Abstract
BACKGROUND
Thymectomy has been a mainstay in the treatment of myasthenia gravis, but there is no conclusive evidence of its benefit. We conducted a multicenter, randomized trial comparing thymectomy plus prednisone with prednisone alone.
METHODS
We compared extended transsternal thymectomy plus alternate-day prednisone with alternate-day prednisone alone. Patients 18 to 65 years of age who had generalized nonthymomatous myasthenia gravis with a disease duration of less than 5 years were included if they had Myasthenia Gravis Foundation of America clinical class II to IV disease (on a scale from I to V, with higher classes indicating more severe disease) and elevated circulating concentrations of acetylcholine-receptor antibody. The primary outcomes were the time-weighted average Quantitative Myasthenia Gravis score (on a scale from 0 to 39, with higher scores indicating more severe disease) over a 3-year period, as assessed by means of blinded rating, and the time-weighted average required dose of prednisone over a 3-year period.
RESULTS
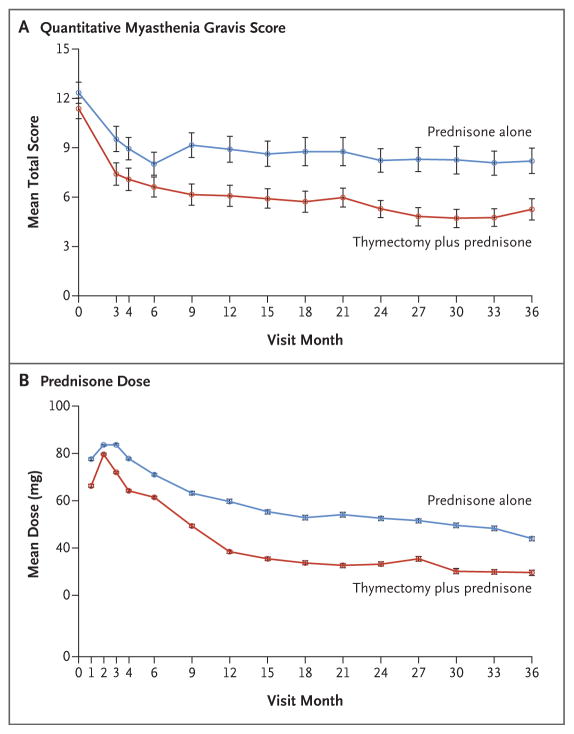
A total of 126 patients underwent randomization between 2006 and 2012 at 36 sites. Patients who underwent thymectomy had a lower time-weighted average Quantitative Myasthenia Gravis score over a 3-year period than those who received prednisone alone (6.15 vs. 8.99, P<0.001); patients in the thymectomy group also had a lower average requirement for alternate-day prednisone (44 mg vs. 60 mg, P<0.001). Fewer patients in the thymectomy group than in the prednisone-only group required immunosuppression with azathioprine (17% vs. 48%, P<0.001) or were hospitalized for exacerbations (9% vs. 37%, P<0.001). The number of patients with treatment-associated complications did not differ significantly between groups (P=0.73), but patients in the thymectomy group had fewer treatment-associated symptoms related to immunosuppressive medications (P<0.001) and lower distress levels related to symptoms (P=0.003).
CONCLUSIONS
Thymectomy improved clinical outcomes over a 3-year period in patients with nonthymomatous myasthenia gravis. (Funded by the National Institute of Neurological Disorders and Stroke and others; MGTX ClinicalTrials.gov number, NCT00294658.)
The First Reported use of Thymectomy in patients with nonthymomatous myasthenia gravis was 75 years ago.1 Of six patients who underwent surgery, three had a favorable response. Subsequent retrospective studies have shown benefits of thymectomy in patients with nonthymomatous myasthenia gravis but with widely varying rates of clinical improvement or remission. A compilation of retrospective studies comparing surgery with medical management did not show a difference in remission rates.2 Two studies that showed clinical improvements after thymectomy indicated that benefit occurred in the first few years after the procedure, but after 5 years, rates of clinical improvement were similar among surgically treated patients and those who were treated medically.3,4 Observational studies have not shown benefits of thymectomy, perhaps because of the effectiveness of modern immunotherapeutic approaches.5
Despite calls for a randomized, controlled study, data are lacking, and uncertainty persists regarding the benefit of thymectomy and the clinical characteristics of the patients who should be offered the procedure.6,7 A systematic review8 of articles describing outcomes in 21 cohorts of patients with myasthenia gravis pointed out numerous methodologic flaws that prevented definite conclusions to be drawn regarding the benefits of thymectomy in patients with nonthymomatous myasthenia gravis.
Glucocorticoids have been widely used for the treatment of myasthenia gravis either as the sole therapy or with thymectomy.9 Although adverse effects are not common with thymectomy, the procedure can cost up to $80,00010 and can be associated with operative complications that need to be weighed against benefits. Glucocorticoids and other immunosuppressive agents place patients at risk for adverse events, some of which are life-threatening, and affect quality of life. Therefore, establishing the role of thymectomy in patients receiving glucocorticoids to manage myasthenia gravis would guide decisions regarding treatment and the costs of health care.
We conducted the Thymectomy Trial in Non-Thymomatous Myasthenia Gravis Patients Receiving Prednisone Therapy (MGTX), an international, randomized, single-blind (rater-blinded) trial, to determine whether extended transsternal thymectomy combined with a standardized prednisone protocol would be superior to prednisone alone after 3 years, with respect to lessening myasthenic weakness, lowering the total dose of prednisone, and enhancing quality of life. Extended transsternal thymectomy was chosen because it provides reproducible resection of the maximal amount of thymic tissue with low morbidity and a limited risk of phrenic-nerve injury.11
METHODS
TRIAL OVERSIGHT
The trial was designed and overseen by an executive committee that included lead investigators and biostatisticians (see the Supplementary Appendix, available with the full text of this article at NEJM.org). During the proposal and trial-design phase, feedback was received from reviewers and staff at the National Institute of Neurological Disorders and Stroke (NINDS). There was no commercial support for this trial. The NINDS funded the trial and assembled a data and safety monitoring board to monitor the trial activities independently.
