Abstract
Several studies have reported hyperactivation in frontal and striatal regions in individuals with reading disorder (RD) during reading-related tasks. Hyperactivation in these regions is typically interpreted as a form of neural compensation and related to articulatory processing. Fronto-striatal hyperactivation in RD can however, also arise from fundamental impairment in reading related processes, such as phonological processing and implicit sequence learning relevant to early language acquisition. We review current evidence for the compensation hypothesis in RD and apply large-scale reverse inference to investigate anatomical overlap between hyperactivation regions and neural systems for articulation, phonological processing, implicit sequence learning. We found anatomical convergence between hyperactivation regions and regions supporting articulation, consistent with the proposed compensatory role of these regions, and low convergence with phonological and implicit sequence learning regions. Although the application of large-scale reverse inference to decode function in a clinical population should be interpreted cautiously, our findings suggest future lines of research that may clarify the functional significance of hyperactivation in RD.
Keywords: Dyslexia, compensation, implicit learning, articulation
1. Introduction
Reading disorder (RD, also known as developmental dyslexia or reading disability) is a developmental disorder associated with phonological processing difficulty and impaired development of fluent reading (S. E. Shaywitz & Shaywitz, 2005). Quantitative meta-analyses of neuroimaging studies comparing RD and typical readers have identified decreased task-related blood oxygen level dependent (BOLD) signal in occipito-temporal and temporo-parietal brain regions within RD (Linkersdörfer, Lonnemann, Lindberg, Hasselhorn, & Fiebach, 2012; Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008; Richlan, Kronbichler, & Wimmer, 2009), consistent with the role of these regions in phonological (Vigneau et al., 2006) and orthographic processing (e.g. Cohen & Dehaene, 2004; Price & Devlin, 2011). Temporo-parietal reductions in grey matter volume in RD adults (Richlan, Kronbichler, & Wimmer, 2013) and in at-risk pre-readers (Black et al., 2012; Raschle, Chang, & Gaab, 2011) along with evidence that candidate RD alleles modulate cortical morphology and activation in left temporo-parietal (Meda et al., 2008; Pinel et al., 2012) and occipito-temporal regions (Cope et al., 2012; Meda et al., 2008) suggest that the primary neurodevelopmental origin of RD lies in posterior brain systems. While most studies thus far have focused primarily on dysfunction in temporo-parietal and occipito-temporal regions, meta-analyses also support increased activation in RD relative to controls in several fronto-striatal regions, including the bilateral striatum (including caudate and putamen) and globus pallidus, thalamus, left inferior frontal gyrus (IFG), left insula and left precentral gyrus (Maisog et al., 2008; Richlan et al., 2009; Richlan, Kronbichler, & Wimmer, 2011). The fronto-striatal hyperactivation patterns seen in RD have often been interpreted as reflecting compensation engaged to reduce the impact of a primary phonological processing deficits arising from the temporo-parietal region (e.g. Richlan et al., 2009) although the cognitive and neurobiological mechanisms that could lead to compensation are poorly understood.
Regions of altered activation between RD and typical readers are frequently interpreted in terms of the hypothesized primary phonological or orthographic deficits that characterize RD, or as compensation for impairments in these processes. However, differences in activation are found in many regions that may support a broader array of cognitive functions, e.g. the inferior frontal gyrus (IFG), basal ganglia and thalamus and precentral gyrus. We apply a new method for interpreting RD activation differences, based on large-scale automated meta-analysis (Yarkoni et al., 2011; see §2), to evaluate support for these interpretations. In particular, we consider the evidence for interpreting hyperactivation as neural compensation, alongside alternative interpretations. Notably, we consider whether hyperactivation could be attributable to impaired phonological representations or reflect abnormal fronto-striatal function that is not exclusive to the language system.
1.1 Fronto-Striatal Hyperactivation as Compensation
Findings of regional hyperactivation in clinical samples are frequently interpreted in terms of neural “compensation.” This term suffers from imprecise usage in the RD literature, but a positive relationship between task performance and brain activation within regions showing greater activation in patients relative to typical individuals is a defining characteristic of neural compensation (Cabeza, Anderson, Locantore, & McIntosh, 2002; Hillary, 2008). In other words, neural compensation occurs when there is additional recruitment of neural resources (presumably observed as an increase in BOLD signal) to support task performance. This additional activation can be seen either in regions normally associated with a task, or observed as activation in additional regions not typically associated with the task in normal individuals. In the case of RD, fronto-striatal hyperactivation could reflect an increased reliance on these systems during reading to compensate for impairments in posterior brain regions (e.g. Shaywitz et al., 2002; Richlan et al., 2009, Hoeft et al., 2007). This interpretation is consistent with views of fronto-striatal regions as components of the articulatory system (Paulesu et al., 1993) recruited during compensatory subvocalization during reading. If hyperactivation in RD can be explained as articulatory compensation, we expect relatively specific co-localization between articulatory and hyperactive brain regions. In addition, consistent with compensation, levels of activation in these regions should show a positive relationship with performance.
Few studies of RD have directly examined the relationship between performance and hyperactivation in fronto-striatal regions and those that have do not clearly support a compensatory interpretation. Bach et al. (2010) examined the relationship between activation in the bilateral IFG during letter substitution/lexical decision and offline measures of reading and phonological skill. Although a positive correlation between reading performance and left IFG/insula activation was found when combining both RD and typical children, left IFG activity was reduced in RD relative to typical controls, suggesting activation in the left IFG during the task was related to performance, but not compensatory for the RD group. However, activation in the right IFG was correlated with phonological ability in the RD group only, suggesting that additional recruitment of the right IFG might serve a compensatory function. Bach et al. found hyperactivation in the RD group in the left pre/post central gyrus, a region more consistently found to be overactive in RD. On the other hand, Ingvar et al., (2002) found that decreased reading skill across Swedish RD and control adults was associated with increased activation in the right IFG/insula and globus pallidus during silent reading, which the authors interpret as an impairment in right-lateralized prosodic processing.
These two studies provide conflicting evidence for a compensatory role of right frontal language regions in RD, which may be due to differing demands of the in-scanner tasks. During lexical decision, activity in the right IFG could reflect a compensatory increase in the inhibition processes required during lexical selection processes supported by this region as in Bach et al (2010). This may be distinct from the role of the right IFG during the more naturalistic reading task that does not require inhibitory processes employed by Ingvar et al (2002).
Right IFG involvement in phonological processing also appears to have a differential developmental trajectory in RD and typically developing readers. Shaywitz et al. (2002) found cross-sectional age-related activation increases in the bilateral IFG and putamen/thalamus in RD, but not typical, children. Activation in these regions was not reported to be related to reading skill, so it is unclear whether the failure to establish a more lateralized frontal network for phonological processing in RD reflects compensation. However, right IFG activation has been found to positively correlate with individual improvement in reading skill after two years in RD but not typical children (Hoeft et al., 2011).
Treatment studies have also reported RD hyperactivation in fronto-striatal regions. For example, Meyler et al. (2008) found hyperactivation in RD children in fronto-striatal regions following a mixture of reading interventions. Although this emergent hyperactivation could reflect neural compensation as a result of intervention, this cannot be distinguished from developmental changes that might reflect an RD-specific neurobiology, unrelated to compensation, since all RD children received intervention and the sample sizes did not allow meaningful comparisons between types of interventions. In a quantitative meta-analysis of eight imaging studies that included an intervention component, Barquero, Davis, & Cutting, (2014) reported intervention-related activation increases in the right IFG/insula, left IFG and left thalamus/basal ganglia and other regions during reading tasks. As Barquero et al. note, these regions could reflect a wide range of processes that may contribute to gains in reading ability. To the extent that RD imaging studies have addressed the link between hyperactivation and compensation, much of the focus has been on the role of the right IFG. Although developmental and intervention studies collectively suggest a compensatory role for this region, discrepancies in the synchronic relationship between right IFG activation and performance remain. Furthermore, the nature of this compensation (if present), in terms of neural mechanisms remains unclear.
1.2. Non-Compensatory Phonological Processing
Reading disorders are typically associated with reduced activity in temporo-parietal regions related to phonological processing (particularly in adults; Richlan et al., 2011), but increased activation in fronto-striatal regions, e.g. the IFG, precentral gyrus and striatum, has also been reported (Hoeft et al., 2007; Kronbichler et al., 2006; Wimmer et al., 2010). In addition to its significance during reading development (McNorgan, Alvarez, Bhullar, Gayda, & Booth, 2011), the striatum remains involved in phonological processing into adulthood (Abdullaev & Melnichuk, 1997; Abdullaev, 1998; Booth, Wood, Lu, Houk, & Bitan, 2007; Crosson et al., 2003; Tettamanti et al., 2005). Although regional increases in activation are often interpreted as being compensatory, hyperactivation could also indicate dysregulation of fronto-striatal pathways. For example, presumed fronto-striatal dysregulation associated with increased activation has been reported in developmental stuttering (Giraud et al., 2008).
Recently, models of increased temporal sampling rates have been advanced to account for the phonological deficits seen in RD. The frequency of ongoing neural oscillations in auditory brain regions closely matches the rate of formant transitions in speech and may correspond to neural coding of phonemic features (Boemio, Fromm, Braun, & Poeppel, 2005; Morillon et al., 2010). In RD, these oscillations occur at a higher frequency, which could reflect a finer-grained temporal representation, i.e., so that speech information is ‘packaged’ into more units in RD than in typical readers (Lehongre, Morillon, Giraud, & Ramus, 2013; Lehongre, Ramus, Villiermet, Schwartz, & Giraud, 2011). As a consequence, RD may process sequences of greater length during phonological processing than typical readers (Giraud & Poeppel, 2012). Under this model, increases in fronto-striatal regions in RD could reflect an additional, but non-compensatory, demand for sequence or phonological processing in the basal ganglia due to processing phonological representations at a finer level of detail than typical readers. In this case we also expect neuroanatomical correspondence between regions associated with phonological processing and hyperactivity in RD.
1.3. Implicit sequence learning and RD
Fronto-striatal pathways are comprised of several functionally and anatomically segregated loops that are involved in a wide range of behaviors, including motor control (Alexander & Crutcher, 1990; Parent & Hazrati, 1995), language (Abdullaev & Melnichuk, 1997; Crosson et al., 2003; Tettamanti et al., 2005), learning (Packard & Knowlton, 2002) and sensory processing (Brown, Schneider, & Lidsky, 1997; Geiser, Notter, & Gabrieli, 2012). Considering the range of cognitive processes supported by fronto-striatal circuitry, it is also possible that anomalous activation may arise as a neurobiological difference in one or more core fronto-striatal systems that can account for behavioral impairments in reading as well as non-linguistic processes, such as learning.
The ability to extract information from complex linguistic sequences is thought to be a critical capacity for language acquisition that may recruit more domain general skills (Christiansen & Chater, 2008; Christiansen, Conway, & Onnis, 2012; Conway & Pisoni, 2008). In the case of beginning readers, implicit learning mechanisms, supported in part by fronto-striatal circuits (Conway & Pisoni, 2008; Middleton & Strick, 2000), may play an important role in early reading acquisition when children learn initial grapheme-to-phoneme mappings. Studies suggest that both children and adults make use of implicit statistical learning mechanisms when acquiring and using these mappings (Deacon, Conrad, & Pacton, 2008; Samara & Caravolas, 2014; Treiman & Kessler, 2006). Activity in the caudate has been found to positively correlate with later reading ability in young (~9 years of age), but not older children (McNorgan et al., 2011), consistent with the use of this region in early learning and application of phonological and orthographic rules.
Evidence for impairment in implicit sequence learning in RD has been mixed, but a recent meta-analysis supports a moderate level of impairment on serial reaction time tasks (SRTT), a form of implicit sequence learning, in RD, particularly children (Lum, Ullman, & Conti-Ramsden, 2013). Learning deficits in RD and other language disordered adults have been found in other forms of sequence learning, including artificial grammar learning (Plante, Gomez, & Gerken, 2002), but may not extend to implicit learning tasks that do not require sequence learning (Howard, Howard, Japikse, & Eden, 2006). Ullman (2004) suggested that some characteristics of dyslexia arise from impairment in implicit learning mechanisms mediated by fronto-striatal pathways, partially compensated by the use of explicit memory systems. Nicolson and colleagues (Nicolson & Fawcett, 2011; Nicolson, Fawcett, Brookes, & Needle 2010) have also emphasized implicit and motor learning difficulties in dyslexia, but suggest the cerebellum as a neurobiological basis for these and reading deficits. Only two functional neuroimaging studies have examined implicit sequence learning in reading disorder and the collective interpretation of these results is limited by substantial task differences (Menghini, Hagberg, Caltagirone, Petrosini, & Vicari, 2006; Nicolson, Fawcett, Berry, Jenkins, & Dean, 1999). While some form of cerebellar involvement in dyslexia is generally supported by structural and functional imaging (e.g. Linkersdörfer et al., 2012), it is unclear whether this finding is related to automatization as Nicolson and Fawcett suggest, or to phonological processing (Booth et al., 2007; Crosson et al., 2003).
