Immune thrombocytopenia (ITP) is characterized by platelet destruction and megakaryocyte dysfunction. Platelet-derived growth factor (PDGF) improves growth and survival in various cell types and promotes mesenchymal stem cell (MSC) proliferation. PDGF-BB protects MSCs derived from ITP patients against apoptosis, senescence, and immunomodulatory defects. This protective effect of PDGF-BB is likely mediated via the p53/p21 pathway, thus potentially providing a new therapeutic approach for ITP.
Keywords: Mesenchymal stem cells, Platelet-derived growth factor-BB, Apoptosis, Senescence, Immunosuppression
Abstract
Immune thrombocytopenia (ITP) is characterized by platelet destruction and megakaryocyte dysfunction. Mesenchymal stem cells (MSCs) from ITP patients (MSC-ITP) do not exhibit conventional proliferative abilities and thus exhibit defects in immunoregulation, suggesting that MSC impairment might be a mechanism involved in ITP. Platelet-derived growth factor (PDGF) improves growth and survival in various cell types. Moreover, PDGF promotes MSC proliferation. The aim of the present study was to analyze the effects of PDGF-BB on MSC-ITP. We showed that MSC-ITP expanded more slowly and appeared flattened and larger. MSC-ITP exhibited increased apoptosis and senescence compared with controls. Both the intrinsic and extrinsic pathways account for the enhanced apoptosis. P53 and p21 expression were upregulated in MSC-ITP, but inhibition of p53 with pifithrin-α markedly inhibited apoptosis and senescence. Furthermore, MSCs from ITP patients showed a lower capacity for inhibiting the proliferation of activated T cells inducing regulatory T cells (Tregs) and suppressing the synthesis of anti-glycoprotein (GP)IIb-IIIa antibodies. PDGF-BB treatment significantly decreased the expression of p53 and p21 and increased survivin expression in MSC-ITP. In addition, the apoptotic rate and number of senescent cells in ITP MSCs were reduced. Their impaired ability for inhibiting activated T cells, inducing Tregs, and suppressing the synthesis of anti-GPIIb-IIIa antibodies was restored after PDGF-BB treatment. In conclusion, we have demonstrated that PDGF-BB protects MSCs derived from ITP patients against apoptosis, senescence, and immunomodulatory defects. This protective effect of PDGF-BB is likely mediated via the p53/p21 pathway, thus potentially providing a new therapeutic approach for ITP.
Significance
Immune thrombocytopenia (ITP) is characterized by platelet destruction and megakaryocyte dysfunction. Platelet-derived growth factor (PDGF) improves growth and survival in various cell types and promotes mesenchymal stem cell (MSC) proliferation. PDGF-BB protects MSCs derived from ITP patients against apoptosis, senescence, and immunomodulatory defects. This protective effect of PDGF-BB is likely mediated via the p53/p21 pathway, thus potentially providing a new therapeutic approach for ITP.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by antibody-mediated platelet destruction [1]. The pathogenesis of ITP is complicated and involves increased platelet destruction and impaired platelet production due to the breakdown of self-tolerance [2]. Abnormal polarization between T helper (Th)1 cells and Th2 cells in the peripheral blood has been considered to play an important role in the pathogenesis of ITP [3]. In addition, decreased levels and functional defects of regulatory T cells (Tregs) are involved in the breakdown of self-tolerance in ITP [4, 5]. Recently, mesenchymal stem cells (MSCs) have been documented to be important immunoregulators because they have potent immunosuppressive effects, regulating both adaptive and innate immune responses [6].
MSCs residing in the bone marrow have long been believed to participate in regulating the balance between hematopoietic stem cell self-renewal and differentiation [7]. In addition to their self-renewal and multidifferential potential, MSCs play crucial roles in immune modulatory functions [8]. Previous large-scale studies have demonstrated the immunosuppressive potential possessed by MSCs. MSCs can inhibit both T- and B-lymphocyte proliferation and activation [9, 10] and reduce the expression of costimulatory molecules among antigen-presenting cells [11]. Furthermore, in vitro studies have shown that MSCs themselves trigger the generation of Tregs, which can negatively regulate immune reactions [12].
In the process of elucidating the pathogeneses of autoimmune diseases, several studies have reported functional impairment of MSCs in patients diagnosed with systemic lupus erythematosus [13], rheumatoid arthritis [14], and aplastic anemia [15], and its use is being explored in the setting of autoimmune disorders. In accordance with these autoimmune diseases, MSCs in ITP patients have also been verified to have reduced proliferative capacity and to have lost their immunosuppressive function. Pérez-Simón et al. demonstrated that bone marrow MSCs (BM-MSCs) from patients with chronic ITP showed impaired proliferative capacity and less ability to inhibit activated T-cell proliferation compared with cells from normal controls [16]. Furthermore, a study by Zhang et al. also confirmed the defectiveness of BM-MSCs in patients with chronic ITP, as evidenced by an increased apoptotic cell rate, an impaired ability to proliferate, and defective immune-inhibiting potential and Treg-inducing ability [17]. It has been universally acknowledged that the production of autoantibodies is the main pathogenic event in the development of ITP; however, few reports are currently available on whether MSCs could affect the generation of antiplatelet antibodies. As an important immunomodulatory cell subset, MSCs have been proposed to play an important role in immune modulation. Therefore, the observed defects in the BM-MSCs might constitute an underlying mechanism in the pathogenesis of ITP. The defects in MSCs might also constitute an important target in the treatment of ITP.
Platelet-derived growth factor (PDGF) isoforms are important mitogens for different types of mesenchymal cells, and these isoforms stimulate the proliferation, survival, and differentiation of cells [18]. PDGF-BB is a dimeric protein of approximately 30 kDa, which belongs to the PDGF/vascular endothelial growth factor family. It can activate both α- and β-PDGF receptors to elicit multiple biological functions. PDGF-BB can protect various cell types from apoptosis and can induce cell cycle and proliferation [19, 20]. However, whether PDGF-BB can correct the defects in MSCs remains unknown.
In the present study, we assessed the role of PDGF-BB in MSCs isolated from ITP patients. We found that MSC-ITP exhibited enhanced apoptosis and senescence and an impaired capacity for immunosuppression, which could suggest the possible involvement of MSCs in the pathogenesis of ITP. We demonstrated that PDGF-BB is capable of protecting ITP MSCs against apoptosis and senescence by modulating the p53/p21 pathway and restoring their immunosuppressive capacity, thus indicating the potential therapeutic utility of PDGF-BB in patients with ITP.
Materials and Methods
Patients
BM samples were obtained from 35 patients with newly diagnosed ITP who met the previously reported criteria [21], and 31 age- and sex-matched healthy donors were included as normal controls. The body mass index distribution (range) was similar between the ITP patients and the healthy donor (control) individuals. All the patients and controls provided consent to participate in the study, which was approved by the Ethics Committee of the Peking University People’s Hospital and conducted in accordance with the Declaration of Helsinki.
Isolation, Expansion, and Characterization of MSCs
Bone marrow mononuclear cells from the patients and normal controls were isolated by Ficoll gradient and cultured in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com), with 10% defined fetal bovine serum (Thermo Fisher Scientific Life Sciences), and 100 U/ml penicillin/streptomycin. The cultures were maintained at 37°C in a 5% CO2 incubator, and the medium containing nonadherent cells was replaced every 3–4 days of the culture period. When the cultures reached 80% confluence, the cells were detached using 0.25% trypsin-EDTA. The cells were seeded in flasks at 1 × 106 cells per 25 cm2 and cultured for another 4–5 days to obtain the next passage of MSCs.
