Abstract
Echinocandin-resistant clinical isolates of Candida albicans have been reported, and key-hot spot mutations in the FKS1 gene, which encodes a major glucan synthase subunit, have been identified in these (caspofungin-resistant [CAS-R]) strains. Although these mutations result in phenotypic resistance to echinocandins in planktonic cells, there is little data on antifungal susceptibilities of CAS-R C. albicans strains within biofilms. Thus, we analyzed biofilms formed by 12 C. albicans CAS-R clinical strains in which we previously identified FKS1 hot-spot mutations and compared the sessile antifungal and paradoxical activity of anidulafungin (ANID), caspofungin (CAS), and micafungin (MICA). Biofilms were formed in a 96-well static microplate model and assayed using both tetrazolium-salt reduction and crystal violet assays, as well as examination by scanning electron microscopy. We first sought to assess biofilm formation and structure in these fks1 mutants and found that the biofilm mass and metabolic activities were reduced in most of the fks1 mutants as compared with reference strain SC5314. Structural analyses revealed that the fks1 mutant biofilms were generally less dense and had a clear predominance of yeast and pseudohyphae, with unusual “pit”-like cell surface structures. We also noted that sessile minimum inhibitory concentrations (MICs) to ANID, CAS, and MICA were higher than planktonic MICs of all but one strain. The majority of strains demonstrated a paradoxical effect (PE) to particular echinocandins, in either planktonic or sessile forms. Overall, biofilms formed by echinocandin-resistant clinical isolates demonstrated varied PEs to echinocandins and were structurally characterized by a preponderance of yeast, pseudohyphae, and pit-like structures.
Keywords: antifungal therapy, biofilms, Candida albicans
Introduction
The echinocandins anidulafungin (ANID), caspofungin (CAS), and micafungin (MICA) comprise a class of antifungal agents that target the glucan synthase enzyme, which is involved in cell wall synthesis of many medically important fungi. In randomized clinical trials, the echinocandins have demonstrated excellent efficacy against Candida albicans and non-Candida albicans Candida species involved in invasive and disseminated infections [1–4]. Despite excellent overall in vitro activity against Candida biofilms, differences in echinocandin efficacy in in vitro and animal models have been reported, although the clinical significance of these differences remains unclear [5–8]. Furthermore, a paradoxical effect (PE), defined as an attenuation of activity of these antifungals at higher concentrations despite potency at lower levels, has been observed in in vitro and animal models. However, the extent of this effect varies depending on the specific echinocandin, fungal species, and model used in the study [9–13]. It should be noted that there is a paucity of systematic, direct comparative studies that define the differences in echinocandin activity against Candida biofilms. In a prior comparative in vitro investigation from our lab, we saw a marked PE with CAS, a moderate one with ANID, but none with MICA when used against C. albicans reference strain SC5314 [14].
From a resistance standpoint, clinical and laboratory isolates of C. albicans and non-Candida albicans Candida species with high levels of resistance to echinocandins have now been described, and while the number of reported echinocandin-resistant clinical isolates is small, the numbers are increasing [15–20]. Key mutations in the C. albicans FKS1 gene encoding a major subunit of the glucan synthase enzyme in these caspofungin-resistant (CAS-R) mutants have been identified [21,22]. In collaboration with the Fungus Testing Laboratory (Department of Pathology, University of Texas Health Science Center at San Antonio), we obtained a collection of Candida clinical isolates with in vitro CAS-resistance (minimum inhibitory concentration [MIC] > 2 μg/ml), including 12 C. albicans isolates, and identified characteristic mutations in FKS1 using both pyrosequencing and Sanger sequencing in these strains [23].
Although certain mutations in fks1 “hot-spot” regions result in phenotypic resistance to the echinocandins, it is unclear whether the same echinocandin activities and PE occur when the yeast occurs in biofilms. Moreover, little is known regarding the overall characteristics of biofilms produced by these CAS-R fks1 mutant strains. Therefore, we sought to compare the in vitro activity of ANID, CAS, and MICA against C. albicans CAS-R clinical isolates and to characterize the occurrence of the PE of each echinocandin within biofilms formed by these strains. In addition, we sought to define structural characteristics of biofilms formed by these echinocandin-resistant C. albicans strains.
