This study assessed the in vitro influence of a clinical-grade human multipotent adult progenitor cell (MAPC) product (MultiStem) on the cytotoxic function of CD8+ T cells (CTLs) by evaluating the immunogenicity of MAPCs and the susceptibility of MAPCs toward CTL-mediated lysis and by analyzing the mechanism of MAPC-mediated modulation of CTL functionality. These MAPCs were found to be an immune-modulating population that inhibits CTL activation and effector responses.
Keywords: MultiStem, Clinical-grade human multipotent adult progenitor cells, Cytotoxic T cells, Stem cell-based immune modulation, T cell-mediated cytotoxicity
Abstract
MultiStem cells are clinical-grade multipotent adult bone marrow-derived progenitor cells (MAPCs), with extensive replication potential and broader differentiation capacity compared with mesenchymal stem cells. Human MAPCs suppress T-cell proliferation induced by alloantigens and mutually interact with allogeneic natural killer cells. In this study, the interaction between MultiStem and CD8+ cytotoxic T lymphocytes (CTLs) was addressed for the first time. In an in vitro setting, the immunogenicity of MultiStem, the susceptibility of MultiStem toward CTL-mediated lysis, and its effects on CTL function were investigated. MultiStem was nonimmunogenic for alloreactive CTL induction and was—even after major histocompatibility complex class I upregulation—insensitive to alloantigen-specific CTL-mediated lysis. Furthermore, MultiStem reduced CTL proliferation and significantly decreased perforin expression during the T-cell activation phase. As a consequence, MultiStem dose-dependently impaired the induction of CTL function. These effects of MultiStem were mediated predominantly through contact-dependent mechanisms. Moreover, MultiStem cells considerably influenced the expression of T-cell activation markers CD25, CD69, and human leukocyte antigen-DR. The MultiStem-induced CD8−CD69+ T-cell population displayed a suppressive effect on the induction of CTL function during a subsequent mixed-lymphocyte culture. Finally, the killer activity of activated antigen-specific CTLs during their cytolytic effector phase was also diminished in the presence of MultiStem. This study confirms that these clinical-grade MAPCs are an immune-modulating population that inhibits CTL activation and effector responses and are, consequently, a highly valuable cell population for adoptive immunosuppressive therapy in diseases where damage is induced by CTLs.
Significance
Because multipotent adult progenitor cells (MAPCs) are among the noteworthy adult mesenchymal stem cell populations for immune therapy and have the advantage over mesenchymal stem cells (MSCs) of large-scale manufacturing and banking potential and thus prompt availability, it is important to understand how MAPCs interact with immune cells to validate their widespread therapeutic applicability. Cytotoxic immune effector cells play a crucial role in immune homeostasis and in the pathogenesis of some autoimmune diseases. This study assessed for the first time the in vitro influence of a clinical-grade human MAPC product (MultiStem) on the cytotoxic function of CD8+ T cells (CTLs) by evaluating the immunogenicity of MAPCs and the susceptibility of MAPCs toward CTL-mediated lysis and by analyzing the mechanism of MAPC-mediated modulation of CTL functionality. These results may represent a highly relevant contribution to the current knowledge and, in combination with the results of future phase II/III trials using MultiStem, could lead to an intriguing continuation of stem cell-based research for immunotherapy.
Introduction
During the last decade, stem cell-based therapy has made enormous progress in the treatment of various diseases. Stem cells are unspecialized self-renewing cells that can undergo multilineage differentiation and are classified according to their differentiation potential [1]. Besides their capacity to regenerate damaged tissues, adult stem cells also possess a diverse array of immune-modulatory characteristics. Mesenchymal stem cells (MSCs) are a prototype of adult nonhematopoietic stem cells, which can be isolated from various tissues and are able to differentiate into several mesenchymal cell types [2–6]. Human MSCs (hMSCs) have been proven to be a non-major histocompatibility complex (non-MHC)-restricted immunosuppressive cell population in vitro because they suppress allogeneic T-cell responses and impair differentiation and maturation of dendritic cells (DCs) [7–11]. hMSCs also interfere with natural killer (NK) cell and B-cell proliferation and function [12, 13]. Several phase I and II studies have already shown the feasibility and safety of in vivo use of hMSCs in immune-related diseases, and phase III studies are being initiated to explore their therapeutic efficacy.
Cytotoxic T lymphocytes (CTLs) become capable of killing cancer cells or virus-infected cells after activation through the CD8-mediated recognition of specific antigens presented in a MHC class I-dependent way by specialized antigen-presenting cells (APCs). This leads to clonal expansion of antigen-specific CTLs, which—upon the second encounter of infected cells—exert their killer effector function. Targeted cell death by activated CTLs is induced by the release of perforin and granzyme via granules or by proapoptotic Fas ligand-Fas receptor (FasL-FasR) clustering, ultimately triggering the apoptosis-inducing caspase cascade [14]. Despite high expression of MHC class I molecules on their surface, MSCs escape CTL-mediated lysis [15]. Conversely, MSCs inhibit CTL formation and prevent CTL-mediated lysis of target cells when added during the primary activation phase [16].
Multipotent adult progenitor cells (MAPCs) are another population of bone marrow-derived adherent progenitor cells [17, 18]. In contrast to hMSCs, human MAPCs (hMAPCs) can also differentiate into functional endothelium in vitro and in vivo and can be expanded for a significantly longer time [19, 20]. This extensive proliferation capacity has led to large-scale manufacturing and banking of MAPCs, allowing the production of uniform clinical doses without the use of multiple donors [21]. Clinical trials can thus be performed by treating several allogeneic patients with a single batch of MultiStem cells derived from one unique patient, so that the results will not depend on the quality of the different isolations. Currently, such clinical-grade MAPCs are infused as an allogeneic off-the-shelf adoptive cell product (MultiStem) resulting in a potentially more advantageous cellular therapy in the context of tissue regeneration and cardiovascular, neurological and immune-related diseases. Third-party MultiStem has been proven safe and well-tolerated in phase I studies in patients with acute myocardial infarction [22] and as an adjuvant cell therapy after bone marrow transplantation in patients with hematological malignancies [23]. Moreover, phase I safety testing has also started in the context of immune modulation after liver transplantation [24] and acute respiratory distress syndrome. Recently, a multicenter phase II study [25] showed that ischemic stroke patients receiving intravenous administration of MultiStem achieved a significantly higher rate of excellent improvement in three clinical rating scales compared with placebo subjects at 1-year follow-up [26]. The effect was even more pronounced when patients were treated within 36 hours of symptom onset—extending the time frame of intervention relative to current stroke care—as was observed in the 90-day interim analysis. Initial results from another phase II trial in refractory ulcerative colitis demonstrated consistent safety of MultiStem, but a single administration was insufficient to achieve a substantial and durable benefit in patients [27].
Given the fact that MAPCs are among the noteworthy mesenchymal cell populations for clinical usage and have the advantage over MSCs of having large-scale manufacturing and banking potential and therefore prompt availability, it is important to understand how these clinical-grade MAPCs (MultiStem) interact with immune cells to validate their widespread therapeutic applicability. We found that hMAPCs are nonimmunogenic for alloreactive T-cell proliferation; they impair allogeneic proliferative T-cell responses, and they mutually interact with allogeneic NK cells in vitro [28, 29]. In this study, the influence of MultiStem cells on the cytotoxic function of CD8+ T cells was assessed by evaluating the immunogenicity of MultiStem and the susceptibility of the stem cell population toward CTL-mediated lysis and by analyzing the MultiStem-mediated modulation of CTL functionality.
