Graphical abstract
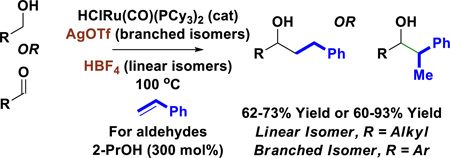
Feedstocks to Building Blocks. Transfer hydrogenative coupling of styrene with primary alcohols delivers branched or linear adducts from benzylic or aliphatic alcohols when modified by AgOTf or HBF4, respectively. Related 2-propanol mediated reductive couplings also are described.
Keywords: Homogenous Catalysis, Cross-Coupling, C-C Bond Formation, Ruthenium, Hydrogenation
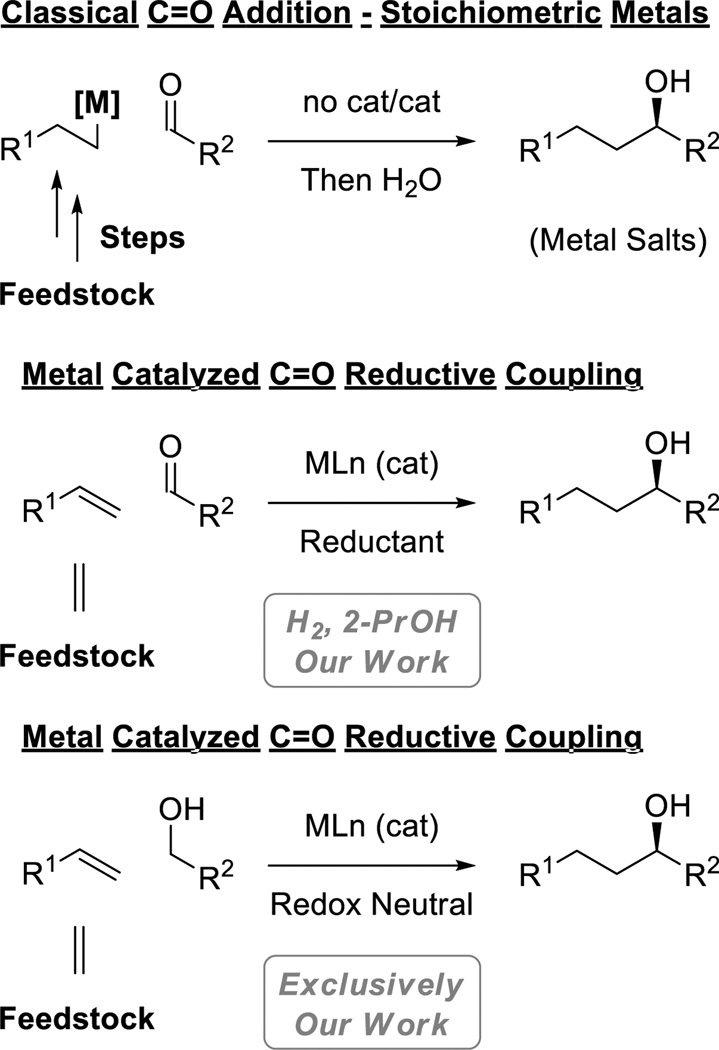
Beginning with the work of Butlerov and Grignard, the use of premetalated C-nucleophiles in carbonyl addition has opened vast volumes of chemical space and is now a longstanding cornerstone of chemical synthesis.[1,2] The catalytic reductive coupling of π-unsaturated reactants with carbonyl compounds represents an alternative to classical carbonyl addition that potentially avoids stoichiometric organometallic reagents and the issues of safety, selectivity and waste that attend their use.[3,5c] Hydroformylation,[4] the largest volume application of homogenous catalysis, is a powerful example of metal catalyzed reductive coupling that illustrates several important characteristics of a scalable process: the abilty to transform abundant chemical feedstocks in a byproduct-free manner and, less obviously, the importance of utilizing terminal reductants that are less costly than the coupling partners themselves. Accordingly, the discovery and development of transfer hydrogenative carbonyl additions wherein alcohols serve dually as reductants and carbonyl precursors represent a major focus of research in our laboratory (Figure 1).[5]
Figure 1.
