Abstract
Objective
To test that cerebral atrophy is associated with increased risk for development of chronic subdural haematoma (cSDH), this study performed volumetric analysis of computed tomography (CT) brain scans from patients who were diagnosed with cSDH on subsequent CT scans and their age-matched controls.
Methods
Volumetric analysis was performed on CT scans acquired a mean of 209 days prior to cSDH diagnosis in 19 patients. Cerebral atrophy present on these scans was then compared to 76 age-matched control patients randomly selected from cSDH-free subjects.
Results
There was a higher degree of atrophy in cSDH patients (n =19, 14.3% ± 5.4%) than in age-matched control patients (n =76, 11.9% ± 5.5%; p =0.044). Logistical regression demonstrated that atrophy was found to be a significant predictor of cSDH at all ages (OR =1.11, 95% CI =[1.01, 1.23], p =0.05). For younger subjects ≤65 years of age (n =50), atrophy was an even stronger predictor of cSDH (OR =1.17, 95% CI =[1.02, 1.34], p =0.026).
Conclusions
Cerebral atrophy is associated with the development of cSDH and this association is greater in patients ≤65 years of age.
Keywords: Cerebral atrophy, chronic subdural haematoma, volumetric analysis
Introduction
Chronic subdural haematoma (cSDH) is a common neurosurgical condition, with an incidence of 1–13 per 100 000 people per year [1–3]. One-third of affected patients are older than 80 years of age [4]. The incidence of cSDH has increased by 36% between 1998 and 2007 [4]. Patients with cSDH are at risk for intracerebral haemorrhage [5], seizures [5] and exacerbation of comorbidities associated with the interruption of anticoagulant therapy [6]. Up to 20% of patients have poor neurologic outcomes, resulting in significant disability [4, 5,7–9]. One-year mortality after cSDH is 32% [10] and patient prognosis is worse when there is conversion to acute SDH [3, 4, 9, 11].
Unlike acute subdural haematoma, 29–38% of patients treated surgically for cSDH have no memorable precipitating trauma [11, 12]. The majority of patients with cSDH have sufficiently mild trauma that there is no loss of consciousness [13]. Several potential pre-disposing factors have been identified, including alcohol abuse and anticoagulant or anti-platelet therapy [3, 11, 12, 14].
Patients with extensive cortical atrophy have been anecdotally noted to sustain a higher risk of cSDH. It was hypothesized that cerebral atrophy would be associated with increased risk for cSDH formation. To test this hypothesis, volumetric analysis of computed tomography (CT) brain scans were performed from patients who were diagnosed with cSDH on subsequent CT scans and their age-matched controls using an automated 3D segmentation algorithm [15]. CT images were used due to their lower cost and greater availability in the outpatient setting relative to MRI.
Methods
Scan selection
Institutional Review Board approval was obtained. The institutional network was searched in the ICD and purpose of visit (POV) narrative field for the keywords ‘subdural’ and ‘haemorrhage’ or ICD-9 code 432.1. 457 patients were identified and the visit date from the first recorded POV entry including a diagnosis of a subdural haemorrhage was used as a date of diagnosis.
The search was then narrowed to those patients who had undergone a non-contrast head CT prior to the admission prompted by a cSDH. This reduced the 457 patients to 185 patients.
The control population was all patients who had undergone a single non-contrast head CT for any reason and did not have a subdural haematoma. There were 6757 control patients suitable for analysis. All of the patient scans for inclusion in this study were performed between May 2005 and April 2011.
Randomization
Nineteen unique cSDH patients (including two women) and 76 age-matched (±2 years) controls (four women) were randomly selected for analysis via the following algorithm to assure that the sample chosen was representative of the entire population and that the statistical conclusions are valid:
the identifying numbers of all suitable subjects (i.e. those satisfying the age constraint) were placed in the excel sheet, one per row, and the rows were numbered;
a random number generator function (REF) in excel was used to determine a row number in the list; and
the corresponding ID was marked as selected and removed from the list. The procedure was iterated with age constraints appropriately adjusted until the entire sample of 19 patients and 76 controls was selected. Patients and random controls were individually age-matched (±2 years) at a ratio of four controls per cSDH patient.