Materials and Methods
Isolation and Culture of MultiStem
Research-grade human MAPCs (MultiStem) were isolated by Athersys/ReGenesys (Athersys, Cleveland, OH, http://www.athersys.com; ReGenesys, Heverlee, Belgium, http://www.regenesys.eu) from bone marrow (BM) of five different healthy volunteers (donors 1–5; supplemental online Table 1). Informed consent for BM collection was obtained in accordance with the guidelines of the Medical Ethics Committee of the University Hospitals Leuven. Isolation and cultivation of the MultiStem clinical product were based on MAPC protocols as previously described [20, 21]. Briefly, MultiStem cells were generated by plating the total cell fraction at 0.5 × 106 cells per cm2 in medium consisting of 60% Dulbecco’s modified Eagle’s medium low-glucose (Lonza, Verviers, Belgium, http://www.lonza.com), 40% MCDB-201 (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), supplemented with 50 nM dexamethasone, 10−4 M L-ascorbic acid, 0.5× linoleic acid-bovine serum albumin (Sigma-Aldrich), 1× insulin-transferrin-selenium, and 100 U/ml penicillin/streptomycin, along with 18% fetal bovine serum (Serum Supreme) (Lonza), 10 ng/ml human platelet-derived growth factor, and 10 ng/ml human epidermal growth factor (R&D Systems, Minneapolis, MN, https://www.rndsystems.com). MultiStem cultures were maintained under hypoxic conditions (5% O2) at a density of 2,000 cells per cm2 per 1× fibronectin-coated (Sigma-Aldrich) T75 culture flask (Corning, Corning, NY, http://www.corning.com). The cells were split every 2–3 days, were not clonally derived, and were used at population doubling 25–35.
Isolation and Activation of (CD8+ Cytotoxic) T Lymphocytes
All subjects donating blood were healthy volunteers of both sexes, aged 20–60 years. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Axis-Shield, Oslo, Norway, http://www.axis-shield.com) density gradient centrifugation (specific gravity, 1.077 g/ml). T cells were further purified by using two rounds of a complement-mediated depletion of all non-T cells with lympho-KWIK-T reagent (One Lambda, Los Angeles, CA, https://www.onelambda.com) and one round of a mixture of complement-fixing anti-NK cell monoclonal antibody (anti-CD16) of the IgM subclass (BD Biosciences, Erembodegem, Belgium, http://www.bdbiosciences.com), as previously described [28]. Purified T cells were resuspended in complete culture medium consisting of RPMI 1640 supplemented with 2 mmol/l l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Lonza), and 10% autologous heat-inactivated plasma. In some experiments, CD8+ CTLs were negatively selected from T-cell fractions by using the human CD8+ T cell isolation kit (Miltenyi Biotec, Leiden, The Netherlands, http://www.miltenyibiotec.com) according to the manufacturer’s instructions.
To obtain alloantigen-specific T cells, purified T cells or CD8+ CTL fractions were cocultured with either allogeneic-irradiated (40 Gy) Epstein-Barr virus (EBV)-transformed B cells (supplemental online data) or allogeneic-irradiated (30 Gy) PBMCs for 7 days in complete culture medium in flat-bottomed 24-well plates (Greiner Bio-One, Wemmel, Belgium, https://www.gbo.com) at a stimulator:responder (S:R) ratio ranging from 1:2 to 1:20. For 4-day polyclonal activation, culture plates were first coated with anti-CD3 monoclonal antibody (mAb) (5 µg/ml UCHT-1; obtained from hybridoma cultures; American Type Culture Collection [ATCC], Manassas, VA, http://www.atcc.org), and afterward 1 µg/ml anti-CD28 (Sanquin, Amsterdam, The Netherlands, http://www.sanquin.nl) was added, together with the T cells [30].
Cytotoxicity Assays
The viability of MultiStem in the presence of activated T cells was tested with the standard 4-hour 51Cr-release method [29]. Therefore, MultiStem cells were labeled with 100 µCi 51Cr (PerkinElmer, Zaventem, Belgium, http://www.perkinelmer.com) per 106 cells and seeded at 5 × 103 cells per well in V-bottomed 96-well plates (Greiner Bio-One). As a control target cell, EBV+ B cells from the corresponding MultiStem donor, which were also used to prime the naive T cells, were labeled with 200 µCi 51Cr per 106 cells. The lytic potential of alloantigen-specific effector CTLs was tested by coculturing cells at effector:target ratio of 10:1 in a total volume of 200 µl of complete medium. Spontaneous 51Cr-release of the target cells was verified, and saponin (Merck, Darmstadt, Germany, http://www.emdgroup.com) was added to measure the maximal 51Cr-release. Release of 51Cr was measured by a Topcount γ counter (Packard Instrument Co., Meriden, CT). Results are expressed as percentage 51Cr-release, calculated as [(experimental release – spontaneous release)/(maximal release – spontaneous release)] × 100.
CTL activity was also checked regardless of antigen specificity of the CTLs with an anti-CD3-redirected cytotoxicity system, as previously described [30]. Briefly, NK-resistant P815 cells (ATCC) were used as 51Cr-labeled target cells (100 µCi of 51Cr per 106 cells) in the presence of an anti-CD3 mAb, being 2 µg/ml OKT3 (muromonab-CD3; Janssen-Cilag, Berchem, Belgium, http://www.cilag.ch) or 10 µg/ml UCHT1. By bridging the effector CTL to the target cell FcγR, the anti-CD3 mAb permits detection of CTL activity regardless of antigen specificity. Results are expressed as percentage of anti-CD3-dependent specific 51Cr-release (% SR), calculated as [(total release in the presence of anti-CD3 – experimental background release in the absence of anti-CD3)/(maximal release – spontaneous release)] × 100.
Immune Regulation by MultiStem
To analyze the immunogenicity of MultiStem, we stimulated T cells with either allogeneic-irradiated MultiStem or PBMCs (both 30 Gy) from the same donor for 7 days at S:R ratio 1:2. Afterward, CTL response was measured. The modulating capacity of MultiStem cells was tested during either the activation phase or the lytic effector phase of T cells. Therefore, MultiStem cells were added at the beginning of the 7-day stimulation period or the 4-hour 51Cr-release assay at a suppressor:responder ratio ranging from 1:1 to 1:100. Afterward, the proliferative and CTL responses were analyzed by 16-hour [3H]thymidine incorporation (1 µCi per well; PerkinElmer; measured on a Tri-Carb 2100TR Liquid Scintillation Counter; PerkinElmer) and by standard 51Cr-release assay, respectively. Proliferation results were analyzed as mean counts per minute of quadruplicate wells and were expressed as percentage response related to the control response in the absence of MultiStem.
In some experiments, 100 U/ml exogenous human recombinant interleukin-2 (rIL-2) (TECIN; Hoffmann-La Roche, Nutley, NJ, http://www.roche.com) was added to the coculture of immune cells and stem cells. The effect of MultiStem on T-cell priming for memory response was tested by stimulating T cells in a primary mixed-lymphocyte culture (MLC) with allogeneic EBV+ B cells in the presence or absence of MultiStem cells. After a 3-day resting period, T cells were restimulated in the absence of MultiStem cells with the same alloantigens in a secondary MLC for 4 days.
The involvement of soluble factors in the immune regulation by MultiStem was evaluated by using 24-well plate Thincert inserts (Greiner Bio-One) with a semipermeable membrane (pore size 0.4 µm) to separate MultiStem cells from the MLC during the T-cell activation phase (range 1:2 to 1:10 MultiStem:T cells). To address the specific role of indoleamine 2,3-dioxygenase (IDO) or prostaglandin E2 (PGE2) as immune suppressive mediators, their respective blocking molecules were added to the coculture system, being 200 µM/ml 1-methyl-trypthophan (1-MT; Sigma-Aldrich) or 2 µg/ml indomethacin (Cayman Chemical Company, Ann Arbor, MI, https://www.caymanchem.com). To examine the role of galectin-1 (Gal-1), we reduced its expression level in MultiStem cells by means of calcium phosphate-mediated transfection (ProFection Mammalian Transfection System; Promega, Leiden, The Netherlands, http://www.promega.com) with 40 µM human Gal-1-targeting modified small interfering RNA (siRNA; sequence 5′-GCUGCCAGAUGGAUACGAAdTdT-3′; GE Healthcare Bio-Sciences AB, Uppsala, Sweden, http://www.gelifesciences.com) according to the manufacturer’s instructions. Production and secretion of Gal-1 was analyzed by means of fluorescence microscopy (1 µg/ml anti-human Gal-1 mAb; Peprotech, London, U.K., http://www.peprotech.com), Western blot (0.2 µg/ml), and enzyme-linked immunosorbent assay (ELISA) on cell supernatant [31]. Transfected MultiStem cells were added as modulating cells in immune-suppressive assays at day 4 after transfection.
The role of the contact-dependent mechanisms programmed death ligand-1 (PD-L1), PD-L2, and programmed death-1 (PD-1) signaling pathway and apoptosis-inducing FasL/FasR interaction was investigated by adding their neutralizing mAbs as follows: 5 µg/ml anti-PD-1, 2 µg/ml anti-PD-L1, 2 µg/ml anti-PD-L2 (eBioscience, San Diego, CA, http://www.ebioscience.com), and 5 µg/ml anti-FasL (BD Biosciences).