1.4 Use of automated meta-analyses to infer function
We consider the possible role of the fronto-striatal system in RD in terms of learning mechanisms, phonological processing and articulation. In an effort to interpret fronto-striatal activation increases in poor readers, we use manual and automated coordinate-based meta-analyses of functional imaging studies to investigate the similarity between brain regions involved in these three processes and fronto-striatal regions implicated in dyslexia. Specifically we attempt to distinguish three hypotheses regarding fronto-striatal regions implicated in dyslexia: (1) These regions are related to articulatory processing, possibly reflecting compensation for phonological difficulties (Richlan et al., 2011; Tettamanti et al., 2005); (2) These regions are secondarily related to phonological processing (Abdullaev & Melnichuk, 1997; Abdullaev, 1998; Booth et al., 2007; Crosson et al., 2003; McNorgan et al., 2011; Tettamanti et al., 2005); and (3) These regions contribute to non-linguistic impairments, including in implicit sequence learning, which may also make learning to read more difficult. The later is motivated by emerging evidence, discussed above, that dyslexia may be associated with deficits in procedural learning systems. Deficits in these systems may lead to difficulties in implicating learning grapheme-phoneme associations (Lum et al., 2013; Ullman, 2004) or even establishing phonological categories (Gabay & Holt, 2015). Although testing this hypothesis requires direct study of implicit learning mechanisms in RD and a causal role for such deficits, establishing whether hyperactive regions correspond to those supporting implicit learning remains instructive. For example, hyperactivation during reading or rhyming tasks within the implicit learning network could indicate a failure to consolidate implicitly learning phonological or orthographic information into other memory systems, providing indirect support for procedural learning deficit hypotheses.
In each case, we assume that, if RD hyperactivation is associated with a given hypothesized process, the brain regions exhibiting hyperactivation during reading tasks will be shared with those regions implicated in supporting the hypothesized process outside of the RD literature. Thus quantitatively assessing the convergence between regions supporting each of these hypothesized processes and RD regions and their specificity provides some level of insight into which, if any, of these processes are viable explanations for hyperactivity in RD.
Declarative memory systems have also been proposed to play a compensatory role in RD (e.g. Ullman & Pullman, 2015), which may predict hyperactivation patterns consistent with declarative memory in general (e.g. medial temporal regions) or specific declarative memory systems (e.g. semantics). However, compensation through declarative memory is proposed to be highly task-dependent and more evident during learning (Ullman & Pullman, 2015), so a failure to find evidence for declarative memory involvement in RD would also be consistent with Ullman’s proposal, given the nature of the tasks used in RD imaging studies. As there is little neuroimaging evidence to support differential recruitment of medial temporal regions in RD, and Ullman’s proposal is essentially unfalsifiable using the meta-analytic techniques available to us, as we are unable to analyze memory function within other cognitive domains, we do not include declarative memory as a process of interest.
2. Methods
To examine brain regions that are associated with articulatory and phonological processing, automated meta-analyses were conducted using the Neurosynth database (Yarkoni et al., 2011). The Neurosynth database (Yarkoni et al., 2011) contains meta-analytic maps for 525 terms, automatically derived from coordinates reported in 5,808 papers from selected journals with a high incidence of functional neuroimaging papers. For each term and voxel within a 2mm resolution space, the posterior probability of a term occurring in the text of a study given reported coordinates within 10mm of the voxel is calculated, using a uniform prior on term occurrences. The size of the database provides a reasonable basis for estimating activation base rates, which is necessary for formal reverse inference and enables probabilistic inference about which cognitive processes (summarized as terms in the database) may be associated with brain states (Poldrack, 2011). While this approach reflects unavoidable bias in the literature coverage of cognitive processes and null results, it is not biased by manual selection of the literature. This approach is also unlikely to identify regions that may be critical for one of the cognitive processes of interest, but have a high base rate of activation (e.g. the striatum or insula). Automated meta-analysis of sequence learning (57 studies) captures studies beyond the implicit sequence learning tasks reported to be impaired in RD, e.g. word segmentation studies (Karuza et al., 2013), studies in dyslexia (Danelli et al., 2013) and explicit sequence learning studies (Yang & Li, 2012).
To identify regions of fronto-striatal hyperactivation associated with RD, coordinate-based meta-analysis of RD studies was performed using manual literature search and data entry. Activation foci in the Neurosynth database are not distinguishable based on comparisons of interest or the directionality of the activation. Thus, although the term ‘dyslexia’ is included in the Neurosynth database, a manual meta-analysis of studies comparing poor and typical readers was necessary to identify regions of hyperactivation in RD.
We then quantitatively investigated convergence with RD fronto-striatal regions and regions identified in the articulatory, phonological and implicit learning meta-analyses. This analysis was supplemented by a more qualitative reverse inference analysis of putative functional networks linked to RD fronto-striatal regions, also conducted using the Neurosynth database.
2.1 Meta-Analysis of RD
RD imaging studies (Table 1) were identified as in previous work (Richlan et al., 2009, 2011). Briefly, PubMed was searched using the keywords ‘dyslexia’ and ‘imaging’. Studies were included if (1) stimuli were letter strings of words or pseudowords, or single letters, (2) tasks were reading or reading-related (e.g., rhyme judgments), and (3) group comparisons (dyslexics vs. controls) were reported in a standard stereotactic space (Talairach or MNI).
Table 1.
Sources and key characteristics of studies included in the reading meta-analysis.
| First author (year) |
N | Native language |
Age mean (SD or range) |
No. foci | ||||
|---|---|---|---|---|---|---|---|---|
| Modality | RD | TR | Task type | Hyper | Hypo | |||
| Bach (2010) | fMRI | 14 | 18 | German | 8.3 (0.4) | Letter substitution | 14 | 2 |
| Blau (2010) | fMRI | 18 | 16 | Dutch | 9.4 (0.4) | Letter-speech-sound integration |
0 | 2 |
| Blau (2009) | fMRI | 13 | 13 | Dutch | 25.2 (4.6) | Letter-speech-sound integration |
0 | 3 |
| Booth (2007) | fMRI | 15 | 17 | English | 10.6 (2.2) | Semantic association judgment |
1 | 1 |
| Brambati (2006) | fMRI | 13 | 11 | Italian | 30.5 (range 13– 63) |
Silent reading | 0 | 9 |
| Cao (2006) | fMRI | 14 | 14 | English | 11.6 (range 8– 14) |
Word rhyme judgment |
0 | 6 |
| Georgiewa (1999) | fMRI | 17 | 17 | German | 14.0 (range 9– 17) |
Word reading | 2 | 2 |
| Gruenling (2004) | fMRI | 17 | 21 | German | 13.6 (1.5) | Pseudoword Rhyming |
32 | 3 |
| Hoeft (2006) | fMRI | 10 | 20 | English | 11.2 (0.5) | Word rhyme judgment |
0 | 6 |
| Hoeft (2007) | fMRI | 19 | 19 | English | 14.4 (2.4) | Word rhyme judgment |
7 | 4 |
| Hu (2010) | fMRI | 19 | 18 | Chinese, English | 14.0 (range 12.1–16) |
Semantic association judgment |
1 | 6 |
| Ingvar (2002) | PET | 9 | 9 | Swedish | 23.5 (range 20– 28) |
Reading silently and aloud |
3 | 3 |
| Kronbichler (2006) | fMRI | 13 | 15 | German | 15.5 (.58) | Sentence verification | 13 | 2 |
| Kronschnabel (2013) |
fMRI | 13 | 22 | German | 16 (.7) | Implicit reading | 0 | 4 |
| Maurer (2011) | fMRI | 11 | 16 | German | 11.4 (0.4) | Target detection | 1 | 13 |
| McCrory (2005) | PET | 8 | 10 | English | 20.2 (1.9) | Word reading and picture naming |
0 | 1 |
| Meyler (2008) | fMRI | 23 | 12 | English | 10.8 (0.5) | Sentence evaluation | 2 | 6 |
| Monzalvo (2012) | fMRI | 23 | 23 | French | 9.8 (range 9.1– 10.5) |
Implicit word reading |
0 | 1 |
| Paulesu (1996) | PET | 5 | 5 | English | 26.2 (1.9) | Letter pair rhyme judgment |
0 | 6 |
| Pecini (2011) | fMRI | 13 | 13 | Italian | 24 (13) | Rhyme generation | 0 | 4 |
| Richlan (2010) | fMRI | 15 | 18 | German | 18.0 (1.1) | Phonological lexical decision |
18 | 15 |
| Rumsey (1997) | PET | 17 | 14 | English | 26.0 (6.5) | Reading aloud | 7 | 13 |
| Schulz (2009) | fMRI | 15 | 15 | German | 11.5 (0.4) | Sentence evaluation | 0 | 9 |
| Tanaka (2011a) | fMRI | 31 | 26 | English | 10.3 (1.1) | Word rhyme judgment |
0 | 2 |
| Tanaka (2011b) | fMRI | 38 | 36 | English | 12.7 (3.9) TR; 14 (1.8) RD |
Word rhyme judgment |
0 | 2 |
| Temple (2001) | fMRI | 24 | 15 | English | 10.6 (1.4) | Visual letter matching and line matching |
1 | 5 |
| Van der Mark (2009) |
fMRI | 18 | 24 | German | 11.4 (0.6) | Phonological lexical decision |
0 | 8 |
| Wimmer (2010) | fMRI | 20 | 19 | German | 20.6 (6.8) | Phonological lexical decision |
10 | 3 |
Abbreviations: Reading disorder (RD); typical reader (TR)
Signed Differential Mapping (SDM) version 4.13 with anisotropic kernels (Radua et al., 2014) was used to generate meta-analytic maps from foci reported in the above studies. Coordinates reported in Talairach space were converted to MNI space (Lancaster et al., 2007). When available, peak t-values were converted to effect sizes and convolved with a smoothing kernel. When peak values were not available, the suggested effect size of d=±1.26 (one-sample) or d=±1.57 (two-sample) was used (Radua et al., 2012). Default values were used for analysis, including a 20 mm FWHM anisotropic kernel. The anisotropic kernel applies a spatial deformation that takes into account the correlations between nearby voxels, based on a grey matter template. The resulting meta-analytic maps were thresholded at a height of p < .005 (uncorrected) and cluster extent of 10 voxels, which is approximately equivalent to the corrected p-value of .05 (Radua et al., 2012) used to threshold Neurosynth-based meta-analytic and functional connectivity maps (described below). Monte Carlo simulations (n=500) were used to obtain empirical p-values. Conjunctions between the resulting hyper- (RD > Typical reader) or hypoactivation (Typical reader > RD) maps and meta-analytic maps for implicit learning, articulation or phonological processing (described below) was used to investigate the similarity between regions implicated in poor reading and those involved in cognitive processes of interest (articulatory processing, phonological processing, and implicit learning). Although this type of analysis cannot conclusively support the existence of a common neurobiological basis that can account for both poor reading and e.g. implicit sequence learning, convergence between meta-analyses may suggest regions or networks that can be targeted for future empirical investigation.
2.2 Automated Meta-Analysis of Articulatory and Phonological Networks
We extracted reverse inference brain images from Neurosynth for each of the terms ‘articulatory’ and ‘phonological’, our two a priori cognitive processes of interest. The reverse inference map for articulation was derived from 27 studies containing (with frequency greater than 1 occurrence per 1000 words) the term 'articulatory' but not ‘phonological’. The reverse inference map for phonology was constructed from 123 studies containing the term ‘phonological’ and excluding ‘articulatory’. Studies with the terms ‘reading’ or ‘dyslexia’ were excluded from both maps to avoid biasing comparisons with the dyslexic meta-analytic maps. All maps were false discovery rate (FDR) corrected at P < .05.
2.3 Manual Meta-Analysis of Implicit Sequence Learning
The reverse inference map for sequence learning was constructed from 57 studies containing both the terms ‘sequence’ and ‘learning.’