To confirm the human MSC phenotype, plastic adherent cells were analyzed for the expression of surface-specific antigens using flow cytometry (supplemental online Fig. 1) [22]. The cells were stained with the following fluorescein isothiocyanate (FITC)-conjugated, allophycocyanin (APC)-conjugated, peridinin chlorophyll protein (PerCP)-conjugated or phycoerythrin (PE)-conjugated monoclonal antibodies: CD14, CD19, CD34, CD45, CD105, CD90, CD73, and human leukocyte antigen (HLA)-DR. The FITC-, PE-, APC-, and PerCP-conjugated isotypes were used as negative controls. The analysis was performed using a flow cytometry machine (Beckman Coulter Inc., Brea, CA, http://www.beckmancoulter.com). The capacity of the MSCs to differentiate along osteogenic, chondrogenic, and adipogenic lineages was assessed (supplemental online Fig. 2), as described previously [22], using commercially available kits (osteogenesis differentiation kit, chondrogenesis differentiation kit, and adipogenesis differentiation kit [Thermo Fisher Scientific Life Sciences]), according to the manufacturer's instructions. After 21 days, osteogenic differentiation was determined by the deposition of mineral nodules, which was detected using alizarin red S staining. Adipogenesis was measured by the accumulation of lipid-containing vacuoles using Oil Red O staining. Chondroblast differentiation was demonstrated using Alcian blue staining.
Cell Proliferation Assay
The MSC proliferation assay was performed using the Cell Counting Kit-8 (CCK-8) assay kit (Dojindo Molecular Technologies, Inc., Rockville, MD, http://www.dojindo.com), according to the manufacturer's protocol. Generally, cells were seeded onto 96-well cell culture cluster plates at a concentration of 2 × 104 cells per well in volumes of 100 µl and grown overnight. Cell Counting Kit-8 reagents were added to a subset of wells and incubated for 2 hours at 37°C, and the absorbance of the samples was measured using an enzyme-linked immunosorbent assay plate reader at a wavelength of 450 nm.
Cell Morphological Changes
4′,6-Diamidino-2-phenylindole (DAPI) dye (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) was used to assess the morphological cell changes (supplemental online Fig. 3). MSCs were fixed for 30 minute in phosphate-buffered saline (PBS) containing 1% glutaraldehyde at room temperature and then washed twice with PBS and exposed to 5 mg/ml DAPI for 30 minutes at room temperature. The cells were observed with a fluorescence microscope.
Senescence-Associated β-Galactosidase Assay
MSCs from ITP patients and normal controls were passaged into six-well culture plates at a density of 5 × 104 cells per well. After 24 hours, a senescence-associated β-galactosidase assay was performed as described in the assay instructions (Cell Biolabs, Inc., San Diego, CA, http://www.cellbiolabs.com). In brief, the cells were washed twice with PBS and fixed into the wells using 1 ml of 1× fixing solution per well. Next, the cells were incubated at room temperature for 15 minutes. After washing with PBS, the cells were incubated in freshly prepared senescence-associated β-galactosidase detection solution at 37°C without CO2 and were protected from the light for approximately 18 hours. The percentage of senescent cells was obtained by counting the number of blue-stained cells and the total cells per field under a light microscope (supplemental online Fig. 4).
Cell Cycle Analysis
For cell cycle analysis, cultured cells were collected and fixed in 70% ethanol for 30 minutes. After washing with PBS and then treating with RNase A (50 μg/ml in PBS; Sigma-Aldrich) for 30 minutes, the cells were incubated with propidium iodide (50 μg/ml; Sigma-Aldrich) for 15 minutes and were analyzed using a flow cytometry machine (Beckman Coulter, Inc.).
Annexin V
Apoptosis was determined by detecting phosphatidylserine exposure on the cell plasma membrane with the fluorescent dye from an Annexin V-FITC Apoptosis Detection Kit (Sigma-Aldrich) according to the manufacturer’s protocols. In brief, the cells were harvested and washed in ice-cold PBS, resuspended in 300-ml binding buffer, and incubated with 5 ml of Annexin V-FITC solution for 30 minutes at 4°C in dark conditions. This step was followed by further incubation with 5 ml of propidium iodide for 5 minutes. Next, the samples were immediately analyzed by flow cytometry (Beckman Coulter, Inc.). Approximately 5 × 105 cells were analyzed in each sample.
Mitochondrial Membrane Potential Assay
The mitochondrial membrane potential (MMP) was determined using the JC-1 Mitochondrial Membrane Potential Assay Kit (Beyotime Institute of Biotechnology, Beijing, China, http://www.bio-equip.cn). JC-1 exists predominantly in the monomeric form in cells with depolarized mitochondria and fluoresces green at 490 nm. Cells with polarized mitochondria predominantly contained JC-1 in an aggregated form and fluorescence reddish-orange. In brief, MSCs were seeded onto six-well plates. After experimental treatment, the cells were washed twice with PBS. Next, 1 ml of staining dye per well (culture medium, JC-1 working dye at a 1:1 ratio) was added, and the cells were incubated at 37°C for 20 minutes. The cells were washed twice with cold JC-1 staining buffer and examined under a fluorescence microscope.
Western Blotting
The cells were washed twice with ice-cold PBS and extracted in lysis buffer (Sigma-Aldrich) for 45 minutes on ice. Equal amounts of protein were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were then blocked with Tris buffered saline Tween (TBST) containing 5% nonfat dry milk for 1 hour. The membranes were then incubated overnight with monoclonal antibodies against B-cell lymphoma 2 (Bcl-2; catalog no. 2870; Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com), Bcl-2-associated X protein (Bax; catalog no. ab32503; Abcam, Cambridge, UK), caspase-3 (catalog no. 9665; Cell Signaling Technology), caspase-9 (catalog no. ab32539; Abcam), factor-associated suicide (Fas; catalog no. ab126821; Abcam), Fas ligand (FasL; catalog no. ab87023; Abcam), caspase-8 (catalog no. ab181580; Abcam), p21 (catalog no. 2947; Cell Signaling Technology), p53 (catalog no. 2527; Cell Signaling Technology), and survivin (catalog no. 2808; Cell Signaling Technology). The antibodies against Bcl-2, caspase-3, p21, p53, and survivin were purchased from Cell Signaling Technology. The remaining antibodies were purchased from Abcam. The membranes were then washed with TBST and incubated with horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody for 1 hour. The blots were developed using an enhanced chemiluminescence kit. To assay the distribution of the p53 and p21 proteins, the cytoplasmic and nuclear proteins from cultured cells were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Chemical Co., Dallas, TX), respectively. β-Tubulin was used as the internal control for the cytoplasmic and nuclear proteins. The cytoplasmic and nuclear fractions were used for Western blot analysis, as described previously (supplemental online Figs. 5–7). The relative protein expression was normalized to β-tubulin.
Immunofluorescence Assay
MSCs were washed once with PBS and fixed in 4% paraformaldehyde for 15 minutes. After permeabilization and blocking, they were incubated with fluorescein isothiocyanate-conjugated phalloidin. The stained cells were then observed under a fluorescent microscope.
T-Lymphocyte Proliferation Assays and Analysis of Tregs by Coculture With BM-MSCs
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation from venous blood obtained from 2 healthy subjects. CD4+ T cells isolated from the PBMCs of normal controls were purified using positive selection with a CD4+ T-cell isolation kit [16, 23] (MicroBeads; Miltenyi Biotec, Bergisch Gladbach, Germany). Purified T lymphocytes (2 × 105 per well) were cocultured with MSCs (2 × 104 per well) in 96-well plates at a ratio of 10:1 in 0.2 ml of Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/ml penicillin/streptomycin triplicate with or without mitogen-induced stimulation by phytohemagglutinin (PHA). On day 3, T lymphocytes were pulsed for 16 hours with 0.5 mCi [3H]-thymidine. These cells were harvested onto glass-fiber filter paper and dried, and the incorporated [3H]-thymidine was measured using a liquid scintillation counter. Data are expressed as the mean counts per minute of triplicate samples. The supernatants were collected to analyze Th1 and Th2 cytokine release analysis (interferon [IFN]-γ and interleukin [IL]-4, and IL-10, respectively) performed by enzyme-linked immunosorbent assay (ELISA; Mabtech, Cincinnati, OH, http://www.mabtech.com). For the analysis of Tregs by coculture with MSCs, MSCs were seeded at 2 × 104 cells per well in triplicate in 96-well plates. Purified CD4+ T cells (2 × 105 per well, at a ratio of 1:10 MSC/CD4+ T cells) were cultured in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, and 100 U/ml penicillin/streptomycin. After 5 days of coculture, nonadherent cells were harvested and evaluated for the proportion of Tregs present by flow cytometry using PE-Cy7-conjugated anti-CD4, PE-conjugated anti-CD25, and antigen-presenting cell-conjugated anti-Foxp3 (all from BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com) antibodies.
