Abstract
The prokaryotic ubiquitin-like protein (Pup) based proteasomal system in the pathogen Mycobacterium tuberculosis (Mtb) is essential for its survival in a mammalian host. The Pup ligase enzyme, PafA, conjugates Pup to a suite of proteins targeted for proteasomal degradation, and is necessary for persistent infection by Mtb. We report the design and application of fluorescent probes toward elucidating the mechanisms of Pup and substrate recognition by PafA. Our studies reveal that the C-terminal 26-amino acid sequence of Pup is the minimal ligase recognition motif in Mtb. Specific hydrophobic residues within this sequence that are known to be important for Pup interaction with proteasomes are also critical for the activation of Pup by PafA.
Keywords: Prokaryotic ubiquitin-like protein, fluorescent probes, Pup ligase, minimal sequence
Mycobacterium tuberculosis (Mtb) is a human pathogen and the main causative agent of tuberculosis (TB). Globally, tuberculosis and HIV are the leading causes of death due to infection and about one-third of the world’s population carries the opportunistic tuberculosis bacilli.1 The emergence of extensively drug-resistant forms of TB in recent years seriously challenges current therapeutic strategies and signals the need to identify and characterize new drug targets in Mtb.2 Initial investigations of the mycobacterial 20S proteasome suggest that along with transcription and translation, inhibition of bacterial protein degradation may also prove to be an effective antibacterial strategy. Indeed, several studies have elucidated the necessity of functional proteasomes for Mtb resistance to oxidative challenge in vitro3 and for persistent infection in mice.4,5 Furthermore, the treatment of non-replicating Mtb with proteasome-inhibiting oxathiazol-2-one compounds was shown to be bactericidal in laboratory cultures.6 These results have firmly established the proteasomal system as a suitable target for Mtb inhibition. The recent discovery of a ubiquitin-like protein modification pathway in Mtb that tags proteins for degradation by 20S proteasomes has revealed several additional targets for the inhibition of protein turnover.7,8 The prokaryotic ubiquitin-like protein (Pup) is a short 64-amino acid polypeptide that is conjugated to proteasomal substrates by the proteasome accessory factor A (PafA) ligase.9 Given the high degree of conservation of Pup and PafA homologues within the actinomycete class of bacteria and the essential role of PafA in maintaining Mtb infection8 we are interested in elucidating the mechanisms of Pup and substrate recognition by PafA. This understanding may be parlayed into the design of rational inhibitors of PafA and will also shed light on the evolutionary origins of the complex protein ubiquitylation machinery in higher organisms.
Proteasomal substrates in eukaryotes are typically tagged for degradation by conjugation of a lysine side-chain ε-amine with the C-terminus of the protein ubiquitin. Ubiquitylation is catalyzed by the E1–E3 family of ligases and begins with E1-catalyzed activation of the α-carboxylate of the C-terminal Gly in ubiquitin as a ubiquitin-adenylate.10 The activated ubiquitin is transferred to a side-chain thiol in the E1 and subsequently to an E2 ligase. In some instances, the E2-ubiquitin thioester further participates in trans-thioesterification with a side-chain thiol in an E3 ligase. Finally, the ubiquitin-C-terminal thioester undergoes nucleophilic attack by a lysine side-chain or, in some instances the N-terminus of protein substrates, to form a stable amide linkage. In contrast with ubiquitin, the small protein Pup is ribosomally synthesized with a C-terminal Gln residue that is deamidated by the deamidase of Pup (Dop) to produce a C-terminal Glu (Figure 1a).9 The newly formed γ-carboxylate is conjugated with lysine side-chain ε-amines in substrates (Figure 1b, top row). Another key difference between Pup and ubiquitin is that the build-up of polymeric chains of Pup is not observed on protein substrates, unlike poly-ubiquitin chains that are typically observed on eukaryotic targets and are required for their proteosomal degradation.