Materials and methods
Strains and antifungal agents used
Starter cultures were routinely grown at 30°C in 1% yeast extract, 2% peptone, 2% glucose broth supplemented with uridine (80 μg/ml). Wild-type C. albicans SC5314 was used as a reference strain [24], while C. albicans ATCC10231, ATCC14053 (fluconazole-resistant), and ATCC24433 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and used as control strains. Twelve C. albicans fks1 mutant clinical isolates were acquired from the University of Texas Health Sciences Center at San Antonio Fungus Testing Laboratory (San Antonio, TX, USA) and used throughout the study. Characterization of these mutants demonstrated reduced CAS susceptibility (MIC > 2 μg/ml) and were found to be CAS-resistant when MICs were determined in the presence of 50% human serum [23]. FKS1 mutations in these strains were determined by both pyrosequencing and Sanger sequencing [23], as indicated in Tables 1 and 2. The echinocandin powders, that is, ANID (Pfizer Inc., New York, NY), CAS (Merck & Co, Inc., Whitehouse Station, NJ), and MICA (Astellas Pharma, Inc., Deerfield, IL, USA) were purchased from the hospital pharmacy and reconstituted in sterile water.
Table 1.
. Comparison of echinocandin activity on planktonic (pMIC80) and sessile (sMIC80) cells of Candida albicans fks1 mutants.
| Anidulafungin (μg/ml) |
Caspofungin (μg/ml) |
Micafungin (μg/ml) |
||||
|---|---|---|---|---|---|---|
| C. albicans strain (mutation) | pMIC80 | sMIC80 | pMIC80 | sMIC80 | pMIC80 | sMIC80 |
| 2762 (F641S) | ≤0.25 | 2 | 2 | 8 | 1 | 8 |
| 32746 (F641S) | 0.5 | 2 | 2 | ≥128 | 1 | >128 |
| 4254 (F641S) | ≤0.25 | ≥128 | 2 | ≥128 | 1 | ≥128 |
| 4715 (F641S) | ≤0.25 | 2 | 2 | 8 | 1 | 4 |
| 41509 (F641S) | 0.5 | 4 | 64 | 8 | 2 | 8 |
| 42996 (F641S) | ≤0.25 | 4 | 2 | 4 | 1 | 8 |
| 43001 (F641S) | ≤0.25 | 2 | 2 | 8 | 1 | 8 |
| 41301 (F641S) | ≤0.25 | ≥128 | 2 | ≥128 | 1 | ≥128 |
| 42379 (S645P) | 0.5 | 4 | 4 | ≥128 | 2 | 16 |
| 53264 (S645P) | 0.5 | 4 | 8 | 16 | 2 | 16 |
| 42286 (S645P/S) | ≤0.25 | 4 | 2 | 4 | 1 | 8 |
| 5415 (S645P/H) | ≤0.25 | 2 | 2 | ≥128 | 1 | 8 |
| SC5314 (WT) | ≤0.25 | ≤0.25 | 1 | 2 | ≤0.25 | ≤0.25 |
The MIC80 represents the lowest echinocandin concentration that inhibited 80% of the metabolic activity of the cells.
Abbreviations: MIC, minimum inhibitory concentration; WT, wild-type FKS1.
Table 2.
. Comparison of paradoxical effect concentrations of planktonic and sessile cells of Candida albicans fks1 mutants.
| Anidulafungin (μg/ml) |
Caspofungin (μg/ml) |
Micafungin (μg/ml) |
||||
|---|---|---|---|---|---|---|
| C. albicans strain (mutation) | Planktonic | Biofilm | Planktonic | Biofilm | Planktonic | Biofilm |
| 2762 (F641S) | ≥128 | ≥128 | — | 64 | — | 32 |
| 32746 (F641S) | ≥128 | — | — | — | — | — |
| 4254 (F641S) | 64 | — | — | — | ≥ 128 | — |
| 4715 (F641S) | ≥128 | — | — | 64 | ≥ 128 | — |
| 41509 (F641S) | — | — | — | ≥128 | ≥128 | ≥128 |
| 42996 (F641S) | — | — | — | 64 | — | — |
| 43001 (F641S) | — | — | — | 64 | — | — |
| 41301 (F641S) | — | — | — | — | — | — |
| 42379 (S645P) | ≥128 | — | — | — | — | — |
| 53264 (S645P) | — | — | — | 64 | ≥128 | — |
| 42286 (S645P/S) | ≥128 | 64 | — | 64 | ≥128 | 64 |
| 5415 (S645P/H) | — | 32 | — | — | — | — |
| SC5314 (WT) | ≥128 | — | — | — | ≥128 | — |
The paradoxical effect concentration was defined as the highest concentration at which the metabolic activity of the cells was observed to increase at two dilutions above the MIC80. A dash denotes that no paradoxical effect was observed.