Detection of IgG Anti-GPIIb-IIIa Antibodies in the In Vitro Coculture System
An in vitro assay was conducted to analyze the effect of MSCs on IgG anti-GPIIb-IIIa antibody synthesis by PBMCs, as previously described [24, 25]. In brief, PBMCs (2 × 105 per well) were cocultured with MSCs (2 × 104 per well) in complete medium in 96-well plates with antigen (trypsin-digested GPIIb-IIIa) in the presence of pokeweed mitogen (1 mg/ml) for 10 days. The levels of IgG anti-GPIIb-IIIa antibody in undiluted culture supernatants were measured using an anti-GPIIb-IIIa ELISA, as previously described.
Statistical Analysis
The data are expressed as the mean ± SD and were analyzed with the commercially available statistical software package SPSS, version 13.0 (SPSS, Chicago, IL, http://ibm.com). One-way analysis of variance with Šidák’s multiple comparison correction was used to compare the mean in the T-cell proliferation assay and Tregs induction assay and the detection of anti-GPIIb-IIIa antibody, IL-4, IL-10, and IFN-γ. The t test was applied in the remaining comparisons. Statistical significance was defined as p < .05.
Results
MSC-ITP Showed Increased Apoptosis and Senescence
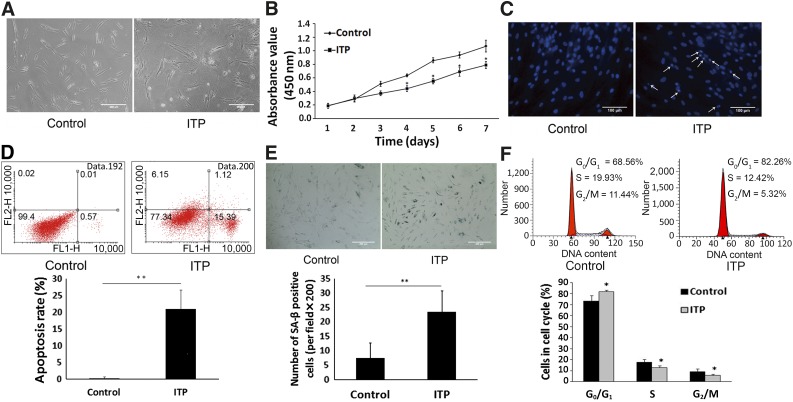
Bone marrow-derived MSCs were successfully isolated, and the culture was expanded from all 25 ITP patients and 21 controls. Flow cytometry analysis demonstrated that MSCs from both healthy donors and ITP patients expressed CD105, CD73, and CD90 and lacked expression of CD14, CD19, CD34, CD45, and HLA-DR. MSCs displayed similar differentiation ability toward osteoblasts, adipocytes, and chondroblasts in vitro after inductive conditions. As shown in Figure 1A, MSCs from the controls (MSC-control) expanded and acquired spindle shape morphology during culture. In contrast, MSCs from the ITP patients (MSC-ITP) expanded more slowly and appeared larger and flattened. Only a few acquired the typical morphology during culture. Furthermore, CCK-8 assays showed a lower proliferative capacity among MSC-ITP compared with MSC-control (Fig. 1B). The apoptosis of MSCs was morphologically determined using DAPI staining. As shown in Figure 1C, MSC-control had normal round and regular nuclei. In contrast, fragmentation and condensation of the nuclei, which are characteristic of apoptotic cells, were observed in MSC-ITP. We further assessed the apoptotic cell rate using Annexin V. As shown in Figure 1D, the rate of apoptosis was higher in the MSC-ITP group than in the MSC-control group (20.92% ± 5.73% vs. 0.27% ± 0.43%; p < .01). The MSC-ITP cells showed increased senescence, as demonstrated by senescence β-galactosidase staining (23.50 ± 7.35 vs. 7.50 ± 5.23; p < .01) (Fig. 1E). Flow cytometry showed an increase in G0/G1 cells in the MSC-ITP (81.74% ± 1.41% vs. 73.19% ± 5.05%; p < .05) (Fig. 1F). These defects were consistently observed at passage 5 and could not be recovered in culture.
Figure 1.
Mesenchymal stem cells (MSCs) from ITP patients showed increased apoptosis and senescence. (A): Morphology of MSCs from control (MSC-control) and ITP patients (MSC-ITP) under a light microscope (magnification ×200; scale bars = 200 μm). (B): The growth curves of MSC-control (n = 8) and MSC-ITP (n = 8) at passage two. MSC-ITP grew progressively slower compared with controls. (C): 4′,6-Diamidino-2-phenylindole staining showed increased fragmentation and condensation of the nuclei of MSC-ITP (control, n = 16; ITP, n = 16; magnification ×400; scale bars = 100 μm). White arrows indicate the fragmented and condensed nuclei of apoptotic cells. (D): Increased apoptotic cell rate of MSC-ITP determined by flow cytometry (control, n = 16; ITP, n = 16). (E): The number of SA-β-positive cells obviously increased in the MSC-ITP (control, n = 16; ITP, n = 16; magnification ×200; scale bars = 200 μm). (F): Cell cycle determined by flow cytometry. MSCs showed a greater fraction in quiescence of the G0/G1 phase in ITP patients (control, n = 16; ITP, n = 16). The MSCs used in each assay were at passage three (except for the Cell Counting Kit-8 assay). *, p < .05; **, p < .01. Error bars indicate SD. Abbreviations: ITP, immune thrombocytopenia; n, number of unique donors in each group; SA-β, senescence-associated β-galactosidase.