Each trial site received approval from a local institutional review board or ethics committee, and each patient provided written informed consent before enrollment. Data were collected by investigative teams at each trial site. Data analysis and regulatory enforcement were performed by staff at the Data Coordinating Center, Department of Biostatistics, University of Alabama at Birmingham. Data were managed at the University of Alabama at Birmingham with the use of a Web-based system. Notification of serious adverse events and visit tracking were performed electronically. The first author wrote the first draft of the manuscript, with assistance from the executive committee. All the authors vouch for the accuracy of the data and analyses reported. The trial was conducted and reported with fidelity to the protocol, available at NEJM.org.
TRIAL DESIGN
MGTX was a multicenter, international, rater-blinded, randomized trial.12 Training meetings for investigators were held in 2006 in the United States and the United Kingdom, and all the investigators were required to pass a certification test to ensure the best possible adherence to the protocol. Thoracic surgeons were certified after viewing a video of the surgical approach and passing a test to show that they understood the required procedure for excision. Centers were asked to complete a screening questionnaire for every patient with myasthenia gravis who was encountered at their sites.
The original inclusion criteria were a duration of myasthenia gravis of less than 3 years, an age of 18 to 60 years, a serum acetylcholine-receptor–antibody level of more than 1.00 nmol per liter (elevated levels of 0.50 to 0.99 nmol per liter were accepted if the diagnosis was confirmed by a positive edrophonium test, abnormal repetitive nerve stimulation, or abnormal single-fiber electromyography), and a Myasthenia Gravis Foundation of America clinical classification11 of II to IV (class I indicates weakness only in ocular muscles, class II mild generalized disease, class III moderate generalized disease, class IV severe generalized disease, and class V a crisis requiring intubation). Participants could be taking appropriate anticholinesterase therapy with or without oral glucocorticoids. Exclusion criteria were thymoma on computed tomography or magnetic resonance imaging of the chest, previous thymectomy, immunotherapy other than prednisone, pregnancy or lactation, unwillingness to avoid pregnancy, contraindications to glucocorticoids, and substantial medical illness that would preclude participation.
In October 2008, which was 2 years after enrollment began, the eligibility criterion regarding disease duration was increased from less than 3 years to less than 5 years and the age ceiling was raised from 60 years to 65 years to enhance recruitment. In October 2009, the sample size was reduced from 200 to 150 participants to reflect a lower-than-expected recruitment rate and better-than-expected retention of participants.
TRIAL PROCEDURES
Participants were stratified according to trial site with the use of a Web-based randomization system in which participants were assigned, in a 1:1 ratio, to undergo thymectomy plus receive the standardized prednisone protocol or to receive the same prednisone protocol alone. The receipt of prednisone began immediately, and surgery was performed within 30 days after randomization. The randomization date was set as month 0 (Fig. S1 in the Supplementary Appendix). To preserve rater blinding, participants were seen exclusively until month 4, as they recovered from surgery, by a neurologist who was aware of the trial-group assignments. At all visits, participants wore black, high-collared, obscuring pullover shirts to conceal transsternal incisions; participants were under strict instructions not to reveal their assigned trial group to the evaluators.
Thymectomy was performed by means of a median sternotomy with the goal of an en bloc resection of all mediastinal tissue that could anatomically contain gross or microscopic thymus (or both).11 Photographs of the specimens were transmitted to the data coordinating center and analyzed by the surgical coordinator to judge the extent of resection and compare it with a detailed operative report. Operative details are summarized in the Supplementary Appendix. Data from patients who crossed over to the other group and from patients who had thymoma discovered at surgery were handled according to an intention-to-treat model.
Participants who were not already receiving prednisone at baseline received an alternate-day dose of oral prednisone starting at 10 mg, which was increased in 10-mg steps to 100 mg on alternate days or to 1.5 mg per kilogram of body weight, whichever was lower. For participants who were already taking prednisone, the dose could be increased up to 120 mg in those who did not reach minimal-manifestation status by month 4. Minimal-manifestation status was defined as having “no symptoms or functional limitations from myasthenia gravis, but there may be some weakness on examination of some muscles” (see the Supplementary Appendix).11 The prednisone dose was maintained until minimal-manifestation status was reached and the Quantitative Myasthenia Gravis score13 (on a scale from 0 to 39, with higher scores on each of 13 items indicating more severe weakness; a reduction of 2.3 points correlates with improved clinical status) was less than 14 and had also fallen at least 1 point below baseline, as determined by an evaluator who was unaware of the trial-group assignment.
The alternate-day prednisone dose was then reduced by 10 mg every 2 weeks until a level of 40 mg was reached, with subsequent slowing of the taper to 5 mg every month, as long as the minimal-manifestation status was maintained. If minimal-manifestation status was lost, the alternate-day prednisone dose was increased by 10 mg every 2 weeks until the status was restored. Tapering could resume 4 weeks later.
Once prednisone tapering commenced, the total dose of pyridostigmine could not exceed 240 mg per day. Plasmapheresis or intravenous immune globulin was permitted at the discretion of the unblinded neurologist in patients whose condition was unstable, but it was not permitted in order to maintain minimal-manifestation status. Patients who did not have minimal-manifestation status at 12 months or who had an unacceptable level of side effects with prednisone could receive azathioprine at a dose of 2.5 mg per kilogram per day or another immunosuppressant such as cyclosporine if azathioprine caused unacceptable side effects.
TRIAL OUTCOMES
The dual primary outcome was the time-weighted average Quantitative Myasthenia Gravis score and the time-weighted average required dose of prednisone over a 3-year period; this dual outcome involved a staged approach to incorporating the potential effect of thymectomy on the clinical response to therapy and its influence on long-term requirements for glucocorticoid use. The rationale for the dual outcome was that an improved clinical status could correlate with a greater prednisone dose and a poorer clinical status with a lower dose. The first stage of analysis compared the clinical outcomes between groups according to the time-weighted average Quantitative Myasthenia Gravis score over a period of 3 years, which was roughly equivalent to the average score over time.14 On the basis of the results of the between-group comparison of the clinical outcomes (clinical improvement, worsening, or status unchanged), we analyzed the difference in the total required dose of prednisone over the period of 3 years (see below).
Prednisone was administered in blister packs containing 10-mg tablets, with separate sheets provided for each dose. Alternate-day dosing between visits was recorded by the patient in a diary. At each clinic visit, pill counts were derived from returned blister packs to determine intake and were compared with the diaries. Pill cutters were provided for 5-mg dosing, and unused half pills were returned to the blister-pack sheets. Pill counts formed the basis for determining the cumulative prednisone exposure, but for confirmation purposes, a secondary analysis that used the prescribed dose was performed, which was independent of adherence by the patients.