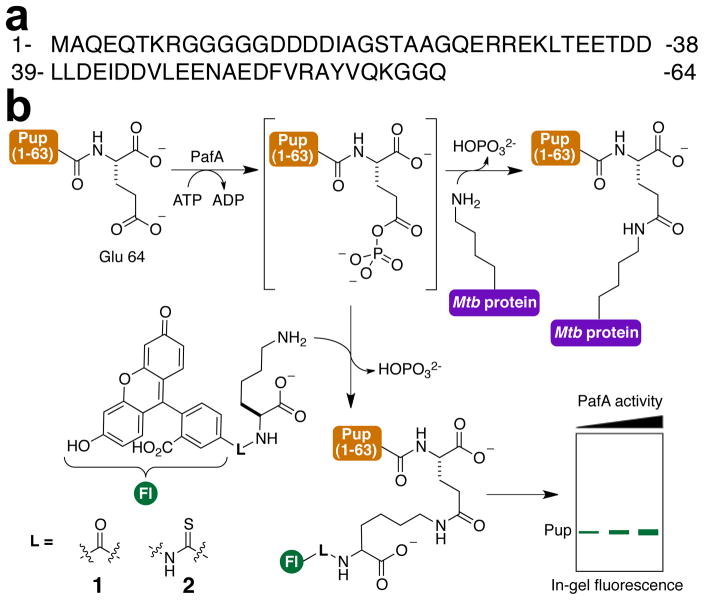
Figure 1. Mechanism based probes of PafA activity.
(a) Sequence of the Pup polypeptide. (b) Scheme depicting PupE conjugation with 1 and 2 catalyzed by PafA.
Initial mechanistic studies of PafA established a key difference from the family of ubiquitin ligases in that PafA utilizes the terminal phosphate of ATP to activate Pup by generating a γ-carboxy phosphoanhydride at its C-terminal glutamate (Figure 1b).11 This high-energy intermediate species, which is observable by MALDI-TOF MS, is proposed to undergo subsequent nucleophilic attack by the lysine side-chain. Several proteomic studies have demonstrated that ~130 different proteins in Mtb and the closely related Mycobacterium smegmatis are pupylated at internal lysine sites.12–15 However, there is no known consensus sequence or conserved structure at the sites of pupylation and substrates are involved in many different pathways including metabolism, cell wall and membrane biosynthesis, transcription regulation and even proteolysis.16 The structure of a PafA homologue from the actinomycete Corynebacterium glutamicum (Cglu) was recently reported,17 but the absence of bound Pup or a protein substrate precludes knowledge of the precise mechanisms underlying PafA function.
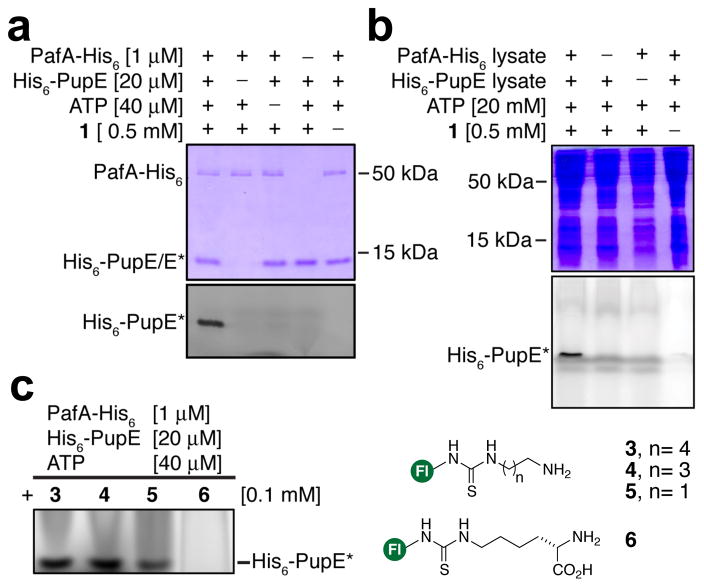
As a first step in our mechanistic studies we sought to identify a PafA-specific chemical probe that allows (1) direct and quantitative visualization of its activity, and (2) is modular and therefore amenable to structure-activity studies of PafA specificity. In this regard, we noted that the PafA-catalyzed reaction is similar to transglutaminase-mediated amide bond formation between glutamine and lysine side-chains. Several fluorescent amines have been employed as substrates for transglutaminases18,19 and we envisioned a similar approach for PafA. Therefore, we first tested Lys conjugated with fluorescein-5-carboxylic acid at its α-amine as a substrate for pupylation with purified N-terminally His6-tagged and deamidated Pup (His6-PupE) and C-terminally His6-tagged PafA (Figures 1b and S1–S2). The amide-linked probe, 1, and thiourea linked analogue, 2, were both robust substrates for pupylation in vitro, which was easily detected by in-gel fluorescence following SDS-PAGE (Figure 2a and S3). The nature of linkage between the fluorophore and amine in our probes did not influence the efficiency of labeling, which facilitated the rapid synthesis of multiple probes. Moreover, these probes were highly specific for PafA activity, which permitted the detection of Pup in complex protein mixtures (Figure 2b). Although the response was not saturated, PafA activity was readily detected with probe concentrations as low as 5 μM (Figure S4). The lack of signal saturation is consistent with the previously reported