MSC-ITP Exhibited Impaired Mitochondrial Function and Death Receptor Pathway
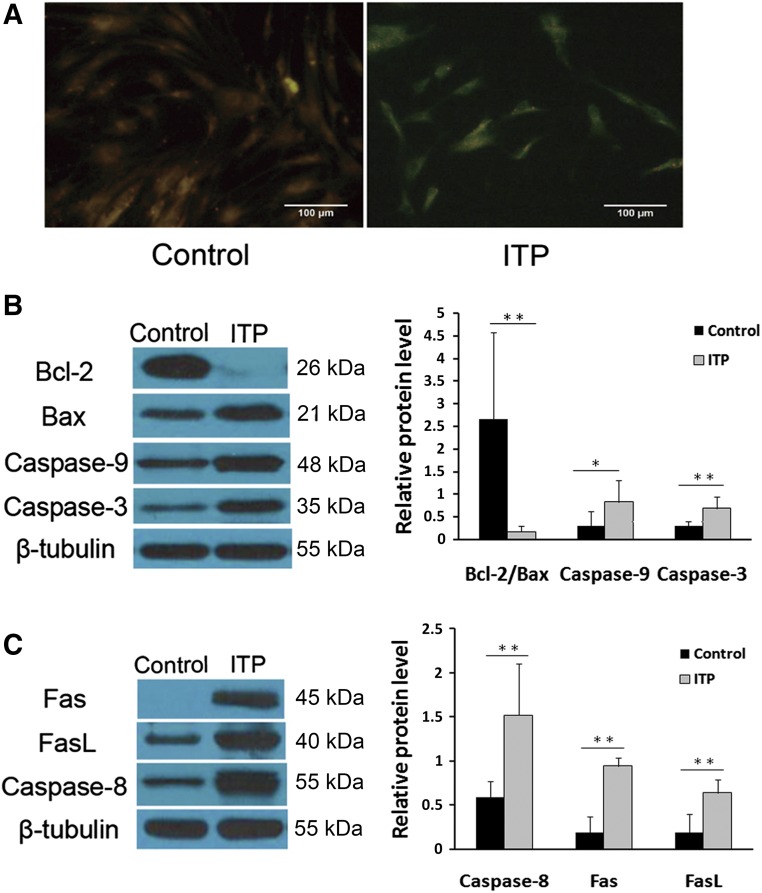
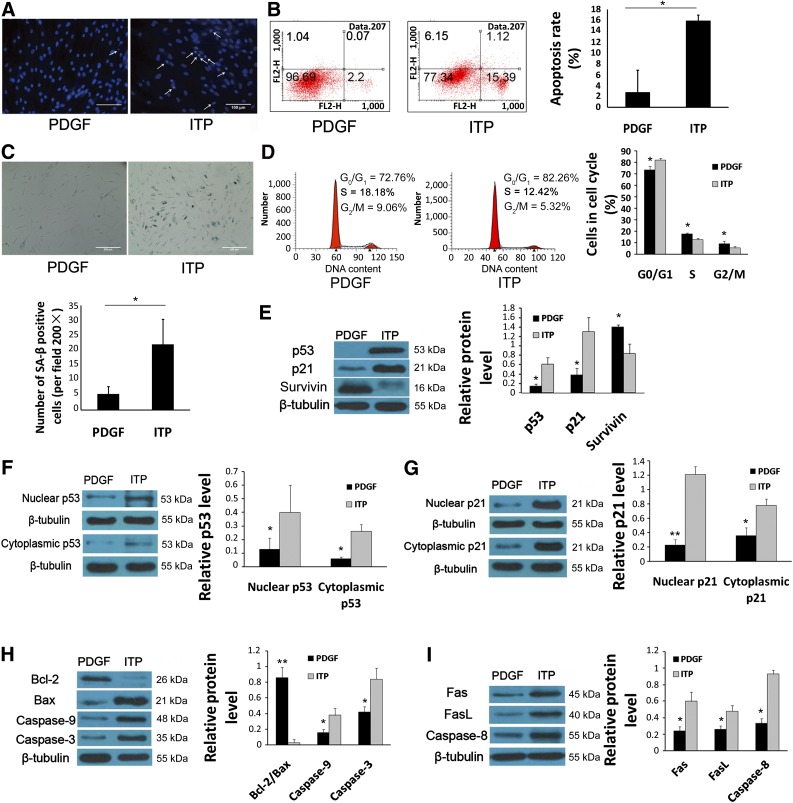
The mitochondrion is one of the key regulators of apoptosis. To investigate the changes in mitochondrial functions during the apoptosis of MSC-ITP, the MMP, Bcl-2/Bax ratio, and caspase-9 and caspase-3 activation were measured. MSC-control exhibited reddish-orange staining, indicative of coupled mitochondria with a normal MMP (Fig. 2A). In contrast, MSC-ITP developed a diffuse green staining pattern, representative of a reduced MMP (Fig. 2A). Furthermore, at the protein level, a decreased Bcl-2/Bax ratio (p < .01), increased caspase-9 activation (p < .05), and increased caspase-3 activation (p < .05) (Fig. 2B) were confirmed by Western blot analysis of MSC-ITP compared with MSC-control. We next explored whether the death receptor pathway was involved in the apoptosis of MSCs. More activation of caspase-8 was detected by Western blot analysis in MSC-ITP (p < .05) (Fig. 2C). Simultaneously, the expression of Fas (p < .05) and FasL (p < .05) was significantly higher in MSC-ITP than in normal controls at the protein level (Fig. 2C).
Figure 2.
Mitochondria and the death receptor pathway were involved in excessive apoptosis of mesenchymal stem cells (MSCs) from ITP patients (MSC-ITP). (A): JC-1 staining showed decreased mitochondrial membrane potential in MSC-ITP (control, n = 6; ITP, n = 6; magnification ×400; scale bars = 100 μm). MSCs from control subjects showed reddish-orange staining indicative of polarized mitochondria predominantly containing JC-1 in an aggregated form. MSC-ITP exhibited green staining, indicating depolarized mitochondria with a monomeric form of JC-1. A decreased Bcl-2/Bax ratio, increased cleaved caspase-9, -8, and -3 (B), and increased expression of Fas and FasL (C) were detected by Western blot (control, n = 8; ITP, n = 8). The MSCs used in each assay were at passage three. *, p < .05; **, p < .01. Error bars indicate SD. Abbreviations: Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Fas, factor-associated suicide; FasL, Fas ligand; ITP, immune thrombocytopenia; n, number of unique donors in each group.
The p53/p21 Pathway Plays an Important Role in Apoptosis and Senescence of MSC-ITP
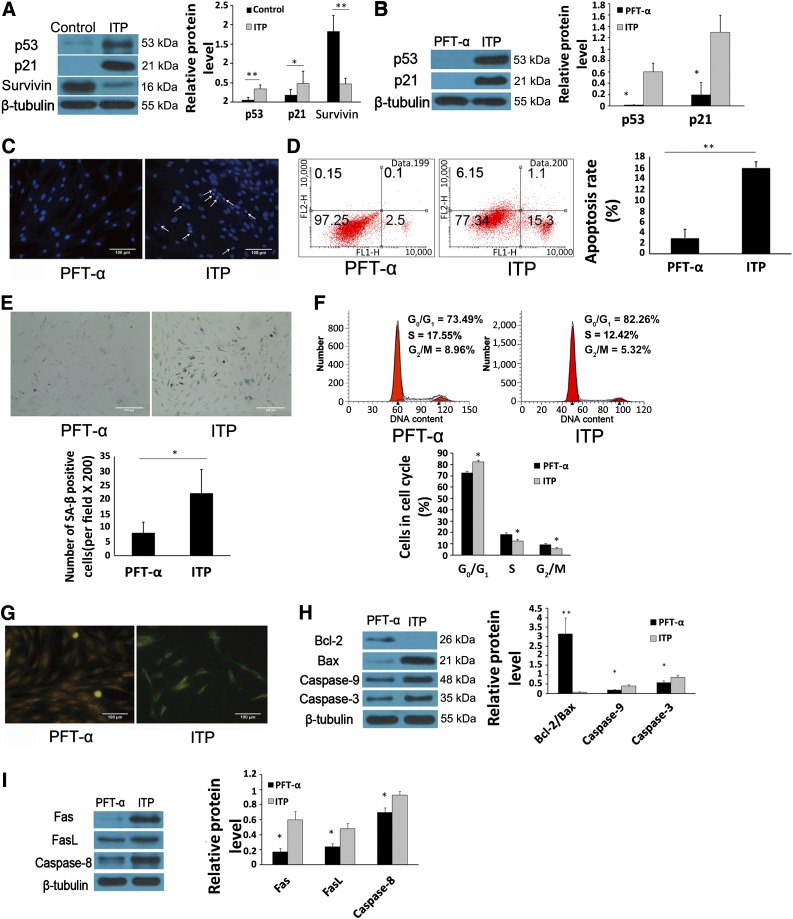
P53 is a critical mediator of cell survival in response to stimulation by several factors. In the present study, we found that the expression of p53 (p < .05) and p21 (p < .05) (Fig. 3A) had increased and the expression of survivin (p < .01) (Fig. 3A) was decreased in MSC-ITP. To further assess the role of the p53/p21 pathway in the apoptotic progress of MSC-ITP, the p53/p21 pathway was inhibited with the p53 inhibitor pifithrin-α (PFT-α). Treatment with PFT-α markedly inhibited the expression of p53 and p21 in MSC-ITP (Fig. 3B). In addition, PFT-α exerted a protective effect on MSC-ITP, as demonstrated by the decreased apoptosis and senescence in MSC-ITP. Improved nuclear morphology was observed in MSC-ITP with p53 inhibition (Fig. 3C). Also, PFT-α reduced the apoptotic rate at a concentration of 50 µg/l (2.91% ± 1.61% vs. 15.92% ± 1.05%, p < .01) (Fig. 3D). Senescence in MSC-ITP was reversed with PFT-α treatment, as evidenced by the significantly decreased number of senescent cells (8.00 ± 3.85 vs. 22.00 ± 8.37; p < .05) (Fig. 3E) and G0/G1 cells (73.49% ± 2.86% vs. 82.26% ± 1.47%; p < .05) (Fig. 3F). Moreover, treatment with PFT-α ameliorated the depolarization of MMP in MSC-ITPs as indicated by the change from green to orange fluorescence with JC-1 staining (Fig. 3G). Western blot analysis showed that the Bcl-2/Bax ratio was upregulated (Fig. 3H; p < .01). Caspase-9 and caspase-3 activation was downregulated with the presence of PFT-α (Fig. 3H; p < .05). Treatment with PFT-α also decreased Fas, FasL, and caspase-8 expression in MSC-ITP at the protein level (Fig. 3I; p < .05).