To address safety and quality of life, secondary analyses focused on serious adverse events, including days of hospitalization over the 3-year period, on the results of surveys of the patients that were adapted from the cardiac-transplantation literature15 to assess 36 treatment-associated complications and 29 symptoms associated with glucocorticoids (see the Supplementary Appendix), and on results of the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36). An order of importance of secondary-outcome measures was not prespecified. Measures pertaining to quality of life were to be used for further analysis if the tiered primary outcome was inconclusive, such as if the time-weighted Quantitative Myasthenia Gravis score favored the thymectomy group but at the cost of a higher prednisone exposure. Other secondary outcomes were the Myasthenia Gravis Activities of Daily Living score (on a scale from 0 to 24, with higher scores indicating more severe disease; a reduction of 2 points correlates with improved clinical status),16,17 the proportion of participants who achieved minimal-manifestation status, and the use of nonglucocorticoid immunosuppressants, plasma exchange, and intravenous immune globulin.
Laboratory monitoring included a complete blood count and glucose, glycated hemoglobin, and potassium levels measured at least monthly from month 0 to month 3 and then every 3 months thereafter. Laboratory monitoring that was performed in patients who were treated with azathioprine or cyclosporine is described in the Supplementary Appendix.
STATISTICAL ANALYSIS
The trial was powered to detect a 30% difference in the time-weighted prednisone dose between the groups, which resulted in a proposed sample size of 150 participants on the basis of Student’s t-test for two independent samples, at a 5% significance level, assuming a mean-to-standard-deviation ratio of 2.0. The denominator that was used to compute the time-weighted average for the Quantitative Myasthenia Gravis score and the prednisone dose was the number of days from randomization to the last visit. Computations used the trapezoidal method. The intention-to-treat method was used for all outcome analyses.
For the first stage of the primary analysis, the protocol specified that first a 99.5% confidence interval for the difference (prednisone-only group minus the thymectomy group) in the time-weighted average Quantitative Myasthenia Gravis score would be assessed. If the confidence interval contained zero, the clinical outcome would be considered as not being better in one group than the other. The second stage would determine superiority on the basis of the exposure to prednisone with the use of a two-sided Student’s t-test of the time-weighted average prednisone dose, at a type I error rate of 0.05, given the clinical outcome. Three imputation methods were used for missing data (see the Supplementary Appendix).
The protocol prespecified the analysis of three subgroups (defined according to previous or no previous glucocorticoid use, sex, and age at disease onset of <40 years vs. ≥40 years). We conducted a post hoc analysis of subgroups defined according to an age at enrollment of less than 50 years versus 50 years or older. Details of the analysis are provided in the Supplementary Appendix. There were no planned adjustments for multiple secondary outcomes.
At the outset of the trial, investigators were asked to predict whether the trial would show a favorable effect for thymectomy (Table S1 in the Supplementary Appendix). These responses remained sealed until the closure of the trial.
RESULTS
PARTICIPANTS
Details regarding recruitment of the participants are provided in Figure S1 in the Supplementary Appendix. A total of 67 centers in 18 countries on six continents (North America, South America, Europe, Africa, Asia, and Australia) participated, with 36 centers conducting recruitment; 32 participating centers were in the United States. A total of 6958 persons underwent screening, 3003 of whom were in the United States. A total of 6727 patients did not meet the inclusion criteria. The main reasons for exclusion were duration of disease of 5 years or more (3129 participants [47%]), an age that did not meet specifications (2842 [42%]), use of nonglucocorticoid immunosuppressive drugs (1977 [29%]), and previous thymectomy or chest surgery (1901 [28%]); multiple reasons for exclusion could have applied to individual patients. Of the 231 eligible patients, 105 declined to participate, and 126 underwent randomization between September 2006 and November 2012 (Fig. S1 in the Supplementary Appendix). There were no significant between-group differences in the characteristics at baseline (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Participants at Baseline.*
| Characteristic | Prednisone Alone (N = 60) | Thymectomy plus Prednisone (N = 66) |
|---|---|---|
| Female sex — no. (%) | 39 (65) | 50 (76) |
| Age — yr | ||
| Median | 33 | 32 |
| Range | 18–64 | 18–63 |
| Race or ethnic group — no. (%)† | ||
| Asian | 4 (7) | 6 (9) |
| Black | 6 (10) | 7 (11) |
| Hispanic | 17 (28) | 17 (26) |
| Non-Hispanic white | 30 (50) | 31 (47) |
| Other | 3 (5) | 5 (8) |
| Therapy at enrollment — no. (%) | ||
| Pyridostigmine | 56 (93) | 60 (91) |
| Glucocorticoid | 47 (78) | 49 (74) |
| Previous therapy — no. (%) | ||
| Intravenous immune globulin | 13 (22) | 12 (18) |
| Plasma exchange | 7 (12) | 9 (14) |
| MGFA class — no. (%)‡ | ||
| IIa | 25 (42) | 25 (38) |
| IIb | 14 (23) | 18 (27) |
| III | 20 (33) | 21 (32) |
| IV | 1 (2) | 2 (3) |
| Duration of disease — yr | ||
| Median | 1.14 | 1.08 |
| Range | 0.15–4.38 | 0.02–4.41 |
| QMG score§ | 12.35±4.90 | 11.40±5.12 |
| Prednisone use at baseline | ||
| No. of patients (%) | 47 (78) | 49 (74) |
| Dose — mg | 42.49±23.52 | 43.43±28.92 |
Plus–minus values are means ±SD. There were no significant between-group differences in the characteristics at baseline. Percentages may not sum to 100 because of rounding.
Race and ethnic group were self-reported. Other race included mixed race, Native American, and Alaskan Native.
Myasthenia Gravis Foundation of America (MGFA) class II indicates mild generalized weakness, class III moderate generalized weakness, and class IV severe generalized weakness. A notation of “a” denotes predominantly limb or axial weakness, and “b” predominantly bulbar weakness.
Three participants in the group that received prednisone alone and one in the thymectomy group withdrew without completing the baseline Quantitative Myasthenia Gravis (QMG) test. QMG scores range from 0 to 39, with higher scores indicating more severe disease.