Statistical Analysis
Statistics were calculated with Prism software (Version 5.0; GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com). Statistical significance was calculated with (un)paired t tests for comparisons between two groups. Values of p < .05 were considered significant.
Results
Human MultiStem Cells Are Nonstimulatory for Allogeneic T Cells in Vitro
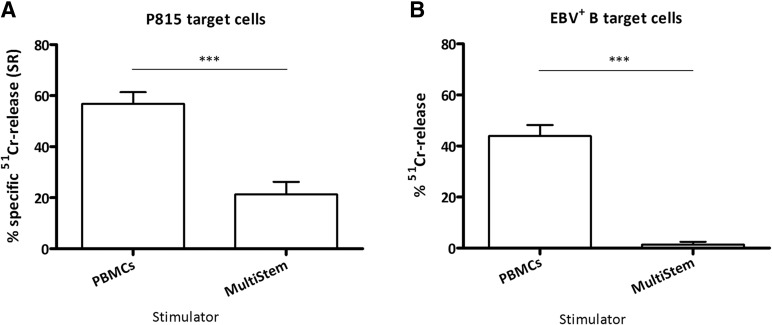
In previous work, we demonstrated that hMAPCs did not induce alloreactive T-cell proliferation or T helper 1 (Th1)/Th2 cytokine production when cocultured in vitro [28]. To assess whether MultiStem could induce the cytotoxic effector function in T cells, responder CD3+ T cells were stimulated with irradiated allogeneic MultiStem on the one hand, and irradiated allogeneic PBMCs of the MultiStem donor on the other hand as a control APC population. Standard 51Cr-release assay revealed that PBMC-stimulated T cells efficiently killed 51Cr-labeled P815 target cells in the presence of an anti-CD3 mAb (mean ± SEM % 51Cr-release: 56.75% ± 4.63%; n = 5; Fig. 1A). In contrast, MultiStem induced only a minimal anti-CD3-redirected cytotoxic response (21.32% ± 4.91%; n = 5). In the alloantigen-specific cytotoxicity assay, EBV+ target B cells were not lysed when T cells were prestimulated with MultiStem (1.39% ± 1.11%; n = 3; Fig. 1B), compared with prestimulation with PBMCs (43.89% ± 4.34%; n = 3). The MultiStem cells or PBMCs were from the same donor as the EBV+ target B cells used for the cytotoxicity assay. These results suggest the lack of immunogenicity of MultiStem cells in the in vitro setting.
Figure 1.
MultiStem does not induce cytotoxic activity in T cells. Freshly isolated responder CD3+ T cells were stimulated with either allogeneic-irradiated (30 Gy) peripheral blood mononuclear cells or allogeneic-irradiated (30 Gy) MultiStem (PBMCs and MultiStem were from the same donor) at a stimulator:responder ratio of 1:2 for 7 days. Coculture was followed by an assessment of anti-CD3-redirected cytotoxic activity against murine P815 mastocytoma target cells (A) or alloantigen-specific cytotoxic activity against Epstein-Barr virus-transformed B cells (B) at an effector:target ratio of 10:1 in a standard 51Cr-release assay. Data are expressed as mean ± SEM percentage of anti-CD3-dependent specific 51Cr-release (% SR) of five independent experiments with four different T cell donors and three different PBMC/MultiStem donors (donors 1, 2, and 3) (A) and mean ± SEM % 51Cr-release of three independent experiments with two different T cell donors and two different PBMC/MultiStem/B cell donors (donors 1 and 2) (B). Statistical significance was calculated with the unpaired t test. ∗∗∗, p < .001. Abbreviations: EBV, Epstein-Barr virus; P815, murine P815 mastocytoma target cells; PBMCs, peripheral blood mononuclear cells; SR, specific 51Cr-release.
MultiStem Is Insensitive to Alloantigen-Specific CTL-Mediated Lysis
The interaction between activated CD8+ CTLs and allogeneic MultiStem was addressed by first investigating the susceptibility of the stem cell population to CTL-mediated killing. Purified CD3+CD8+ T cells were stimulated with allogeneic-irradiated EBV+ B cells for 7 days, followed by an assessment of CTL activity against murine P815 cells (n = 6), alloantigen-specific MultiStem (n = 6), and EBV+ B cells (n = 3) as MHC-specific control target cells. As shown in Figure 2, activated T cells killed anti-CD3-coated P815 cells (36.30% ± 4.85%), whereas MultiStem cells (from the same donor as the EBV+ B cells used for stimulation) were insensitive to an alloantigen-specific CTL attack (2.54% ± 1.72%). Moreover, the T-cell antigen-specific cytotoxic activity (13.50% ± 3.71%) against EBV+ B cells was significantly higher than the specific lysis of MultiStem. In some experiments (n = 4), MultiStem cells were pretreated with interferon-γ (IFN-γ) (100 U/ml; Roche Diagnostics, Vilvoorde, Belgium, http://www.roche.com) for 48 hours to increase MHC class I molecule expression [28, 29]. However, this upregulation did not result in a higher sensitivity to CTL-mediated lysis, confirming the immune-privileged status of these clinical-grade hMAPCs (data not shown).
Figure 2.
Activated T cells do not lyse allogeneic MultiStem cells. Results of anti-CD3-redirected and alloantigen-specific cytotoxic activity of irradiated (40 Gy) EBV+ B cell-stimulated CD8+ cytotoxic T lymphocytes (CTLs) (stimulator:responder ratio of 1:10 for 7 days) against respectively anti-CD3-coated P815 target cells, MultiStem, or EBV+ B cells at an effector:target ratio of 10:1. MultiStem cells and EBV+ B target cells were from the same donor as the EBV+ B cells used for CTL activation. Target cell killing is expressed as mean ± SEM percentage of 51Cr-release of n independent experiments (P815 targets, n = 6; MultiStem targets, n = 6; EBV+ B targets, n = 3). Four different CTL donors and three different MultiStem/B-cell donors (donors 1, 2, and 3) were used. Statistical significance was calculated with the paired t test. ∗, p < .05; ∗∗∗, p < .001. Abbreviations: EBV, Epstein-Barr virus; P815, murine P815 mastocytoma target cells.
MultiStem Cells Impair Proliferation, Perforin Expression, and Cytotoxic Function of CD8+ T Cells
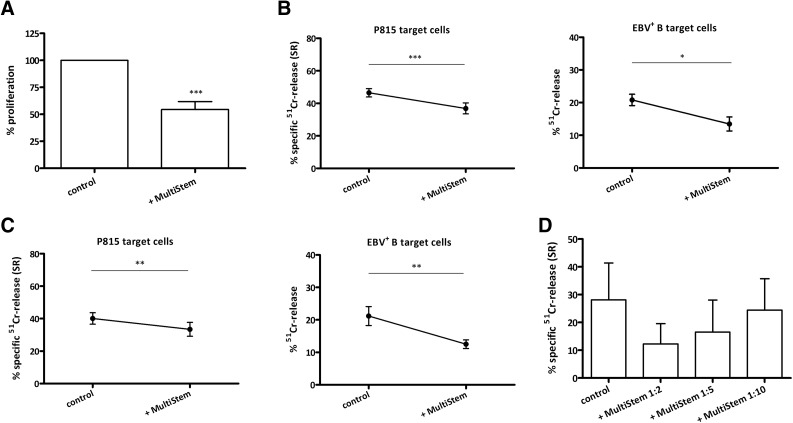
In the next set of experiments, the interference of MultiStem with the clonal expansion of antigen-specific CD8+ T cells or with the generation of CTL function was investigated. Therefore, CD3+CD8+ T cells were primed with allogeneic EBV+ B cells for 7 days. Irradiated third-party MultiStem cells were added to the MLC either at the beginning of the 7-day activation phase or the 4-hour cytolytic effector phase. In the presence of MultiStem, CTLs had a lower proliferative response (Fig. 3A). MultiStem-modulated CTLs demonstrated a slightly, but significantly, reduced lytic capacity in both cytotoxicity systems compared with CTLs that were not exposed to MultiStem during the activation phase (anti-CD3-redirected: 36.86% ± 3.39% versus 46.48 ± 2.55%; n = 32; alloantigen-specific: 13.47% ± 2.15% versus 20.82% ± 1.74%; n = 9; Fig. 3B). The presence of MultiStem during the cytotoxic effector phase also significantly diminished the killing of both target cell populations (anti-CD3-redirected: 33.44% ± 4.27% versus 40.10% ± 3.50%; n = 18; alloantigen-specific: 12.50 ± 1.33% versus 21.18% ± 2.92%; n = 15; Fig. 3C). These data suggest that MultiStem impairs both CD8+ T-cell proliferation and induction of CTL activity, and the lytic T-cell effector function itself.