KM of ~23 mM for free Lys11 and demonstrates the advantage of employing a highly sensitive fluorescent readout. With a modular and specific probe in hand we first focused on understanding the substrate specificity of PafA. In this regard, we noted that a significant difference between pupylation and ubiquitylation is that ubiquitin may be attached to lysine side-chain ε-amines as well as at the N-terminal α-amine of proteins.20 However, pupylation has only been observed at lysine side-chains.15,21 In order to understand the basis for the amine-specificity of PafA, we synthesized a series of fluorescent amine substrates, 3–5 (Figure 2c), where the distance of the amine from the bulky fluorophore was varied. Activity assays with PafA and Pup revealed that longer chain amines were better substrates and that the extent of pupylation decreased with shortening distance of the amine from the bulky fluorophore (Figures 2c and S5). Pupylation was, however, only observed at amines attached to primary carbons. Thus, the α-amine of Nε-FITC-L-Lys, 6, was not significantly pupylated (Figures 2c and S5). The free amino acids Ala and Gly were also tested in pupylation assays followed by liquid chromatography and electrospray ionization mass spectrometry (LC-ESI-MS). Similar to our results with 6, Ala was not measurably pupylated. However, free Gly in which the α-amine is attached to a primary carbon was pupylated by PafA (Figure S6). Importantly, PafA specificity for the ε-amine of Lys did not change at higher pHs (Figure S7) suggesting that selectivity arises from structural requirements in the ligase and not from the chemical step. The recently reported structure of Cglu PafA with bound ADP and Mg2+ revealed a shallow open surface where substrates may bind (Figure S8).17 Our results indicate that the reactive phospho-anhydride of Pup is accessible to linear amines, and that branching at the carbon adjacent to the nucleophilic amine may interfere with the favored Bürgi–Dunitz angle of nucleophilic attack.22 A subset of ubiquitin E2 ligases have also been shown to inherently select against amines attached to secondary carbons23 and it is likely that the selectivity observed in PafA is an evolutionary precursor to that observed in E2 ligases.
Figure 2. Application of fluorescent substrates to probe PafA activity.
(a) 15% SDS-PAGE showing labeling of His6-PupE by probe 1 in an ATP and PafA-His6 dependent manner. Top-gel is coomassie stained while the bottom gel slice shows in-gel fluorescence. (b) 15% SDS-PAGE showing the specificity of 1 for labeling His6-PupE in cellular lysates. Top gel is coomassie stained while the bottom gel shows in-gel fluorescence. (c) Ingel fluorescence of His6-PupE modified by the indicated probes 3–6. His6-PupE* indicates the probe-labeled fluorescent peptide. Fl, denotes fluo-rescein.