Eight patients who were randomly assigned to the thymectomy group declined surgery. Eight patients who were randomly assigned to the prednisone-only group underwent thymectomy outside the protocol (Fig. S1 in the Supplementary Appendix). Histologic findings are reported in Table S2 in the Supplementary Appendix. One thymoma was discovered in a patient in the thymectomy group.
ANALYSIS OF TWO-STAGE PRIMARY OUTCOME
In the first stage of the analysis, the time-weighted average Quantitative Myasthenia Gravis scores were significantly lower (indicating improved clinical status) in the thymectomy group than in the prednisone-only group through month 36 (P<0.001) (Fig. 1A and Table 2). The estimated difference in the mean score between the thymectomy group and the prednisone-only group was 2.85 points (99.5% confidence interval [CI], 0.47 to 5.22).
Figure 1. Quantitative Myasthenia Gravis Score and Prednisone Dose, According to Treatment Group.
Quantitative Myasthenia Gravis scores range from 0 to 39, with higher scores on each of 13 items indicating more severe disease; a reduction of 2.3 points correlates with improved clinical status. I bars indicate standard errors.
Table 2.
Primary and Subgroup Analyses of the Primary Outcomes.*
| Outcome | Prednisone Alone | Thymectomy plus Prednisone | Estimated Difference (95% CI)† | P Value‡ | ||
|---|---|---|---|---|---|---|
| value | no. of patients | value | no. of patients | |||
| Primary analyses | ||||||
|
| ||||||
| Time-weighted average QMG score over 3-yr period | 8.99±4.93 | 56 | 6.15±4.09 | 62 | 2.85 (0.47 to 5.22) | <0.001 |
|
| ||||||
| Time-weighted average alternate-day prednisone dose over 3-yr period (mg) | 60±27 | 56 | 44±21 | 61 | 16 (7 to 25) | <0.001 |
|
| ||||||
| Subgroup analyses | ||||||
|
| ||||||
| Time-weighted average QMG score | ||||||
|
| ||||||
| Prednisone use at enrollment | 0.86 | |||||
|
| ||||||
| Yes | 9.10±5.06 | 46 | 6.30±3.89 | 47 | 2.80 (0.11 to 5.49) | 0.004 |
|
| ||||||
| No | 8.84±4.60 | 9 | 5.66±4.79 | 15 | 3.18 (−3.03 to 9.39) | 0.12 |
|
| ||||||
| Sex | 0.57 | |||||
|
| ||||||
| Female | 9.73±5.16 | 38 | 6.47±4.13 | 46 | 3.26 (0.34 to 6.18) | 0.002 |
|
| ||||||
| Male | 7.45±4.11 | 18 | 5.23±3.95 | 16 | 2.22 (−1.96 to 6.40) | 0.12 |
|
| ||||||
| Age at disease onset | 0.74 | |||||
|
| ||||||
| <40 yr | 9.60±5.32 | 34 | 6.50±4.41 | 42 | 3.10 (−0.13 to 6.33) | 0.007 |
|
| ||||||
| ≥40 yr | 7.85±3.50 | 18 | 5.33±2.79 | 18 | 2.52 (−0.65 to 5.69) | 0.02 |
|
| ||||||
| Time-weighted average alternate-day prednisone dose (mg) | ||||||
|
| ||||||
| Prednisone use at enrollment | 0.37 | |||||
|
| ||||||
| Yes | 61±28 | 46 | 44±22 | 46 | 17 (7 to 28) | 0.002 |
|
| ||||||
| No | 48±19 | 9 | 42±20 | 15 | 7 (−10 to 24) | 0.40 |
|
| ||||||
| Sex | 0.32 | |||||
|
| ||||||
| Female | 59±25 | 37 | 46±23 | 45 | 13 (3 to 24) | 0.01 |
|
| ||||||
| Male | 61±32 | 19 | 38±15 | 16 | 23 (6 to 40) | 0.009 |
|
| ||||||
| Age at disease onset | 0.94 | |||||
|
| ||||||
| <40 yr | 61±27 | 33 | 45±22 | 41 | 16 (4 to 27) | 0.007 |
|
| ||||||
| ≥40 yr | 56±28 | 19 | 41±21 | 18 | 15 (−1 to 32) | 0.07 |
CI denotes confidence interval.
We used 95% confidence intervals in all analyses except for analyses involving the QMG score, for which we used 99.5% confidence intervals, per protocol.
P values for between-group comparisons are based on two independent sample Student’s t-tests. P values for interaction with treatment were based on fitting a general linear model separately for each variable.
Analyses in the second stage showed that over a period of 36 months, the time-weighted average prednisone dose was significantly lower in the thymectomy group than in the prednisone-only group (Fig. 1B and Table 2). The average alternate-day dose was 44 mg in the thymectomy group, as compared with 60 mg in the prednisone-only group (estimated difference, 16 mg; 95% CI, 7 to 25; P<0.001). Less than 1% of the trial visits in the prednisone-only group were missed, as compared with 1% of those in the thymectomy group. None of the imputation methods for the missing data changed the underlying findings.
SUBGROUP ANALYSIS
The tests for interactions were not significant in any of the three subgroup analyses, and therefore, conclusions cannot be drawn about differential benefits in the subgroups. A total of 26 participants had not previously used glucocorticoids. Use of the drug was not clear in 4 patients, 3 of whom were in the prednisone-only group. Among participants who had not taken glucocorticoids previously, the between-group differences in the Quantitative Myasthenia Gravis score and the prednisone dose were not significant; among patients with previous exposure to glucocorticoids, the between-group differences were significant (Table 2). Among women, the between-group differences in the two outcomes were significant; among men, the difference in prednisone dose was significant. Thymectomy was associated with a significantly lower time-weighted average Quantitative Myasthenia Gravis score (indicating clinical improvement) than prednisone alone both in participants with disease onset at less than 40 years of age and in those with disease onset at 40 years of age or older (Table 2).
SECONDARY OUTCOME MEASURES
The survey regarding treatment-associated complications showed no significant difference between the two treatment groups over a period of 3 years (P = 0.73). The survey regarding treatment-associated symptoms favored the thymectomy group over the prednisone-alone group in the number of participants with symptoms (P<0.001), in the total number of symptoms (P<0.001), and in the distress level related to symptoms (P = 0.003) over the 3-year period. No significant between-group differences were seen in either the physical or the mental component of the SF-36. Details regarding these outcomes are provided in Tables S5, S6, and S7 in the Supplementary Appendix.