The CT scans analysed for the cSDH group were performed a mean of 209 days (range from 12–683 days; median =80 days) prior to the scan resulting in a diagnosis of cSDH.
CT scanning
All patient scans were helical CT obtained on Toshiba Aquilion 16 or Aquilion 64 scanners (Toshiba, Tustin, CA). Data from three different scanners was used for this study. Acquisition parameters were as follows: peak tube voltage 120 kVp, x-ray tube current 300 mA, radiation exposure time 500–1000 ms, number of detectors 16 or 64, field of view 20–25 cm, soft-tissue reconstruction kernel FC64, acquisition matrix size 512 × 512 × 30–50, isotropic in-plane resolution 0.390–0.468 mm and axial-slice thickness 3–5 mm.
Volumetric analysis
Cortical atrophy A was defined as the ratio (expressed in %) of the cerebrospinal fluid (CSF) volume to intracranial space (ICS) volume: A =100 × vol(CSF)/vol(ICS). Segmentation of brain tissue was performed using Bridge Burner, a fully-automated and validated 3D segmentation algorithm [15]. Segmentation of the ICS was based on soft-tissue intensity thresholding, connectivity and constrained growth [15]. The CSF volume was segmented by selecting all ICS voxels with attenuation values within the fluid range, i.e. from −4 Hu to +16 Hu (Figure 1). The ratio of the number of CSF voxels to the number of ICS voxels gives A. A representative image is included as Figure 1.
Figure 1.
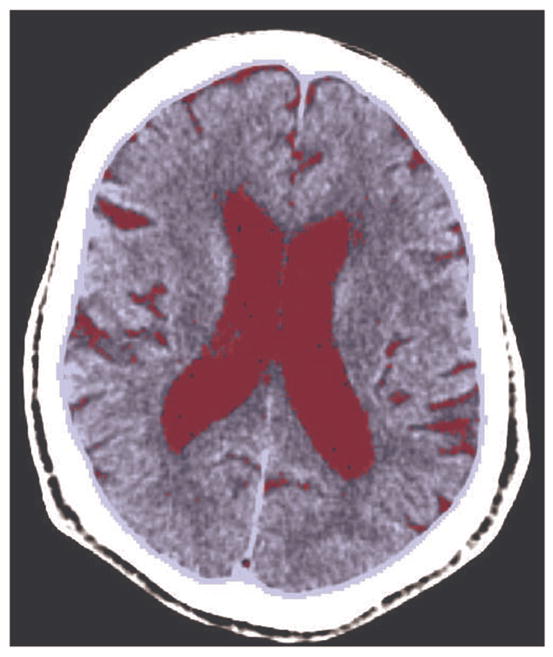
Segmentation results on a representative CT slice: the brain tissue is shown in blue and the CSF containing regions are in red. The intracranial cavity is the sum of CSF and the brain volumes. Segmentation is performed on ~20–30 cerebral slices.
To reduce the processing time while incurring minimal error in A (absolute difference <0.5%), the original image matrix was reduced from (512 × 512 × 30–50) to (256 × 256 × 30–50) by in-plane averaging. User interaction was limited to excluding axial slices below the petrous portion of the temporal bone. This was done to focus the measurements on cerebral atrophy, which included the two lateral and third ventricles and all peripheral (sulcal) atrophy. The atrophy in the cerebellum, 4th ventricle and spinal canal were not measured.
Statistics
Logistic regression was used to calculate the association between A, the continuous predictor variable, on cSDH, the dichotomous outcome variable. After establishing the significance of the logistic model, the odds ratio (OR) of A as a risk factor for cSDH was assessed. Univariate analysis was performed using the F-test, Student t-test, chi-square test and Wald test. All values are expressed as mean ± SD and differences were considered statistically significant if the associated p-value was less than 0.05.