Figure 3.
P53 participated in the process of increased apoptosis and senescence of mesenchymal stem cells (MSCs) from ITP patients (MSC-ITP). (A): Western blot (control, n = 8; ITP, n = 8) showed that expression of p53 and p21 was increased and the expression of survivin was decreased in MSC-ITP. (B): The p53 inhibitor, PFT-α, markedly inhibited the expression of p53 and p21 in MSC-ITP (n = 4). P53 inhibition downregulated apoptosis, as evidenced by morphological nuclear changes (n = 8; magnification ×400; scale bars = 100 μm) (C) and the decreased apoptosis rate (n = 8) (D). Inhibiting p53 expression reduced the senescence-associated β-galactosidase staining-positive cells (n = 8; magnification ×200; scale bar = 200 μm) (E) and G0/G1 cells (n = 8) (F). (G): PFT-α treatment prevented decreased mitochondrial membrane potential in MSC-ITP, as shown by the change from green to orange staining of JC-1 (n = 6; magnification ×400; scale bars = 100 μm). Decreased Bcl-2/Bax ratio, activation of caspase-9, -8, and -3 (H) and increased expression of Fas, FasL, and caspase-8 (I) in MSC-ITP, detected by Western blot, were ameliorated by PFT-α treatment (n = 4). The MSCs used in each assay were at passage three. *, p < .05; **, p < .01. Error bars indicate SD. Abbreviations: Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Fas, factor-associated suicide; FasL, Fas ligand; ITP, immune thrombocytopenia; n, number of unique donors in each group; PFT-α, pifithrin-α.
P53 and p21 Were Mainly Localized in the Nuclear Fraction of MSC-ITP
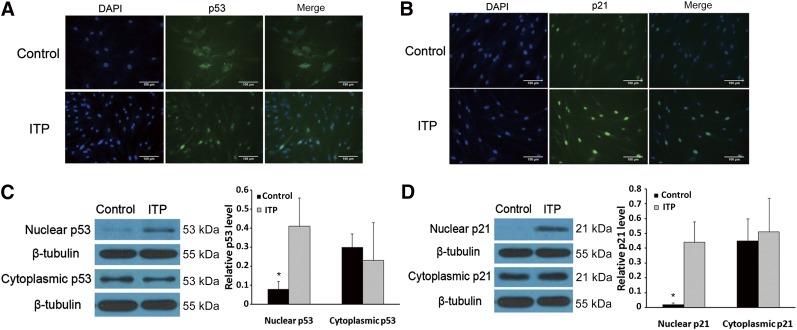
Recent studies have found that proteins and their functions are based on subcellular localization. The p53 and p21 proteins, which accumulate in the nuclei, are necessary for cell cycle arrest. In our study, using immunofluorescence staining, we observed that p53 (Fig. 4A) and p21 (Fig. 4B) were mainly localized in the nuclei of the MSC-ITPs. In contrast, lower levels were found in the cytoplasm of the MSC-ITP than in that of the MSC-control. To further detect the expression of these proteins, we used separation of the nuclei and cytoplasm and Western blot analysis. We found that p53 and p21 were expressed more in the nuclei of the MSC-ITP than in the normal controls (Fig. 4C, 4D). These results suggest that the effects of p53 and p21 on MSC-ITP might be based on their subcellular localization.
Figure 4.
Analysis of the location and expression of p53 and p21 in the cytoplasm and nuclei of mesenchymal stem cells (MSCs) from ITP patients (MSC-ITP). (A, B): Immunofluorescence staining to analyze the location of p53 and p21 in MSCs (n = 8; magnification ×400; scale bars = 100 μm). (C, D): Western blot analysis of cytoplasmic and nuclear p53 and p21 expression (n = 4). The MSCs used in each assay were at passage three. *, p < .05. Error bars indicate SD. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ITP, immune thrombocytopenia; n, number of unique donors in each group.
MSC-ITP Exhibited an Impaired Capability of Inhibiting T-Cell Proliferation, Inducing Tregs, and Suppressing Anti-GPIIb-IIIa Antibodies
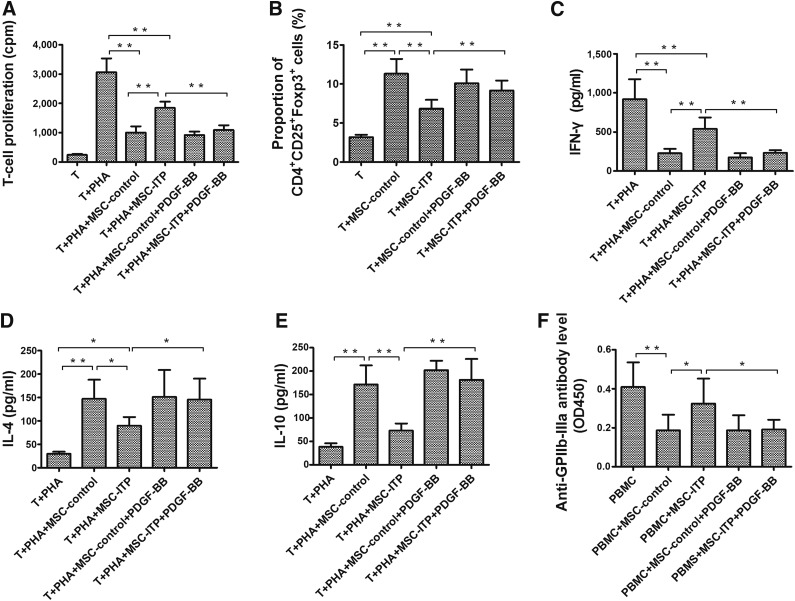
To evaluate the immunomodulatory effect of MSCs on T cells, we stimulated T cells with PHA in the presence of MSCs from healthy donors versus ITP. As shown in Figure 5A, PHA-induced T-cell proliferation was inhibited by MSCs from both ITP patients (p < .01) and normal controls (p < .01). However, the capacity of MSC-ITP in suppressing T-lymphocyte proliferation was significantly lower than that of the MSC-control (p < .01). Previous studies have suggested that MSCs could induce T cells with a regulatory phenotype when cocultured with CD4+ T cells, and the decreased levels and functional defects of Tregs have been shown to participate in the breakdown of self-tolerance in ITP. We next investigated the Treg-inducing function of MSCs from ITP patients and normal controls. As shown in Figure 5B, a significant increase occurred in the proportion of CD4+CD25+Foxp3+ Tregs in CD4+ T cells cocultured with BM-MSCs from both groups compared with CD4+ T cells cultured alone. However, the proportion of CD4+CD25+Foxp3+ Tregs in the patient group was significantly lower than that in the control group (Fig. 5B; p < .01). Additionally, ELISA showed that MSCs from both groups significantly inhibited the expression of IFN-γ (Fig. 5C) and enhanced the expression of IL-4 (Fig. 5D) and IL-10 (Fig. 5E). However, less IFN-γ and more IL-4 and IL-10 were detected in the coculture system in the MSC-control group compared with the MSC-ITP group. The production of antiplatelet antibodies has been regarded as the main pathogenic mechanism of ITP. We next evaluated whether MSCs could inhibit the production of anti-GPIIb-IIIa antibody. As shown in Figure 5F, coculture with the MSCs from healthy donors significantly reduced the absorbance of anti-GPIIb-IIIa antibody in the supernatant compared with the culture of PBMCs alone in the presence of digested GPIIb-IIIa (p < .01). Coculture with the MSCs from ITP patients resulted in a relatively lower amount of anti-GPIIb-IIIa antibodies, but the difference did not reach statistical significance. These data suggest that MSC-ITP might play a dysfunctional role in immunoregulation.
Figure 5.