Table 3 lists data regarding adverse events. Fewer patients were hospitalized for exacerbations of myasthenia gravis in the thymectomy group than in the prednisone-only group (9% vs. 37%, P<0.001). The mean (±SD) cumulative number of hospital days was 8.4±8.6 in the thymectomy group, as compared with 19.2±24.5 in the prednisone-only group (P = 0.09).
Table 3.
Adverse Events.*
| Variable | Prednisone Alone (N = 60) | Thymectomy plus Prednisone (N = 66) | P Value |
|---|---|---|---|
| No. of events | 93 | 48 | <0.001† |
| ≥1 event — no. of patients (%) | 33 (55) | 25 (38) | 0.05‡ |
| Event | |||
| Life-threatening event — no. of patients (%) | 7 (12) | 1 (2) | 0.03§ |
| Disability or incapacity — no. of patients (%)¶ | 2 (3) | 8 (12) | 0.10 |
| Event requiring medical or surgical intervention — no. of patients (%) | 5 (8) | 9 (14) | 0.40 |
| Death — no. of patients (%) | 1 (2) | 0 | 0.48 |
| Complication due to thymectomy — no. of patients (%) | NA | 1 (2) | — |
| Hospitalization — no. of patients (%) | 31 (52) | 15 (23) | <0.001‡ |
| Hospitalization for exacerbation of myasthenia gravis — no. of patients (%) | 22 (37) | 6 (9) | <0.001‡ |
| Cumulative no. of hospital days | 19.2±24.5 | 8.4±8.6 | 0.09 |
| Reason for hospitalization according to MedDRA term — no. (%) | |||
| Gastrointestinal disorder | 2 (3) | 2 (3) | 1.00 |
| Hepatobiliary disorder | 1 (2) | 0 | 0.48 |
| Infection or infestation | 7 (12) | 4 (6) | 0.35 |
| Injury, poisoning, or procedure complication | 0 | 2 (3) | 0.50 |
| Metabolism or nutrition disorder | 0 | 1 (2) | 1.00 |
| Nervous system disorder | 22 (37) | 8 (12) | 0.001‡ |
| Respiratory, thoracic, or mediastinal disorder | 2 (3) | 1 (2) | 0.60 |
| Surgical or medical procedure | 7 (12) | 0 | 0.005§ |
| Vascular disorder | 1 (2) | 0 | 0.48 |
Plus–minus values are means ±SD. MedDRA denotes Medical Dictionary for Regulatory Activities, and NA not applicable.
The P value is based on Poisson regression that included all 126 patients.
The P value is based on a chi-square test that included all 126 patients.
The P value is based on Fisher’s exact test that included all 126 patients.
Causes of disability or incapacity in the group that received prednisone alone included worsening swallowing difficulties and myasthenia gravis; causes in the thymectomy group included osteoporotic thoracic fracture, ocular-muscle involvement due to relapsing myasthenia gravis, post-thymectomy diaphragmatic hemiparesis, rib fracture, impending myasthenic crisis, ankle fracture, tear of left knee meniscus, and low back pain with possible stenosis.
When we compared prednisone requirements on the basis of prescribed dose instead of pill counts, the pattern favoring thymectomy over prednisone alone persisted (43 mg vs. 59 mg, P = 0.001) (see the Supplementary Appendix). Similarly, other findings favored thymectomy over prednisone alone, including the time-weighted average score on the Myasthenia Gravis Activities of Daily Living scale (2.24 vs. 3.41, P = 0.008), azathioprine use (17% vs. 48% of participants, P<0.001), and the percentage of patients who had minimal-manifestation status at month 36 (67% vs. 47%, P = 0.03) (Table S4 in the Supplementary Appendix). In an analysis that maintained the prednisone dose at the time that azathioprine was added through month 36, we found that prednisone exposure was 33% lower in the thymectomy group than in the prednisone-only group (average alternate-day requirement, 46 mg vs. 68 mg; difference, 22 mg; 95% CI, 11 to 34; P<0.001). Other secondary outcomes (use of plasma exchange, use of intravenous immune globulin, Myasthenia Gravis Activities of Daily Living score, minimal-manifestation status, and hospitalizations at time points before 3 years) are summarized in the Supplementary Appendix.
UNBLINDING
Over the 9-year period of trial visits, six episodes of self-reported unblinding of the rater occurred. One participant became pregnant, and trial personnel were informed of the treatment-group assignment for safety reasons. In the other cases, two episodes of unblinding occurred when patients crossed over to the thymectomy group and three when unblinded personnel performed assessments because the blinded evaluator was unavailable.
DISCUSSION
This trial provides evidence supporting the use of thymectomy for improving clinical outcomes and reducing the need for immunosuppressive therapy in patients with myasthenia gravis. Over a period of 3 years, thymectomy was associated with a more favorable outcome than was prednisone alone, with a difference in Quantitative Myasthenia Gravis scores that correlated with clinically significant improvement.13,14 In this trial, thymectomy was associated with a score that was 2.85 points lower than that with medical therapy alone. The minimal clinically important difference for this scale has not been determined, but in a study assessing the validity of this scale, scores were 2.3 points lower in patients who were assessed by neurologists as having had clinical improvement over time.14 The time-weighted average required alternate-day prednisone dose was significantly lower in the thymectomy group than in the prednisone-only group.
The finding that prednisone doses at month 36 were higher in each group than might be predicted (according to routine practice) could be related to the protocol requirement to maintain minimal manifestation of disease and lower use of glucocorticoid-sparing agents. Patients in the prednisone-only group were more likely than those in the thymectomy group to be treated with azathioprine. We do not believe that even greater use of glucocorticoid-sparing immunosuppressive drugs in the prednisone-only group would have negated the improved clinical outcomes that were associated with thymectomy. Randomized trials of mycophenolate mofetil did not show improved clinical outcomes when the drug was added to prednisone in patients with myasthenia gravis.18,19 The inability of this trial to show significant between-group differences among men with respect to the Quantitative Myasthenia Gravis score or in the subgroup of patients who had not taken prednisone previously with respect to either of the coprimary outcomes may relate to the small number of patients but is worth further exploration.