Figure 3.
MultiStem impairs the proliferation and (induction of) cytotoxicity of CD8+ T cells. (A): Purified CD8+ cytotoxic T lymphocytes (CTLs) were stimulated with irradiated allogeneic EBV-transformed B cells (stimulator:responder [S:R] ratio of 1:10) during 7 days with or without irradiated third-party MultiStem at a suppressor:responder ratio of 1:2. Proliferation was measured on day 6 with [3H]thymidine incorporation. Results are expressed as mean ± SEM % proliferation relative to control (average counts per minute: 46,801) of quadruplicates in five experiments with five CTL and two MultiStem donors (donors 2 and 5). (B): Results of anti-CD3-redirected cytotoxicity of B cell-stimulated CD8+ CTLs against P815 targets (effector:target [E:T] ratio of 10:1; left) or alloantigen-specific activity against target B cells (right) with or without MultiStem (E:T ratio of 1:2) during the 7-day activation phase. Data are expressed as mean ± SEM percentage of anti-CD3-dependent specific 51Cr-release (% SR) of 32 experiments with 19 CTL and 4 MultiStem donors (donors 1–4; left) or percentage of 51Cr-release of 9 experiments with 6 CTL and 3 MultiStem donors (donors 1–3; right). (C): Same experimental setup as (B), but MultiStem was only present during the 4-hour cytotoxic effector phase. Left: A total of 18 experiments with 10 CTL donors and 4 MultiStem donors (donors 1–4). Right: A total of 15 experiments with 8 CTL donors and 4 MultiStem donors (donors 1–4). (D): Results of anti-CD3-redirected cytotoxicity of B cell-stimulated CD3+ T cells (S:R ratio of 1:20) against P815 targets with or without MultiStem at different ratios during the 7-day activation phase. Data are pooled from three experiments with two T-cell and two MultiStem donors (donors 2 and 5). Statistical significance was calculated with the paired t test. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: EBV, Epstein-Barr virus; P815, murine P815 mastocytoma target cells; SR, specific 51Cr-release.
To verify whether MultiStem had a similar suppressive effect on the cytotoxicity induction of the total CD3+ T cell fraction, we performed a similar experiment with total T cells. In fact, MultiStem addition led to comparable inhibition of cytotoxicity induction of CD3+ T cells. This effect was dose-dependent (Fig. 3D) and characteristic for MultiStem, because the addition of MultiStem was compared with the addition of an unrelated endothelial cell line (human umbilical vein endothelial cells). The latter did not impair T-cell cytotoxicity induction (supplemental online Fig. 1). Of note, the suppressive effect was not enhanced by IFN-γ-pretreatment of MultiStem (data not shown). The possibility that MultiStem could indirectly suppress T-cell activation and functioning through interference with the EBV+ B cells was investigated. Flow-cytometric analysis revealed no change in surface marker expression (CD54, CD58, CD80, CD86, human leukocyte antigen [HLA]-ABC, and HLA-DR) of the stimulator EBV+ B cells after exposure to MultiStem (data not shown). This finding was confirmed by exploring the suppressive effect of MultiStem on the cytolytic activity of polyclonal activated (anti-CD3/CD28) T cells. The presence of MultiStem resulted in a significantly reduced cytotoxic activity (anti-CD3-redirected: 38.33% ± 5.18% in the presence of MultiStem versus 69.04% ± 3.39% in the control condition; n = 3 [p < .005]). These data indicate that the inhibitory effect of MultiStem on CTL generation is mediated via direct interaction with the T cells.
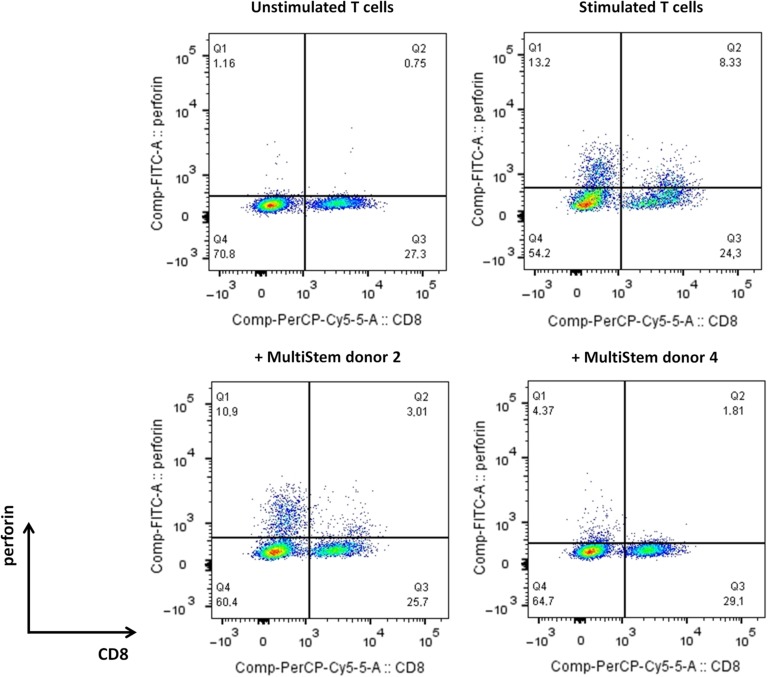
Next, the influence of MultiStem addition during the T-cell stimulation phase on the perforin expression was studied (Fig. 4). Flow-cytometric analysis revealed that the amount of perforin-positive CD8+ T cells was reduced when MultiStem was present during the T-cell activation phase (mean ± SEM % CD8+perforin+ cells of stimulated CD3+ lymphocytes: 4.13% ± 0.99% in the presence of MultiStem versus 8.17% ± 1.34% in the absence of MultiStem; n = 6). MultiStem had also an inhibitory effect on perforin expression in CD8− T cells.
Figure 4.
MultiStem impairs the expression of perforin in CD8+ T cells. Flow-cytometric analysis of irradiated (40 Gy) Epstein-Barr virus-positive B cell-stimulated CD3+ T cells (stimulator:responder ratio of 1:20) for intracellular perforin expression after a 7-day stimulation period in the absence (upper) or presence (lower) of irradiated (30 Gy) third-party MultiStem cells (suppressor:responder ratio of 1:2). Results are expressed as percentage of positive cells in the CD3+ lymphocyte gate of one representative experiment with one T-cell donor and two different MultiStem donors (donors 2 and 4), out of three independent experiments. Abbreviations: Comp, compensation; cy5, cyanine 5; FITC-A, fluorescein isothiocyanate-A; PerCP, peridinin chlorophyll protein; Q1, quarter 1.
MultiStem Cells Mediate T-Cell Cytotoxicity Suppression Through Contact-Dependent Mechanisms
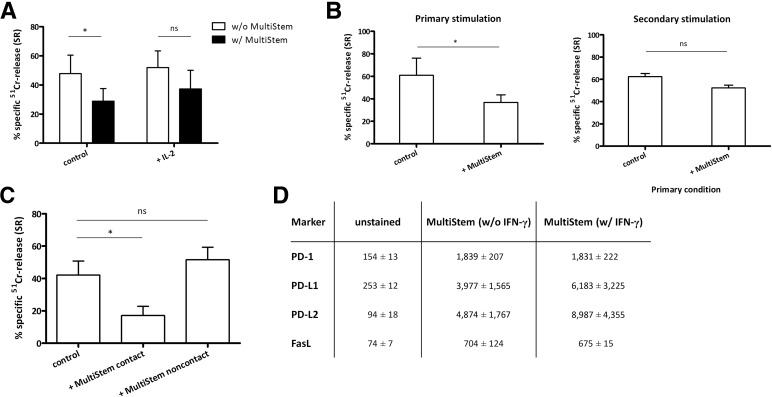
To investigate the mechanism of the cytotoxicity-reducing effect of MultiStem cells, we first verified whether the addition of MultiStem resulted in T-cell apoptosis, anergy, or tolerance. As explained above, MultiStem blocked the induction of CD8+ CTL activity in the MLC. Addition of exogenous rIL-2 only partially restored cytotoxic activity of the alloantigen-primed T cells (Fig. 5A). To further explain the lack of rescued CTL activity in spite of IL-2 addition, the induction of CTL apoptosis by means of Annexin V/PI staining was analyzed. In the presence of MultiStem, no induction of T-cell apoptosis could be detected (data not shown). Furthermore, we also restimulated CD8+ CTLs, which were suppressed during a primary MLC by third-party MultiStem (p = .01), with the same alloantigens in a secondary MLC in the absence of MultiStem and then analyzed their cytotoxic function. This showed that these CTLs still displayed a secondary memory immune response similar to control T cells that had been prestimulated in the absence of MultiStem (p = .18; Fig. 5B). These findings show that MultiStem cells do not induce apoptosis and do not render T cells anergic.