Only 14 studies in the Neurosynth database included all of the terms ‘implicit’, ‘sequence’ and ‘learning’ with a frequency greater than 1 per 1000 words, which may produce unreliable results. Although it would be feasible to construct an automated reverse inference map based on the 57 studies containing both the terms ‘sequence’ and ‘learning’, it is not possible to reliably dissociate implicit sequence learning from sequence learning in general, including more explicit forms of learning, using Neurosynth. As it is implicit sequence learning, as opposed to other forms of learning, that is hypothesized to be impaired in RD, we employed a manual meta-analysis of implicit sequence learning tasks, described below. Furthermore, the larger Neurosynth map for sequence learning (not reported here) largely encompasses the manually-derived meta-analytic map for implicit sequence learning (with the exception of superior temporal regions). Preliminary analyses, also not reported here, also indicated poor convergence between the Neurosynth sequence learning map and RD regions.
The manual meta-analysis focused on non-linguistic implicit sequence learning studies. Functional neuroimaging studies of implicit learning were identified by searching PubMed with the keywords ‘implicit learning’, ‘procedural learning’, ‘sequence learning’, ‘serial reaction time task’ or ‘serial response time task’ occurring in conjunction with ‘positron emission tomography’, ‘PET’, ‘functional MRI’, ‘fMRI’, ‘BOLD’, ‘magnetic resonance imaging’, ‘functional neuroimaging’, ‘blood flow’, or ‘blood oxygen’. Studies were included if they (1) reported standard space coordinates for at least one neurologically normal group, (2) reported results from a task that required participants to respond to a non-linguistic, non-random sequence of stimuli without being explicitly informed of the existence of the sequence, (3) did not exclusively report paradigm manipulation contrasts (e.g. dual vs. single task conditions or explicit vs. implicit instruction) and (4) reported whole-brain results at a single statistical threshold. Three studies (Martis, Wright, McMullin, Shin, & Rauch, 2004; Woodward, Tibbo, & Purdon, 2007; Zedkova, Woodward, Harding, Tibbo, & Purdon, 2006) reported activations from regions of interest in addition to reporting whole brain results outside the ROI. Peaks within the ROIs were included if they met the whole brain significance criteria. Several papers reported results separately for young and old (>60 years) adults (Bo, Peltier, Noll, & Seidler, 2011; Daselaar, Rombouts, Veltman, Raaijmakers, & Jonker, 2003; Dennis & Cabeza, 2011; Rieckmann, Fischer, & Bäckman, 2010). Since adult studies of dyslexia primarily recruit younger adults, only coordinates from the young adult populations within these studies were included in the meta-analysis. Coordinates from subjects with specialized musical training (Landau & D’esposito, 2006) were also excluded. Coordinates from 28 studies and 362 subjects were entered into the meta-analysis (Table 2).
Table 2.
Sources and key characteristics of studies included in the implicit learning meta-analysis. Studies in bold were also included in the Neurosynth ‘sequence learning’ analysis.
| First author (year) |
No. of Foci |
N | Age | Response Hand |
Modality | Contrast |
|---|---|---|---|---|---|---|
|
Learning-related contrasts |
||||||
| Bischoff-Grethe (2004) | 54 | 16 | 19.8 | Right | fMRI | Learning related BOLD change during learning |
| Grafton (1995) | 10 | 12 | 22.0 | Right | PET | rCBF change over learning blocks |
| Grafton (2002) | 31 | 8 | 26.0 | Left | PET | |
| Hazeltine (1997) | 17 | 11 | 21.5 | Right | PET | rCBF change over learning blocks |
| Honda (1998) | 2 | 14 | 36.0 | Right | PET | correlation between RT and rCBF during learning |
| Marvel (2007) | 9 | 11 | 29.5 | Right | PET | Correlation between rCBF and RT reduction |
| Muller (2004) | 29 | 8 | 28.1 | Dominant | fMRI | Learning > single button response |
| Rieckmann (2010) | 35 | 14 | 24.7 | Bilateral | fMRI | Sequence > Random changes between first and second half |
| Strangman (2005) | 12 | 9 | 26.5 | Right | fMRI | Slope over learning |
| van der Graaf (2006) | 11 | 8 | 23.1 | Bilateral | fMRI | Final [Seq-Random] > Intial [Seq-Random] |
| Wadden (2013) | 6 | 10 | 64.7 | Right | fMRI | Correlation with temporal precision |
|
Sequence vs Random contrasts |
||||||
| Berns (1997) | 3 | 10 | Right | PET | 2nd grammar > first grammar | |
| Bo (2011) | 23 | 14 | 21.4 | Right | fMRI | Sequence > Random (pre+post), averaged across conditions and time |
| Daselaar (2003) | 21 | 26 | 32.4 | Bilateral | fMRI | Sequence > Random |
| Dennis (2011) | 9 | 12 | 22.0 | not spec | fMRI | Sequence > Random |
| Doyon (1996) | 11 | 14 | 38.7 | Right | PET | Highly learned > random |
| Gheysen 2010 | 20 | 22 | 22.0 | Bilateral | fMRI | Sequence > Random |
| Gobel (2011) | 11 | 18 | 24.0 | Bilateral | fMRI | Sequence > Random and New |
| Kumari (2002) | 14 | 6 | 31.8 | Right | fMRI | Pattern > Random |
| Landau (2006) | 9 | 8 | 20.6 | Bilateral | fMRI | Sequence > Random |
| Martis (2004) | 6 | 27 | 28.3 | Bilateral | fMRI | Sequence > Random |
| Naismith 2010 | 21 | 20 | 50.6 | Unilateral | fMRI | Sequence > Random |
| Rauch (1995) | 6 | 7 | 26.7 | Bilateral | PET | Sequence > Random |
| Rauch (1997) | 10 | 10 | 27.5 | Bilateral | fMRI | Sequence > Random |
| Ursu (2009) | 13 | 10 | 24.4 | Bilateral | fMRI | No Conflict > Conflict |
| Werheid (2003) | 7 | 7 | 52.9 | Bilateral | fMRI | Sequence > Random |
| Woodward (2007) | 17 | 15 | 31.3 | Bilateral | fMRI | Sequence > Random |
| Zedkova (2006) | 10 | 15 | 31.3 | Bilateral | fMRI | Sequence > Random |
The selected studies reported results from of two distinct analyses: sequence-related and learning-related analysis because performance changes may have a partially distinct brain basis from sequence encoding (Seidler et al., 2002). We therefore also examined sequence-related analysis that compared blocks of a learned sequence with random or novel sequence blocks and learning-related analysis that examined changes in regional cerebral blood flow (rCBF) or blood-oxygen-level dependent (BOLD) signal across the course of the experiment or correlations between rCBF/BOLD signal with behavioral performance over blocks. Since the theoretical relevance of implicit sequence learning mechanisms in RD is based on the ability to extract and use statistical information in grapheme-phoneme mappings, we focus primarily on the sequence-related results. These results compare sequences with meaningful statistical information to those that do not, providing information about the regions relevant to distinguishing these two types of sequences. In contrast, the learning-related comparisons are sensitive to other factors, such as motor execution, that may contribute to behavioral performance in implicit sequence learning tasks. Each of these two meta-analyses was conducted using SDM, following the procedure described in §2.1.
2.4 Convergence in neural processes involved in Articulation, Phonology, Implicit Learning and RD
Spatial convergence between RD regions and regions involved in the process of interest was quantified using logistic regression. For each of the binarized hypo- or hyperactive RD maps, we fitted a multiple logistic regression model to predict the value of each in-brain voxel based on the value of corresponding voxels in the binarized articulatory, phonological, and implicit sequence learning maps. The contribution of each term to the model (i.e. the extent to which each predictor map improved prediction of voxels in the hypo or hyperactivation RD map beyond the other predictor maps) was assessed using a likelihood ratio test between the full model and a reduced model without the term. To examine the dependence of the results on the choice of statistical threshold, these models were fitted with a range of statistical thresholds applied to the RD meta-analysis (height p < .01, < .005 and < .001; k = 10 for all), while keeping the threshold fixed at the a priori threshold for the predictor articulatory, phonological and implicit sequence learning maps. The threshold of p < .005, k = 10 used for manual meta-analysis is approximately equivalent to the FDR-corrected p-value of .05 (Radua et al., 2012). The automated meta-analysis thresholds (articulatory and phonological maps) were fixed at p < .05 (FDR corrected).
2.5 Developmental differences
Comparing across studies, children and adults have been found to exhibit different RD-related patterns of brain activity, particularly for hyperactivation patterns, with adults showing greater hyperactivation in the striatum (Richlan et al., 2011). To examine possible age-related differences, we also conducted separate RD meta-analyses for children and adults and examined convergence with the articulatory, phonological and implicit sequence learning maps, as described above, within each age group.
3. Results
3.1 Regions associated with poor reading
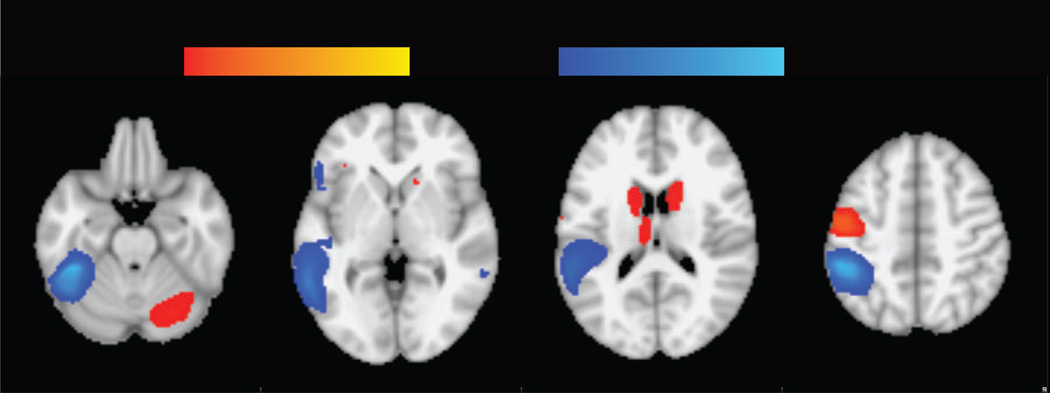
Consistent with previous meta-analyses (Linkersdörfer et al., 2012; Maisog et al., 2008; Richlan et al., 2009), results of the RD meta-analysis showed a broad left temporo-parietal and occipto-temporal region of hypoactivation (Typical > RD readers) in RD and additional clusters of hypoactivation in the left IFG and right middle temporal gyrus (MTG). Hyperactivation (RD > Typical readers) in RD was found in several fronto-striatal-cerebellar regions also consistent with previous meta-analyses, including the bilateral caudate body, left pre/postcentral gyrus, left IFG/insula and right cerebellum (Figure 1, Table 3). Hyperactivation within the basal ganglia and thalamus was found only adults.
Figure 1.
Meta-analysis of RD, showing regions of hypoactivation (cold color) and hyperactivation (warm color) in RD. Thresholded at p < .005 (uncorrected) with a cluster extent threshold of 10 voxels, equivalent to p < .05 FDR-corrected.
Table 3.
Results of RD meta-analysis.
| Region | Peak (MNI) | Peak SDM-Z |
Cluster size (voxels) |
||
| Typical > RD | x | y | z | ||
| L FGA,C | −40 | −40 | −18 | 4.39 | 8046 |
| L SMGA,C | −50 | −38 | 46 | 4.25 | * |
| L ITGA,C | −54 | −58 | −10 | 3.32 | * |
| L MTGC | −60 | −52 | −2 | 3.20 | * |
| L IFG OpA | −48 | 12 | 6 | 1.66 | 217 |
| L IFG TriA | −54 | 26 | 2 | 1.57 | * |
| R MTGC | 60 | −44 | −2 | 1.59 | 60 |
| RD > Typical Readers | |||||
| R Crus IA,C | 26 | −70 | −30 | 1.54 | 1344 |
| L PreCGA,C | −50 | −10 | 50 | 2.52 | 1253 |
| L PostCGA | −58 | 6 | 22 | 1.46 | * |
| R Caudate bodyA | 16 | 14 | 10 | 1.40 | 423 |
| CingulateC | −6 | −12 | 28 | 1.26 | 288 |
| L CaudateA | −18 | 0 | 20 | 1.30 | 277 |
| L ThalamusA | −8 | −16 | 20 | 1.37 | 110 |
| L InsulaC | −32 | 26 | −8 | 1.07 | 21 |
| L IFG TriC | −34 | 30 | 2 | 1.10 | 11 |
Significant clusters thresholded at a height of p < .005 and cluster extent > 10 voxels.