MSCs from ITP patients showed impaired immunosuppressive properties. (A): Effects of MSCs on PHA-induced T-cell proliferation. Data expressed as the absorbance at 450 mm (n = 10). (B): The proportion of CD4+CD25+Foxp3+ Tregs in CD4+ T cells cocultured with MSCs or CD4+ T cells cultured alone detected by flow cytometry (n = 10). (C): Determination of IFN-γ in coculture system by enzyme-linked immunosorbent assay (ELISA; n = 10). (D): Determination of IL-4 in coculture system by ELISA (n = 10). (E): Determination of IL-10 in coculture system by ELISA (n = 10). (F): Effect of MSCs on the antigen (digested GPIIb-IIIa)-induced IgG anti-GPIIb-IIIa antibody production by PBMCs. IgG anti-GPIIb-IIIa antibody levels in undiluted culture supernatants were measured by anti-GPIIb-IIIa ELISA, and the data are presented as absorbance (n = 10). The MSCs used in each assay were at passage three. *, p < .05; **, p < .01. Error bars indicate SD. Abbreviations: GP, glycoprotein; IFN-γ, interferon-γ; IL, interleukin; ITP, immune thrombocytopenia; MSCs, mesenchymal stem cells; PBMCs, peripheral blood mononuclear cells; PDGF, platelet-derived growth factor; PHA, phytohemagglutinin; Tregs, regulatory T cells.
PDGF-BB Treatment Protected MSC-ITP From Apoptosis and Senescence by Downregulating p53 Expression and Restored the Immunomodulatory Effects
Because PDGF-BB has been demonstrated to prevent apoptosis in various types of cells, we examined whether PDGF-BB protected MSCs from apoptosis and senescence. Third-passage MSCs were treated with PDGF-BB at a concentration of 50 ng/ml for 24 hours. PDGF-BB showed a favorable protective effect on MSC-ITP. As shown in Figure 6A, the morphology of the nuclei was improved, and the apoptotic rate of MSC-ITPs was downregulated (2.76% ± 4.02% vs. 15.92% ± 1.05%; p < .05) (Fig. 6B) by treatment with PDGF-BB. Senescence in MSC-ITP was reversed with PDGF-BB treatment, as evidenced by the significantly decreased number of senescent cells (5.30 ± 2.50 vs. 22.00 ± 8.37; p < .05) (Fig. 6C) and G0/G1 cells (73.49% ± 2.86% vs. 82.26% ± 1.47%; p < .05) (Fig. 6D) in MSC-ITP. Given that the p53/p21 pathway was involved in mediating the apoptosis and senescence of MSC-ITP, we next explored whether PDGF-BB exerted protective effects by regulating the p53/p21 pathway. The results showed that PDGF-BB significantly decreased the expression of p53 and p21 and increased the expression of survivin at the protein level (Fig. 6E). PDGF-BB also partly reversed the abnormal subcellular localization of p53 (Fig. 6F) and p21 (Fig. 6G) in MSC-ITP. Given that the excess apoptosis and senescence was reversed, we next evaluated whether the impaired immunosuppression capacity was restored in PDGF-BB-treated MSC-ITP. After coculture of CD4+ T cells with PDGF-BB-treated MSC-ITP, the T-cell proliferation induced by PHA was significantly reduced (Fig. 5A; p < .01) and the number of Tregs was increased (Fig. 5B; p < .01) compared with coculture with MSC-ITP. After PDGF-BB treatment, the capacity of MSC-ITP to inhibit the production of anti-GPIIb-IIIa antibodies was restored (Fig. 5F; p < .05). These results indicate that PDGF-BB can reverse the defect of MSC-ITP in vitro.
Figure 6.
PDGF-BB treatment reversed the excessive apoptosis and senescence of mesenchymal stem cells (MSCs) from ITP patients (MSC-ITP) by regulating the p53/p21 pathway. PDGF-BB mitigated the apoptosis and senescence of MSC-ITP, as demonstrated by morphological nuclear changes (n = 8; magnification ×400; scale bars = 100 μm) (A), flow cytometry (n = 8) (B, D), and SA-β staining (n = 8; magnification ×200; scale bars = 200 μm) (C). (E): PDGF-BB treatment decreased the protein expression of p53 and p21 and increased the expression of survivin in MSC-ITP (n = 4). The abnormal subcellular localization of p53 (F) and p21 (G) in MSC-ITP was reversed by PDGF (n = 4). Decreased Bcl-2/Bax ratio, activation of caspase-9 and -3 (H) and increased expression of Fas, FasL, and caspase-8 (I) in MSC-ITP, detected by Western blot, were reversed by PDGF treatment (n = 4). The MSCs used in each assay were at passage three. *, p < .05; **, p < .01. Error bars indicate SD. Abbreviations: Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Fas, factor-associated suicide; FasL, Fas ligand; ITP, immune thrombocytopenia; n, number of unique donors in each group; PDGF, platelet-derived growth factor; SA-β, senescence-associated β-galactosidase.
Discussion
In the present study, we have presented evidence on increased apoptosis and senescence and the impaired immunomodulatory capacity of MSC-ITP. We also demonstrated that PDGF-BB attenuated apoptosis and senescence via the p53/p21 pathway and restored the immunomodulatory capacity of MSCs derived from ITP patients, thus providing new insight into the pathogenesis of ITP.
ITP is characterized by platelet destruction within the spleen and megakaryocyte destruction in the bone marrow [1]. Autoantibodies induced by the polarization of Th1 and T-cell cytotoxicity resulted in excessive platelet destruction and suppressed platelet production [26]. An imbalance between immune stimulation and regulation indicated by abnormal regulatory T cells was involved in the pathogenesis of ITP [4, 27]. MSCs have been documented to display not only stem cell multipotency [28] but also robust immunosuppressive properties [29]. MSCs from healthy individuals could inhibit the conversion of Th2 to Th1 by increasing IL-4 and decreasing IFN-γ levels in effector T cells [30] and inducing the generation of regulatory T cells [31]. However, MSCs from ITP patients were found to lose their conventional proliferation capacity and thus were defective in immunoregulation [16, 17]. Also, in either an animal model or human ITP patients, the transplantation of MSCs derived from bone marrow or the umbilical cord has improved platelet production and attenuated megakaryocyte dysfunction by reversing the shift in the Th1/Th2 cytokine balance [32–35], suggesting that MSC impairment might be involved in the pathogenesis of ITP.
MSCs from ITP patients have been shown to have less proliferative capacity and a higher rate of apoptosis; however, the mechanism for these abnormalities remains unknown. In the present study, we not only investigated the enhanced apoptosis and senescence of MSCs from ITP patients, we also explored the underlying molecular changes that give rise to deficiencies in the proliferation and survival of MSC-ITP. Apoptosis and senescence are two mechanisms that prevent replication when cells undergo irreversibly accumulated damage [36]. Cellular senescence is an irreversible form of cell cycle arrest that prevents the proliferation of damaged cells or cells that have surpassed their capacity to proliferate [37]. Apoptosis, also called programmed cell death, is a cell-autonomous mechanism that relies on the pathway-controlled activation of caspases and nucleases, leading to the death of injured cells without affecting neighboring cells [38]. The intrinsic pathway of apoptosis regulates the activity of the Bcl-2 family proteins that control the integrity of the mitochondrial membrane [39]. The release of proapoptotic factors from the mitochondria, such as cytochrome C, into the cytoplasm promotes the activation of the initiator caspase-9, which in turn activates effector caspases, such as caspase-3 and caspase-7 [40]. The extrinsic pathway relies on the activation of death receptors, which promote the recruitment and activation of initiator caspase-8 via adaptor proteins, such as the Fas-associated death domain. Strong caspase-8 activity can directly activate effector caspases [41]. Pérez-Simón et al. found that caspase-9 was overexpressed in MSCs from ITP patients compared with controls [16]. Given this indication, we also detected the molecular change along the intrinsic apoptosis pathway. We found not only excessive apoptosis of MSC-ITP but also the specific pathway by which apoptosis was regulated. MSC-ITP showed a decreased Bcl-2/Bax ratio, resulting in decreased MMP and consequently activating caspase-9 and caspase-3, indicating the activation of the mitochondrial pathway. Moreover, the death receptor pathway was involved in upregulated apoptosis in MSC-ITP, as demonstrated by increased Fas and FasL expression and caspase-8 activation. These results showed that both intrinsic and extrinsic pathways are involved in excessive apoptosis of MSC-ITP.