Figure 5.
MultiStem-mediated suppression of T-cell cytotoxicity is contact-dependent. (A): Purified CD8+ cytotoxic T lymphocytes (CTLs) were stimulated with irradiated allogeneic Epstein-Barr virus-positive B cells (stimulator:responder [S:R] ratio of 1:10) with or without irradiated third-party MultiStem (ratio of 1:2) and with or without interleukin-2 (IL-2) for 7 days. Data are expressed as mean ± SEM percentage of SR of P815 targets (effector:target ratio of 10:1) and pooled from four experiments with three CTL and two MultiStem donors (donors 2 and 4). (B): Standard experimental setup was used (left). Right: After a 3-day resting period, CTLs from the primary mixed-lymphocyte culture, cultured with or without MultiStem, were restimulated during 4 days with the same alloantigens in the absence of MultiStem. Results are expressed as mean ± SEM percentage of SR against P815 targets of three experiments with two CTL and two MultiStem donors (donors 2 and 4). (C): Results of anti-CD3-redirected cytotoxicity of B cell-stimulated CD3+ T cells (S:R ratio of 1:20) against P815 targets with or without MultiStem, in direct contact or separated by a transwell system, during the 7-day activation phase. Data are pooled from six experiments with five T-cell and two MultiStem donors (donors 2 and 5). (D): Flow-cytometric analysis of MultiStem, pretreated or not with interferon-γ, for the PD-1 receptor and its ligands programmed death ligands 1 and 2 and for the ligand of the Fas pathway. Average mean fluorescence intensity values ± SD of three experiments (MultiStem donors 2, 4, and 5) are shown. The background signal of unstained cells is included. Statistical significance was calculated with the paired t test. ∗, p < .05. Abbreviations: FasL, Fas ligand; IFN-γ, interferon-γ; IL-2, interleukin 2; ns, not significant; PD-1, programmed death-1; SR, specific 51Cr-release; w/, with; w/o, without.
The contact-dependency of the effect of MultiStem cells was analyzed by means of transwell inserts to separate MultiStem from total CD3+ T cells during the activation phase. As shown in Figure 5C, a suppressive effect of MultiStem was found only in the condition in which both cell populations were in close proximity. Moreover, neither blocking IDO activity nor inhibiting PGE2 synthesis was able to restore cytotoxicity, indicating that these two soluble molecules play no role in MultiStem-mediated T-cell cytotoxicity suppression (data not shown). To identify surface receptors or ligands that could be responsible for this contact-dependent pathway, MultiStem surface expression of the PD-L1/PD-L2/PD-1 molecules and of FasL was verified. These molecules were all expressed in variable amounts, with the highest expression of PD-L1 and PD-L2 ligands upon IFN-γ pretreatment (Fig. 5D). They were not identified as mediators of the MultiStem-related cytotoxicity suppression on the basis of functional blocking experiments with mAbs (data not shown).
MultiStem Exerts an Indirect Effect on T-Cell Cytotoxicity by Enriching the CD8−CD69+ Suppressor Population
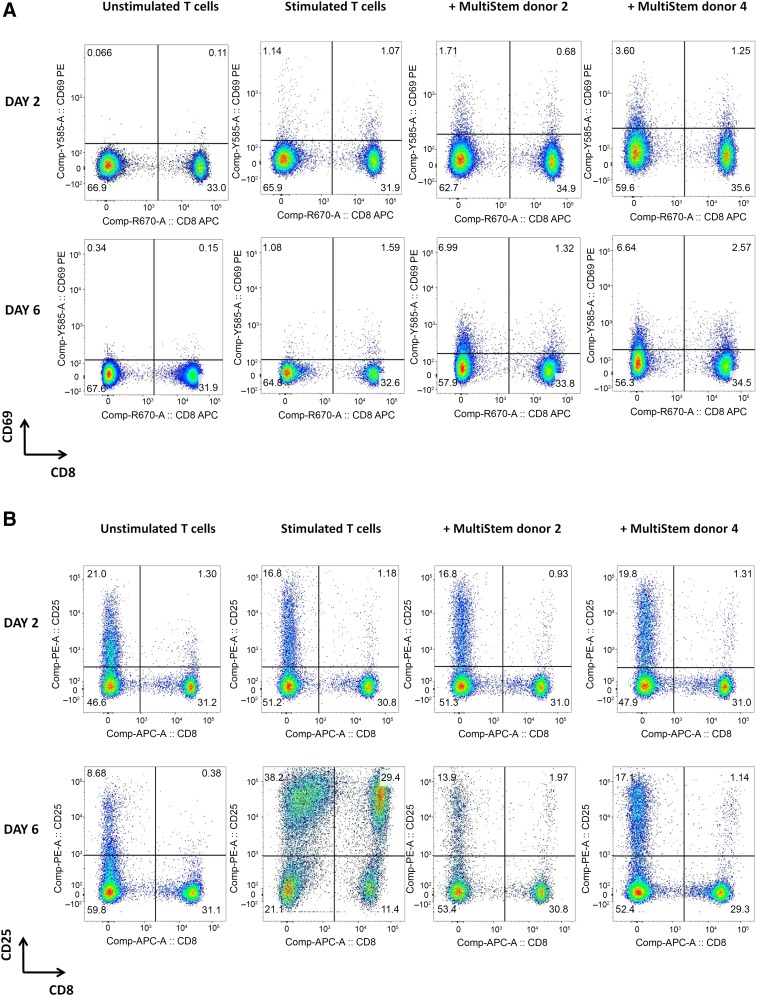
In the next set of experiments, the influence of MultiStem on CD3+ T-cell activation marker expression was examined. After 2 days of stimulation, a slightly increased expression of the early activation marker CD69 on both CD8− and CD8+ fractions was observed, which was even more pronounced on CD8− cells in the presence of MultiStem (percentage of CD8−CD69+ fraction of CD3+ lymphocytes: 1.71% and 3.60% in the presence of, respectively, MultiStem donors 2 and 4 versus 1.14% in the absence of MultiStem; Fig. 6A). The expression level on stimulated T cells further increased after 6 days in the MultiStem-modulated conditions, especially in the CD8− fraction (percentage of CD8−CD69+ fraction of CD3+ lymphocytes: 6.99% and 6.64% versus 1.08%). Furthermore, CD25 (interleukin-2 receptor α [IL-2Rα] chain; Fig. 6B) upregulation on stimulated T cells was reduced after 6 days of coculture with MultiStem, compared with the control condition without MultiStem. This downregulation was most pronounced for CD8+ cells (percentage of CD8−CD25+ fraction of CD3+ lymphocytes: 13.9% and 17.1% versus 38.2%; percentage of CD8+CD25+ cells: 1.97% and 1.14% versus 29.4%). The relative amount of regulatory T cells (Tregs; CD8−CD25hiCD127dim) was lower in the MultiStem-modulated conditions (supplemental online Fig. 2; percentage of CD25hiCD127dim fraction of CD8− lymphocytes: 3.52% and 4.37% versus 27.8%), which was confirmed by a significantly reduced expression of the Treg-specific forkhead box P3 (FoxP3) transcription factor by CD8− T cells (percentage of FoxP3+ fraction of CD8− lymphocytes: 5.54% and 5.15% versus 14.5%). Thirdly, HLA-DR (Fig. 6C) upregulation obviously declined after 6 days in MultiStem-modulated T cells, compared with the control condition (percentage of CD8−HLA-DR+ fraction of CD3+ lymphocytes: 1.53% and 0.89% versus 21.7%; percentage of CD8+HLA-DR+ cells: 0.39% and 0.14% versus 20.6%). Finally, we also analyzed activation marker expression after restimulation of T cells, which were suppressed during a primary MLC by third-party MultiStem. In the absence of MultiStem during the secondary MLC, these restimulated T cells displayed a recovery of CD25 expression and a normalization of CD69 expression (data not shown). In summary, these observations indicate an important effect of MultiStem on the activation and differentiation of T cells, which is lost after MultiStem withdrawal.