Thymectomy for the treatment of myasthenia gravis is based on several lines of evidence that support a central role of the thymus in the pathogenesis of the disease.20,21 Thymomas are present in 10% of patients with myasthenia gravis, and thymectomy is considered to be mandatory to prevent further spread.21 Up to 70% of the remaining patients with myasthenia gravis have hyperplastic thymic changes that are not seen in healthy persons.20,22 However, the success of immunotherapy has raised questions regarding whether such an operation is necessary.2 A U.S. database suggests that hospital admissions for thymectomy in patients with myasthenia gravis fell dramatically after 2000.23 Planned analysis of trial data to assess for correlation between histologic findings and response to thymectomy have not yet been conducted.
Extended transsternal thymectomy requires median sternotomy and is associated with a resection of 85 to 95% of thymic tissue.11,24 The MGTX did not test less-invasive thymectomy approaches that have similar effectiveness and shorter postoperative recovery times and better cosmesis.25–29 One concern is that surgeons who use minimally invasive techniques may leave behind ectopic thymic tissue in perithymic and pericardial fields.24 In some studies, ectopic thymus has had a negative effect on outcomes at a follow-up of more than 7 years.30 Furthermore, numerous reports describe patients with myasthenia gravis as not having clinical improvement after incomplete thymic resections.31–33 Randomized trials to compare resectional techniques are needed.
Potential limitations of our trial include its single-blind nature and the pill-count method. It was deemed unethical to subject control patients to a transsternal sham thymectomy. A clinical measure alone could not be used as the primary end point since it would not account for the prednisone use that would be required to achieve minimal-manifestation status, which can occur with glucocorticoids alone.9 We used pill counts to measure prednisone intake, but pill counts are not a precise measure of actual intake (for comparison with other methods, see the Supplementary Appendix).
In conclusion, this randomized, medication-controlled, rater-blinded trial showed a benefit of thymectomy in patients with myasthenia gravis over a period of 3 years with respect to clinical outcomes, requirements for prednisone and azathioprine therapy, the number of symptoms and the distress level related to immunosuppressive agents, and the need for hospitalization to manage disease exacerbations.
Supplementary Material
Acknowledgments
Supported by a grant (U01 NS042685) from the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health, and by the Muscular Dystrophy Association and the Myasthenia Gravis Foundation of America.
Dr. Wolfe reports receiving lecture fees and fees for serving on advisory boards from Grifols and Baxalta, consulting fees from Alpha Cancer Technologies, argenx, and UCB, and grant support from CSL Behring and Alexion Pharmaceuticals; Dr. Kaminski, receiving fees for serving on data and safety monitoring boards from Genentech and Novartis, consulting fees from Alnylam Pharmaceuticals, UCB, Chugai Pharma, Rubius Therapeutics, RA Pharmaceuticals, and Momenta Pharmaceuticals, receiving serum samples from Rubius Therapeutics, receiving study medication from Genentech, and holding a patent related to technology to focus a complement inhibitor on the neuromuscular junction for the treatment of myasthenia gravis (U.S. patent no. 8,961,981); Dr. Evoli, receiving fees for serving on an advisory board from CSL Behring and for serving as a scientific-award jury member from Grifols; Dr. Beydoun, receiving consulting and lecture fees from Grifols and Baxalta and grant support from GlaxoSmithKline, Alexion Pharmaceuticals, and CSL Behring; Dr. Vincent, receiving grant support from GlaxoSmithKline, and royalties from patents related to MuSK antibodies for diagnosis of myasthenia gravis (U.K. patent no., PCT/GB01/02661; licensed to Athena Diagnostics) and LGI1 and CASPR2 antibodies for autoimmune encephalitis (U.K. patent no., PCT/GB2009/051441; licensed to Euroimmun); Dr. Yoshikawa, receiving grant support from Astellas Pharma; Dr. Waddington-Cruz, receiving consulting fees from Pfizer, Genzyme, and Ionis Pharmaceuticals (formerly Isis), travel support from Pfizer, and grant support from Pfizer, Ionis Pharmaceuticals, and Alnylam Pharmaceuticals; Dr. Pulley, receiving personal fees from Grifols and Mallinckrodt Pharmaceuticals; Dr. Verschuuren, receiving consulting fees from Alexion Pharmaceuticals and Argenx, paid to his institution, and holding a patent (U.S. patent no. 14/486,400; royalties are paid to his institution) related to therapy for anti-MuSK myasthenia gravis; and Dr. Cutter, receiving fees for serving on data and safety monitoring boards from Biogen Idec, Teva Neuroscience, Pfizer, Sanofi, Celgene, Gilead Sciences, Neuren Pharmaceuticals, ModigeneTech, Opko Biologics, Ono Pharmaceuticals, Merck Serono, GlaxoSmithKline, Horizon Pharma, Reata Pharmaceuticals, and PTC Therapeutics, fees for serving on a steering committee from MedImmune, fees for serving on advisory boards from EMD Serono, Novartis, Questcor Pharmaceuticals, Genentech, and Janssen Pharmaceutica, consulting fees from Teva Neuroscience, Genzyme, Genentech, Transparency Life Sciences, Roche, Opexa Therapeutics, Somahlution, Savara Pharmaceuticals, and Nivalis Therapeutics, fees from a law firm for providing a legal opinion regarding a patent infringement case on behalf of Galderma, and grant support to his institution from Teva Neuroscience. No other potential conflict of interest relevant to this article was reported.
We thank the members of the data and safety monitoring board (Steven Keller, M.D. [chair], Alan Dyer, Ph.D., Donald Schotland, M.D., John Winer, M.D., and Peter Gilbert, Sc.M. [ex officio]); Audrey Penn, M.D., of the NINDS, Johan Aarli, M.D., Robert Griggs, M.D., Robert Lisak, M.D., and Lewis Rowland, M.D., for advocating for the trial in its early stages; and the patients who participated in this trial and were willing to be randomly assigned to either a surgical group or a nonsurgical group and to adhere to a trial protocol that lasted for years.