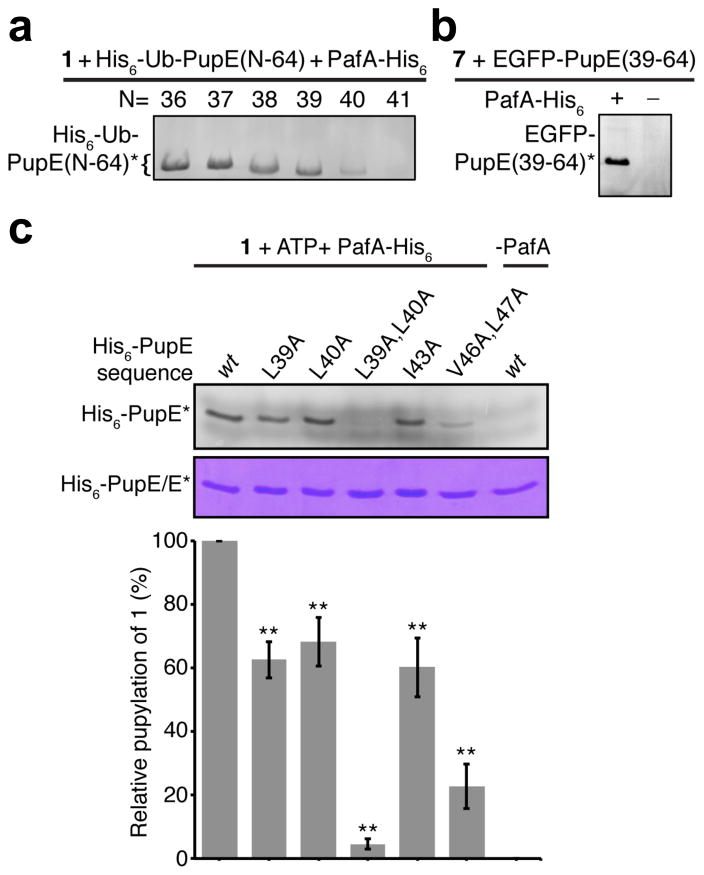
We next turned our attention to investigating how Mtb PafA binds Pup. Unlike the well-folded ubiquitin, Pup is disorderd in buffered solutions, with no structural motifs seen in circular dichroism spectra and minimal helicity inferred from NMR experiments.24–26 Darwin and co-workers have demonstrated that an N-terminally truncated Pup(31–64) peptide is sufficient for pupylation in vivo.27 Given the strong conservation of the Pup C-terminal sequence in actinobacteria (Figure S9), we wondered what minimal sequence of Pup is sufficient for PafA-mediated conjugation with substrates. Therefore, we tested the C-terminal 34 amino acids of Pup and N-terminal truncants thereof in in vitro pupylation assays with PafA and probe 1. In order to facilitate soluble expression of the short fragments (M.W.< 4,000 Da) and to simplify visualization of the assay products by SDS-PAGE, an N-terminal ubiquitin (Ub) fusion tag was employed.28 We first confirmed that full-length His6-Ub-PupE(1–64) was efficiently labeled with probe 1 at Glu64 by PafA (Figure S10), and then proceeded to test Pup fragments in pupylation assays. Starting from His6-Ub-PupE(31–64) we truncated five N-terminal amino acids at a time. This allowed us to narrow down the residues critical for pupylation to a region between amino acids 36 to 41 in Pup (Figure S10). With this knowledge in hand, four additional Pup N-terminal truncants starting from His6-Ub-PupE(37–64) were prepared and tested with PafA and probe 1 (Figure 3a). Due to the high specificity of probe 1, each of the His6-Ub-Pup fragments could be expressed in E. coli and directly tested in cell lysates containing PafA and probe 1 without additional purification steps. Interestingly, we noted a gradual and significant decrease in labeling by 1 when the Pup sequence was truncated from His6-Ub-PupE(39–64) to His6-Ub-PupE(41–64) (Figure 3a). Furthermore, assays with synthetic PupE(40–64) and PupE(41–64) peptides followed by LC-ESI-MS analysis confirmed that the former is labeled to some degree while the latter is not (Figure S11). These results demonstrated that the C-terminal 26 amino acid PupE(39–64) sequence is the minimal recognition motif for PafA in Mtb.