Local maxima within a cluster.
Abbreviations: fusiform gyrus (FG), supramarginal gyrus (SMG), inferior temporal gyrus (ITG), middle temporal gyrus (MTG), inferior frontal gyrus (IFG), precentral gyrus (preCG), postcentral gyrus (PostCG).
Labels are assigned based on the FSL Harvard-Oxford atlas.
Regions appearing in the adult meta-analysis.
Regions appearing the child meta-analysis.
3.2 Regions associated with articulation and phonology
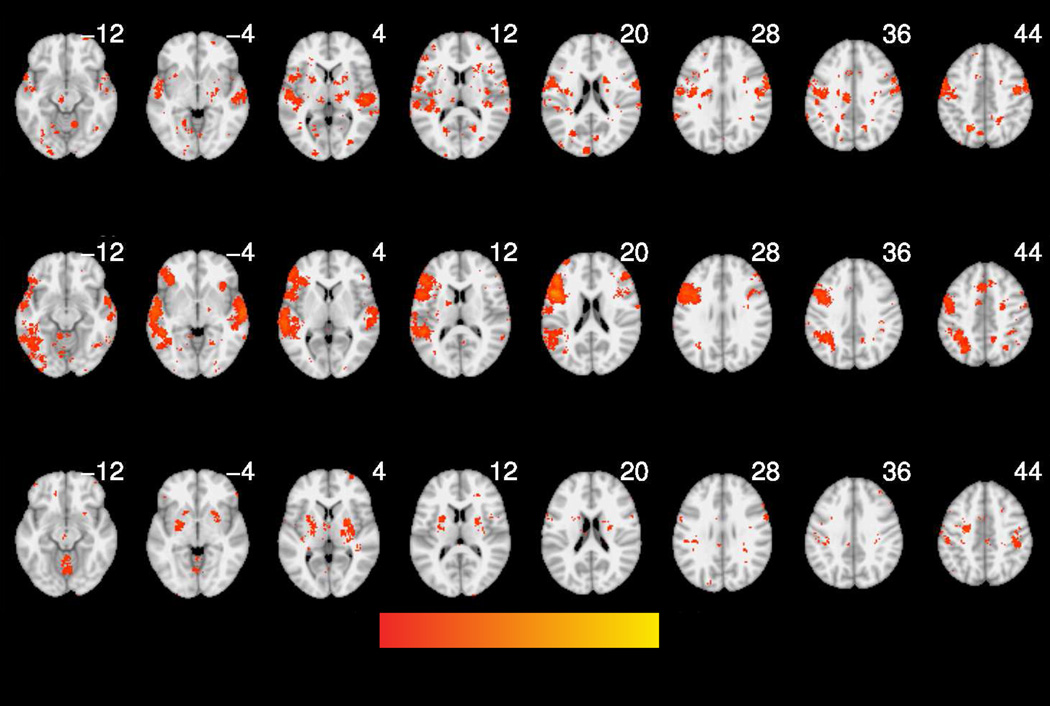
Reverse inference maps for each of the Neurosynth-based analyses are shown in Figure 2. Regions associated with articulation included the bilateral thalamus, multiple bilateral regions of the cerebellum and large bilateral clusters in the lateral precentral gyri, extending into the postcentral gyri and portions of the left frontal operculum. Regions associated with phonological processing were generally left-lateralized and included large portions of the left lateral precentral gyrus, IFG and supramarginal/angular gyrus and bilateral superior temporal gyrus (STG).
Figure 2.
Reverse inference maps (derived from Neurosynth) for articulation (a), phonology (b), and sequence learning (c). Thresholded at p < .05 (FDR-corrected).
3.3 Regions associated with implicit sequence learning
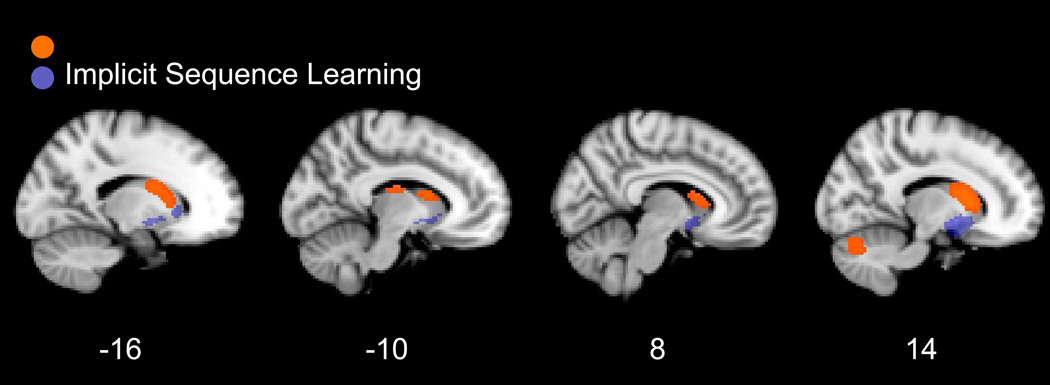
Regions associated with implicit sequence learning in our manual meta-analysis included the bilateral striatum, left superior temporal gyrus and left supramarginal gyrus (Table 4; Figure 3).
Table 4.
Results of supplementary implicit sequence learning meta-analysis
| Region | x | y | z | Peak SDM-Z |
Cluster size (voxels) |
|---|---|---|---|---|---|
| Sequence > Random | |||||
| Right lentiform nucleus |
14 | 2 | −6 | 3.50 | 1453 |
| Left caudate head | −10 | 12 | −4 | 2.74 | * |
| Right amygdala | 24 | −2 | −16 | 2.72 | * |
| Right hippocampus | 30 | −8 | −14 | 2.41 | * |
| Left pallidum | −14 | 6 | −4 | 2.40 | * |
| Right caudate head | 8 | 16 | −2 | 2.27 | * |
| Left STG | −52 | −8 | −4 | 2.42 | 498 |
| Left SMG | −54 | −54 | 28 | 2.21 | * |
| Left insula | −40 | −6 | −4 | 2.12 | * |
| Left orbital IFG | −46 | 32 | −16 | 2.15 | 105 |
Significant clusters thresholded at a height of p < .005 and cluster extent > 10 voxels.
Local maxima within a cluster.
Abbreviations: supramarginal gyrus (SMG), superior temporal gyrus (STG), inferior frontal gyrus (IFG).
Labels are assigned based on the FSL Harvard-Oxford atlas.
Figure 3.
Supplementary manual meta-analysis of implicit sequence learning. Thresholded at p < .005 (uncorrected) with a cluster extent threshold of 10 voxels.
3.2 Functional Role of Hypoactivation Map in RD
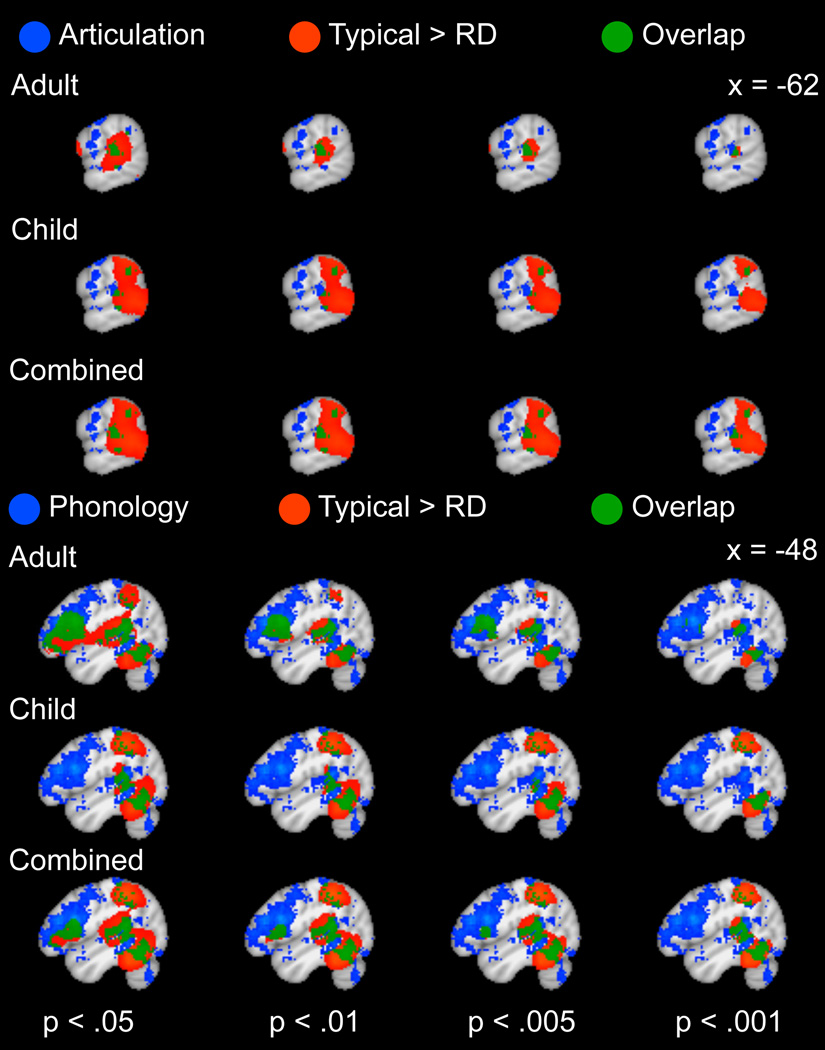
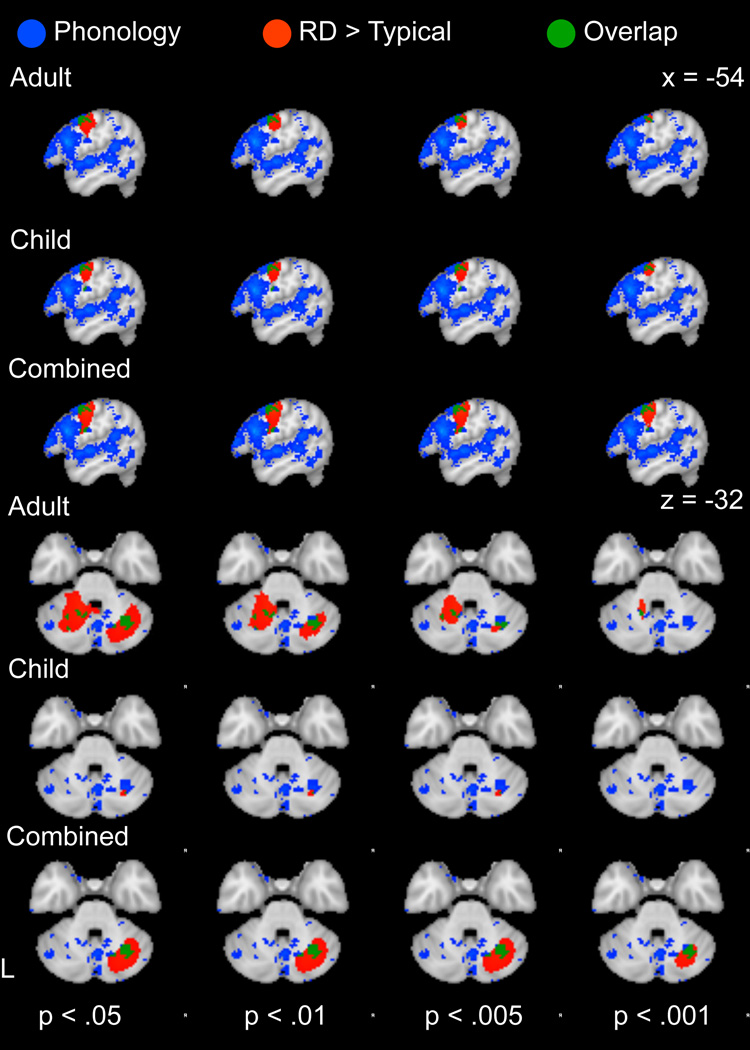
Consistent with evidence for a phonological processing deficit, the phonological map (derived from Neurosynth meta-analysis) overlapped with temporo-parietal hypoactivation regions (Figure 4) and substantially improved the fit of the hypoactive logistic regression models (X(1)2 = 5949, 6764, 7669 for children, adults and both ages, respectively) compared to a reduced model with articulatory and (implicit) sequence learning maps as predictors. Although articulatory (also derived from Neurosynth) and implicit sequence learning maps also improved the fit of the hypoactive logistic model relative to reduced models without these terms, the contribution of these maps to the model fit was an order of magnitude lower than that of phonological predictor (Table 5). Below, we focus on comparisons between hyperactivation regions and regions recruited for articulatory and phonological processing and implicit sequence learning.