APPENDIX
The authors’ full names and academic degrees are as follows: Gil I. Wolfe, M.D., Henry J. Kaminski, M.D., Inmaculada B. Aban, Ph.D., Greg Minisman, M.A., Hui-Chien Kuo, M.S., Alexander Marx, M.D., Philipp Ströbel, M.D., Claudio Mazia, M.D., Joel Oger, M.D., J. Gabriel Cea, M.D., Jeannine M. Heckmann, M.B., Ch.B., Ph.D., Amelia Evoli, M.D., Wilfred Nix, M.D., Emma Ciafaloni, M.D., Giovanni Antonini, M.D., Rawiphan Witoonpanich, M.D., John O. King, M.D., Said R. Beydoun, M.D., Colin H. Chalk, M.D., Alexandru C. Barboi, M.D., Anthony A. Amato, M.D., Aziz I. Shaibani, M.D., Bashar Katirji, M.D., Bryan R.F. Lecky, M.D., Camilla Buckley, M.D., Angela Vincent, M.B., B.S., Elza Dias-Tosta, M.D., Ph.D., Hiroaki Yoshikawa, M.D., Ph.D., Márcia Waddington-Cruz, M.D., Ph.D., Michael T. Pulley, M.D., Ph.D., Michael H. Rivner, M.D., Anna Kostera-Pruszczyk, M.D., Robert M. Pascuzzi, M.D., Carlayne E. Jackson, M.D., Guillermo S. Garcia Ramos, M.D., Jan J.G.M. Verschuuren, M.D., Janice M. Massey, M.D., John T. Kissel, M.D., Lineu C. Werneck, M.D., Ph.D., Michael Benatar, M.D., Ph.D., Richard J. Barohn, M.D., Rup Tandan, M.D., Tahseen Mozaffar, M.D., Robin Conwit, M.D., Joanne Odenkirchen, M.P.H., Joshua R. Sonett, M.D., Alfred Jaretzki, III, M.D., John Newsom-Davis, M.D., and Gary R. Cutter, Ph.D.
The authors’ affiliations are as follows: the Department of Neurology, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, Buffalo (G.I.W.), the Department of Neurology, University of Rochester Medical Center, Rochester (E.C.), and the Section of General Thoracic Surgery, Columbia University Medical Center, New York (J.R.S., A.J.) — all in New York; the Department of Neurology, George Washington University School of Medicine and Health Sciences, Washington, DC (H.J.K.); the Department of Biostatistics, University of Alabama at Birmingham, Birmingham (I.B.A., G.M., H.-C.K., G.R.C.); the Institute of Pathology, University Medical Center Mannheim, University of Heidelberg, Mannheim (A.M.), the Institute of Pathology, University of Göttingen, Göttingen (P.S.), and the Department of Neurology, Johannes Gutenberg University, Mainz (W.N.) — all in Germany; the Department of Neurology, University of Buenos Aires, Buenos Aires (C.M.); the Division of Neurology, University of British Columbia, Vancouver (J. Oger), and the Department of Neurology, McGill University, Montreal (C.H.C.) — both in Canada; the Department of Neurology, University of Chile, Santiago (J.G.C.); the Division of Neurology, Department of Medicine, University of Cape Town, Cape Town, South Africa (J.M.H.); the Department of Neurology, Catholic University (A.E.), and the Department of Neurosciences, Mental Health and Sensory Organs, Sapienza University of Rome (G.A.) — both in Rome; the Division of Neurology, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (R.W.); the Department of Neurology, University of Melbourne, Melbourne, VIC, Australia (J.O.K.); the Department of Neurology, University of Southern California, Los Angeles (S.R.B.), and the Department of Neurology, University of California Irvine Medical Center, Orange (T.M.) — both in California; the Department of Neurology, Medical College of Wisconsin, Milwaukee (A.C.B.); the Department of Neurology, Harvard Medical School, Boston (A.A.A.); Nerve and Muscle Center of Texas, Houston (A.I.S.), and the Department of Neurology, University of Texas Health Science Center, San Antonio (C.E.J.) — both in Texas; the Department of Neurology, Case Western Reserve University, Cleveland (B.K.), and the Department of Neurology, Ohio State University Wexner Medical Center, Columbus (J.T.K.) — both in Ohio; Walton Centre for Neurology and Neurosurgery, Liverpool (B.R.F.L.), and Nuffield Department of Clinical Neurosciences, Oxford University, Oxford (C.B., A.V., J.N.-D.) — both in the United Kingdom; Unit of Neurology, University of Brasilia, Brasilia (E.D.-T.), the Department of Neurology, Federal University, Rio de Janeiro (M.W.-C.), and the Department of Neurology, Universidade Federal do Parana, Curitiba (L.C.W.) — all in Brazil; the Department of Neurology, Kanazawa University, Kanazawa, Japan (H.Y.); the Department of Neurology, University of Florida, Jacksonville (M.T.P.), and the Department of Neurology, University of Miami, Miami (M.B.) — both in Florida; the Department of Neurology, Georgia Regents University, Augusta (M.H.R.); the Department of Neurology, Medical University of Warsaw, Warsaw, Poland (A.K.-P.); the Department of Neurology, Indiana School of Medicine, Indianapolis (R.M.P.); the Department of Neurology and Psychiatry, Instituto Nacional de la Nutrición, Tlalpan, Mexico (G.S.G.R.); the Department of Neurology, Leiden University Medical Center, Leiden, the Netherlands (J.J.G.M.V.); the Department of Neurology, Duke University Medical Center, Durham, NC (J.M.M.); the Department of Neurology, University of Kansas Medical Center, Kansas City (R.J.B.); the Department of Neurological Sciences, University of Vermont College of Medicine, Burlington (R.T.); and the Division of Extramural Research, National Institutes of Health, National Institute of Neurological Disorders and Stroke, Bethesda, MD (R.C., J. Odenkirchen).