Figure 3. Identification of a minimal sequence and residues in Pup critical for pupylation.
(a) In-gel fluorescence from 15% SDS-PAGE gel showing PafA-His6 catalyzed labeling of the indicated His6-Ub-PupE fragments by probe 1 in cellular lysates. (b) In-gel fluorescence from 15% SDS-PAGE gel showing the PafA-His6 dependent labeling of EGFP-PupE(39–64) by Nα-TAMRA-L-Lys (7) in cellular lysates. (c) 15% SDS-PAGE gel of PafA mediated labeling of wild-type (wt) and mutant full-length His6-PupE polypeptides by probe 1. Top-gel slice shows in-gel fluorescence of labeled proteins and the bottom gel slice shows coomassie staining as a loading control. The bar-graph below shows quantitation of in-gel fluorescence of each mutant relative to wt Pup and is normalized for protein loading. Error bars, s.d. (n= 3), Student’s two-tailed t-test, ** P < 0.05. Asterisks indicate the probe-labeled fluorescent peptides/proteins in each gel.
An additional and surprising result from our experiments was the observation that the addition of the small protein ubiquitin to the N-terminus of the minimal recognition motif, PupE(39–64), did not inhibit PafA activity. In order to test this motif as a general tag for protein labeling by PafA, we appended it to the C-terminus of the ~27 kDa enhanced green fluorescent protein (EGFP). To our delight the EGFP-PupE(39–64) fusion was labeled by Lys conjugated at its α-amine with tetramethylrhodamine (Nα-TAMRA-L-Lys, 7) in a PafA dependent manner (Figure 3b). Protein labeling strategies for cell-surface imaging have been extensively developed with short peptide modifying enzymes such as the mammalian transglutaminases19 and Sfp phosphopantetheinyl transferase.29 The ability to genetically append the PupE(39–64) sequence to proteins without inhibiting PafA function is promising for its application as an orthogonal cell-surface protein labeling strategy that will complement and expand the repertoire of currently available techniques.
Having identified the PupE(39–64) sequence as the minimal recognition motif for PafA we turned our attention to identifying residues within this sequence that are critical for pupylation. We first focused on Leu39 and Leu40 that are present in the minimal sequence but are absent in the poor substrate PupE(41–64) (Figure 1a and Figure 3a). Site-directed Ala mutagenesis of Leu39 and Leu40 in full-length His6-PupE, either individually or in combination, showed dramatic effects on the extent of labeling with probe 1, with the His6-PupE(L39A,L40A) double mutant showing the least labeling (Figure S12–S13 and 3c). This indicates a key role for Leu39 and Leu40 in catalysis by PafA. In order to understand the role of these residues in the first chemical step- the phosphorylation of PupE by PafA- we employed a previously reported TLC-based radioassay that measures the production of α-32P-ADP from α-32P-ATP.11 Assays with PafA, α-32P-ATP, and a tag-less version of Pup, G-PupE(1–64), or the minimal sequence G-PupE(39–64) (Figure S14) revealed that both substrates were phosphorylated at similar rates (Figure S15). On the other hand, the rate of phosphorylation of the G-PupE(L39A,L40A) mutant was indistinguishable from the background hydrolysis of ATP by PafA in the absence of Pup (Figure S15). This indicates that Leu39 and Leu40 are critical for recognition and efficient phosphorylation of PupE by PafA. Several hydrophobic residues in PupE, including Leu39 and Leu40, were also proposed to make key van der Waals contacts with the coiled-coil domain of the proteasomal ATPase Mpa.30 Although PafA does not share significant homology with Mpa, our results clearly showed that both proteins engage overlapping regions of PupE (Mpa interacts with residues 21–51 and PafA interacts with residues 39–64 of PupE). Therefore, we wondered if the same hydrophobic residues in PupE, namely Leu39, Leu40, Ile43, Val46 and Leu47 play roles in both Mpa binding and PafA activity. Site-directed Ala mutations of each of the hydrophobic residues also led to decreased labeling with probe 1 (Figure 3c and S16) indicating that they are important for PafA activity. However, the most dramatic effect was seen for the His6-PupE(L39A,L40A) double mutant. Thus, PupE employs the same hydrophobic residues to bind Mpa and PafA, with Leu39 and Leu40 contributing most significantly to the latter interaction.