Abbreviations: MIC, minimum inhibitory concentration; WT, wild-type FKS1.
Assessment of in vitro growth and filamentation
in vitro growth rates were assayed in liquid media by first diluting cells from overnight cultures to an optical density of 0.05, read at a wavelength of 600 nm (OD600nm), in complete synthetic media supplemented with uridine (80 μg/ml). The cells were then grown at 30°C using a Synergy H1m (BioTek Instruments, Inc., Winooski, VT, USA) with double orbital shaking at fast speed and 2 mm frequency, with OD600 nm readings taken at 15-min intervals. Filamentation was assessed on solid RPMI-1640 media with L-glutamine and buffered with 0.165M 4-morpholinepropanesulfonic acid at pH 7.0. Overnight cultures were washed with 1× phosphate-buffered saline (PBS) and portions spotted to agar plates that were then incubated at 37°C for 5 d. Colonies were visualized, and the degree of filamentation was assessed based on presence/absence and density of hyphal structures.
Determination of planktonic MICs using XTT
We determined planktonic MICs using the Clinical and Laboratory Standards Institute M27-A3 broth microdilution method [25], incubating at 35°C for 24 h. However, rather than reading turbidity, we determined the MICs using the XTT-reduction assay [26], where the reduction of the tetrazolium salt, (2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide) (XTT), is followed spectrophotometrically. The effects of the antifungal agents were then expressed as the percent of metabolic activity relative to the metabolic activity of the untreated planktonic cells.
Biofilm formation and XTT assay
For biofilm studies, cells were washed twice in 1× PBS and resuspended at a density of 1 × 106 cells/ml in RPMI-1640 supplemented with L-glutamine (US Biological, Swampscott, MA, USA) and buffered to pH 7.0 with 0.165M MOPS (Sigma, St. Louis, MO, USA; buffered RPMI-1640). The methods used for C. albicans biofilm formation in polystyrene 96-well microtiter plates and the XTT-reduction assay to determine the metabolic activities of the biofilms were performed as previously described [26]. Serial, twofold dilutions indexed to base 2 of the echinocandin (from 0.25 to 128 μg/ml) were added to preformed (24 h) biofilms formed in buffered RPMI-1640 and incubated at 37°C for 24 h. Each experiment was independently performed three times, in quadruplicate. Drug-free biofilm wells containing only buffered RPMI-1640 were used as untreated controls. The production of formazan was measured spectrophotometrically at an optical density of 490 nm on a microplate reader (BioTek ELx808; BioTek Instruments, Inc., Winooski, VT, USA). The effects of the antifungal agents were then expressed as the percent of metabolic activity relative to the metabolic activity of the untreated biofilms (controls).
Crystal violet assay
Biofilm mass was quantified using the crystal violet assay as previously described [27]. Biofilms were grown in triplicate at a concentration of 1 × 106 cells/ml in buffered RPMI-1640 at 37°C for 24 h. Biofilms and controls (SC5314 and empty wells) were washed three times with 200 μl of PBS. Next, 50 μl of crystal violet solution (0.6 g crystal violet, 10 ml isopropanol, 10 ml methanol, and 180 ml water) was added to each well and allowed to stand at room temperature for 5 min, after which any excess crystal violet dye was removed by washing the biofilms three times with 200 μl of water. Then 100 μl of ethanol was added to each well and mixed thoroughly by gently pipetting up and down until the crystal violet solution was uniform in color; 75 μl of the ethanol/crystal violet solution was then transferred to a new 96-well microtiter plate and the light absorbance of the crystal violet solution read at 630 nm.
Light and scanning electron microscopy
Light micrographs of the biofilms formed in the presence of each test reagent were acquired using an inverted microscope (Micromaster Digital Inverted Microscope with Infinity Optics; Fisher Scientific, Pittsburgh, PA, USA) and data acquisition software (Micron 2.0.0; Westover Scientific, Mill Creek, WA, USA). Sample preparation for scanning electron microscopy (SEM) was performed on biofilm samples formed on a coverslip (Thermanox; Nalge Nunc International, Rochester, NY, USA) after 24 h incubation of a 0.5-ml inoculum containing 1 × 106 cells/ml in buffered RPMI-1640 medium as previously described [28]. Scanning electron micrographs were obtained using a scanning electron microscope (Hitachi S-800; Hitachi High Technologies America, Inc., Pleasanton, CA, USA) set at 20 kV with images acquired using Quartz PCI software (Quartz Imaging Corporation, Vancouver, BC, Canada).
