro. The domain of dystrophin restoration due to healthy mesoangioblasts nuclei spanned almost the entire myotube (with an average domain length of 210–240 μm).
Cell delivery in vivo is a complex process and reproducing all the phases (e.g., delivery, migration, fusion to host tissue) in vitro will be very challenging. However, these phases may be dissected in separate steps and an in vitro assay may be developed for each as a predictive tool of the corresponding in vivo performance. For example, Boiden chambers coated with endothelium, under various experimental conditions, may mimic crossing of the blood vessel in vivo [23].
However, how a mesoangioblast nucleus (or a nucleus of other types of myogenic progenitors) contributes to dystrophin synthesis and, consequently, which is the minimal ratio of delivered/survived and fused cells versus resident myofiber nuclei needed to rise dystrophin level above the 30% threshold is a fundamental question in this context. Indeed, in any cell therapy protocol, the preparation of donor cells to be injected requires their manipulation ex vivo and it is widely known that this step could be crucial to obtain good results. In this scenario, we hypothesize that our microscaled in vitro model could be used as a quality control test of donor cell batches and could help the prediction of clinical outcomes. In this respect, it is interesting to note that preliminary results from the phase I/II trial [14] indicated that on the patient with highest engraftment, donor dystrophin (detected by Western blot analysis) was expressed, albeit at a very low level, when donor DNA (analyzed by satellite microchimerism) was approximately 1% of total DNA in the biopsy specimen.
Another important aspect to consider is the very large number of variables that may be tested to enhance the efficacy of cell therapy, such as pretreatment of donor cells and/or of host muscle cells with molecules that may enhance differentiation, protein synthesis, or fusion. Obviously, it would be impossible to test all these variables in patients, and preclinical experimental studies on animal models may not reveal subtle differences between mouse and human cells [24, 25]. Our model could be used as an in vitro standard for testing the extent of dystrophin restoration in parallel with preclinical studies and before initiating clinical trials.
Conclusion
We have demonstrated that the evaluation of a fundamental clinical outcome, such as dystrophin expression, conducted on skeletal muscle tissue-on-a-chip could be of valuable support during preclinical phases or clinical trials.
Supplementary Material
Acknowledgments
This work was supported by Telethon GGP08140 and AFM 15462 and 16028 to N.E., and Optistem FP7 IP 223098 and Biodesign FP7 IP 262948 and Fundacio La Marato to G.C. E.S. was supported by University of Padova Grant Giovani Studiosi 2010 (DIRPRGR10), “Amici del cuore di Montebelluna” and “ASCM.” S.Z. was supported by Città della Speranza.
Author Contributions
E.S. and S.Z.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; A.Z. and F.S.T.: collection and/or assembly of data, data analysis and interpretation; F.L.V.: collection and/or assembly of data; G.C.: conception and design, financial support, final approval of manuscript; N.E.: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
F.S.T. has a pending patent application (PCT/GB2013/050112), has provided speaking and consulting services to Takeda Pharmaceuticals International Inc. via UCL Consultants, neither of which is related to this work, and has received a grant from Takeda Pharmaceuticals International’s New Frontier Science program to University College London. The other authors indicated no potential conflicts of interest.
References
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti S, Hoshiya H, Tedesco FS. Repair or replace? Exploiting novel gene and cell therapy strategies for muscular dystrophies. FEBS J. 2013;280:4263–4280. doi: 10.1111/febs.12178. [DOI] [PubMed] [Google Scholar]
- 4.Goyenvalle A, Seto JT, Davies KE, et al. Therapeutic approaches to muscular dystrophy. Hum Mol Genet. 2011;20(R1):R69–R78. doi: 10.1093/hmg/ddr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goemans NM, Tulinius M, van den Akker JT, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 6.Cirak S, Arechavala-Gomeza V, Guglieri M, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: An open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neri M, Torelli S, Brown S, et al. Dystrophin levels as low as 30% are sufficient to avoid muscular dystrophy in the human. Neuromuscul Disord. 2007;17:913–918. doi: 10.1016/j.nmd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Tedesco FS, Hoshiya H, D’Antona G, et al. Stem cell-mediated transfer of a human artificial chromosome ameliorates muscular dystrophy. Sci Transl Med. 2011;3:96ra78. doi: 10.1126/scitranslmed.3002342. [DOI] [PubMed] [Google Scholar]
- 9.Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 10.Cappellari O, Cossu G. Pericytes in development and pathology of skeletal muscle. Circ Res. 2013;113:341–347. doi: 10.1161/CIRCRESAHA.113.300203. [DOI] [PubMed] [Google Scholar]
- 11.Sampaolesi M, Torrente Y, Innocenzi A, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 12.Sampaolesi M, Blot S, D’Antona G, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 13.Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 14.Cossu G, Previtali SC, Napolitano S, et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med. 2015;7:1513–1528. doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Stolpe A, den Toonder J. Workshop meeting report Organs-on-Chips: Human disease models. Lab Chip. 2013;13:3449–3470. doi: 10.1039/c3lc50248a. [DOI] [PubMed] [Google Scholar]
- 16.Liang P, Lan F, Lee AS, et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engle SJ, Puppala D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell. 2013;12:669–677. doi: 10.1016/j.stem.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Lesko LJ, Schmidt S. Individualization of drug therapy: History, present state, and opportunities for the future. Clin Pharmacol Ther. 2012;92:458–466. doi: 10.1038/clpt.2012.113. [DOI] [PubMed] [Google Scholar]
- 19.Luni C, Serena E, Elvassore N. Human-on-chip for therapy development and fundamental science. Curr Opin Biotechnol. 2014;25:45–50. doi: 10.1016/j.copbio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Serena E, Zatti S, Reghelin E, et al. Soft substrates drive optimal differentiation of human healthy and dystrophic myotubes. Integr Biol (Camb) 2010;2:193–201. doi: 10.1039/b921401a. [DOI] [PubMed] [Google Scholar]
- 21.Zatti S, Zoso A, Serena E, et al. Micropatterning topology on soft substrates affects myoblast proliferation and differentiation. Langmuir. 2012;28:2718–2726. doi: 10.1021/la204776e. [DOI] [PubMed] [Google Scholar]
- 22.Tonlorenzi R, Dellavalle A, Schnapp E, et al. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol. 2007;3:2B.1.1–2B.1.29. doi: 10.1002/9780470151808.sc02b01s3. [DOI] [PubMed] [Google Scholar]
- 23.Giannotta M, Benedetti S, Tedesco FS, et al. Targeting endothelial junctional adhesion molecule-A/ EPAC/ Rap-1 axis as a novel strategy to increase stem cell engraftment in dystrophic muscles. EMBO Mol Med. 2014;6:239–258. doi: 10.1002/emmm.201302520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willmann R, De Luca A, Benatar M, et al. Enhancing translation: Guidelines for standard pre-clinical experiments in mdx mice. Neuromuscul Disord. 2012;22:43–49. doi: 10.1016/j.nmd.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng J, Muntoni F, Morgan JE. Stem cells to treat muscular dystrophies - where are we? Neuromuscul Disord. 2011;21:4–12. doi: 10.1016/j.nmd.2010.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.