Footnotes
References
- 1.Blalock A, Harvey AM, Ford FR, Lilienthal JL. The treatment of myasthenia gravis by removal of the thymus gland. JAMA. 1941;117:1529–33. [Google Scholar]
- 2.McQuillen MP, Leone MG. A treatment carol: thymectomy revisited. Neurology. 1977;27:1103–6. doi: 10.1212/wnl.27.12.1103. [DOI] [PubMed] [Google Scholar]
- 3.Oosterhuis HJ. Observations of the natural history of myasthenia gravis and the effect of thymectomy. Ann N Y Acad Sci. 1981;377:678–90. doi: 10.1111/j.1749-6632.1981.tb33766.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez M, Gomez MR, Howard FM, Jr, Taylor WF. Myasthenia gravis in children: long-term follow-up. Ann Neurol. 1983;13:504–10. doi: 10.1002/ana.410130506. [DOI] [PubMed] [Google Scholar]
- 5.Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141–9. doi: 10.1002/mus.20950. [DOI] [PubMed] [Google Scholar]
- 6.Lanska DJ. Indications for thymectomy in myasthenia gravis. Neurology. 1990;40:1828–9. doi: 10.1212/wnl.40.12.1828. [DOI] [PubMed] [Google Scholar]
- 7.Cea G, Benatar M, Verdugo RJ, Salinas RA. Thymectomy for non-thymomatous myasthenia gravis. Cochrane Database Syst Rev. 2013;10:CD008111. doi: 10.1002/14651858.CD008111.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:7–15. doi: 10.1212/wnl.55.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Pascuzzi RM, Coslett HB, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol. 1984;15:291–8. doi: 10.1002/ana.410150316. [DOI] [PubMed] [Google Scholar]
- 10.Healthcare Cost and Utilization Project: nationwide inpatient sample. Rockville, MD: Agency for Healthcare Research and Quality; ( http://www.hcup-us.ahrq.gov/) [Google Scholar]
- 11.Jaretzki A, III, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Newsom-Davis J, Cutter G, Wolfe GI, et al. Status of the thymectomy trial for nonthymomatous myasthenia gravis patients receiving prednisone. Ann N Y Acad Sci. 2008;1132:344–7. doi: 10.1196/annals.1405.014. [DOI] [PubMed] [Google Scholar]
- 13.Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the Quantitative Myasthenia Gravis score. Ann N Y Acad Sci. 1998;841:769–72. doi: 10.1111/j.1749-6632.1998.tb11015.x. [DOI] [PubMed] [Google Scholar]
- 14.Bedlack RS, Simel DL, Bosworth H, Samsa G, Tucker-Lipscomb B, Sanders DB. Quantitative Myasthenia Gravis score: assessment of responsiveness and longitudinal validity. Neurology. 2005;64:1968–70. doi: 10.1212/01.WNL.0000163988.28892.79. [DOI] [PubMed] [Google Scholar]
- 15.Moons P, De Geest S, Abraham I, Cleemput JV, Van Vanhaecke J. Symptom experience associated with maintenance immunosuppression after heart transplantation: patients’ appraisal of side effects. Heart Lung. 1998;27:315–25. doi: 10.1016/s0147-9563(98)90052-8. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia Gravis Activities of Daily Living profile. Neurology. 1999;52:1487–9. doi: 10.1212/wnl.52.7.1487. [DOI] [PubMed] [Google Scholar]
- 17.Muppidi S, Wolfe GI, Conaway M, Burns TM. MG-ADL: still a relevant outcome measure. Muscle Nerve. 2011;44:727–31. doi: 10.1002/mus.22140. [DOI] [PubMed] [Google Scholar]
- 18.Muscle Study Group. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008;71:394–9. doi: 10.1212/01.wnl.0000312373.67493.7f. [DOI] [PubMed] [Google Scholar]
- 19.Sanders DB, Hart IK, Mantegazza R, et al. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology. 2008;71:400–6. doi: 10.1212/01.wnl.0000312374.95186.cc. [DOI] [PubMed] [Google Scholar]
- 20.Conti-Fine BM, Diethelm-Okita B, Ostlie N, Wang W, Milani M. Immunopathogenesis of myasthenia gravis. In: Kaminski HJ, editor. Myasthenia gravis and related disorders. New York: Humana Press; 2009. pp. 43–70. [Google Scholar]
- 21.Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 22.Berrih-Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. 2014;52:90–100. doi: 10.1016/j.jaut.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. 2009;72:1548–54. doi: 10.1212/WNL.0b013e3181a41211. [DOI] [PubMed] [Google Scholar]
- 24.Jaretzki A., III Thymectomy for myasthenia gravis: analysis of the controversies regarding technique and results. Neurology. 1997;48(Suppl 5):S52–63. [Google Scholar]
- 25.Lee CY, Kim DJ, Lee JG, Park IK, Bae MK, Chung KY. Bilateral video-assisted thoracoscopic thymectomy has a surgical extent similar to that of transsternal extended thymectomy with more favorable early surgical outcomes for myasthenia gravis patients. Surg Endosc. 2011;25:849–54. doi: 10.1007/s00464-010-1280-y. [DOI] [PubMed] [Google Scholar]
- 26.Mack MJ, Landreneau RJ, Yim AP, Hazelrigg SR, Scruggs GR. Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg. 1996;112:1352–9. doi: 10.1016/s0022-5223(96)70151-4. [DOI] [PubMed] [Google Scholar]
- 27.Mantegazza R, Baggi F, Bernasconi P, et al. Video-assisted thoracoscopic extended thymectomy and extended transsternal thymectomy (T-3b) in non-thymomatous myasthenia gravis patients: remission after 6 years of follow-up. J Neurol Sci. 2003;212:31–6. doi: 10.1016/s0022-510x(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 28.Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg. 2011;141:673–7. doi: 10.1016/j.jtcvs.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Shrager JB, Deeb ME, Mick R, et al. Transcervical thymectomy for myasthenia gravis achieves results comparable to thymectomy by sternotomy. Ann Thorac Surg. 2002;74:320–6. doi: 10.1016/s0003-4975(02)03722-0. [DOI] [PubMed] [Google Scholar]
- 30.Ponseti JM, Gamez J, Vilallonga, et al. Influence of ectopic thymic tissue on clinical outcome following extended thymectomy in generalized seropositive nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg. 2008;34:1062–7. doi: 10.1016/j.ejcts.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 31.Jaretzki A, III, Penn AS, Younger DS, et al. “Maximal” thymectomy for myasthenia gravis: results. J Thorac Cardiovasc Surg. 1988;95:747–57. [PubMed] [Google Scholar]
- 32.Masaoka A, Monden Y, Seike Y, Tanioka T, Kagotani K. Reoperation after transcervical thymectomy for myasthenia gravis. Neurology. 1982;32:83–5. doi: 10.1212/wnl.32.1.83. [DOI] [PubMed] [Google Scholar]
- 33.Miller RG, Filler-Katz A, Kiprov D, Roan R. Repeat thymectomy in chronic refractory myasthenia gravis. Neurology. 1991;41:923–4. doi: 10.1212/wnl.41.6.923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.