In conclusion, we have demonstrated the successful design and application of specific and modular fluorescent probes toward understanding the mechanism of function of PafA, the sole Pup ligase in Mtb. Our studies have revealed mechanistic similarities between PafA and ubiquitin E2 ligases and provide a rationale for the selective pupylation of Lys side-chain ε-amines. The fluorescent probe 1 was also used to identify residues 39–64 in PupE as the minimal recognition motif for PafA. Surprisingly, this minimal motif could be appended to the C-terminus of small and large proteins, such as ubiquitin and EGFP, respectively, without compromising PafA activity. Finally, similar to the ubiquitin system, where a hydrophobic patch composed of Leu8, Ile44 and Val70 is a docking site for many ubiquitin-binding proteins,31 Leu39 and Leu40 are part of a docking site for Pup binding to both Mpa and PafA. This is the first identification of a common hydrophobic protein interaction surface on Pup that is similar to what is already known for ubiquitin in eukaryotes. Studies are currently underway to identify the structure of the PupE(39–64) peptide when bound to PafA, which will guide the future design of specific inhibitors of the Pup-PafA interaction.
Supplementary Material
Acknowledgments
This work was generously supported by the Department of Chemistry at the University of Washington, Seattle. We are grateful to Professors Michael H. Gelb and Rachel E. Klevit for insightful suggestions. We thank Professor Wilfred A. van der Donk, Dr. Leah M. Miller and members of the Chatterjee lab for critical reading of the manuscript. Denis Smirnov is thankful for a Mary Gates Research Scholarship and a UW Amgen Scholarship. Finally, we thank Professors Dustin J. Maly and Pradipsinh K. Rathod for allowing us to use their phosphorimaging equipment.
Footnotes
The authors declare no competing financial interest.
Supporting figures and detailed experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Lawn SD, Zumla AI. Lancet. 2011;378:57. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 2.Zignol M, Hosseini MS, Wright A, Lambregts-van Weezenbeek C, Nunn P, Watt CJ, Williams BG, Dye C. J Infect Dis. 2006;194:479. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- 3.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. Science. 2003;302:1963. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 4.Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. Nat Med. 2007;13:1515. doi: 10.1038/nm1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandotra S, Lebron MB, Ehrt S. PLoS Pathog. 2010;6:e1001040. doi: 10.1371/journal.ppat.1001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin G, Li D, de Carvalho LPS, Deng H, Tao H, Vogt G, Wu K, Schneider J, Chidawanyika T, Warren JD, Li H, Nathan C. Nature. 2009;461:621. doi: 10.1038/nature08357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns KE, Liu WT, Boshoff HIM, Dorrestein PC, Barry CE. J Biol Chem. 2008;284:3069. doi: 10.1074/jbc.M808032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce MJ, Mintseris J, Ferreyra J, Gygi SP, Darwin KH. Science. 2008;322:1104. doi: 10.1126/science.1163885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Striebel F, Imkamp F, Sutter M, Steiner M, Mamedov A, Weber-Ban E. Nat Struct Mol Biol. 2009;16:647. doi: 10.1038/nsmb.1597. [DOI] [PubMed] [Google Scholar]