Definitions
Antifungal activity was defined as a statistically significant reduction in the metabolic activity of C. albicans planktonic cells or biofilms treated with an echinocandin compared with the metabolic activity of the untreated cells. MIC80 represents the lowest echinocandin concentration that inhibited 80% of metabolic activity of either planktonic cells (pMIC80) or biofilms (sMIC80). A PE was defined as regrowth at two dilutions above the MIC80 [29]. For strains exhibiting a PE, the concentration at which the metabolic activity was the greatest was termed the PE concentration (PEC).
Statistical analyses
The metabolic activities of the treatment groups were compared to the controls using one-way analysis of variance and Tukey multiple comparisons post-test. Differences were considered significant at P < 0.05. The relationship between the crystal violet and XTT assay values was calculated using the Pearson product correlation. Statistical analyses were performed, as well as graphs produced, with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) and Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Results
Analyses of biofilm mass and metabolic activity
Biofilm mass and metabolic activity were assessed and categorized as follows: poor (≤25% mass/metabolic activity relative to SC5314), moderate (between 25% and 75% mass/metabolic activity relative to SC5314), and strong (≥75% mass/metabolic activity relative to SC5314). There were variable biofilm masses, as measured by crystal violet assay, among the fks1 mutant strains in comparison with SC5314 (Fig. 1). Two fks1 mutant strains (32746 and 5415) were categorized as poor biofilm producers, four (2762, 4254, 4715, and 42286) and the three ATCC reference strains were moderate biofilm producers, and six fks1 mutant strains (41509, 42379, 42996, 43001, 53264, and 41301) were categorized as strong biofilm producers. In addition to biofilm mass, the metabolic activities within the biofilms were ascertained using the XTT assay (Fig. 2), which indicated that, with the exception of strains 53264 and 41301, all of the fks1 strains had poor to moderate metabolic activity compared with wild-type SC5314. Two of the ATCC reference strains had strong metabolic activity similar to that found in SC5314, but ATCC 10231 had little metabolic activity even though its biofilm mass was comparable to that of the other ATCC strains. Overall correlation between the XTT-reduction assay and crystal violet assay was positive and highly statistically significant (correlation coefficient 0.47; P < 0.0001). However, it should be noted that results of the crystal violet and XTT assays from several of the individual strains were discordant in terms of biofilm mass and biofilm activity. We were unable to identify a clear association between FKS1 hot-spot mutation in these strains and degree of biofilm formation.
Figure 1.
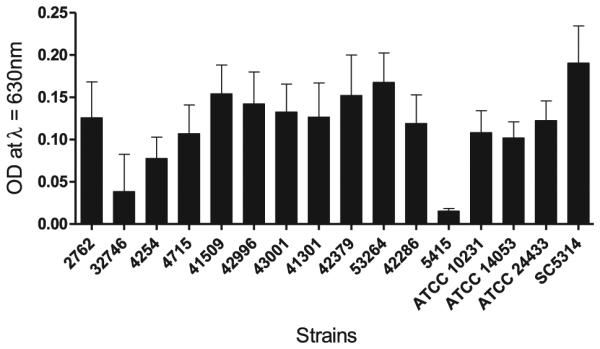
Assessment of biofilm mass of Candida albicans reference strains and fks1 clinical isolates. Biofilms were grown in triplicate at a concentration of 1 × 106 cells/ml in buffered RPMI-1640 at 37°C for 24 h. Biofilm mass was quantified using the crystal violet assay. Light absorbance was measured in a plate reader at OD 630 nm. Each experiment was performed independently three times.
Figure 2.
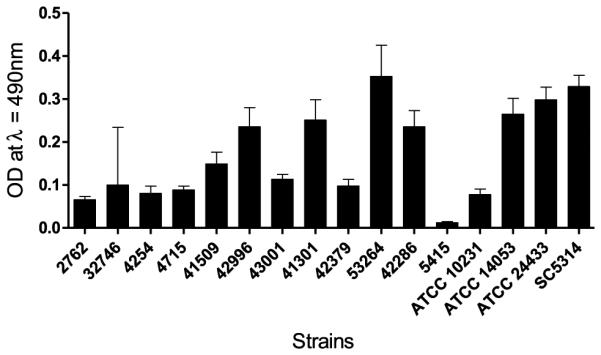
Assessment of biofilm metabolic activity of Candida albicans reference strains and fks1 clinical isolates. Biofilms were grown in quadruplicate at a concentration of 1 × 106 cells/ml in buffered RPMI-1640 at 37° C for 24 h. The XTT assay was used to assay sessile metabolic activity. Formation of the colored formazan was subsequently measured at OD 490 nm. Each experiment was performed independently three times.