Figure 6.
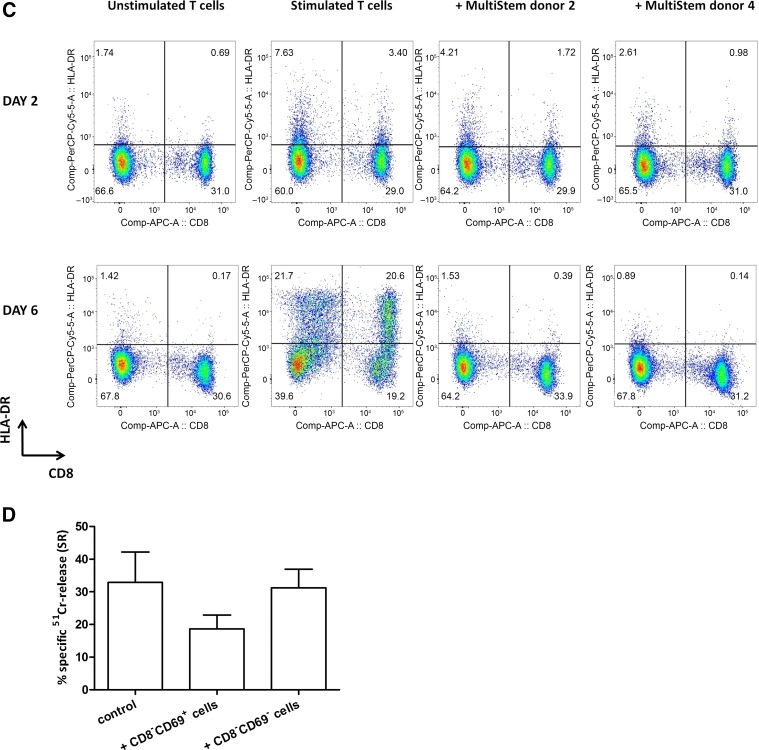
T-cell activation marker expression is altered in the presence of MultiStem. (A–C): Flow-cytometric analysis of CD3+ T lymphocytes for expression of T-cell activation markers CD69 (A), CD25 (B), and HLA-DR (C) on days 2 and 6 of a 6-day stimulation period with irradiated (40 Gy) allogeneic Epstein-Barr virus-positive (EBV+) B cells (stimulator:responder [S:R] ratio of 1:20) in the absence [(un)stimulated T cells] or presence (+MultiStem donor 2/4) of irradiated (30 Gy) third-party MultiStem cells (suppressor:responder ratio of 1:1). Data are presented as percentage of cells within the CD3+ lymphocyte gate. One representative experiment with one T-cell donor and two different MultiStem donors (donors 2 and 4) out of three independent experiments is shown. (D): Freshly isolated CD3+ T cells were primed with irradiated (40 Gy) allogeneic EBV+ B cells (S:R ratio of 1:20) in the presence of irradiated (30 Gy) third-party MultiStem cells (ratio of 1:2). After 6 days of stimulation, two populations were sorted from the coculture (CD45+CD3+CD8−CD69+ T cells and CD45+CD3+CD8−CD69− T cells). These fractions were added at a ratio of 1:2 to a subsequent mixed-lymphocyte culture with freshly isolated responder T cells and irradiated allogeneic EBV+ B cells (S:R ratio of 1:20). After 7 days, anti-CD3-redirected cytotoxicity was analyzed. Data are expressed as mean ± SEM percentage of anti-CD3-dependent specific 51Cr-release (% SR) of P815 target cells (effector:target ratio 10:1). Results are pooled from two independent experiments, in which two different T-cell donors and two MultiStem donors (donors 2 and 3) were used. Abbreviations: APC, allophycocyanin; Comp, compensation; cy5, cyanine 5; HLA-DR, human leukocyte antigen-DR; PE, phycoerythrin; SR, specific 51Cr-release.
The persistent and increasing CD69 expression on activated CD8− T cells in the presence of MultiStem, whereas other activation markers were reduced, was remarkable. Because of the recently discovered immune-regulatory role of CD69 [32, 33], functional tests were performed to analyze the regulatory activity of this CD69-positive population. Standard 51Cr-release assay revealed that the MultiStem-induced CD8−CD69+ T cell population had an inhibitory effect on the induction of CTL activity, in contrast to their CD69-negative counterparts (Fig. 6D). Given the fact that galectin-1, a β-galactoside-binding immune-suppressive protein, has recently been discovered as a ligand for CD69 on DCs [34], the presence of Gal-1 on MultiStem was also studied. Fluorescence microscopy revealed Gal-1 expression (Fig. 7A), and this was confirmed by Western blot analysis and ELISA (data not shown). To elucidate the role of Gal-1 in MultiStem-mediated immune modulation, siRNA transfection was used to decrease its expression level by MultiStem cells (data not shown). Functional immune-suppressive assays showed that Gal-1 could not be identified as a mediator of MultiStem-mediated CD3+ T-cell cytotoxicity suppression (Fig. 7B). These results indicate that MultiStem enriches the CD8−CD69+ T cell population during coculture, which displays immune-suppressive properties.
Figure 7.
MultiStem cells express Gal-1. (A): Fluorescent microscopic analysis of MultiStem (donor 4) for galectin-1 (yellow). Nuclear control staining with 4′,6-diamidino-2-phenylindole (blue) is included. Magnification, ×400. (B): Purified CD3+ T cells were stimulated with irradiated (40 Gy) allogeneic Epstein-Barr virus-transformed B cells (stimulator:responder ratio of 1:20) during 7 days in the absence (control) or presence of irradiated (30 Gy) third-party MultiStem cells at suppressor:responder ratio of 1:1. MultiStem cells were transfected with scrambled small interfering RNA or with siRNA targeted against Gal-1 and were added to the culture at day 4 after transfection. After 7 days, anti-CD3-redirected cytotoxicity was analyzed. Data are expressed as mean ± SEM percentage of anti-CD3-dependent specific 51Cr-release (% SR) of P815 target cells (effector:target ratio of 10:1). Results are pooled from four independent experiments with two different T cell donors and two different MultiStem donors (donors 2 and 4). Statistical significance was calculated with the paired t test. Abbreviations: Gal-1, galectin-1; ns, not significant; siRNA, small interfering RNA; SR, specific 51Cr-release.
Discussion
The results presented in this manuscript provide valuable insights into the immunological effects of clinical-grade hMAPCs (MultiStem) on CD8+ T cells. Given the fact that adult stem cell populations show interesting potential in the context of immune therapy and that cytotoxic immune effector cells play a crucial role in immune homeostasis and in the pathogenesis of some autoimmune diseases, our in vitro data on MultiStem-mediated CTL modulation are highly relevant. A detailed knowledge of the immunogenic and immune-modulatory capacities and the accompanying mechanism of immune regulation of this clinical-grade MAPC-derived stem cell product is indispensable for designing, understanding, and optimizing future clinical trials in immune-related diseases.
We have demonstrated that MultiStem cells are immune-privileged because they do not induce an alloreactive CTL response and are able to escape antigen-specific immune recognition by activated T cells. MultiStem cells alter CD8+ T-cell activation marker expression, decrease alloantigen-induced CD8+ T-cell proliferation, and suppress induction of CTL activity by significantly impairing the perforin expression after T-cell priming. This modulatory effect is dose-dependent and cell contact-dependent, and results in a diminished killing effect during the CTL effector phase. Besides this inhibitory effect on the functional activity of CD8+ T cells, when added to a total T-cell population, MultiStem also exerts an additional indirect suppressive effect through the induction of a CD69-positive population among the CD8− T cells, which displays regulatory properties. When added only during the cytotoxic effector phase, MultiStem cells are still capable of slightly inhibiting the lytic activity of activated T cells.