- 10.Hershko A, Ciechanover A. Annu Rev Biochem. 1998;67:425. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 11.Guth E, Thommen M, Weber-Ban E. J Biol Chem. 2011;286:4412. doi: 10.1074/jbc.M110.189282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watrous J, Burns K, Liu WT, Patel A, Hook V, Bafna V, Barry CE, Bark S, Dorrestein PC. Mol Biosyst. 2010;6:376. doi: 10.1039/b916104j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen C, Akhter Y, Jeon AHW, Schmitt-Ulms G, Meyer HE, Stefanski A, Stühler K, Wilmanns M, Song YH. Mol Syst Biol. 2010;6:386. doi: 10.1038/msb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Festa RA, McAllister F, Pearce MJ, Mintseris J, Burns KE, Gygi SP, Darwin KH. PLoS ONE. 2010;5:e8589. doi: 10.1371/journal.pone.0008589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung CW. BMC Bioinf. 2012;13:40. doi: 10.1186/1471-2105-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delley CL, Striebel F, Heydenreich FM, Ozcelik D, Weber-Ban E. J Biol Chem. 2012;287:7907. doi: 10.1074/jbc.M111.331124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozcelik D, Barandun J, Schmitz N, Sutter M, Guth E, Damberger FF, Allain FH, Ban N, Weber-Ban E. Nat Commun. 2012;3:1014. doi: 10.1038/ncomms2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternack R, Laurent HP, Ruth T, Kaiser A, Schon N, Fuchsbauer HL. Anal Biochem. 1997;249:54. doi: 10.1006/abio.1997.2139. [DOI] [PubMed] [Google Scholar]
- 19.Lin CW, Ting AY. J Am Chem Soc. 2006;128:4542. doi: 10.1021/ja0604111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciechanover A. Methods Mol Biol. 2005;301:255. doi: 10.1385/1-59259-895-1:255. [DOI] [PubMed] [Google Scholar]
- 21.Sutter M, Damberger FF, Imkamp F, Allain FH, Weber-Ban E. J Am Chem Soc. 2010;132:5610. doi: 10.1021/ja910546x. [DOI] [PubMed] [Google Scholar]
- 22.Bürgi HB, Dunitz JD, Lehn JM, Wipff G. Tetrahedron. 1974;30:1563. [Google Scholar]
- 23.Pickart CM, Rose IA. J Biol Chem. 1985;260:1573. [PubMed] [Google Scholar]
- 24.Liao S, Shang Q, Zhang X, Zhang J, Xu C, Tu X. Biochem J. 2009;422:207. doi: 10.1042/BJ20090738. [DOI] [PubMed] [Google Scholar]
- 25.Sutter M, Striebel F, Damberger FF, Allain FHT, Weber-Ban E. FEBS Lett. 2009;583:3151. doi: 10.1016/j.febslet.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Solomon WC, Kang Y, Cerda-Maira F, Darwin KH, Walters KJ. J Mol Biol. 2009;392:208. doi: 10.1016/j.jmb.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns KE, Pearce MJ, Darwin KH. J Bacteriol. 2010;192:2933. doi: 10.1128/JB.01639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt TR, Jonnalagadda S, Monia BP, Sternberg EJ, Marsh JA, Stadel JM, Ecker DJ, Crooke ST. Proc Natl Acad Sci USA. 1989;86:2540. doi: 10.1073/pnas.86.8.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin J, Lin AJ, Golan DE, Walsh CT. Nat Protocols. 2006;1:280. doi: 10.1038/nprot.2006.43. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Darwin KH, Li H. Nat Struct Mol Biol. 2010;17:1352. doi: 10.1038/nsmb.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicke L, Schubert HL, Hill CP. Nat Rev Mol Cell Biol. 2005;6:610. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.