Assessment of in vitro growth and filamentation
We assessed both in vitro growth and filamentation capacity of the strains, but found there was no correlation between in vitro growth rates (doubling times) and the degree of biofilm formation in these strains (data not shown). The degree of filamentation of each strain was also assayed on solid RPMI-1640 agar. There was no apparent correlation in the degree of filamentation and the mutations in FKS1 or in the degree of biofilm formation (data not shown).
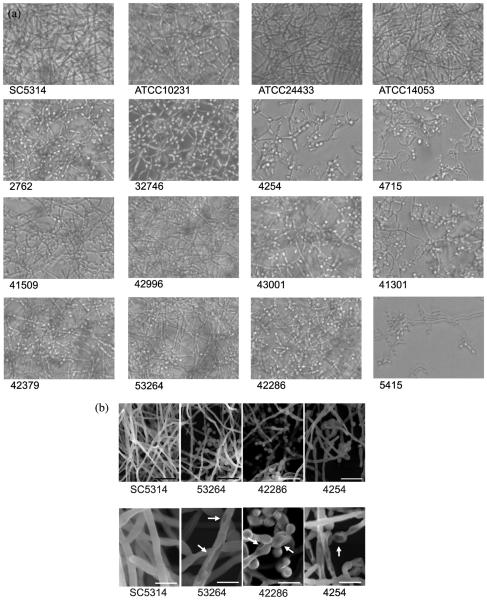
Structural analyses using light and scanning electron microscopy
Direct visualization of SC5314 biofilm morphology revealed a dense, filamentous growth with minimal yeast forms present (Fig. 3a). Similar filamentous growth was observed for the strong biofilm-producing fks1 mutant strains 42379, 42996, and 53264, but there were more yeast forms observed in the preformed (24 h) biofilms in comparison with SC5314 (Fig. 3a). The fks1 mutant strains that produced poor to moderate biofilm growth demonstrated less dense filamentous growth with many yeast forms present (Fig. 3a).
Figure 3.
Ultrastructural assessment of biofilm morphology using scanning electron microscopy. (A) Scanning electron microscopy of representative Candida albicans fks1 mutants that form poor (4254), moderate (42286), and strong (53264) biofilms compared with reference strain SC5314. (B) Scanning electron microscopy view of pit-like cell surface structures identified on select C. albicans fks1 mutants.
In order to assess biofilm ultrastructure in detail, SEM studies were conducted with C. albicans strains SC5314 and a representative fks1 mutant with poor (4254), moderate (42286), and strong (53264) biofilm growth (Fig. 3b). SC5314 formed a dense, filamentous biofilm with minimal yeast forms present in the mature biofilm. While the fks1 mutant produced a strong biofilm (53264) with filamentous growth, there was a marked increase of yeast forms present that was more clearly seen using SEM. The other fks1 mutants similarly produced biofilms with a predominance of yeast forms and pseudohyphae. Interestingly, we observed pits on the cell surface of fks1 mutants 42286 and 4254, both of which formed less robust biofilms (Fig. 3b). These pit-like structures were consistent throughout the biofilms formed by these fks1 mutants, suggesting a structural defect in the cell surface of these strains, although the specific origin of these defects is not clear. Strain 53264 also had some pit-like structures, but the structures appeared less frequently than in the other two fks1 mutant strains (Fig. 3b).
Effect of echinocandins on biofilm metabolic activity
The effect of echinocandins on preformed C. albicans fks1 biofilms was assayed using the XTT-reduction assay. Notably, among all C. albicans fks1 strains, ANID demonstrated the greatest antifungal activity against preformed biofilms with the lowest sMIC80. In 11 of 12 C. albicans fks1 strains (Table 1), sMICs of C. albicans biofilms to ANID, MICA, and CAS were at least two- to three-fold higher than the MICs of their planktonic counterpart (pMIC). In some strains, there was a dramatic increase of sMIC compared with pMIC for CAS; in contrast, in strain 41509, the pMIC was higher than in the sMIC to CAS (Table 1).