Figure 4.
(a) Comparison between regions involved in phonological processing (blue), identified using reverse inference with the Neurosynth database and cortical regions of decreased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green. (b) Comparison between regions involved in articulatory processing (blue), identified using reverse inference with the Neurosynth database and cortical regions of decreased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green. The results of the RD meta-analysis are presented at a voxel threshold of p < .05 to p < .001 to illustrate the stability of the overlap.
Table 5.
Model fit summaries.
| P < .01 | P < .005 | P < .001 | |
|---|---|---|---|
| X2 | X2 | X2 | |
| Children | |||
| Hyperactivation | |||
| Articulation | 636*** | 644*** | 351*** |
| Phonology | 185*** | 116*** | 112*** |
| IL | 67*** | 43*** | 14*** |
| Hypoactivation | |||
| Articulation | 384*** | 358*** | 350*** |
| Phonology | 6409*** | 5949*** | 5020*** |
| IL | 82*** | 56*** | <1 |
| Adults | |||
| Hyperactivation | |||
| Articulation | 480*** | 223*** | 19*** |
| Phonology | 173*** | 234*** | 413*** |
| IL | 4* | 1 | 1 |
| Hypoactivation | |||
| Articulation | 403*** | 21*** | 2 |
| Phonology | 8120*** | 6764*** | 2435*** |
| IL | 150*** | 154*** | 121*** |
| Combined | |||
| Hyperactivation | |||
| Articulation | 491*** | 568*** | 542*** |
| Phonology | 27*** | 40*** | 128** |
| IL | 73*** | 67*** | 43*** |
| Hypoactivation | |||
| Articulation | 7** | 44*** | 158*** |
| Phonology | 8064*** | 7669*** | 6709*** |
| IL | 157*** | 140*** | 64*** |
Model term significance:
p<.001***;
p<.01**;
p<.05*
Abbreviations: implicit sequence learning [manual](IL)
3.3 Functional Role of Hyperactivation Map in RD
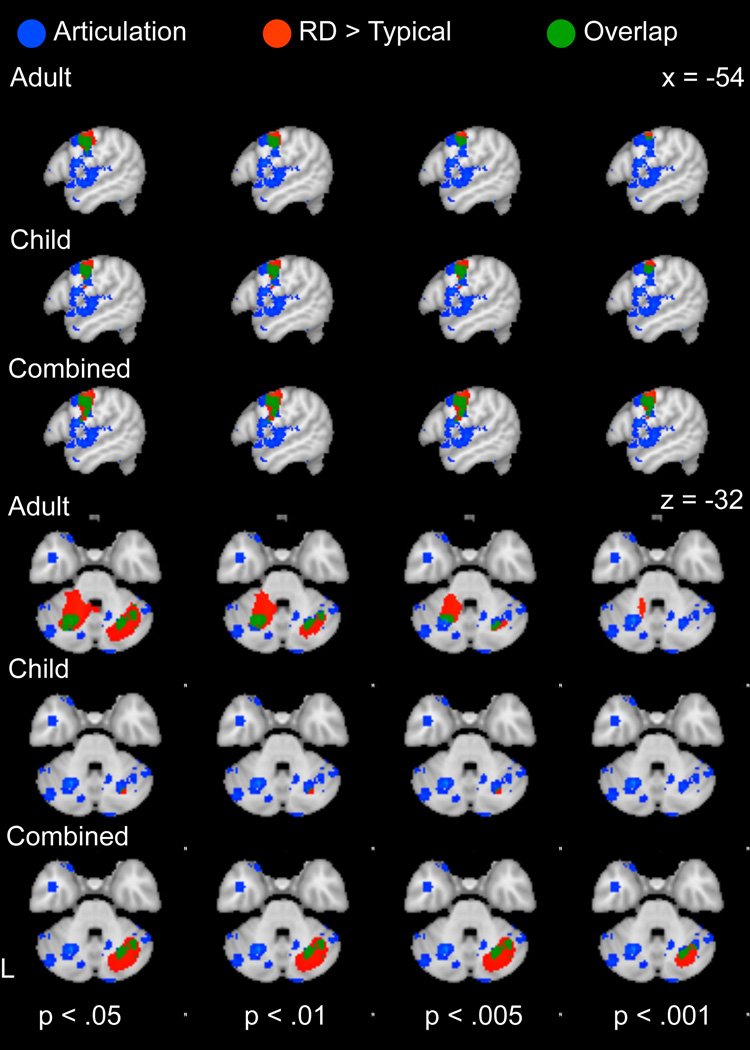
3.3.1 Spatial Overlap between RD Hyperactivation and Articulatory Processing Maps
To evaluate suggestions that RD-related hyperactivation reflects articulatory compensation, we first examined the convergence between hyperactive regions and regions active during articulation, as identified in Neurosynth. The articulatory reverse inference map intersected with several regions of RD-related hyperactivation, including the left precentral gyrus and right cerebellum (Figure 5a). Subcortical articulatory regions were primarily in the bilateral putamen and thalamus, but overlapped with the RD meta-analysis in the right caudate (Figure 5b). Of the three a priori maps we examined, the articulatory map contributed more to model fit (X(1)2 = 568) than the phonological (X(1)2 = 40 or implicit sequence learning (X(1)2 = 67) at a threshold of p < .005, k = 10 for hyperactive RD map, when combining across ages (Table 5). Similar results were obtained for other thresholds applied to the RD map (Table 5). When considering adults and children separately, a more ambiguous pattern emerged. The association between articulation and hyperactivation remained high in children across a range of thresholds, but a threshold-dependent association was found for hyperactivation in adults, with the contribution of the articulatory map decreasing at higher thresholds (but remaining significant).
Figure 5.
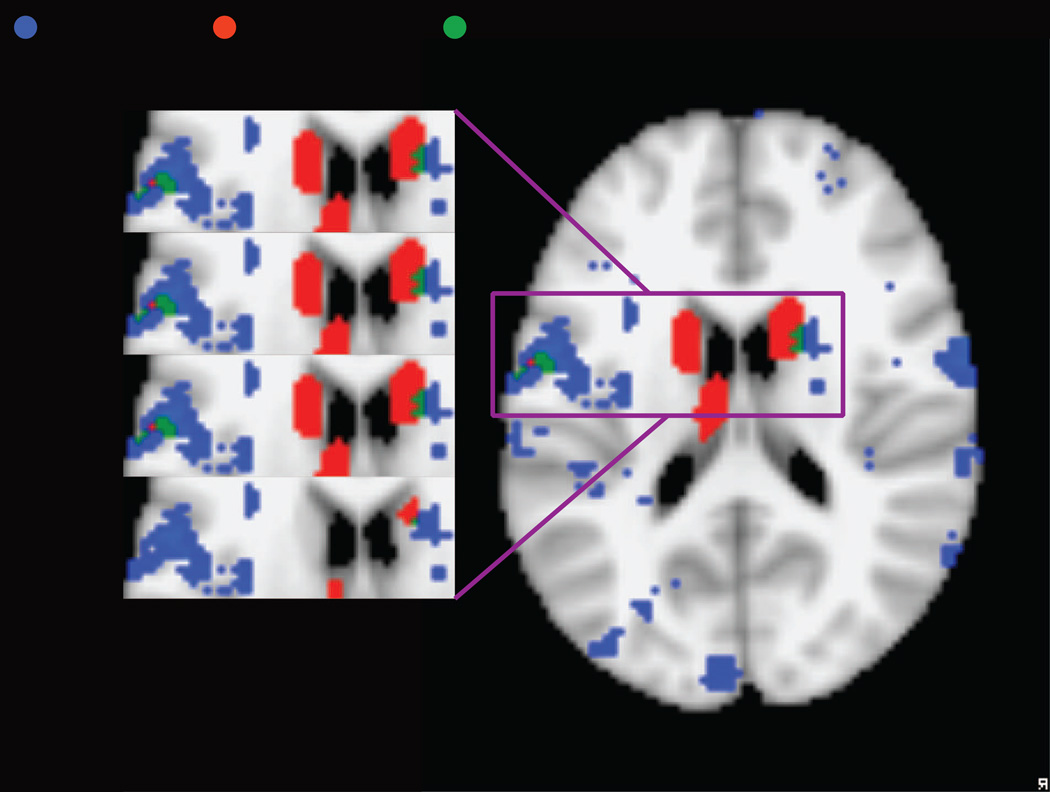
(a) Comparison between cortical and cerebellar regions involved in articulatory processing (blue), identified using reverse inference with the Neurosynth database and cortical regions of increased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green. (b) Detail of overlap between subcortical and IFG/insula regions involved in articulatory processing (blue), identified using reverse inference with the Neurosynth database and cortical regions of increased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green. The results of the RD meta-analysis are presented at a voxel threshold of p < .05 to p < .001 to illustrate the stability of the overlap.
3.3.2 Spatial Overlap between RD Hyperactivation and Phonological Processing Maps
To investigate phonological processing as an alternative source of compensatory activity, a similar analysis was conducted using the phonological reverse inference map from Neurosynth. As mentioned above in 3.3a, inclusion of the phonological map contributed less to the fit of the logistic model (X(1)2 = 40) than the articulatory map (X(1)2 = 568). The phonological map overlapped with RD-related hyperactivation in the left precentral gyrus, right cerebellum and left caudate (Figure 6).
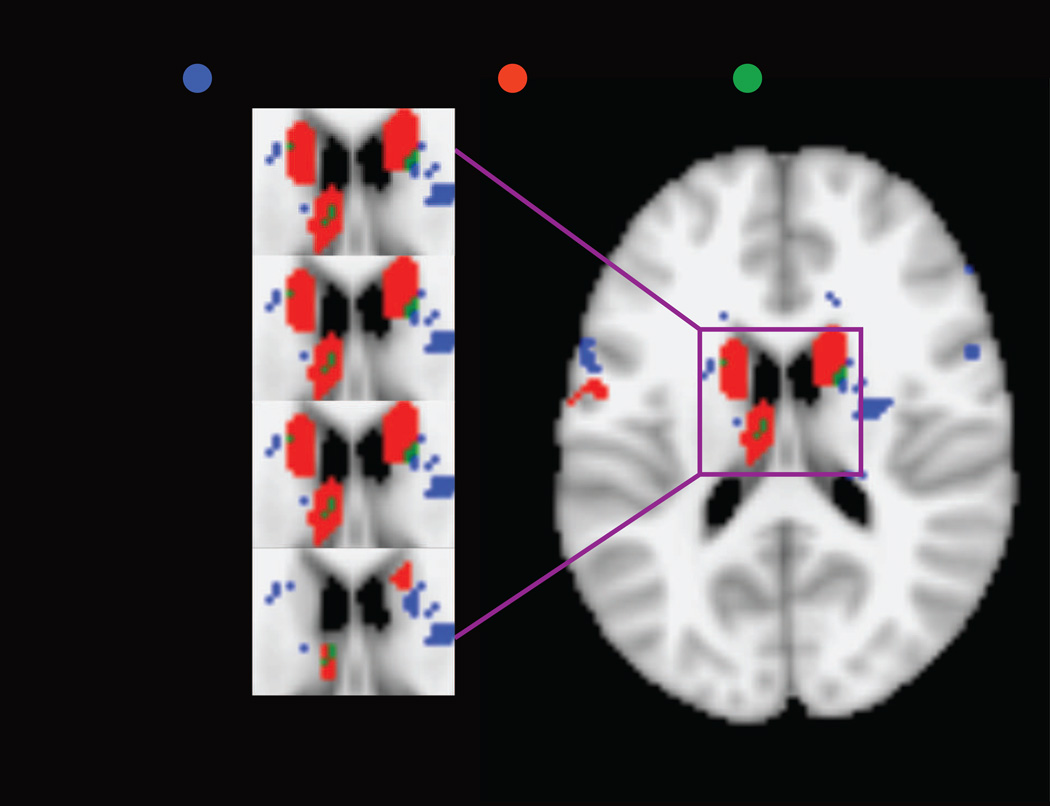
Figure 6.