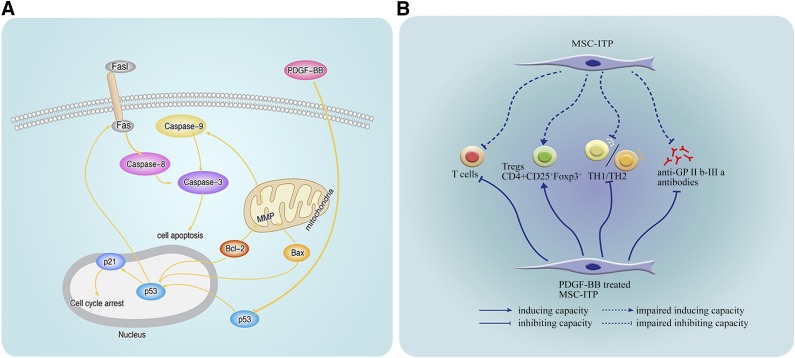
Recently, it was shown that p53 plays an important regulatory role in both apoptosis and senescence [42]. It senses DNA damage and, in response, induces and promotes senescence or apoptosis in the case of extensive damage [43, 44]. It has been reported that cell cycle progression is regulated by cyclins and cyclin-dependent kinase (CDK), such as cyclin E and CDK2, and this regulation is negatively inhibited by p53 and the CDK inhibitor p21, a target gene of the p53 protein [45]. It has been confirmed that overexpression of p53 causes the arrest of cell growth [46]. At the same time, p53 acts at multiple levels of the intrinsic and extrinsic pathways through the induction of multiple apoptotic target genes and through transcription-independent mechanisms [47, 48]. To investigate whether p53 participates in the apoptosis and senescence of MSC-ITP, p53 and p21 expression was measured at the protein level. The findings revealed upregulation of p53 and p21 in MSC-ITP. Blocking p53 with PFT-α reversed senescence and ameliorated apoptosis by suppressing both intrinsic and extrinsic pathways. The results indicated that the p53 pathway is responsible for the enhanced apoptosis and senescence in MSC-ITP (Fig. 7A).
Figure 7.
Comprehensively, PDGF-BB mediated protection on mesenchymal stem cells (MSCs) from ITP patients. (A): PDGF-BB protects MSC-ITP against apoptosis and senescence. In MSC-ITP, p53 translocated from the cytoplasm to the nucleus and altered Bcl-2 family proteins, which promotes caspase-9 and then caspase-3 activation. P53 also upregulated the expression of FasL and Fas, activating caspase-8 and caspase-3 and causing apoptosis in MSCs. P53 increased the expression of p21 and caused cell cycle arrest. PDGF-BB is capable of protecting MSC-ITP against senescence and apoptosis by inhibiting the p53/p21 pathway. (B): PDGF-BB restores the immunosuppressive capacity of MSC-ITP. PDGF-BB reversed the impaired capacity for inhibiting T-cell proliferation and inducing Tregs in MSC-ITP. PDGF-BB also reversed the shift in the Th1/Th2 cytokine balance and maintained the capacity for suppressing the production of anti-GPIIb-IIIa antibodies in the coculture system. Abbreviations: Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Fas, factor-associated suicide; FasL, Fas ligand; GP, glycoprotein; MMP, mitochondrial membrane potential; MSC-ITP, MSCs from patients with immune thrombocytopenia; PDGF, platelet-derived growth factor; Th, T helper cell.
Activation of autoreactive CD4+ T cells on recognition of GPIIb-IIIa peptides presented by antigen-presenting cells is a critical step for triggering and maintaining the pathogenic antiplatelet autoantibody response [26]. In the present study, we found that MSCs from ITP patients have a defect in inhibiting T-cell activation and inducing Tregs, consistent with the findings from previous studies [16]. Unbalanced Th1/Th2 activity was demonstrated via cytokine secretion profiles in previous studies of ITP patients. Th1 secretes cytokines IL-2 and IFN-γ, and Th2 secretes cytokines IL-4 and IL-10 [49, 50]. Significantly upregulated IFN-γ levels are indicative of a Th1-predominant immune response, also known as Th1 polarization. Th1 polarization is closely related to the clinical outcome of ITP [50, 51]. MSCs from both patients with ITP and normal controls enhanced protein IL-4 and IL-10 expression, and IFN-γ expression was reduced. MSC-ITP was defective in promoting IL-4 and IL-10 secretion and inhibiting IFN-γ secretion compared with that of the healthy controls. These data indicate that MSCs lose their immunomodulatory ability, which is needed for maintaining the immune balance. Because ITP is characterized by the production of autoantibody against targeted antigens, we further assessed whether these defects in MSCs could affect the production of antiplatelet antibodies. As expected, MSCs from healthy individuals were able to suppress the production of anti-GPIIb-IIIa antibodies in the in vitro coculture system, but the MSCs from ITP patients showed a weaker capacity in this respect, providing a stronger indication that the functional defects of MSC-ITP could possibly be involved in the pathogenesis of ITP. Because the impairment of MSCs, including excess apoptosis and senescence and defects in immunoregulation, might play a role in the development of ITP, correcting the defects of MSCs could represent a potential alternative therapeutic approach for the treatment of ITP.
PDGF isoforms and PDGF receptors have important functions in the regulation of growth and survival of various cell types [52]. It was demonstrated that PDGF treatment potently ameliorated photoreceptor degeneration via the suppression of apoptotic pathways [53]. Fingas et al found that myofibroblast-derived PDGF-BB could promote survival signaling in cholangiocarcinoma cells [54]. Vantler et al. showed that PDGF-BB had the capacity to protect cardiomyocytes from apoptosis and to improve the contractile function of engineered heart tissue [19]. Moreover, the potential of PDGF to induce proliferation in MSCs has been documented in a variety of studies [55]. Sotoca et al. showed that PDGF efficiently promoted proliferation and maintained the multipotency of healthy human MSCs [56]. The potential of PDGF to induce proliferation in MSCs was further confirmed by the upregulation of proliferation- and cell cycle-related genes [57]. Considering that PDGF is a potent growth-promoting factor for MSCs, we assessed whether PDGF could alleviate apoptosis and senescence in patients with ITP. We found that PDGF-BB could mitigate the apoptosis and senescence of MSC-ITP by downregulating p53 expression and consequently maintain the immunomodulatory effects.
Conclusion
We have shown that MSC-ITP display enhanced senescence and apoptosis and defects in immunoregulation, which could implicate the possible involvement of MSCs in the pathogenesis of ITP. We demonstrated that PDGF-BB is capable of protecting MSCs derived from ITP patients against apoptosis and senescence by inhibiting the p53/p21 pathway and maintaining the immunosuppression capacity, indicating that PDGF-BB could serve as a novel potential therapeutic alternative for ITP.
Supplementary Material
Acknowledgments
This work was supported by The National Key Technology Support Program (Grant 2012BAI38B03), National Natural Science Foundation of China (Grants 81270643 and 81470343), and Seeding Grant for Medicine and Engineering Sciences of Peking University (Grant BMU20140389). This work was also supported by grants from the National Natural Science Foundation of China (Grant 81300439) and the Specialized Research Fund for the Doctoral Program of Higher Education (Grant 20130001120078).