Characterization of the PE
A PE was observed within most fks1 biofilms at higher echinocandin concentrations (Table 2). CAS was associated with a PE in 7 of the 12 (58.3%) fks1 strains in our in vitro biofilm assay, with the PE occurring at concentrations of 64 μg/ml or higher. ANID produced a PE in 3 of 12 (25.0%) fks1 strains, and MICA produced a PE in 3 of 12 (25.0%) fks1 strains (Table 2). A PE also occurred in a number of strains in the planktonic form, depending on the echinocandin used (Table 2). Although some strains had a PE in planktonic form, this did not necessarily correlate with a PE in biofilm form. Thus, the planktonic PE is not predictive of a biofilm PE.
Discussion
In this study, we demonstrated in vitro differences in echinocandin activity against biofilms formed by mutant clinical isolates of C. albicans fks1, but without any clear correlation with the type of fks1 hot-spot mutation carried by each strain. Overall, ANID was found to have the lowest sMIC80 within biofilms of the three echinocandins tested. In vivo differences in echinocandin efficacy have recently been described in C. glabrata, which could be correlated with specific hot-spot mutations [5]. In this mouse model of disseminated C. glabrata infection, MICA had wild-type activity against a C. glabrata Fks2p-S663F mutant strain, in contrast to ANID and CAS. Thus, there appears to be increasing evidence of echinocandin-, species-, strain-, and mutation-specific differences in antifungal activity.
Although the echinocandins as a class retain excellent in vitro activity against Candida biofilms [30–32], paradoxical activity within biofilms and of the planktonic cells of Candida species has been described [6]. In a series of 60 Candida bloodstream isolates grown in planktonic form, the prevalence of paradoxical growth in the presence of CAS, ANID, and MICA was 60%, 40%, and 0%, respectively [10]. Of 127 C. dubliniensis and 103 C. albicans isolates, a PE was observed in 63% of C. dubliniensis strains with MICA; in 90% of C. dubliniensis, and in 14% of C. albicans isolates with CAS. Note that no PE was reported with ANID [11]. Within biofilms, Melo et al. described a PE in 30 clinical Candida spp. isolates in an in vitro biofilm model with CAS [13]. When compared with these strains grown in planktonic form, the PE was overall seen more frequently within biofilms, although the presence of a PE within individual strains in planktonic form did not correlate with occurrence of a PE when grown as a biofilm. In our in vitro studies, we also saw a PE in both planktonic and sessile forms of the strains tested. As with these previously published studies, there was no direct correlation between the PE within planktonic or sessile forms or the echinocandin used. Specifically, the PE profile observed in the planktonic form is not predictive of a PE in the biofilm form. In a neutropenic mouse model of invasive pulmonary aspergillosis, a modest PE (increased fungal burden) occurred with CAS but not MICA [12]. However, in a murine model of disseminated candidiasis, C. albicans strains that demonstrated an in vitro PE of CAS did not display a consistent PE in vivo [33]. It should be noted that the PE can be eliminated in the presence of 50% serum in vitro [34,35], and thus the findings of this study must be interpreted cautiously from a clinical standpoint.
There are limited data on the mechanisms responsible for the PE. Potential mechanisms responsible for this phenomenon include an increase in cell wall chitin content [36], upregulation of the protein kinase C cell wall integrity pathway, and involvement of the calcineurin pathway [37]. Interestingly, brief exposures to caspofungin resulted in killing of C. albicans in vitro due to a prolonged antifungal effect but did not result in a PE, suggesting a compensatory response occurs with prolonged exposure to drug [34]. Of note, the PE has not been associated with mutations in known resistance-related regions or with increased expression of FKS1 in the yeast form [38]. In the biofilm-related experiments presented here, a PE occurred in the presence of the echinocandins with no clear association with the type of FKS1 hot-spot mutation. However, it should be noted that these clinical isolates are not fully characterized and other, as yet unknown, genetic factors may contribute to the resulting PE profile.
There is a paucity of data regarding the ability of echinocandin-resistant strains to form biofilms. Given the importance of glucan synthase in cell wall biogenesis and maintenance, we postulated that these fks1 clinical isolates may be less able to form biofilms. Overall, the fks1 mutants tended to form biofilms of reduced metabolic activity and density than the robust biofilm-forming reference strain SC5314. These results were less pronounced when compared with additional ATCC clinical isolates, as they also formed less dense biofilms. In the strains we examined, there was no clear association between the type of hot-spot mutation and the degree of biofilm formation. It should be noted that these strains are not of the same genetic background, and thus a direct comparison between strains is not formally possible. Differences in each strain’s biofilm-forming capacity is likely due to multiple factors and not simply differences in FKS1 activity. While many of these strains have the same FKS1 hot-spot mutation, they have differing biofilm-forming phenotypes.