(a) Comparison between cortical and cerebellar regions involved in phonological processing (blue), identified using reverse inference with the Neurosynth database and cortical regions of increased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green. (b) Detail of overlap between subcortical and IFG/insula regions involved in phonological processing (blue), identified using reverse inference with the Neurosynth database and cortical regions of increased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green. The results of the RD meta-analysis are presented at a voxel threshold of p < .05 to p < .001 to illustrate the stability of the overlap.
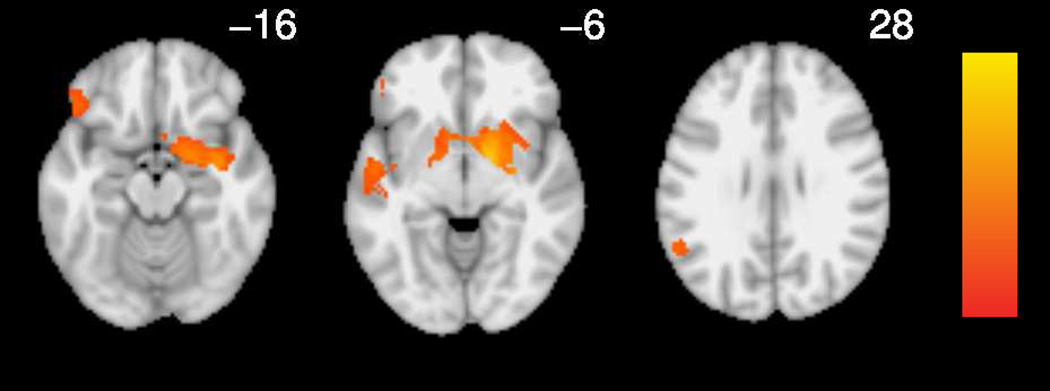
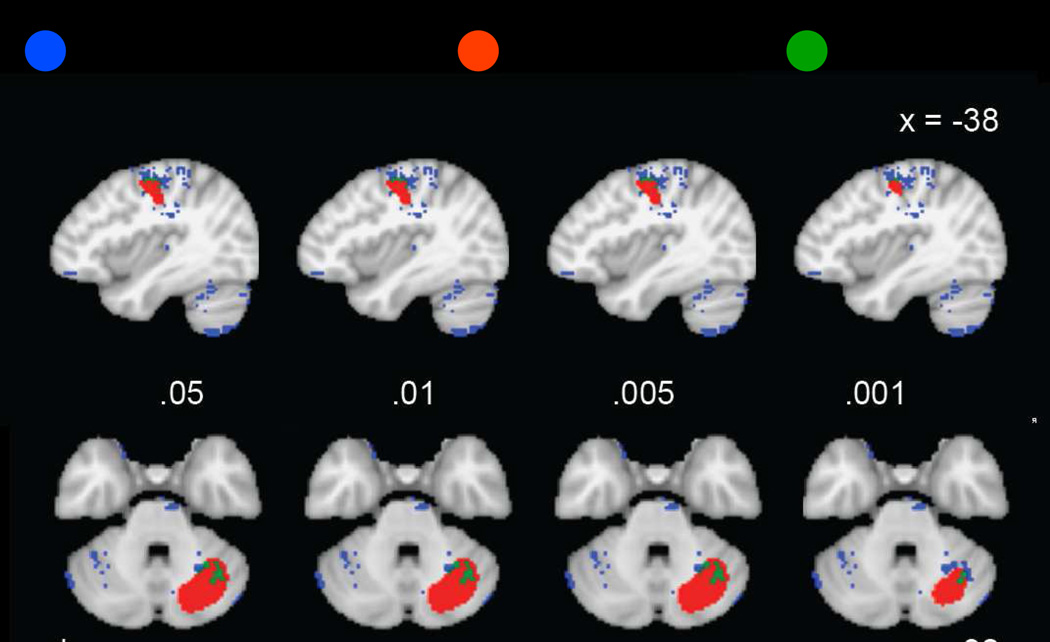
3.3.3 Spatial Overlap between RD Hyperactivation and Implicit Sequence Learning Maps
Finally, we investigated the possibility that increased fronto-striatal activation in RD is related to non-linguistic processing deficits that have also been reported in RD, specifically implicit sequence learning. The intersection of the thresholded binarized meta-analytic maps of hyperactivation in RD identified small clusters of overlap in the left striatum for the implicit sequence learning map (Figure 7). Aside from this small cluster that showed overlap in the caudal head of the left caudate, striatal regions identified in the implicit sequence learning and RD-related hyperactivation meta-analyses did not overlap. Activations were largely restricted to the head of the caudate nucleus for implicit sequence learning and to the caudate body for RD-related hyperactivation (Figure 8), regions which have distinct functions and connectivity (Robinson et al., 2012). Inclusion of the implicit sequence learning map contributed less to the fit of the logistic model (X(1)2 = 67) than the articulatory map (X(1)2 = 568).
Figure 7.
Detail of overlap between subcortical and IFG/insula regions involved in sequence learning (blue), identified using reverse inference with the Neurosynth database and cortical regions of increased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green. The results of the RD meta-analysis are presented at a voxel threshold of p < .05 to p < .001 to illustrate the stability of the overlap.
Figure 8.
Comparison between striatal and cerebellar regions involved in implicit sequence learning (purple), identified using a manual meta analysis of studies reporting a Sequence > Random contrast, database and cortical regions of increased activation in RD relative to typical readers (orange) based on a manual meta-analysis of RD studies.
4. Discussion
A number of functional imaging studies of RD have reported regions of fronto-striatal hyperactivation in RD during reading and phonological processing. While this hyperactivation is commonly discussed in terms of neural compensation to support phonological or articulatory compensation strategies, there is no direct evidence that hyperactivation in these regions is associated with improved performance and indeed reflects compensation. Since these regions also support a variety of cognitive processes, hyperactivation could reflect involvement of processes unrelated to language tasks. To clarify the role of hyperactivation regions, we applied meta-analytic decoding (Poldrack, 2011), using manual and automatically constructed (Yarkoni et al., 2011) meta-analyses, to a manual meta-analysis of RD. We evaluated the convergence between hyperactivation regions and regions recruited during proposed compensatory processes (phonological and articulatory processing) as well as regions involved in implicit sequence learning, a skill that is potentially relevant to reading development and also impaired in RD. Consistent with evidence that RD is largely due to a phonological processing deficit and validating the meta-analytic approach, there was a high degree of convergence between hypoactive RD regions and the reverse inference map for phonological processing, but not for the articulatory or sequence learning maps.
4.1 RD and implicit sequence learning
We found little support for a shared neural basis for RD hyperactivation and sequence learning processes, consistent with previous comparisons (Danelli et al., 2013). However, behavioral studies have supported the presence of sequence learning impairments in RD (Lum et al., 2013), although with considerable variability in how these are expressed. Notably, impairments seem most reliable in children, the stage at which impairment may have the most profound impact on reading ability. In adults, sequence learning impairments may be more task-dependent (Lum et al., 2013). In the present study, overlap between implicit sequence learning and RD hyperactivation was largely found in children, consistent with Lum et al’s meta-analysis of the behavioral literature. Neural differences between RD and typical readers during sequence learning have been found in adults (Menghini et al., 2006; Nicolson et al., 1999), but this has not been examined in children. Impairments in these implicit learning processes may have consequences for acquiring orthographic-phonological mappings when learning to read, and implicit learning performance predicts later reading fluency in a second language orthography (Frost, Siegelman, Narkiss, & Afek, 2013). Although our results do not strongly support a shared neural basis for RD and sequence learning processes, this may well reflect the nature of the reading and phonological tasks included in the RD meta-analysis. These tasks, even in children, are likely to rely on orthographic-phonological mappings that have already been learned and are no longer dependent on implicit sequence learning processes. Regions of hyperactivation in the bilateral caudate are also adjacent to regions of decreased fractional anisotropy (FA) and mean diffusivity in RD (Steinbrink et al., 2008), which may indicate convergent changes in the function and structural connectivity of the striatum in RD, potentially having broad consequences in reading, sequence learning and other domains.
4.2 RD and articulation
Of the processes we examined, articulation was most clearly associated with hyperactivation in RD within children, based on reverse inference maps, while this association was threshold-dependent in adults. The articulatory RI map overlapped with regions of the left precentral gyrus, left IFG/insula and right caudate, potentially consistent with suggestions that there is an increased reliance on articulatory coding in RD (Richlan et al., 2009; B. A. Shaywitz et al., 2002) The means through which articulatory processing could compensate for phonological deficits in RD has not been clearly formulated in the RD literature, particularly in the context of the complex relationship between reading, phonological processing and articulation. Shaywitz et al. (2002) may provide the clearest, if speculative suggestion, that overt movement of the articulators may facilitate developing sound awareness as children learn to read. However, articulatory processing may be more subtly engaged during reading and phonological processing. Without the need to endorse a specific model of lexical access, we consider the general framework of direct (orthography->semantics) access to meaning for skilled readers and familiar words, and indirect (through grapheme-phoneme assembly) access for pseudowords and low frequency words (e.g. Heim et al., 2009; Katz & Feldman, 1983).
A classic body of research (c.f. Besner, 1987) finds that articulatory suppression affects phonological processing during reading, under many conditions. Suppression effects on phonological processing are evident for items that are read, but not heard (Peterson & Johnson, 1971) suggesting articulation may be particularly relevant when orthographic information is phonologically decoded. In typical readers, orthographic similarity between words increases false alarms during rhyme and homophony judgments, supporting a priority for orthographic information (Tree, Longmore, & Besner, 2011) The effect of orthographic similarity was amplified under articulatory suppression for homophone, but not rhyme, judgments. This pattern suggests that articulatory suppression disrupts the alternative grapheme->phoneme pathway. A similar conclusion is supported in sentence comprehension by Coltheart, Avons, & Trollope (1990) RD readers have increased reliance on sublexical analysis (c.f. Martens & de Jong, 2006), and increased covert articulation relative to typical readers is likely to accompany this. In other words, hyperactivation associated with articulation in RD may reflect alternative paths for obtaining meaning from print.
Phonological recoding during reading is also important for learning orthographic mappings (de Jong, Bitter, van Setten, & Marinus, 2009) and this process partially relies on articulatory processing and subvocal articulation (Kyte & Johnson, 2006). RD individuals, with impaired phonological processing, may, in general, increase their reliance on articulatory codes during phonological processing. Thus increased, compensatory, articulatory processing may be found in RD relative to typical readers, even in cases where both groups would be expected to largely rely on sublexical analysis (e.g., rhyming or pseudoword reading). Our finding that RD hyperactivation regions are more strongly associated with articulatory processing in children than adults supports this perspective. Children are still developing grapheme-phoneme mappings and rely more heavily on sublexical analysis than adults (Waters, Seidenberg, & Bruck, 1984). By adulthood, the reliance on sublexical analysis and articulatory processing may be diminished by experience, even in poor readers.
4.2.2 Distinguishing articulation and phonology
Articulatory processing is likely to be engaged during phonological processing (and vice versa), making it difficult to unambiguously consider regions of RD hyperactivation as being more strongly related to one of these processes. In an effort to avoid meta-analytic maps that reflect a mixture of both processes, we constructed Neurosynth meta-analytic maps for each of these terms that excluded the other (e.g. selecting studies with a high frequency of ‘articulation’, but not of ‘phonology’). However, the effectiveness of this approach is dependent on the perspective and approaches used in individual studies. For example, a study could focus on articulatory aspects of a production task and be included in the articulatory meta-analysis, yet also involve a phonological manipulation inherent to the production task. Phonological studies may, by hypothesis, also involve covert articulatory processing. Although such studies may not explicitly control for articulatory processing, the reported results are typically the result of a comparison between conditions of varying phonological or linguistic complexity, which may remove articulatory contributions to some degree.
Most critically, our approach removes potentially highly informative papers that may attempt to explicitly dissociate phonological and articulatory processing. To address this, we reviewed excluded papers (i.e. those containing both ‘articulatory’ and ‘phonological’ with high frequency) and identified two as particularly relevant to distinguishing these processes. (Chen & Desmond, 2005) employed a modified Sternberg paradigm to contrast parametrically varied working memory load (i.e. phonological and articulatory rehearsal) against matched levels of covert articulation without memory demands. Their results suggest a distinction between a frontal-cerebellar articulatory system and parietal-cerebellar phonological memory system. Notably, with respect to hyperactivation in RD, the cerebellar components of these systems are anatomically distinguished, with superior cerebellar regions (VI and Crus I, a region of hyperactivity in RD) associated with articulation.