Results
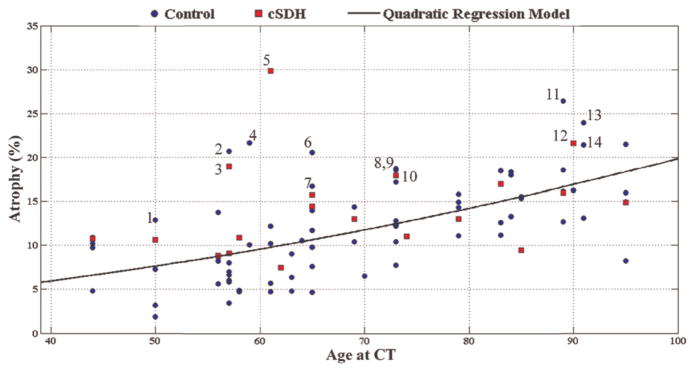
In 76 control patients there was a significant association between age and atrophy (Figure 2). As suggested by the data and by prior studies [16, 17], a quadratic regression model fit this relationship well (Figure 2): A =0.0012x2 + 0.058x + 1.7 (R2=0.372, p <0.0001, F-test). Thus, each year adds ~0.2% additional CSF to the intracranial cavity.
Figure 2.
Percentage cortical atrophy is plotted against age at CT scan in patients who had a chronic subdural haematoma diagnosed on the scan following the one analysed (cSDH group; red square). CT scans of age-matched controls (control group; blue circle) were also analysed. The linear regression model shown was derived from the control group. Fourteen data points with the largest residuals relative to the trend line are marked 1–14 (see Discussion).
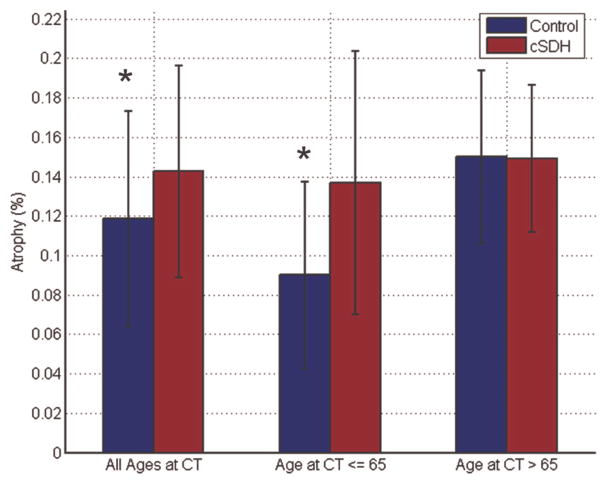
There was a higher degree of atrophy in cSDH patients (n =19, 14.3 ± 5.4%) than in age-matched control patients (n =76, 11.8 ± 5.5%). This difference was statistically significant (p =0.044, Student t-test) (Figures 2 and 3).
Figure 3.
The difference in cerebral atrophy between those who have cSDH and those who do not is significant in patients ≤65 years of age (p =0.01), but not in patients older than 65 years of age.
If the subjects are separated into two groups by age, patients ≤65 (n =50, 10 with cSDH) and patients >65 years old (n =45, nine with cSDH), the difference in cerebral atrophy between those who have cSDH and those who do not is significant in the younger age group, but not in patients older than 65 years (Figure 3). For patients ≤65 years old, controls had 9.0% ± 4.7% atrophy vs 13.7% ± 6.7% in the cSDH group (p =0.007, student t-test).
Logistical regression demonstrated atrophy to be a significant predictor of cSDH (OR =1.11, 95% CI =[1.01, 1.23], p =0.05, Wald test). For younger subjects ≤65 years of age, atrophy was an even stronger predictor of cSDH (OR =1.17, 95% CI =[1.02, 1.34], p =0.026, Wald test).
In examining the data, it was clear that some subjects had atrophy disproportionately greater than the mean for their age-matched controls. The charts of the 14 outliers (marked 1–14 in Figure 2) were reviewed with the most significant increase in atrophy relative to the trend line. Note that six of the seven subjects under the age of 65 with the most atrophy relative to the control trend were alcoholics (Table I).
Table I.