In contrast, from a structural standpoint, the fks1 mutant biofilms clearly had a much greater proportion of yeast and pseudohyphal structures than those of the reference C. albicans strain SC5314, which was characterized by biofilms composed of dense hyphae. Of further interest, at least two of the three selected fks1 mutants studied with SEM demonstrated pit-like structures on their cell surfaces, suggestive of a substantial cell surface defect. The third fks1 mutant strain also had some surface pits, but these pits were less consistent than the mutants that formed biofilms of reduced mass. While the significance of these pits is unknown, they do not appear to be consistent with bud scars, and it is unlikely that they represent artifacts since they were observed throughout the biofilms of the fks1 mutants and not in wild-type control strain SC5314. These pits bear a resemblance to the cell surface defects characterized by SEM in wild-type C. albicans biofilms treated with CAS, which inhibit glucan synthase [39,40].
In summary, we compared the in vitro activity of ANID, CAS, and MICA against biofilms formed by a set of clinically derived fks1 mutant strains in order to characterize antifungal and paradoxical activity of each echinocandin. We identified the occurrence of a strain- and drug-dependent PE in these strains, but there was no correlation between the PE observed in the planktonic form of the fks1 mutant strains and the PE observed in their corresponding biofilm. The fks1 mutant strains demonstrated the lowest sMICs to ANID. We also demonstrated that biofilm growth was altered among the fks1 mutants, with a predominance of yeast and pseudohyphae and of pit-like structures on the cell surface of several representative strains.
Acknowledgments
We thank William Fonzi (Georgetown University) for providing strain SC5314 and Stephen Jett (University of New Mexico) for his assistance with the SEM at the University of New Mexico Health Sciences Center Electron Microscopy Facility.
Funding
This work was supported, in part, by grants from the Society of Infectious Diseases Pharmacists (C.J.W.), the Department of Veterans Affairs (S.A.L.), and the Biomedical Research Institute of New Mexico (S.A.L.).
Footnotes
Declaration of interest
N.P.W. has received research support from Pfizer, Schering-Plough, Merck, Basilea, and Astellas, and has received travel support from Viamet. He has also served as a consultant for Merck, Astellas, and Viamet.
References
- 1.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020–2029. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 3.Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472–2482. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva A, Gotuzzo E, Arathoon EG, et al. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med. 2002;113:294–299. doi: 10.1016/s0002-9343(02)01191-9. [DOI] [PubMed] [Google Scholar]
- 5.Arendrup MC, Perlin DS, Jensen RH, et al. Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FKS resistance mutations. Antimicrob Agents Chemother. 2012;56:2435–2442. doi: 10.1128/AAC.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiederhold NP. Attenuation of echinocandin activity at elevated concentrations: A review of the paradoxical effect. Curr Opin Infect Dis. 2007;20:574–578. doi: 10.1097/QCO.0b013e3282f1be7f. [DOI] [PubMed] [Google Scholar]
- 7.Zaas AK. Echinocandins: A wealth of choice—how clinically different are they? Curr Opin Infect Dis. 2008;21:426–432. doi: 10.1097/QCO.0b013e328307c79c. [DOI] [PubMed] [Google Scholar]
- 8.Spreghini E, Orlando F, Tavanti A, et al. in vitro and in vivo effects of echinocandins against Candida parapsilosis sensu stricto, Candida orthopsilosis and Candida metapsilosis. J Antimicrob Chemother. 2012;67:2195–2202. doi: 10.1093/jac/dks180. [DOI] [PubMed] [Google Scholar]
- 9.Cateau E, Rodier MH, Imbert C. in vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J Antimicrob Chemother. 2008;62:153–155. doi: 10.1093/jac/dkn160. [DOI] [PubMed] [Google Scholar]
- 10.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. Paradoxical effect of echinocandins across Candida species in vitro: Evidence for echinocandin-specific and Candida species-related differences. Antimicrob Agents Chemother. 2007;51:2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischhacker M, Radecke C, Schulz B, Ruhnke M. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur J Clin Microbiol Infect Dis. 2008;27:127–131. doi: 10.1007/s10096-007-0411-4. [DOI] [PubMed] [Google Scholar]
- 12.Lewis RE, Albert ND, Kontoyiannis DP. Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary aspergillosis. J Antimicrob Chemother. 2008;61:1140–1144. doi: 10.1093/jac/dkn069. [DOI] [PubMed] [Google Scholar]