Park, Iverson, & Park (2011) compared brain activation during overt phoneme production across three levels of articulatory complexity (e.g. [ti i]-[ti ye]-[ti ɯi]). Regions sensitive to increasing complexity included the insula/IFG, left SMA, inferior and superior portions of the cerebellum and, for high complexity, the left pre/post central gyrus and IPL. Although there was an associated increase in phonetic complexity across stimulus levels, phonological complexity was relatively consistent across difficulty levels, so this study is suggestive of regions closely linked to articulation.
In addition to these studies, (Riecker et al., 2005)—not included in the Neurosynth database—implicated several regions in articulatory processing, but not phonological perception, by manipulating syllable repetition rate vs. passive listening at corresponding rates. Notably, Riecker et al. identified regions of the bilateral cerebellum, putamen/thalamus and precentral gyrus as sensitive to repetition rate, regions that also appear in the articulatory, but not phonological, Neurosynth maps. Other regions identified by Riecker et al. appear in both articulatory and phonological maps (cingulate) or not at all (frontal pole). Thus, while there is a high degree overlap between the phonological and articulatory Neurosynth maps, the convergence between regions uniquely in the articulatory map and those implicated in articulation by Riecker et al and other studies suggests that our approach has produced partially separable maps for these two processes. In addition, the cerebellar activations associated with articulation in both Riecker et al. and Chen & Desmond’s findings are consistent with those found in the RD hyperactive analysis, supporting the interpretation of hyperactivation in RD as reflecting articulatory processes.
4.3 Limitations
Our use of meta-analytic techniques to identify convergence between RD hyperactivation regions and regions involved in articulation, phonology and implicit learning is a novel approach to further understanding how patterns of hyperactivation may contribute to reading ability in RD or be related to other impairments seen in RD. Although our analysis lends support to claims that hyperactivation indicates compensatory articulatory processing in terms of likely function of these regions, it is important to note that the necessary link between performance and hyperactivation in these regions has not been established. Our analysis also assumes that brain-function relations are similar in both RD and typical readers (who presumably provide the majority of data available in Neurosynth). Thus, we cannot address the possibility of neural rewiring—that regions of hyperactivation have been recruited for cognitive processes in novel ways that may be unique to RD.
Neural reorganization of fronto-striatal hyperactivation regions may be accompanied by differences between RD and typical readers in functional or structural connectivity or morphological differences. Although regions of RD-related hypoactivation co-localize with regions of decreased grey matter volume (GMV) in RD (e.g. Linkersdörfer et al., 2012), there is less evidence for convergence between GMV changes in hyperactivation regions. In our analysis, the hyperactivation in the right cerebellum closely overlapped a grey matter cluster that was found to discriminate RD and typical readers (Pernet, Poline, Demonet, & Rousselet, 2009). Pernet et al. found that grey matter volume in this region fell within a narrow range in typical readers and identified two subgroups of RD associated with either above or below typical GMV, with the high GMV subgroup having better phonological ability. This may indicate a compensatory restructuring of the cerebellum in some RD individuals, perhaps to support articulatory processing, that is associated with both GMV increase and increased task-related BOLD signal.
In terms of functional connectivity, Richards & Berninger (2008) found increased functional connectivity in RD between a left IFG seed and portions of the left precentral gyrus, which overlap regions of hyperactivation reported here. Following explicit training in decoding and grapheme-phonome mappings, this functional connectivity normalized and showed no significant difference from connectivity in typical readers. This finding supports the interpretation of precentral hyperactivation in RD as compensatory. Studies of resting state functional connectivity in RD using seeds placed in typical reading regions (e.g. Koyama et al. 2011, 2010; Schurz et al., 2014), have not identified functional connectivity differences between these regions and the regions of hyperactivation we identified. In addition, hyperactivation regions have not been linked to reading in a meta-analysis of typical adult readers (Bolger, Perfetti, & Schneider, 2005). This may indicate that hyperactivation regions are part of additional functional networks that are not well integrated into the canonical reading system.
4.4 Conclusion
Additional studies that directly examine the relationship between in-scanner task performance and hyperactivation are needed to support neural compensation claims. In addition to establishing an activation-performance link, future studies should also directly examine access to articulatory coding in RD. If hyperactivation does reflect compensatory articulatory processing in RD, this may extend to differential effects in RD and typical readers in tasks that prime articulatory coding (e.g. Klein et al., 2014). Specifically, RD subjects may show increased articulatory priming effects even in lexical decision tasks as a result of increased reliance on articulatory coding during reading.
In summary, we provide the first quantitative analyses of convergence between hyperactive regions in RD and regions related to possible compensatory and primary deficit processes in RD. Our analyses are consistent with the view that articulatory processes may be associated with hyperactivation in RD, however this association is much weaker than the link between phonological processing and regions of hypoactivation in RD. Further studies are needed to understand the role of hyperactivation in RD, particularly whether hyperactivation reflects largely articulatory processes or a mixture of other processes, such as cognitive control, and whether hyperactivation is indeed compensatory.
Figure 9.
Comparison between cortical and cerebellar regions involved in sequence learning (blue), identified using reverse inference with the Neurosynth database and cortical regions of increased activation in RD relative to typical readers (red) based on a manual meta-analysis of RD studies, with overlap in green.
Highlights.
We review interpretations of increased fronto-striatal activity in reading disorder
Convergence between hyperactive brain regions and regions supporting articulation
Current literature does not provide consistent support for compensation hypotheses
Acknowledgments
FH was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants K23HD054720 (PI: F. Hoeft), R01HD067254 (PI: L. Cutting, Vanderbilt U), R01HD065794 (PI: K. Pugh, Haskins Labs), P01HD001994 (PI: J. Rueckl, Haskins Labs), Flora Family Foundation, UCSF Academic Senate Award, and the Extraordinary Brain Series of the Dyslexia Foundation. RH was partially supported by a UCSF Catalyst Award and the UCSF Digital Health Research Projects Award. FR was supported by the Austrian Science Fund (FWF P 23916-B18 and P 25799-B23) and the Austrian Agency for International Cooperation in Education and Research (OeAD PL 11/2015).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullaev YG. Neuronal activity of human caudate nucleus and prefrontal cortex in cognitive tasks. Behavioural Brain Research. 1998;97(1–2):159–177. doi: 10.1016/s0166-4328(98)00037-0. http://doi.org/10.1016/S0166-4328(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Abdullaev YG, Melnichuk KV. Cognitive operations in the human caudate nucleus. Neuroscience Letters. 1997;234(2–3):151–155. doi: 10.1016/s0304-3940(97)00680-0. http://doi.org/10.1016/S0304-3940(97)00680-0. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends in Neurosciences. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. http://doi.org/10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- Bach S, Brandeis D, Hofstetter C, Martin E, Richardson U, Brem S. Early emergence of deviant frontal fMRI activity for phonological processes in poor beginning readers. NeuroImage. 2010;53(2):682–693. doi: 10.1016/j.neuroimage.2010.06.039. http://doi.org/10.1016/j.neuroimage.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Barquero LA, Davis N, Cutting LE. Neuroimaging of reading intervention: A systematic review and activation likelihood estimate meta-analysis. PloS One. 2014;9(1):e83668. doi: 10.1371/journal.pone.0083668. http://doi.org/10.1371/journal.pone.0083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besner D. Phonology, lexical access in reading, and articulatory suppression: A critical review. The Quarterly Journal of Experimental Psychology Section A. 1987;39(908165841):467–478. http://doi.org/10.1080/14640748708401799. [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, Hoeft F. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. NeuroImage. 2012;59(3):3021–3032. doi: 10.1016/j.neuroimage.2011.10.024. http://doi.org/10.1016/j.neuroimage.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo J, Peltier SJ, Noll DC, Seidler RD. Age differences in symbolic representations of motor sequence learning. Neuroscience Letters. 2011;504(1):68–72. doi: 10.1016/j.neulet.2011.08.060. http://doi.org/10.1016/j.neulet.2011.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boemio A, Fromm S, Braun A, Poeppel D. Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nature Neuroscience. 2005;8(3):389–395. doi: 10.1038/nn1409. http://doi.org/10.1038/nn1409. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Human Brain Mapping. 2005;25(1):92–104. doi: 10.1002/hbm.20124. http://doi.org/10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Research. 2007;1133(1):136–144. doi: 10.1016/j.brainres.2006.11.074. http://doi.org/10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Current Opinion in Neurobiology. 1997;7(2):157–163. doi: 10.1016/s0959-4388(97)80003-7. http://doi.org/10.1016/S0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. http://doi.org/10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Christiansen MH, Chater N. Language as shaped by the brain. The Behavioral and Brain Sciences. 2008;31(5) doi: 10.1017/S0140525X08004998. 489-508–58. http://doi.org/10.1017/S0140525X08004998. [DOI] [PubMed] [Google Scholar]
- Christiansen MH, Conway CM, Onnis L. Similar neural correlates for language and sequential learning: Evidence from event-related brain potentials. Language and Cognitive Processes. 2012;27(2):231–256. doi: 10.1080/01690965.2011.606666. http://doi.org/10.1080/01690965.2011.606666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22(1):466–476. doi: 10.1016/j.neuroimage.2003.12.049. http://doi.org/10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Coltheart V, Avons SE, Trollope J. Articulatory suppression and phonological codes in reading for meaning. The Quarterly Journal of Experimental Psychology Section A. 1990;42(908165841):375–399. http://doi.org/10.1080/14640749008401227. [Google Scholar]
- Conway CM, Pisoni DB. Neurocognitive basis of implicit learning of sequential structure and its relation to language processing. Annals of the New York Academy of Sciences. 2008;1145:113–131. doi: 10.1196/annals.1416.009. http://doi.org/10.1196/annals.1416.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope N, Eicher JD, Meng H, Gibson CJ, Hager K, Lacadie C, Gruen JR. Variants in the DYX2 locus are associated with altered brain activation in reading-related brain regions in subjects with reading disability. NeuroImage. 2012;63(1):148–156. doi: 10.1016/j.neuroimage.2012.06.037. http://doi.org/10.1016/j.neuroimage.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, Briggs RW. Left and right basal ganglia and frontal activity during language generation: contributions to lexical, semantic, and phonological processes. Journal of the International Neuropsychological Society : JINS. 2003;9(7):1061–1077. doi: 10.1017/S135561770397010X. http://doi.org/10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Danelli L, Berlingeri M, Bottini G, Ferri F, Vacchi L, Sberna M, Paulesu E. Neural intersections of the phonological, visual magnocellular and motor/cerebellar systems in normal readers: Implications for imaging studies on dyslexia. Human Brain Mapping. 2013;34:2669–2687. doi: 10.1002/hbm.22098. http://doi.org/10.1002/hbm.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SaRB, Veltman DJ, Raaijmakers JGW, Jonker C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiology of Aging. 2003;24(7):1013–1019. doi: 10.1016/s0197-4580(03)00030-7. http://doi.org/10.1016/S0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Deacon SH, Conrad N, Pacton S. A statistical learning perspective on children’s learning about graphotactic and morphological regularities in spelling. Canadian Psychology/Psychologie Canadienne. 2008;49(2):118–124. http://doi.org/10.1037/0708-5591.49.2.118. [Google Scholar]