Past medical history of the 14 subjects most severely afflicted by cerebral atrophy relative to age-matched controls.
| Age | Sex | Tobacco | Alcoholic (≤3/day) | Atrophy (%) | cSDH | Past medical history | Indication for CT scan | |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | denies | yes | 12.9 | no | HTN | boiler fell on patient no LOC |
| 2 | 57 | M | active | yes | 20.7 | no | seizure disorder, HTN | tripped; fell in street no LOC |
| 3 | 57 | M | denies | yes | 19.0 | yes | seizure disorder, HTN, DM | seizure |
| 4 | 59 | M | remote | yes | 21.7 | no | idiopathic CVA at age 18; HTN, DM, afib on coumadin, COPD, GERD | syncopal episode |
| 5 | 61 | M | active | yes | 29.8 | yes | severe TBI at age 19, seizures, PTSD, HBV, HTN agent | seizure |
| 6 | 65 | M | denies | no | 20.5 | no | orange exposure, CHF, afib on coumadin HTN, DM, nephropathy, CHF/CAD on plavix | syncopal episode |
| 7 | 65 | M | active | yes | 16.7 | no | severe TBI at age 57; HTN, DM, PVD | impaired gait, multiple falls |
| 8 | 73 | M | active | no | 18.7 | no | latent syphilis, HTN, DM, DVT on coumadin | fell; too weak to stand |
| 9 | 73 | M | remote | no | 18.6 | yes | HTN, DM | syncopal episode |
| 10 | 73 | M | denies | no | 18.0 | yes | CVA at age 71; HTN, DM | decreased consciousness |
| 11 | 89 | F | denies | no | 26.4 | no | Alzheimer’s dementia, osteoporosis, CAD | fall without LOC |
| 12 | 90 | M | active | yes | 21.6 | yes | dementia since age 83; seizures, abdominal aortic aneurysm | fall without LOC |
| 13 | 91 | M | denies | no | 23.9 | no | acute on chronic renal failure, CAD | syncopal episode |
| 14 | 91 | M | denies | no | 21.4 | no | CVA at age 86, afib on Coumadin, CAD, vascular dementia | syncopal episode |
Afib, atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; DVT, deep venous thrombosis; F, female; GERD, gastroesophageal reflux disorder; HTN, hypertension; LOC, loss of consciousness; M, male; s/p, status post; PVD, peripheral vascular disease; TBI, traumatic brain injury.
Discussion
The major finding of this study is that cerebral atrophy is associated with subsequent development of cSDH. This has been described anecdotally but never demonstrated quantitatively.
The finding is consistent with known mechanisms of pathogenesis for cSDH. Unlike acute subdural haematoma, which is nearly always preceded by compelling trauma, 29–38% of patients treated surgically for cSDH have no memorable precipitating trauma [11, 12]. The vast majority have sufficiently mild trauma that there is no loss of consciousness [13], suggesting that afflicted patients may have an intrinsic susceptibility which has been postulated to be increased cerebral atrophy. Cerebral atrophy enables minor stress or trauma to provoke separation of the dura–arachnoid interface, as is also seen with subdural hygroma [18]. Cat and dog models suggest that, once the dura and arachnoid separate, fibrin, from either serum or exudates, can induce proliferation of granulation tissue on the inner dural surface [19]. Trotter [20] originally hypothesized in 1914 that this proliferation of dural border cells results in production of a neomembrane and subsequent growth of new vessels directly within the subdural space. Subsequent studies show that chronic SDH can result from bleeding from these vessels by repeated micro-haemorrhage from the neomembrane [21].
It has also been hypothesized that atrophy leads to tearing of bridging veins between the rigidly fixed dura and mobile arachnoid layer. Microscopy of post-mortem material demonstrates that the sub-dural portion of the bridging veins has thinner vessel walls with less collagen, resulting in greater fragility than the subarachnoid portion [22]. Thus, studies elucidating the pathogenesis of cSDH support the hypothesis that patients with greater cerebral atrophy are more likely to develop subsequent cSDH.
The association between atrophy and cSDH that has been noted contributes to further understanding of why older patients, who have a higher incidence of brain atrophy [16, 17], are at increased risk for cSDH [1]. The Framingham Heart Study demonstrated a strong correlation between alcoholism and cerebral atrophy [23]. The results suggest that cerebral atrophy may be one feature of alcoholism, along with increased falls and platelet dysfunction, that contributes to greater risk for cSDH [3, 11, 12]. In this study, six out of the seven youngest subjects with the most cerebral atrophy relative to age-matched controls were current alcoholics.