Our data on the immunogenicity and CTL susceptibility of MultiStem are similar to previously published reports on hMSCs. hMSCs do not induce T-cell proliferation, cytotoxic activity, or proinflammatory cytokine production and induce little to no levels of activation markers [10, 15, 35, 36]. Regarding CTL sensitivity, in most studies, hMSCs were not susceptible to alloantigen-specific T cell-mediated lysis, even despite (upregulation of) MHC class I expression [15, 16, 37]. Based on the in vitro data presented here, we conclude that MultiStem cells escape recognition and lysis by the adaptive (MHC-specific CTLs) similar to the innate (killer cell immunoglobulin-like receptor-mismatched resting NK cells) immune system in vitro. Whether this will be the same for the in vivo setting—which eventually will depend on the in situ inflammatory microenvironment, as we have observed in the case of NK-cell mediated lysis [29]—has to be further explored. Moreover, MultiStem might be processed by antigen-presenting host DCs in an in vivo setting. This indirect manner of alloantigen presentation to T cells should be investigated further.
CTL priming in the presence of MultiStem led to an impaired cytotoxic potential against target cells in vitro. Similar to other hMSC studies, they exerted a dose-dependent inhibitory effect on the differentiation of CTL precursors into CTL effectors during the T-cell priming [16, 35, 37]. This suppressive effect was not simply due to sterical cell hindrance or an increased cell density during the MLC, which was confirmed by Potian et al. in the MSC setting [38]. Moreover, the reduced T-cell cytotoxicity was due to a direct effect of MultiStem on the responder T cells, because the stem cells had no influence on the alloantigen-presenting B cells. In contrast to most previous reports, the addition of stem cells to antigen-primed effector CTLs at the time of cytotoxic reaction also diminished target cell killing. Rasmusson et al. observed no suppressive effect of MSCs added at a later time point during the T-cell priming phase (day 3) or during the effector phase, suggesting that MSCs may predominantly inhibit the development and proliferation of antigen-specific CTLs rather than their killer function [16, 37, 39]. Our results imply that MultiStem reduces the total cytotoxic response of CD8+ T cells, not only by inhibiting clonal expansion, but also by directly and dose-dependently impairing the acquirement of cytotoxic capacity of the cells during the activation and effector phase.
The mechanism of stem cell-mediated immune suppression is not yet completely understood. The mode of action differs, depending on the studied immune cell population and immune response. In our study, no induction of tolerance, anergy, or apoptosis of T cells could be demonstrated, in accordance with previously published MSC studies [9, 10, 40]. The inhibitory effect was reversed after removal of stem cells, as shown in restimulation experiments and as has been described for MSCs in the case of T-cell proliferation suppression [9, 10, 39, 41]. We found that third-party MultiStem addition led to a significant alteration in activation-associated surface marker expression of alloantigen-stimulated CD8+ T cells, involving a significant inhibition of CD25 and HLA-DR upregulation on day 6 of the stimulation phase. These results could indicate a dysregulated T-cell activation in the presence of MultiStem, which might be associated with an impaired acquirement of cytotoxic properties. Besides the crucial effect on the survival and expansion of CD4+ and CD8+ T cell subsets, IL-2 exerts a direct and independent enhancing effect on perforin and granzyme expression by CD8+ T cells [42]. Accordingly, the observed MultiStem-mediated lack of CD25 (IL-2Rα chain) expression on CTLs and a consequently lower amount of high-affinity IL-2 receptors leads to a disturbed autocrine IL-2 signaling cascade and lower perforin expression. The reduced CD25 upregulation on CTLs can also explain the lack of IL-2 effects to overcome the cytotoxicity suppression, whereas the priming of T cells remains unaffected.
Another crucial point is the fact that, when the total CD3+ T-cell population instead of CD8+ cells is used as the responding population, MultiStem cells have an additional influence on the CD8− (or CD4+) fraction. MultiStem also inhibits HLA-DR upregulation and reduces CD25 expression, although to a lower extent, in CD4+ T cells. Le Blanc et al. confirmed decreased expression of CD25 on phytohemagglutinin-stimulated T cells on day 3 in the MSC setting [36]. Conversely, CD69 expression remained upregulated over time in the presence of MultiStem during the T-cell priming. Such remarkable and sustained increase in CD69 expression in hMSC-modulated T lymphocytes was already shown, and, accordingly, it was observed in all CD4+ and CD8+ T-cell subsets, including distinct regulatory subsets [43]. Recently, it has been shown that the previously known early activation marker CD69 also has an important regulatory role in the control of immune and inflammatory responses [32, 33]. Saldanha-Araujo et al. have demonstrated that different nuclear factor-κB signaling pathways can regulate the early or late and sustained expression and their respective function [43]. Several groups have demonstrated that a recently discovered subset of regulatory T cells in tumor-bearing mice [44], healthy controls [45], and patients with hepatocellular carcinoma is CD4+CD25−CD69+ [46]. The exact immune-suppressive mechanism of this population has not yet been revealed, but in the murine setting, these cells suppress T-cell proliferation through membrane-bound transforming growth factor-β [44]. Accordingly, we also found a MultiStem-induced CD8−CD69+ population displaying suppressive properties when added as modulating cells to a subsequent MLC. Based on our findings and on the recent literature, we hypothesize that MultiStem addition would be able to induce a suppressor T-cell population, either by differentiation of CD8−CD69+ T cells toward this regulatory phenotype or by an aberrant T-cell activation in the presence of MultiStem.
Several soluble and/or contact-dependent molecules have been proposed as candidate immunosuppressive mediators in the case of hMSCs. Here, MultiStem-mediated suppression of T-cell cytotoxicity was contact-dependent. Although we reported for the first time on the expression of ligands and receptor of the PD-1 pathway and of FasL on MultiStem cells, we were not able to identify one single responsible contact-dependent mechanism. Sotiropoulou et al. confirmed contact-dependency of hMSCs in the case of NK-cell cytotoxicity suppression, whereas, on the contrary, proliferation and cytokine production suppression were mediated by soluble factors [47]. Krampera et al. observed that, in the murine system, MSCs inhibited T-cell responses only when the cell populations were in close proximity to each other [41]. However, other MSC studies have shown CTL cytotoxicity suppression by MSCs in transwell culture systems or via supernatant of cultured MSCs [16, 35]. Our data show that MultiStem modulates cytotoxic function of T cells mainly through (for the moment not further specified) contact-dependent mechanism(s), while soluble factors (IDO and Gal-1) are also partially involved in proliferation modulation [28, 29]. This suggests that suppressive factor(s) of cytotoxicity are not constitutively secreted by MultiStem, but that a dynamic cross-talk between MultiStem and immune cells is required.
Based on the MultiStem-mediated elevated levels of CD69 in T cells and the presence of its ligand Gal-1 [34] in MultiStem, Gal-1/CD69 binding could be an important mechanism of immune modulation by adult stem cells, independent of cytotoxicity suppression. de la Fuente et al. already proved inhibition of Th17 differentiation and function in mice and humans upon CD69 recruitment [34]. In our study, we demonstrated that Gal-1 signaling did not mediate the inhibitory effect of MultiStem on T-cell cytotoxicity. However, T-cell differentiation experiments in the presence of MultiStem are outside the scope of this study.
The overall rationale of this study was not to optimize the MultiStem production process or to compare the functionality and equivalency of different donor-derived stem cell products. Instead, we aimed to analyze the immune-regulatory effect of a standardized and well-characterized product derived from one specific donor. Keeping this in mind, we performed the individual analyses by consistently using one specific stem cell donor during all experiments. To ascertain that no significant interdonor variability of the stem cell batches was present, we always added one (or more) extra cell donors to the individual analyses. Important to note is that we never observed significant functional differences between the five different MultiStem donors.
Whether the in vitro effects described in this manuscript might be extrapolated to a fully integrated and complex in vivo setting has to be evaluated. Upon administration in vivo, the longevity and functionality of (clinical-grade) hMAPCs will primarily be dictated by the inflammatory status and the cytokine balance in the local microenvironment. This underscores the complexity of the issue and the need for more relevant preclinical animal models.