- de Jong PF, Bitter DJL, van Setten M, Marinus E. Does phonological recoding occur during silent reading, and is it necessary for orthographic learning? Journal of Experimental Child Psychology. 2009;104(3):267–282. doi: 10.1016/j.jecp.2009.06.002. http://doi.org/10.1016/j.jecp.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Dennis Na, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiology of Aging. 2011;32(12):2318, e17–e30. doi: 10.1016/j.neurobiolaging.2010.04.004. http://doi.org/10.1016/j.neurobiolaging.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R, Siegelman N, Narkiss A, Afek L. What predicts successful literacy acquisition in a second language? Psychological Science. 2013;24(7):1243–1252. doi: 10.1177/0956797612472207. http://doi.org/10.1177/0956797612472207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay Y, Holt LL. Incidental learning of sound categories is impaired in developmental dyslexia. Cortex. 2015;73:131–143. doi: 10.1016/j.cortex.2015.08.008. http://doi.org/10.1016/j.cortex.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser E, Notter M, Gabrieli JDE. A corticostriatal neural system enhances auditory perception through temporal context processing. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2012;32(18):6177–6182. doi: 10.1523/JNEUROSCI.5153-11.2012. http://doi.org/10.1523/JNEUROSCI.5153-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A-L, Neumann K, Bachoud-Levi A-C, von Gudenberg AW, Euler HA, Lanfermann H, Preibisch C. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain and Language. 2008;104(2):190–199. doi: 10.1016/j.bandl.2007.04.005. http://doi.org/10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Giraud A-L, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nature Neuroscience. 2012;15(4):511–517. doi: 10.1038/nn.3063. http://doi.org/10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Human Brain Mapping. 2009;30(2):392–402. doi: 10.1002/hbm.20512. http://doi.org/10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG. Neuroimaging of working memory dysfunction and the dilemma with brain reorganization hypotheses. Journal of the International Neuropsychological Society : JINS. 2008;14(4):526–534. doi: 10.1017/S1355617708080788. http://doi.org/10.1017/S1355617708080788. [DOI] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Gabrieli JDE. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. http://doi.org/10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, Gabrieli JDE. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. http://doi.org/10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JHJ, Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44(7):1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. article. http://doi.org/10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Ingvar M, af Trampe P, Greitz T, Eriksson L, Stone-Elander S, von Euler C. Residual differences in language processing in compensated dyslexics revealed in simple word reading tasks. Brain and Language. 2002;83(2):249–267. doi: 10.1016/s0093-934x(02)00055-x. [DOI] [PubMed] [Google Scholar]
- Karuza EA, Newport EL, Aslin RN, Starling SJ, Tivarus ME, Bavelier D. The neural correlates of statistical learning in a word segmentation task: An fMRI study. Brain and Language. 2013;127:46–54. doi: 10.1016/j.bandl.2012.11.007. http://doi.org/10.1016/j.bandl.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L, Feldman LB. Relation between pronunciation and recognition of printed words in deep and shallow orthographies. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1983 doi: 10.1037//0278-7393.9.1.157. http://doi.org/10.1037/0278-7393.9.1.157. [DOI] [PubMed] [Google Scholar]
- Klein M, Grainger J, Wheat KL, Millman RE, Simpson MIG, Hansen PC, Cornelissen PL. Early activity in Broca’s area during reading reflects fast access to articulatory codes from print. Cerebral Cortex (New York, N.Y. : 1991) 2014;7:1715–1723. doi: 10.1093/cercor/bht350. http://doi.org/10.1093/cercor/bht350. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. The Journal of Neuroscience. 2011;31(23):8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. http://doi.org/10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. Reading networks at rest. Cerebral Cortex. 2010;20(11):2549–2559. doi: 10.1093/cercor/bhq005. http://doi.org/10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 2006;44(10):1822–1832. doi: 10.1016/j.neuropsychologia.2006.03.010. http://doi.org/10.1016/j.neuropsychologia.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kyte CS, Johnson CJ. The role of phonological recoding in orthographic learning. Journal of Experimental Child Psychology. 2006;93(2):166–185. doi: 10.1016/j.jecp.2005.09.003. http://doi.org/10.1016/j.jecp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. http://doi.org/10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, D’esposito M. Sequence learning in pianists and nonpianists: an fMRI study of motor expertise. Cognitive, Affective & Behavioral Neuroscience. 2006;6(3):246–259. doi: 10.3758/cabn.6.3.246. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17243360. [DOI] [PubMed] [Google Scholar]
- Lehongre K, Morillon B, Giraud A-L, Ramus F. Impaired auditory sampling in dyslexia: further evidence from combined fMRI and EEG. Frontiers in Human Neuroscience. 2013;7:454. doi: 10.3389/fnhum.2013.00454. http://doi.org/10.3389/fnhum.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehongre K, Ramus F, Villiermet N, Schwartz D, Giraud A-L. Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron. 2011;72(6):1080–1090. doi: 10.1016/j.neuron.2011.11.002. http://doi.org/10.1016/j.neuron.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: An ALE meta-analysis. PLoS ONE. 2012;7(8):e43122. doi: 10.1371/journal.pone.0043122. http://doi.org/10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J, Ullman MT, Conti-Ramsden G. Procedural learning is impaired in dyslexia: Evidence from a meta-analysis of serial reaction time studies. Research in Developmental Disabilities. 2013;34(10):3460–3476. doi: 10.1016/j.ridd.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences. 2008;1145(1):237–259. doi: 10.1196/annals.1416.024. http://doi.org/10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Martens VEG, de Jong PF. The effect of word length on lexical decision in dyslexic and normal reading children. Brain and Language. 2006;98(2):140–149. doi: 10.1016/j.bandl.2006.04.003. http://doi.org/10.1016/j.bandl.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Martis B, Wright CI, McMullin KG, Shin LM, Rauch SL. Functional magnetic resonance imaging evidence for a lack of striatal dysfunction during implicit sequence learning in individuals with animal phobia. American Journal of Psychiatry. 2004;161(1):67–71. doi: 10.1176/appi.ajp.161.1.67. [DOI] [PubMed] [Google Scholar]
- McNorgan C, Alvarez A, Bhullar A, Gayda J, Booth JR. Prediction of reading skill several years later depends on age and brain region: implications for developmental models of reading. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2011;31(26):9641–9648. doi: 10.1523/JNEUROSCI.0334-11.2011. http://doi.org/10.1523/JNEUROSCI.0334-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Gelernter J, Gruen JR, Calhoun VD, Meng H, Cope NA, Pearlson GD. Polymorphism of DCDC2 reveals differences in cortical morphology of healthy individuals-A preliminary voxel based morphometry study. Brain Imaging and Behavior. 2008;2(1):21–26. doi: 10.1007/s11682-007-9012-1. http://doi.org/10.1007/s11682-007-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: An fMRI study. NeuroImage. 2006;33(4):1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. http://doi.org/10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JDE, Just MA. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: A longitudinal study of neuroplasticity. Neuropsychologia. 2008;46(10):2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. http://doi.org/10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain and Cognition. 2000;42(2):183–200. doi: 10.1006/brcg.1999.1099. http://doi.org/10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Morillon B, Lehongre K, Frackowiak RSJ, Ducorps A, Kleinschmidt A, Poeppel D, Giraud A-L. Neurophysiological origin of human brain asymmetry for speech and language. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18688–18693. doi: 10.1073/pnas.1007189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. The Lancet. 1999 doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Brookes RL, Needle J. Procedural learning and dyslexia. Dyslexia. 2010;16(3):194–212. doi: 10.1002/dys.408. http://doi.org/10.1002/dys.408. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research. Brain Research Reviews. 1995;20(1):91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Park H, Iverson GK, Park HJ. Neural correlates in the processing of phoneme-level complexity in vowel production. Brain and Language. 2011;119(3):158–166. doi: 10.1016/j.bandl.2011.05.010. http://doi.org/10.1016/j.bandl.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neuroscience. 2009;10(1):67. doi: 10.1186/1471-2202-10-67. http://doi.org/10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LR, Johnson ST. Some effects of minimizing articulation on short-term retention. Journal of Verbal Learning and Verbal Behavior. 1971;10(4):346–354. http://doi.org/10.1016/S0022-5371(71)80033-6. [Google Scholar]
- Pinel P, Fauchereau F, Moreno A, Barbot A, Lathrop M, Zelenika D, Dehaene S. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2012;32(3):817–825. doi: 10.1523/JNEUROSCI.5996-10.2012. http://doi.org/10.1523/JNEUROSCI.5996-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante E, Gomez R, Gerken L. Sensitivity to word order cues by normal and language/learning disabled adults. Journal of Communication Disorders. 2002;35(5):453–462. doi: 10.1016/s0021-9924(02)00094-1. http://doi.org/10.1016/S0021-9924(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72(5):692–697. doi: 10.1016/j.neuron.2011.11.001. http://doi.org/10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. http://doi.org/10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry : The Journal of the Association of European Psychiatrists. 2012;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001. http://doi.org/10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua J, Rubia K, Canales-Rodríguez EJ, Pomarol-Clotet E, Fusar-Poli P, Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Frontiers in Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00013. http://doi.org/10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. NeuroImage. 2011;57(3):742–749. doi: 10.1016/j.neuroimage.2010.09.055. http://doi.org/10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Berninger VW. Abnormal fMRI connectivity in children with dyslexia during a phoneme task: Before but not after treatment. Journal of Neurolinguistics. 2008;21(4):294–304. doi: 10.1016/j.jneuroling.2007.07.002. http://doi.org/10.1016/j.jneuroling.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. http://doi.org/10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56(3):1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. http://doi.org/10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64(4):700–706. doi: 10.1212/01.WNL.0000152156.90779.89. http://doi.org/10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, Bäckman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. NeuroImage. 2010;50(3):1303–1312. doi: 10.1016/j.neuroimage.2010.01.015. http://doi.org/10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PT. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage. 2012;60(1):117–129. doi: 10.1016/j.neuroimage.2011.12.010. http://doi.org/10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara A, Caravolas M. Statistical learning of novel graphotactic constraints in children and adults. Journal of Experimental Child Psychology. 2014;121:137–155. doi: 10.1016/j.jecp.2013.11.009. http://doi.org/10.1016/j.jecp.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Schurz M, Wimmer H, Richlan F, Ludersdorfer P, Klackl J, Kronbichler M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cerebral Cortex (New York, N.Y. : 1991. 2015;25(10):3502–3514. doi: 10.1093/cercor/bhu184. http://doi.org/10.1093/cercor/bhu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Purushotham a, Kim S-G, Uğurbil K, Willingham D, Ashe J. Cerebellum activation associated with performance change but not motor learning. Science (New York, N.Y.) 2002;296:5575, 2043–2046. doi: 10.1126/science.1068524. http://doi.org/10.1126/science.1068524. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Lyon GR. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Biological Psychiatry. 2005;57(11):1301–1309. doi: 10.1016/j.biopsych.2005.01.043. http://doi.org/10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Müller H-P, Juengling FD, Kassubek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46(13):3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. http://doi.org/10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Perani D. Basal ganglia and language: phonology modulates dopaminergic release. Neuroreport. 2005;16(4):397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Tree JJ, Longmore C, Besner D. Orthography, phonology, short-term memory and the effects of concurrent articulation on rhyme and homophony judgements. Acta Psychologica. 2011;136(1):11–19. doi: 10.1016/j.actpsy.2010.08.009. http://doi.org/10.1016/j.actpsy.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Treiman R, Kessler B. Spelling as statistical learning: Using consonantal context to spell vowels. Journal of Educational Psychology. 2006;98(3):642–652. http://doi.org/10.1037/0022-0663.98.3.642. [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 2004;92(1–2):231–270. doi: 10.1016/j.cognition.2003.10.008. http://doi.org/10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pullman MY. A compensatory role for declarative memory in neurodevelopmental disorders. Neuroscience & Biobehavioral Reviews. 2015;51:205–222. doi: 10.1016/j.neubiorev.2015.01.008. http://doi.org/10.1016/j.neubiorev.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. http://doi.org/10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Waters GS, Seidenberg MS, Bruck M. Children’s and adults’ use of spelling-sound information in three reading tasks. Memory & Cognition. 1984;12(3):293–305. doi: 10.3758/bf03197678. http://doi.org/10.3758/BF03197678. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Schurz M, Sturm D, Richlan F, Klackl J, Kronbichler M, Ladurner G. A dual-route perspective on poor reading in a regular orthography: an fMRI study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46(10):1284–1298. doi: 10.1016/j.cortex.2010.06.004. http://doi.org/10.1016/j.cortex.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Tibbo P, Purdon SE. An fMRI investigation of procedural learning in unaffected siblings of individuals with schizophrenia. Schizophrenia Research. 2007;94(1–3):306–316. doi: 10.1016/j.schres.2007.04.026. http://doi.org/10.1016/j.schres.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Yang J, Li P. Brain networks of explicit and implicit learning. PloS One. 2012;7(8):e42993. doi: 10.1371/journal.pone.0042993. http://doi.org/10.1371/journal.pone.0042993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. http://doi.org/10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedkova L, Woodward ND, Harding I, Tibbo PG, Purdon SE. Procedural learning in schizophrenia investigated with functional magnetic resonance imaging. Schizophrenia Research. 2006;88(1–3):198–207. doi: 10.1016/j.schres.2006.06.039. http://doi.org/10.1016/j.schres.2006.06.039. [DOI] [PubMed] [Google Scholar]