Carbonyl addition using non-stabilized carbanions and their equivalents.
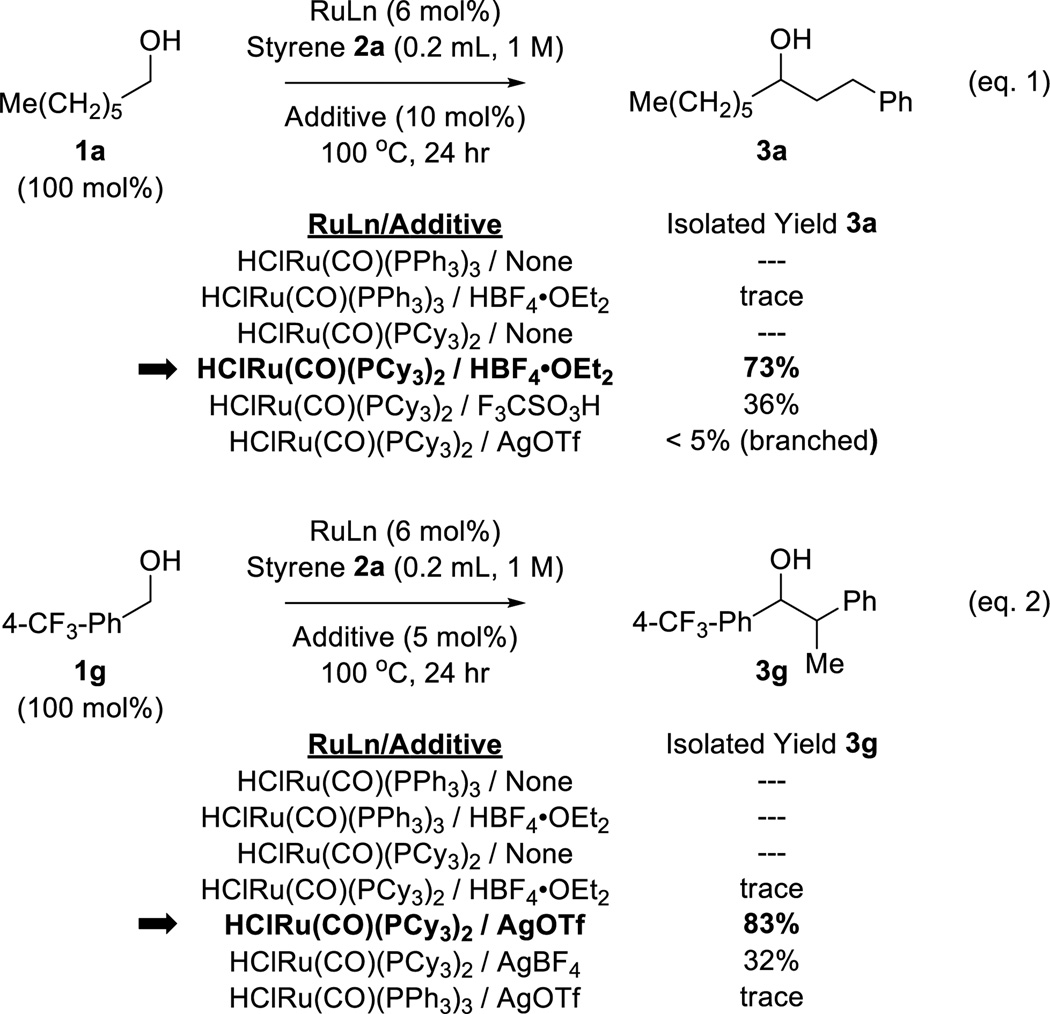
While the transfer hydrogenative coupling of primary alcohols with diverse olefin pronucleophiles have been developed (1,3-dienes[5,7] and 1,3-enynes[5,8]), related reactions of styrene, an abundant petrochemical feedstock (>25 × 106 tons/2010)[6] have proven challenging and are restricted to the use of α-hydroxy-carbonyl compounds,[9] that is, precursors to highly activated vicinal dicarbonyl compounds. We were inspired by Yi’s observation[10] that the addition of HBF4•OEt2 to the ruthenium-hydride complex HClRu(CO)(PCy3)2 dramatically enhances catalytic activity in alkene hydrogenation[10a] and hydrovinylation.[10b] As corroborated by Yi’s mechanistic studies, HBF4 opens a coordination site at ruthenium by protonating a tricyclohexylphosphine ligand.[10] In the hope that such coordinative unsaturation would unlock the transfer hydrogenative coupling of styrene with primary alcohols,[11,12] a series of experiments were conducted using the HClRu(CO)(PCy3)2/HBF4•OEt2 catalyst system (Scheme 1, eq. 1). Whereas exposure of heptanol 1a to styrene 2a in the presence of commercially available HClRu(CO)(PPh3)3 did not lead to products of C-C coupling in the absence or