Hypoxia [24], smoking [25] and other lung pathologies [26] also pre-dispose to cerebral atrophy. Dementia leads to cerebral atrophy in specific patterns depending on its sub-type and aetiology [27]. Further analyses will need to be performed to establish whether there is a causal relationship between these atrophy-inducing factors and cSDH occurrence. These results do not imply that the relationship between atrophy and cSDH is causative. It may not be that atrophy leads to cSDH, but rather that some other condition associated with increased atrophy leads to cSDH. It is also possible that cSDH causes atrophy.
The major limitations of this study include that it is retrospective and that the test subject population is relatively small and not representative of the general population since it is comprised of veterans who are mostly men and with an average age older than other reported cSDH populations. The age-matched non-cSDH controls are chosen from veteran patients who potentially may have more pathology than population-based healthy controls. cSDH has been shown to have male preponderance [28]; however, females represent approximately one-third of patients in most large series [1, 11, 12]. These subject differences limit the generalizability of the data to non-veteran populations. Healthy controls not seeking a CT scan for any medical indication would be expected to have lower cerebral atrophy, further strengthening the hypothesis.
An additional limitation of this study is that it was restricted only to those cSDH sufferers who had a CT scan during the 5 years prior to the visit on which they were diagnosed with cSDH. The control population was defined as people only having one scan. Having had more than one scan may represent a selection bias because these patients were presumably scanned for a reason such as fall/syncopal episode, loss of consciousness, seizure or change in mental status. Thus, this study may be biased because the control population had fewer reasons to have a scan than the test population. Such a bias may limit the generalizability of these results to only those people who have had multiple scans and thus multiple indications for scans.
Despite the limitations of this retrospective study, it is the first to suggest a relationship between cerebral atrophy and subsequent development of cSDH. In future studies it is planned to define further define the relationship between atrophy and cSDH by algorithmic analysis of a cohort of patients analysed volumetrically and followed through time to determine whether the more atrophic patients do indeed have a higher risk of cSDH. A multivariate logistical regression will be performed to identify independent risk factors vs mere co-occurrences. The authors will perform quantification of the subdural haematoma volume rather than the binary measure of either present or absent. If further studies support this results, one may be able to quantify more accurately an individual patient’s risk for cSDH based on quantitative cerebral atrophy assessment. This may impact clinical decision-making with regards to anti-coagulation or other treatment modalities.
Conclusions
In sum, the results suggest that cerebral atrophy is associated with the development of cSDH and this risk is increased in patients less than 65 years of age.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Fogelholm R, Waltimo O. Epidemiology of chronic subdural haematoma. Acta Neurochirurgica (Wien) 1975;32:247–250. doi: 10.1007/BF01405457. [DOI] [PubMed] [Google Scholar]
- 2.Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: Present status on Awaji Island and epidemiological prospect. Neurologia Medico-Chirurgica (Tokyo) 1992;32:207–209. doi: 10.2176/nmc.32.207. [DOI] [PubMed] [Google Scholar]
- 3.Mellergard P, Wisten O. Operations and re-operations for chronic subdural haematomas during a 25-year period in a well defined population. Acta Neurochiurgica (Wien) 1996;138:708–713. doi: 10.1007/BF01411476. [DOI] [PubMed] [Google Scholar]
- 4.Frontera JA, de los Reyes K, Gordon E, Gowda A, Grilo C, Egorova N, Patel A, Bederson JB. Trend in outcome and financial impact of subdural hemorrhage. Neurocritical Care. 2011;14:260–266. doi: 10.1007/s12028-010-9418-2. [DOI] [PubMed] [Google Scholar]
- 5.De Jesus O, Pacheco H, Negron B. Chronic and subacute subdural hematoma in the adult population. The Puerto Rico experience Puerto Rico Health Sciences Journal. 1998;17:227–233. [PubMed] [Google Scholar]