- 13.Melo AS, Colombo AL, Arthington-Skaggs BA. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob Agents Chemother. 2007;51:3081–3088. doi: 10.1128/AAC.00676-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miceli MH, Bernardo SM, Lee SA. in vitro analysis of the occurrence of a paradoxical effect with different echinocandins and Candida albicans biofilms. Int J Antimicrob Agents. 2009;34:500–502. doi: 10.1016/j.ijantimicag.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez S, Lopez-Ribot JL, Najvar LK, et al. Caspofungin resistance in Candida albicans: Correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother. 2004;48:1382–1383. doi: 10.1128/AAC.48.4.1382-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katiyar S, Pfaller M, Edlind T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2006;50:2892–2894. doi: 10.1128/AAC.00349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CD, Lomaestro BW, Park S, Perlin DS. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26:877–880. doi: 10.1592/phco.26.6.877. [DOI] [PubMed] [Google Scholar]
- 18.Moudgal V, Little T, Boikov D, Vazquez JA. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother. 2005;49:767–769. doi: 10.1128/AAC.49.2.767-769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller MA, Boyken L, Hollis RJ, et al. Global surveillance of in vitro activity of micafungin against Candida: A comparison with caspofungin by CLSI-recommended methods. J Clin Microbiol. 2006;44:3533–3538. doi: 10.1128/JCM.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. Candida bloodstream infections: Comparison of species distribution and resistance to echinocandin and azole antifungal agents in intensive care unit (ICU) and non-ICU settings in the sentry antimicrobial surveillance program (2008–2009) Int J Antimicrob Agents. 2011;38:65–69. doi: 10.1016/j.ijantimicag.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother. 2006;50:2058–2063. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49:3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiederhold NP, Grabinski JL, Garcia-Effron G, Perlin DS, Lee SA. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob Agents Chemother. 2008;52:4145–4148. doi: 10.1128/AAC.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 25.Reference Methods for Broth Dilution Antifungal Susceptibility Testing of Yeasts. third. PAClinical and Laboratory Standards Institute; Wayne: 2008. approved standard. CLSI document M27-A3. [Google Scholar]
- 26.Ramage G, Lopez-Ribot JL. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol Med. 2005;118:71–79. doi: 10.1385/1-59259-943-5:071. [DOI] [PubMed] [Google Scholar]
- 27.Bernardo SM, Lee SA. Candida albicans SUR7 contributes to secretion, biofilm formation, and macrophage killing. BMC Microbiol. 2010;10:133. doi: 10.1186/1471-2180-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorumsensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bizerra FC, Melo AS, Katchburian E, et al. Changes in cell wall synthesis and ultrastructure during paradoxical growth effect of caspofungin on four different Candida species. Antimicrob Agents Chemother. 2011;55:302–310. doi: 10.1128/AAC.00633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: An update. Eukaryot Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemons KV, Espiritu M, Parmar R, Stevens DA. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob Agents Chemother. 2006;50:1293–1297. doi: 10.1128/AAC.50.4.1293-1297.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shields RK, Nguyen MH, Du C, et al. Paradoxical effect of caspofungin against Candida bloodstream isolates is mediated by multiple pathways but eliminated in human serum. Antimicrob Agents Chemother. 2011;55:2641–2647. doi: 10.1128/AAC.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szilagyi J, Foldi R, Bayegan S, Kardos G, Majoros L. Effect of nikkomycin Z and 50% human serum on the killing activity of high-concentration caspofungin against Candida species using time-kill methodology. J Chemother. 2012;24:18–25. doi: 10.1179/1120009X12Z.0000000005. [DOI] [PubMed] [Google Scholar]
- 36.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother. 2006;50:3160–3161. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiederhold NP, Kontoyiannis DP, Prince RA, Lewis RE. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: Possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother. 2005;49:5146–5148. doi: 10.1128/AAC.49.12.5146-5148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens DA, White TC, Perlin DS, Selitrennikoff CP. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn Microbiol Infect Dis. 2005;51:173–178. doi: 10.1016/j.diagmicrobio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann SP, VandeWalle K, Ramage G, et al. in vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother. 2002;46:3591–3596. doi: 10.1128/AAC.46.11.3591-3596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira JA, Carr JH, Starling CE, de Resende MA, Donlan RM. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob Agents Chemother. 2009;53:4377–4384. doi: 10.1128/AAC.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]