presence of HBF4•OEt2, HClRu(CO)(PCy3)2/HBF4•OEt2 delivered the product of C-C bond formation 3a in 73% yield as a linear single regioisomer. In the absence of HBF4•OEt2, adduct 3a was not formed. Other Brønsted acids were assayed, but were uniformly less effective. It was reasoned that cationic ruthenium(II) complexes derived from HClRu(CO)(PCy3)2 and AgOTf might also display enhanced catalytic activity due to coordinative unsaturation.[13] Hence, the coupling of 1a and 2a was attempted using HClRu(CO)(PCy3)2 in the presence of AgOTf. Here, the linear regioisomer 3a was not formed, yet a small quantity of the corresponding branched regioisomer was detected. This result supported the feasibility of optimizing a catalytic pathway to branched adducts. While aliphatic alcohols were recalcitrant partners for branch-selective coupling, the coupling of benzylic alcohol 1g with styrene 2a to form the branched adduct 3g was amenable to optimization (Scheme 1, eq. 2).
Scheme 1.
Selected optimization experiments for the ruthenium catalyzed C-C coupling of 1-heptanol 1a and benzyl alcohol 1g with styrene 2a.a
aYields are of material isolated by silica gel chromatography. See Supporting Information for further details.
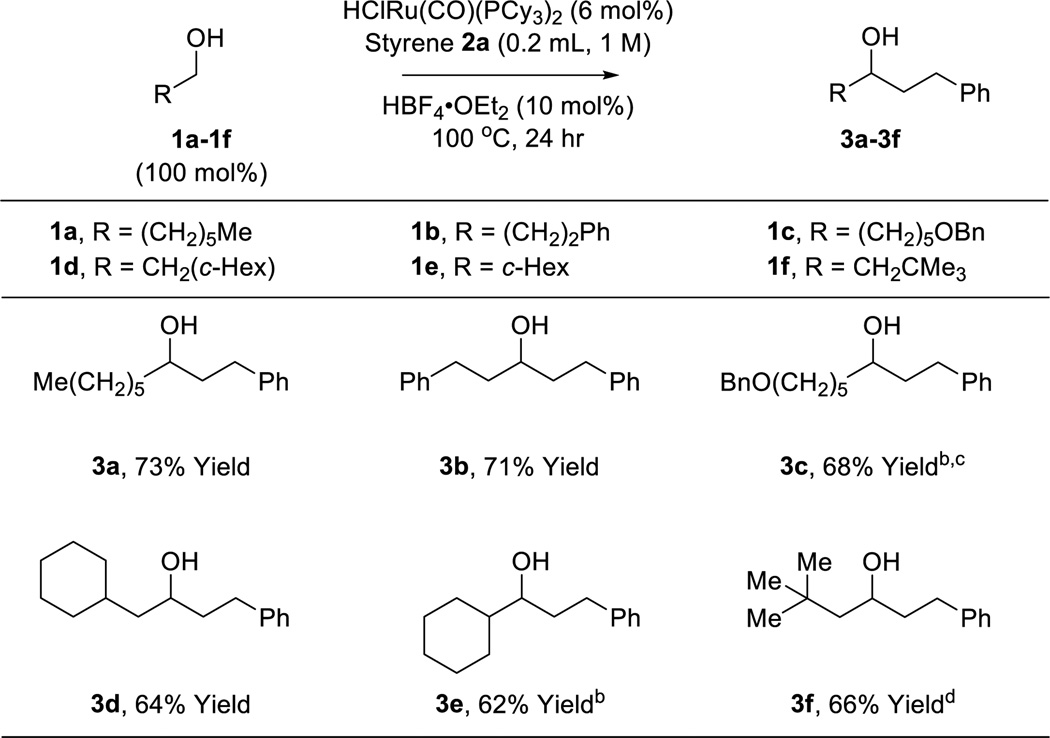
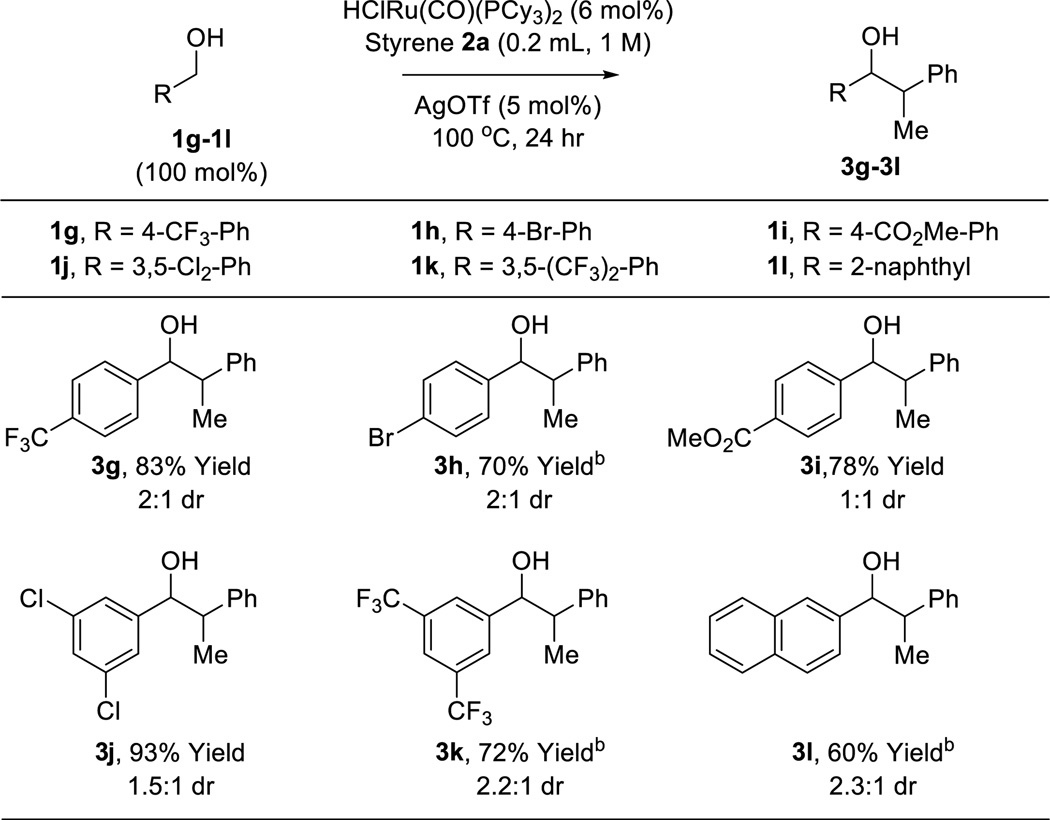
The scope of this regioselective transfer hydrogenative coupling of aliphatic and benzylic alcohols was briefly surveyed (Tables 1 and 2). The reaction of styrene 2a with aliphatic alcohols 1a–1f provided adducts 3a–3f in good yield with complete levels of linear regioselectivity (Table 1). Even alcohols with branching at the β-position, such as cyclohexyl methanol 1e, participate in C-C coupling although higher temperatures (120 °C) are required. In contrast, significantly diminished efficiencies were observed using para-substituted styrenes bearing either electron donating or electron releasing groups. The coupling of benzylic alcohols 1g–1l delivered adducts 3g–3l in good to excellent yields with complete branched regioselectivity (Table 2). Here, electron deficient benzylic alcohols were more efficient partners for coupling, perhaps due to stabilization of the σ-benzylruthenium intermediate (vida infra). Finally, beyond the redox-neutral couplings from the alcohol oxidation level, 2-propanol mediated reductive couplings of styrene 2a with alkyl- and aryl-substituted aldehydes also is possible, as illustrated by the conversion of heptanal (dehydro-1a) to the linear secondary alcohol 3a (eq. 3) and the conversion of dehydro-1g to the branched adduct 3g (eq. 4).