- 6.Phan TG, Koh M, Wijdicks EF. Safety of discontinuation of anticoagulation in patients with intracranial hemorrhage at high thromboembolic risk. Archives of Neurology. 2000;57:1710–1713. doi: 10.1001/archneur.57.12.1710. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: The role for craniotomy reevaluated. Neurosurgery. 1993;33:67–72. doi: 10.1227/00006123-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Iantosca MR, Simon RH. Chronic subdural hematoma in adult and elderly patients. Neurosurgery Clinics of North America. 2000;11:447–454. [PubMed] [Google Scholar]
- 9.Ramachandran R, Hegde T. Chronic subdural hematomas–causes of morbidity and mortality. Surgical Neurology. 2007;67:367–372. doi: 10.1016/j.surneu.2006.07.022. discussion 372–363. [DOI] [PubMed] [Google Scholar]
- 10.Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: Not a benign disease. Journal of Neurosurgery. 2011;114:72–76. doi: 10.3171/2010.8.JNS10298. [DOI] [PubMed] [Google Scholar]
- 11.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurologia Medico-Chirurgica (Tokyo) 2001;41:371–381. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 12.Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, Martinez-Rumbo R. Chronic subdural haematoma: Surgical treatment and outcome in 1000 cases. Clinical Neurology and Neurosurgery. 2005;107:223–229. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Deci DM. Chronic subdural hematoma presenting as headache and cognitive impairment after minor head trauma. West Virginia Medical Journal. 2004;100:106–107. [PubMed] [Google Scholar]
- 14.Lindvall P, Koskinen LO. Anticoagulants and antiplatelet agents and the risk of development and recurrence of chronic subdural haematomas. Journal of Clinical Neuroscience. 2009;16:1287–1290. doi: 10.1016/j.jocn.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Mikheev A, Nevsky G, Govindan S, Grossman R, Rusinek H. Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. Journal of Magnetic Resonance Imaging. 2008;27:1235–1241. doi: 10.1002/jmri.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiology of Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke. 1995;26:1171–1177. doi: 10.1161/01.str.26.7.1171. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS. The pathogenesis and clinical significance of traumatic subdural hygroma. Brain Injury. 1998;12:595–603. doi: 10.1080/026990598122359. [DOI] [PubMed] [Google Scholar]
- 19.Apfelbaum RI, Guthkelch AN, Shulman K. Experimental production of subdural hematomas. Journal of Neurosurgery. 1974;40:336–346. doi: 10.3171/jns.1974.40.3.0336. [DOI] [PubMed] [Google Scholar]
- 20.Trotter W. Chronic subdural haemorrhage of traumatic origin and its relationship to pachymeningitis haemorrhagica interna. British Journal of Surgery. 1914;2:271–291. [Google Scholar]
- 21.Fujisawa H, Ito H, Saito K, Ikeda K, Nitta H, Yamashita J. Immunohistochemical localization of tissue-type plasminogen activator in the lining wall of chronic subdural hematoma. Surgicl Neurology. 1991;35:441–445. doi: 10.1016/0090-3019(91)90177-b. [DOI] [PubMed] [Google Scholar]
- 22.Yamashima T, Friede RL. Why do bridging veins rupture into the virtual subdural space? Journal of Neurology, Neurosurgery and Psychiatry. 1984;47:121–127. doi: 10.1136/jnnp.47.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, Wolf PA. Association of alcohol consumption with brain volume in the Framingham study. Archives of Neurology. 2008;65:1363–1367. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gale SD, Hopkins RO. Effects of hypoxia on the brain: Neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. Journal of the International Neuropsychological Society. 2004;10:60–71. doi: 10.1017/S1355617704101082. [DOI] [PubMed] [Google Scholar]
- 25.Hayee A, Haque A, Anwarullah AK, Rabbani MG. Smoking enhances age related brain atrophy–a quantitative study with computed tomography. Bangladesh Medical Research Council Bulletin. 2003;29:118–124. [PubMed] [Google Scholar]
- 26.Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of Acute Respiratory Distress Syndrome. Brain Injury. 2006;20:263–271. doi: 10.1080/02699050500488199. [DOI] [PubMed] [Google Scholar]
- 27.Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 28.Kanat A, Kayaci S, Yazar U, Kazdal H, Terzi Y. Chronic subdural hematoma in adults: Why does it occur more often in males than females? Influence of patient’s sexual gender on occurrence. Journal of Neurosurgical Sciences. 2010;54:99–103. [PubMed] [Google Scholar]