Conclusion
In summary, we reported for the first time that MultiStem cells exert a direct inhibitory effect on the expansion and functional activity of CD8+ cytotoxic T cells, but they might also have an indirect suppressive effect via CD4+ T cells through the induction of a distinct CD4+CD69+ suppressor population. According to our findings, we can conclude that MultiStem cells have broad immune-suppressive properties, modulating both NK and T cell expansion and—as shown here—also cytotoxic functionality. However, scrutiny is still required, and the immunological mechanism should be further elucidated in vitro and in various animal models to acquire new knowledge about the complex cross-talk between stem cells and the immune system. These results may represent an extensive contribution to the current knowledge and, in combination with the results of future phase II/III trials using MultiStem, will lead to an intriguing continuation of stem cell-based research for immunotherapy.
Supplementary Material
Acknowledgments
We thank Lieve Coorevits, Sven Seys, and Anaïs Van Hoylandt (KU Leuven–University of Leuven) for their excellent technical assistance; Wim Huybrechts (Centre for Human Genetics, University Hospital UZ Leuven) for the production of the Epstein-Barr virus-transformed lymphoblastoid cell lines; the ReGenesys employees for the production and supply of research-grade MultiStem cells; and Thomas Vanwelden (KU Leuven–University of Leuven) for his help with the stem cell cultures. This work was supported by Centre of Excellence Grant EF/05/011 funding, KU Leuven–University of Leuven; the Odysseus Programme of the Flemish Research Foundation (Fonds Wetenschappelijk Onderzoek [FWO], Vlaanderen); European Commission Grant EC-FP6-STREP-STROKEMAP (to C.M.V.); an industrial research and development project grant from the Flemish agency for Innovation by Science and Technology (Agentschap Innoveren & Ondernemen [formerly Instituut voor Innovatie door Wetenschap en Technologie, or IWT], Vlaanderen) (to Athersys, Inc.); and research funding from the Olivia Hendrickx Research Fund (http://www.olivia.be). M.V.W. and V.D.R. were funded by a grant from the IWT. S.W.V.G. and D.M.B. are senior clinical investigators of FWO.
Author Contributions
J. Plessers: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; E.D.: collection and/or assembly of data, data analysis and interpretation; M.V.W.: collection and/or assembly of data, data analysis and interpretation, manuscript revision; V.D.R., J. Pinxteren, and C.M.V.: manuscript revision; D.M.B.: conception and design, data interpretation, manuscript revision; S.W.V.G.: conception and design, data analysis and interpretation, manuscript writing, manuscript revision.
Disclosure of Potential Conflicts of Interest
V.D.R. is an employee of ReGenesys, spouse of an employee of GlaxoSmithKline Biologicals, has uncompensated intellectual property rights, and has stocks and stock options at GlaxoSmithKline Biologicals. J. Pinxteren is a compensated employee for ReGenesys. C.M.V. has uncompensated intellectual property rights with and is a compensated consultant for Athersys/ReGenesys. The other authors indicated no potential conflicts of interest.
References
- 1.Bongso A, Lee EH. Stem cells: Their definition, classification and sources. In: Bongso A, Lee EH, eds. Stem Cells: From Bench to Bedside. Toh Tuck Link, Singapore: World Scientific Publishing, 2005:1–13 [Google Scholar]
- 2.Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 6.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 8.Tolar J, Le Blanc K, Keating A, et al. Concise review: Hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 10.Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 11.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 12.Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 13.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 14.Barry M, Bleackley RC. Cytotoxic T lymphocytes: All roads lead to death. Nat Rev Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson I, Uhlin M, Le Blanc K, et al. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 16.Rasmusson I, Ringdén O, Sundberg B, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 18.Sohni A, Verfaillie CM. Multipotent adult progenitor cells. Best Pract Res Clin Haematol. 2011;24:3–11. doi: 10.1016/j.beha.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Roobrouck VD, Clavel C, Jacobs SA, et al. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29:871–882. doi: 10.1002/stem.633. [DOI] [PubMed] [Google Scholar]
- 20.Reyes M, Dudek A, Jahagirdar B, et al. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boozer S, Lehman N, Lakshmipathy U, et al. Global characterization and genomic stability of human MultiStem, a multipotent adult progenitor cell. J Stem Cells. 2009;4:17–28. [PubMed] [Google Scholar]
- 22.Penn MS, Ellis S, Gandhi S, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: Phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 23.Maziarz RT, Devos T, Bachier CR, et al. Single and multiple dose MultiStem (multipotent adult progenitor cell) therapy prophylaxis of acute graft-versus-host disease in myeloablative allogeneic hematopoietic cell transplantation: A phase 1 trial. Biol Blood Marrow Transplant. 2015;21:720–728. doi: 10.1016/j.bbmt.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Obermajer N, Popp FC, Johnson CL, et al. Rationale and prospects of mesenchymal stem cell therapy for liver transplantation. Curr Opin Organ Transplant. 2014;19:60–64. doi: 10.1097/MOT.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 25.Hess DC, Sila CA, Furlan AJ, et al. A double-blind placebo-controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. Int J Stroke. 2014;9:381–386. doi: 10.1111/ijs.12065. [DOI] [PubMed] [Google Scholar]
- Hess DC. Final results of the B01-02 phase 2 trial testing the safety and efficacy of MultiStem in treatment of ischemic stroke. Presented at: International Stroke Conference; February 16–19, 2016; Los Angeles, CA. [Google Scholar]
- 27.Athersys, Inc. Athersys announces results from phase 2 study of MultiStem®cell therapy for ulcerative colitis. Available at http://www.athersys.com/releasedetail.cfm?ReleaseID=842936; accessed April 28, 2014.
- 28.Jacobs SA, Pinxteren J, Roobrouck VD, et al. Human multipotent adult progenitor cells are nonimmunogenic and exert potent immunomodulatory effects on alloreactive T-cell responses. Cell Transplant. 2013;22:1915–1928. doi: 10.3727/096368912X657369. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs SA, Plessers J, Pinxteren J, et al. Mutual interaction between human multipotent adult progenitor cells and NK cells. Cell Transplant. 2014;23:1099–1110. doi: 10.3727/096368913X665585. [DOI] [PubMed] [Google Scholar]
- 30.Van Gool SW, de Boer M, Ceuppens JL. CD28 ligation by monoclonal antibodies or B7/BB1 provides an accessory signal for the cyclosporin A-resistant generation of cytotoxic T cell activity. J Immunol. 1993;150:3254–3263. [PubMed] [Google Scholar]
- 31.Verschuere T, Van Woensel M, Fieuws S, et al. Altered galectin-1 serum levels in patients diagnosed with high-grade glioma. J Neurooncol. 2013;115:9–17. doi: 10.1007/s11060-013-1201-8. [DOI] [PubMed] [Google Scholar]
- 32.Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 33.González-Amaro R, Cortés JR, Sánchez-Madrid F, et al. Is CD69 an effective brake to control inflammatory diseases? Trends Mol Med. 2013;19:625–632. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Fuente H, Cruz-Adalia A, Martinez Del Hoyo G, et al. The leukocyte activation receptor CD69 controls T cell differentiation through its interaction with galectin-1. Mol Cell Biol. 2014;34:2479–2487. doi: 10.1128/MCB.00348-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angoulvant D, Clerc A, Benchalal S, et al. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 2004;41:469–476. [PubMed] [Google Scholar]
- 36.Le Blanc K, Rasmusson I, Götherström C, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 37.Maccario R, Podestà M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 38.Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: Functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 39.Ramasamy R, Tong CK, Seow HF, et al. The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol. 2008;251:131–136. doi: 10.1016/j.cellimm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 41.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 42.Janas ML, Groves P, Kienzle N, et al. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175:8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- 43.Saldanha-Araujo F, Haddad R, Farias KC, et al. Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: Roles of canonical and non-canonical NF-κB signalling. J Cell Mol Med. 2012;16:1232–1244. doi: 10.1111/j.1582-4934.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han Y, Guo Q, Zhang M, et al. CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 45.Gandhi R, Farez MF, Wang Y, et al. Cutting edge: Human latency-associated peptide+ T cells: a novel regulatory T cell subset. J Immunol. 2010;184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, Feng A, Sun J, et al. Increased CD4(+) CD69(+) CD25(-) T cells in patients with hepatocellular carcinoma are associated with tumor progression. J Gastroenterol Hepatol. 2011;26:1519–1526. doi: 10.1111/j.1440-1746.2011.06765.x. [DOI] [PubMed] [Google Scholar]
- 47.Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.