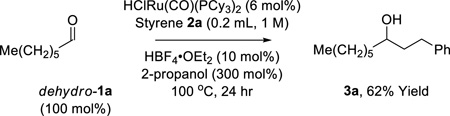 |
(eq. 3) |
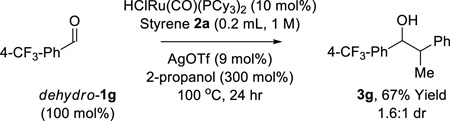 |
(eq. 4) |
Table 1.
Ruthenium catalyzed C-C coupling of aliphatic alcohols 1a–1f with styrene 2a to form secondary alcohols 3a–3f.a
Yields are of material isolated by silica gel chromatography. See Supporting Information for further details.
HClRu(CO)(PCy3)2 (10 mol%), HBF4 (15 mol%).
styrene 2a (0.4 mL), 120 °C.
2-PrOH (100 mol%).
Table 2.
Ruthenium catalyzed C-C coupling of benzylic alcohols 1g–1l with styrene 2a to form secondary alcohols 3g–3l.a
Yields are of material isolated by silica gel chromatography. See Supporting Information for further details.
HClRu(CO)(PCy3)2 (10 mol%), AgOTf (9 mol%).
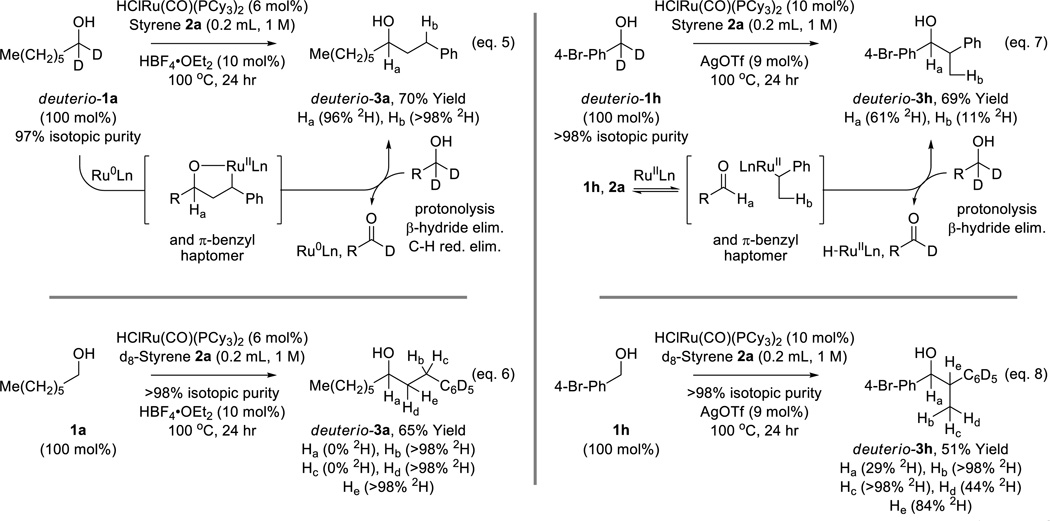
To gain insight into the catalytic mechanism, in particular the origins of linear vs branched regioselectivity, a series of deuterium labelling experiments were performed (Scheme 2). Exposure of deuterio-1a, which is deuterated at the carbinol position (97% 2H), to styrene 2a under standard conditions results in the formation of deuterio-3a (Scheme 2, eq. 5). Complete retention of deuterium at the carbinol methine (96% 2H) is accompanied by complete transfer of deuterium to the benzylic methylene (>98% 2H). These data are consistent with a catalytic mechanism involving carbonyl-styrene oxidative coupling to form an oxaruthenacycle, which upon transfer hydrogenolysis mediated by deuterio-1a delivers deuterio-3a. Here, formation of a benzylic carbon-ruthenium bond defines the regioselectivity of oxaruthenacycle formation and, hence, the linear regioselectivity of C-C coupling. Although the primary alcohol reactant deuterio-1a dehydrogenates upon oxaruthenacycle transfer hydrogenolysis, the secondary alcohol product deuterio-3a appears kinetically unreactive toward dehydrogenation, presumably due to steric effects. A related deuterium labeling experiment in which alcohol 1a is reacted with d8-styrene 2a provides a result consistent with this mechanistic interpretation (Scheme 2, eq. 6). Exposure of deuterio-1h, to styrene 2a under standard conditions for the coupling of benzylic alcohols provides deuterio-3h (Scheme 2, eq. 7). Significant loss of deuterium is observed at the carbinol methine (61% 2H) and the transfer of deuterium to the methyl hydrogen is incomplete (11% 2H). Such loss of deuterium is consistent with a mechanism involving rapid, reversible hydrogen transfer between deuterio-1h and styrene 2a to form aldehyde-benzylruthenium