Author Contributions
J.-m.Z.: experiment performance, data analysis and interpretation, manuscript writing; F.-e.F., Q.-m.W., and X.-l.Z.: experiment performance, data analysis and interpretation, figure creation; H.-x.F.: data analysis and interpretation, manuscript writing; L.-p.X. and K.-y.L.: conception and design, manuscript writing, final approval of manuscript; X.-j.H. and X.-h.Z.: conception and design, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Cines DB, Cuker A, Semple JW. Pathogenesis of immune thrombocytopenia. Presse Med. 2014;43:e49–e59. doi: 10.1016/j.lpm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Semple JW, Provan D, Garvey MB, et al. Recent progress in understanding the pathogenesis of immune thrombocytopenia. Curr Opin Hematol. 2010;17:590–595. doi: 10.1097/MOH.0b013e32833eaef3. [DOI] [PubMed] [Google Scholar]
- 3.Mouzaki A, Theodoropoulou M, Gianakopoulos I, et al. Expression patterns of Th1 and Th2 cytokine genes in childhood idiopathic thrombocytopenic purpura (ITP) at presentation and their modulation by intravenous immunoglobulin G (IVIg) treatment: Their role in prognosis. Blood. 2002;100:1774–1779. [PubMed] [Google Scholar]
- 4.Liu B, Zhao H, Poon MC, et al. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol. 2007;78:139–143. doi: 10.1111/j.1600-0609.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 5.Nishimoto T, Kuwana M. CD4+CD25+Foxp3+ regulatory T cells in the pathophysiology of immune thrombocytopenia. Semin Hematol. 2013;50(suppl 1):S43–S49. doi: 10.1053/j.seminhematol.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Kim N, Im KI, Lim JY, et al. Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: Experiments and practice. Ann Hematol. 2013;92:1295–1308. doi: 10.1007/s00277-013-1796-z. [DOI] [PubMed] [Google Scholar]
- 7.Almeida-Porada G, Porada CD, Tran N, et al. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620–3627. [PubMed] [Google Scholar]
- 8.English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 10.Tabera S, Pérez-Simón JA, Díez-Campelo M, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93:1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- 11.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 12.Tasso R, Ilengo C, Quarto R, et al. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:786–793. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Liu L, Meng D, et al. Enhanced apoptosis and senescence of bone-marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Stem Cells Dev. 2012;21:2387–2394. doi: 10.1089/scd.2011.0447. [DOI] [PubMed] [Google Scholar]
- 14.Mohanty ST, Kottam L, Gambardella A, et al. Alterations in the self-renewal and differentiation ability of bone marrow mesenchymal stem cells in a mouse model of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R149. doi: 10.1186/ar3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Valle M, Podestà M, et al. T-cell suppression mediated by mesenchymal stem cells is deficient in patients with severe aplastic anemia. Exp Hematol. 2005;33:819–827. doi: 10.1016/j.exphem.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Simón JA, Tabera S, Sarasquete ME, et al. Mesenchymal stem cells are functionally abnormal in patients with immune thrombocytopenic purpura. Cytotherapy. 2009;11:698–705. doi: 10.3109/14653240903051558. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Li H, Ma L, et al. The defective bone marrow-derived mesenchymal stem cells in patients with chronic immune thrombocytopenia. Autoimmunity. 2014;47:519–529. doi: 10.3109/08916934.2014.938320. [DOI] [PubMed] [Google Scholar]
- 18.Heldin CH. Autocrine PDGF stimulation in malignancies. Ups J Med Sci. 2012;117:83–91. doi: 10.3109/03009734.2012.658119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vantler M, Karikkineth BC, Naito H, et al. PDGF-BB protects cardiomyocytes from apoptosis and improves contractile function of engineered heart tissue. J Mol Cell Cardiol. 2010;48:1316–1323. doi: 10.1016/j.yjmcc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Cabezas R, Avila MF, Gonzalez J, et al. PDGF-BB protects mitochondria from rotenone in T98G cells. Neurotox Res. 2015;27:355–367. doi: 10.1007/s12640-014-9509-5. [DOI] [PubMed] [Google Scholar]
- 21.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 24.Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102:1393–1402. doi: 10.1172/JCI4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuwana M, Kaburaki J, Kitasato H, et al. Immunodominant epitopes on glycoprotein IIb-IIIa recognized by autoreactive T cells in patients with immune thrombocytopenic purpura. Blood. 2001;98:130–139. doi: 10.1182/blood.v98.1.130. [DOI] [PubMed] [Google Scholar]
- 26.Olsson B, Andersson PO, Jernås M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123–1124. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Heck S, Patel V, et al. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112:1325–1328. doi: 10.1182/blood-2008-01-135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 29.Jorgensen C, Djouad F, Apparailly F, et al. Engineering mesenchymal stem cells for immunotherapy. Gene Ther. 2003;10:928–931. doi: 10.1038/sj.gt.3302019. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 31.Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 32.Ma L, Zhou Z, Zhang D, et al. Immunosuppressive function of mesenchymal stem cells from human umbilical cord matrix in immune thrombocytopenia patients. Thromb Haemost. 2012;107:937–950. doi: 10.1160/TH11-08-0596. [DOI] [PubMed] [Google Scholar]
- 33.Xiao J, Zhang C, Zhang Y, et al. Transplantation of adipose-derived mesenchymal stem cells into a murine model of passive chronic immune thrombocytopenia. Transfusion. 2012;52:2551–2558. doi: 10.1111/j.1537-2995.2012.03642.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin R, Ma H, Ding Z, et al. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev. 2013;22:2836–2848. doi: 10.1089/scd.2013.0166. [DOI] [PubMed] [Google Scholar]
- 35.Fang B, Mai L, Li N, et al. Favorable response of chronic refractory immune thrombocytopenic purpura to mesenchymal stem cells. Stem Cells Dev. 2012;21:497–502. doi: 10.1089/scd.2011.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicencio JM, Galluzzi L, Tajeddine N, et al. Senescence, apoptosis or autophagy? When a damaged cell must decide its path—A mini-review. Gerontology. 2008;54:92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- 37.Campisi J, d’Adda di Fagagna F. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 38.Cagnol S, Chambard JC. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 39.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 40.Ferri KF, Kroemer G. Mitochondria—The suicide organelles. BioEssays. 2001;23:111–115. doi: 10.1002/1521-1878(200102)23:2<111::AID-BIES1016>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 41.Choi C, Benveniste EN. Fas ligand/Fas system in the brain: Regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Zuckerman V, Wolyniec K, Sionov RV, et al. Tumour suppression by p53: The importance of apoptosis and cellular senescence. J Pathol. 2009;219:3–15. doi: 10.1002/path.2584. [DOI] [PubMed] [Google Scholar]
- 43.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 44.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 45.Sherr CJ. G1 phase progression: Cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 46.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 48.Meulmeester E, Jochemsen AG. P53: A guide to apoptosis. Curr Cancer Drug Targets. 2008;8:87–97. doi: 10.2174/156800908783769337. [DOI] [PubMed] [Google Scholar]
- 49.Shan NN, Hu Y, Hou M, et al. Decreased Tim-3 and its correlation with Th1 cells in patients with immune thrombocytopenia. Thromb Res. 2014;133:52–56. doi: 10.1016/j.thromres.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Shao Q, Ning H, Lv J, et al. Regulation of Th1/Th2 polarization by tissue inhibitor of metalloproteinase-3 via modulating dendritic cells. Blood. 2012;119:4636–4644. doi: 10.1182/blood-2011-08-376418. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Zhao H, Xue F, et al. Reduced expression of MIR409-3p in primary immune thrombocytopenia. Br J Haematol. 2013;161:128–135. doi: 10.1111/bjh.12213. [DOI] [PubMed] [Google Scholar]
- 52.Donovan J, Abraham D, Norman J. Platelet-derived growth factor signaling in mesenchymal cells. Front Biosci (Landmark Ed) 2013;18:106–119. doi: 10.2741/4090. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Abu-Asab MS, Yu CR, et al. Platelet-derived growth factor (PDGF)-C inhibits neuroretinal apoptosis in a murine model of focal retinal degeneration. Lab Invest. 2014;94:674–682. doi: 10.1038/labinvest.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–2088. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng J, Ye H, Liu Z, et al. Platelet-derived growth factor-BB accelerates prostate cancer growth by promoting the proliferation of mesenchymal stem cells. J Cell Biochem. 2013;114:1510–1518. doi: 10.1002/jcb.24492. [DOI] [PubMed] [Google Scholar]
- 56.Sotoca AM, Roelofs-Hendriks J, Boeren S, et al. Comparative proteome approach demonstrates that platelet-derived growth factor C and D efficiently induce proliferation while maintaining multipotency of hMSCs. Exp Cell Res. 2013;319:2649–2662. doi: 10.1016/j.yexcr.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 57.Qiu P, Song W, Niu Z, et al. Platelet-derived growth factor promotes the proliferation of human umbilical cord-derived mesenchymal stem cells. Cell Biochem Funct. 2013;31:159–165. doi: 10.1002/cbf.2870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.