pairs in advance of turn-over limiting carbonyl addition. Here, hydrometalation to form a benzylic carbon-ruthenium bond defines the branched regioselectivity of C-C coupling. The related deuterium labeling experiment wherein the non-deuterated alcohol 1h is reacted with d8-styrene 2a corroborates reversible transfer of hydrogen between alcohol 1h and d8-styrene 2a (Scheme 2, eq. 8).
Scheme 2.
General catalytic pathways accounting for linear vs branched regioselectivity as corroborated by deuterium labelling studies.a
aYields are of material isolated by silica gel chromatography. Isotopic composition determined by HRMS, 1H and 2H NMR. See Supporting Information for further details.
In summary, we report the first transfer hydrogenative couplings of styrene with primary alcohols. Remarkably, the ruthenium precatalyst HClRu(CO)(PCy3)2 delivers branched or linear adducts from benzylic or aliphatic alcohols when modified by AgOTf or HBF4, respectively. As corroborated by deuterium labelling studies, linear regioselectivity stems from catalytic mechanisms involving carbonyl-styrene oxidative coupling to form oxaruthenacyclic intermediates, whereas branched regioselectivity is a consequence of pathways involving styrene hydrometalation. Ongoing studies are focused on the transfer hydrogenative coupling of alcohols with other abundant π-unsaturated feedstocks, including α-olefins.[5c]
Supplementary Material
Acknowledgments
Acknowledgment is made to the Robert A. Welch Foundation (F-0038) and the NIH (RO1-GM069445) for partial support of this research.
References
- 1.a) Butlerov AZ. Chem. Pharm. 1863;6:484–497. [Google Scholar]; b) Grignard V. Compt. Rend. 1900;130:1322–1324. [Google Scholar]
- 2.Knochel P, Molander GA. Comprehensive Organic Synthesis. 2nd. 1 and 2. Oxford: Elsevier; 2014. [Google Scholar]
- 3.Krische MJ, editor. Metal Catalyzed Reductive C–C Bond Formation, Topics in Current Chemistry. Vol. 279. Berlin Heidelberg, Germany: Springer; 2007. [Google Scholar]
- 4.For selected reviews on hydroformylation, see: Beller M, Cornils B, Frohning CD, Kohlpaintner CW. J. Mol. Catal. A. 1995;104:17–85. van Leeuwen PWNM, Claver C, editors. Rhodium Catalyzed Hydroformylation. Norwell, MA: Kluwer Academic Publishers; 2000. Breit B, Seiche W. Synthesis. 2001:1–36. Weissermel K, Arpe H-J. Industrial Organic Chemistry. 4th. Weinheim: Wiley-VCH; 2003. pp. 127–144. van Leeuwen PWNM. Homogeneous Catalysis: Understanding the Art. Dordrecht: Kluwer Academic Publishers; 2004.
- 5.Reviews: Ketcham JM, Shin I, Montgomery TP, Krische MJ. Angew. Chem. 2014;126:9294–9302. doi: 10.1002/anie.201403873. Angew. Chem., Int. Ed.2014, 53, 9142–9150. Dechert-Schmitt A-MR, Schmitt DC, Gao X, Itoh T, Krische MJ. Nat. Prod. Rep. 2014;31:504–513. doi: 10.1039/c3np70076c. Nguyen KD, Park BY, Luong T, Sato H, Garza VJ, Krische MJ. Science. 2016;351 doi: 10.1126/science.aah5133. 10.1126/science.aah5133.
- 6.Chen S-S. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc.; 2006. Styrene. [Google Scholar]
- 7.For ruthenium catalyzed transfer hydrogenative coupling of 1,3-dienes, see: Shibahara F, Bower JF, Krische MJ. J. Am. Chem. Soc. 2008;130:6338–6339. doi: 10.1021/ja801213x. Shibahara F, Bower JF, Krische MJ. J. Am. Chem. Soc. 2008;130:14120–14122. doi: 10.1021/ja805356j. Smejkal T, Han H, Breit B, Krische MJ. J. Am. Chem. Soc. 2009;131:10366–10367. doi: 10.1021/ja904124b. Zbieg JR, Moran J, Krische MJ. J. Am. Chem. Soc. 2011;133:10582–10586. doi: 10.1021/ja2046028. Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Science. 2012;336:324–327. doi: 10.1126/science.1219274. McInturff EL, Yamaguchi E, Krische MJ. J. Am. Chem. Soc. 2012;134:20628–20631. doi: 10.1021/ja311208a.
- 8.For ruthenium catalyzed transfer hydrogenative coupling of 1,3-enynes, see: Patman RL, Williams VM, Bower JF, Krische MJ. Angew. Chem. 2008;120:5298–5301. doi: 10.1002/anie.200801359. Angew. Chem. Int. Ed.2008, 47, 5220–5223. Geary LM, Leung JC, Krische MJ. Chem. Eur. J. 2012;18:16823–16827. doi: 10.1002/chem.201202446. Nguyen KD, Herkommer D, Krische MJ. J. Am. Chem. Soc. 2016;138:5238–5241. doi: 10.1021/jacs.6b02279.
- 9.For ruthenium and osmium catalyzed transfer hydrogenative coupling of α-olefins and styrenes, see: Yamaguchi E, Mowat J, Luong T, Krische MJ. Angew. Chem. 2013;125:8586–8589. doi: 10.1002/anie.201303552. Angew. Chem. Int. Ed.2013, 52, 8428–8431. Park BY, Luong T, Sato H, Krische MJ. J. Org. Chem. 2016;81:8585–8594. doi: 10.1021/acs.joc.6b01923.
- 10.a) Yi CS, Lee DW, He Z, Rheingold AL, Lam K-C, Concolino TE. Organometallics. 2000;19:2909–2915. [Google Scholar]; b) Yi CS, He Z, Lee DW. Organometallics. 2001;20:802–804. [Google Scholar]
- 11.For catalytic reductive coupling of styrene with anhydrides, see: Kokubo K, Miura M, Nomura M. Organometallics. 1995;14:4521–4524. Hong Y-T, Barchuk A, Krische MJ. Angew. Chem. 2006;118:7039–7042. Angew. Chem. Int. Ed.2006, 128, 6885–6888. Bandar JS, Ascic E, Buchwald SL. J. Am. Chem. Soc. 2016;138:5821–5834. doi: 10.1021/jacs.6b03086.
- 12.For reductive Prins reaction of electron rich alkenes, see: Zheng Y-L, Liu Y-Y, Wu Y-M, Wang Y-X, Lin Y-T, Ye M. Angew. Chem. 2016;55:6423–6426. Angew. Chem. Int. Ed.2016, 55, 6315–6318.
- 13.Sanchez RP, Jr, Connell BT. Organometallics. 2008;27:2902–2904. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.